Introduction
Pancreatic cancer is a highly aggressive and fatal
malignant digestive tumor for which the 5-year relative survival
rate is 8%. The poor prognosis of this severe disease is mainly due
to the local invasion and distant metastasis at an early stage. In
2018, 55,440 individuals were estimated to be newly diagnosed with
pancreatic cancer, which may result in more than 44,000
cancer-related deaths in the United States (1). In China, pancreatic cancer accounts
for the second leading upward trend of age-standardized mortality
rates from 2000 to 2011 (2).
Routine treatments for this severe disease include surgery,
radiation and chemotherapy. However, at present, approximately 80%
of pancreatic cancer patients who are diagnosed do not qualify for
surgical resection due to early relapse or metastatic spread of the
disease. Therefore, the exploration of risk factors and novel
effective therapeutic options are urgently needed to improve the
treatment outcome in patients with pancreatic cancer (3).
Diabetes mellitus could be both a risk factor and a
consequence of pancreatic cancer. A collaborative analysis from the
International Pancreatic Cancer Case-Control Consortium analyzed
more than 8,000 pancreatic cancer cases and confirmed that
individuals with diabetes mellitus have an almost 2-fold increased
risk of developing pancreatic cancer (4). In a Chinese retrospective cohort
study, both male and female type 2 diabetes mellitus patients had
an increased risk to develop pancreatic cancer compared with the
general population with the standardized incidence ratios of 2.973
and 2.687, respectively (5).
Diabetes mellitus improves following pancreatectomy, suggesting
that diabetes may be induced by pancreatic cancer (6). Our previous studies confirmed that a
hyperglycemic condition could promote the proliferation, invasion,
epithelial-mesenchymal transition (EMT) as well as the metastasis
of pancreatic cancer (7,8).
Epidermal growth factor (EGF) is a polypeptide that
regulates various cellular functions involving proliferation,
survival, differentiation, angiogenesis and metastasis in many
types of cancer. EGF binds with a high affinity receptor located in
the cellular membrane and stimulates rapid activation of protein
kinase activity (9). Overexpression
of epidermal growth factor receptor (EGFR) and ligands has been
shown in pancreatic ductal adenocarcinoma and pancreatic cancer
cells, which is essential for the initiation and progression of
this disease (10). In our previous
study, it was shown that high glucose could promote pancreatic
cancer cell proliferation via the induction of EGF expression and
transactivation of EGFR (11).
Curcumin, a natural polyphenol compound derived from
turmeric, has multiple biologic properties, including health
maintenance and cancer prevention (12). Curcumin exhibits its antitumor
effects via targeting multiple signaling pathways, including
mitogen-activated protein kinase (MAPK), protein kinase B (Akt) and
others (13). In a recent study, it
was indicated that curcumin plays an important role in suppressing
superoxide dismutase-induced EMT of pancreatic cancer by inhibiting
the PI-3K/Akt/NF-κB signaling pathway (14). It was also confirmed that curcumin
could restrain hypoxia-induced EMT via suppression of the hedgehog
signaling pathway in pancreatic cancer cells (15). However, whether curcumin is able to
inhibit high glucose-induced progression of pancreatic cancer and
the related mechanisms have not yet been elucidated.
In the present study, the hypothesis that curcumin
is able to inhibit high glucose-driven EGF-induced invasive and
migratory abilities in pancreatic cancer cells was tested. The
effect of curcumin on high glucose-induced activation of EGFR,
extracellular signal-regulated kinase (ERK) and Akt signaling
pathways was also investigated. In addition, the effect of curcumin
on EGF-modulated activation of EGFR, ERK and Akt, as well as the
expression of uPA and E-cadherin was demonstrated. Results from
this study suggest that curcumin is a potential anticancer agent
for the therapy of pancreatic cancer via the suppression of the
EGF/EGFR signaling pathway and its downstream signaling molecules
including ERK and Akt.
Materials and methods
Cell culture and reagents
Human BxPC-3 pancreatic cancer cell line was
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). The cells were cultured in Gibco™ DMEM (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), which contained 10% dialyzed
heat-inactivated FBS, 100 U/ml penicillin as well as 100 µg/ml
streptomycin in a humidified atmosphere of 5% CO2 at
37°C. Exponentially growing cells in complete medium were treated
with 50 ng/ml EGF, 20 µM curcumin, 10 µM LY 294002 (a PI3K
inhibitor), and/or 50 µM PD 98059 (an ERK inhibitor) in normal
culturing conditions (5.5 mM glucose) or high glucose (25 mM)
conditions for indicated time intervals according to the aim of the
experiment. Curcumin, EGF, PD 98059 and LY294002 were purchased
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Millicell® culture plate inserts for the Transwell assay
were obtained from EMD Millipore (Billerica, MA, USA). Matrigel was
purchased from BD Biosciences (Bedford, MA, USA). Primary
antibodies [dilution at 1:100 in phosphate-buffered saline
(PBS)-Tween-20] against EGF (cat. no. sc-374255), E-cadherin (cat.
no. sc-52328) and uPA (cat. no. sc-59727) were obtained from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). The anti-EGFR (cat. no.
4267), anti-phospho-EGFR (Tyr 1068, cat. no. 2234), anti-Akt (cat.
no. 9272), anti-phospho-Akt (Ser473, cat. no. 4060), anti-ERK (cat.
no. 9102) and anti-phospho-ERK (Thr202/Tyr204, cat. no. 9106) were
purchased from Cell Signaling Technology (Beverly, MA, USA). Other
reagents were purchased from common commercial sources. All drug
solutions were freshly prepared on the day of testing.
MTT proliferation assays
BxPC-3 cells were seeded in 96-well plates at the
density of 1×104 cells per well. The cells were treated
with curcumin, PD 98059 or LY 294002 in EGF or a high glucose
condition. After incubation for 24 h at 37°C, 15 µl of MTT solution
was added to each well and the cells were incubated for 4 h at
37°C. DMSO (100 µl) was then added to each well. The optical
density (OD) value at 490 nm was determined using a
spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Transwell Matrigel invasion assay
The 8.0-µm pore inserts of the Transwell chambers
were coated with 30 µl of Matrigel. After serum starvation for 24
h, BxPC-3 cells were suspended in the top chamber at a
concentration of 5×104 in DMEM containing 1% FBS in the
absence or presence of high glucose, EGF, curcumin, PD 98059 and/or
LY 294002. Simultaneously, in the lower chambers, 500 ml of DMEM
containing 20% FBS was placed. The Matrigel invasion chamber was
incubated for 48 h in a humidified tissue culture incubator. The
non-invading cells were then removed from the upper surface. After
staining with crystal violet, the stained cells on the bottom
surface were counted under an Nikon inverted microscope DIAPHOT-TMD
(Nikon, Tokyo, Japan) to test the invasion ability of the cancer
cells. Three random fields were captured at ×20 magnification
(n=3).
Wound healing assay
BxPC-3 cells (1.0×105 cells/500 µl) were
seeded in 24-well plates. A sterile pipette tip was used to produce
a wound line between the cells that grew to 90–100% confluence and
cellular debris was removed. Cells were allowed to migrate for 24
h. Images were captured at time 0 and 24 h post-wounding under a
Nikon Diaphot TMD inverted microscope (×10 magnification). The
relative distance traveled by the leading edge from 0 to 24 h was
assessed using Photoshop software (Adobe Systems, Inc., San Jose,
CA, USA) (n=5).
Real-time quantitative PCR
(qRT-PCR)
After being extracted from the BxPC-3 cells using
the Fastgen 200 RNA isolation system (Fastgen, Shanghai, China)
according to the manufacturer's protocol, the total RNA was
reverse-transcribed into cDNA using the Fermentas RevertAid™ kit
(MBI Fermentas, Burlington, ON, Canada). The primer sequences were
as follows: uPA-F,5′-TAAGAGCTGGTGTCTGATTG-3′ and uPA-R,
5′-TTGGATGAACTAGGCTAAAA-3′; E-cadherin-F,
5′-ATTCTGATTCTGCTGCTCTTG-3′ and E-cadherin-R,
5′-AGTCCTGGTCCTCTTCTCC-3′; β-actin-F,
5′-GACTTAGTTGCGTTACACCCTTTCT-3′ and β-actin-R,
5′-GAACGGTGAAGGTGACAGCAGT-3′. The PCR reactions consisted of 30 sec
at 95°C, followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec
and 72°C for 30 sec. After each qRT-PCR experiment, a dissociation
curve analysis was conducted. The relative gene expression was
calculated using the previously described 2−∆∆Cq method
(16).
Western blot analysis
Total protein from BxPC-3 cells was extracted from
cultured cells in Radio-immunorrecipitation assay (RIPA) lysis
buffer on ice for 25 min. Insoluble materials were removed by
centrifugation at 4°C with 15,000 × g for 15 min. Subsequently,
supernatants were collected and total protein concentrations were
measured using the BCA assay kit (Pierce, Rockford, IL, USA).
Clarified protein lysates (30–80 µl) from the BxPC-3 cancer cells
were electrophoretically resolved on a denaturing
SDS-polyacrylamide gel (10–12%) and electrotransferred onto
polyvinylidene difluoride (PVDF) membranes. The membranes were
initially blocked with 5% non-fat dry milk in Tris-buffered saline
(TBS) for 2 h and then probed with antibodies against E-cadherin,
uPA, EGF, EGFR, p-EGFR, Akt, p-Akt, ERK, p-ERK and β-actin
(Control). After incubation with the primary antibodies at 4°C
overnight, the membranes were hybridized with secondary goat
anti-mouse (cat. no. 7056) or goat anti-rabbit (cat. no. 7074)
antibodies at 1:1,000 dilution (Cell Signaling Technology, Beverly,
MA, USA) for 2 h at room temperature. Immunopositive bands were
developed using an enhanced chemiluminescence (ECL) detection
system (Amersham, Piscataway, NJ, USA). Immunodetection was
visualized on a Gel Doc 2000 Imaging System (Bio-Rad Laboratories).
All analyses were conducted in triplicate. The data were analyzed
as (p-protein/control)/(total protein/control).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS Inc., Chicago, IL, USA). Data are
presented as the means ± SEM of three replicate assays. Differences
between the groups were analyzed by analysis of variance [ANOVA
followed by least significant difference (LSD)]. Statistical
significance was set at P<0.05. All experiments were repeated
independently at least three times.
Results
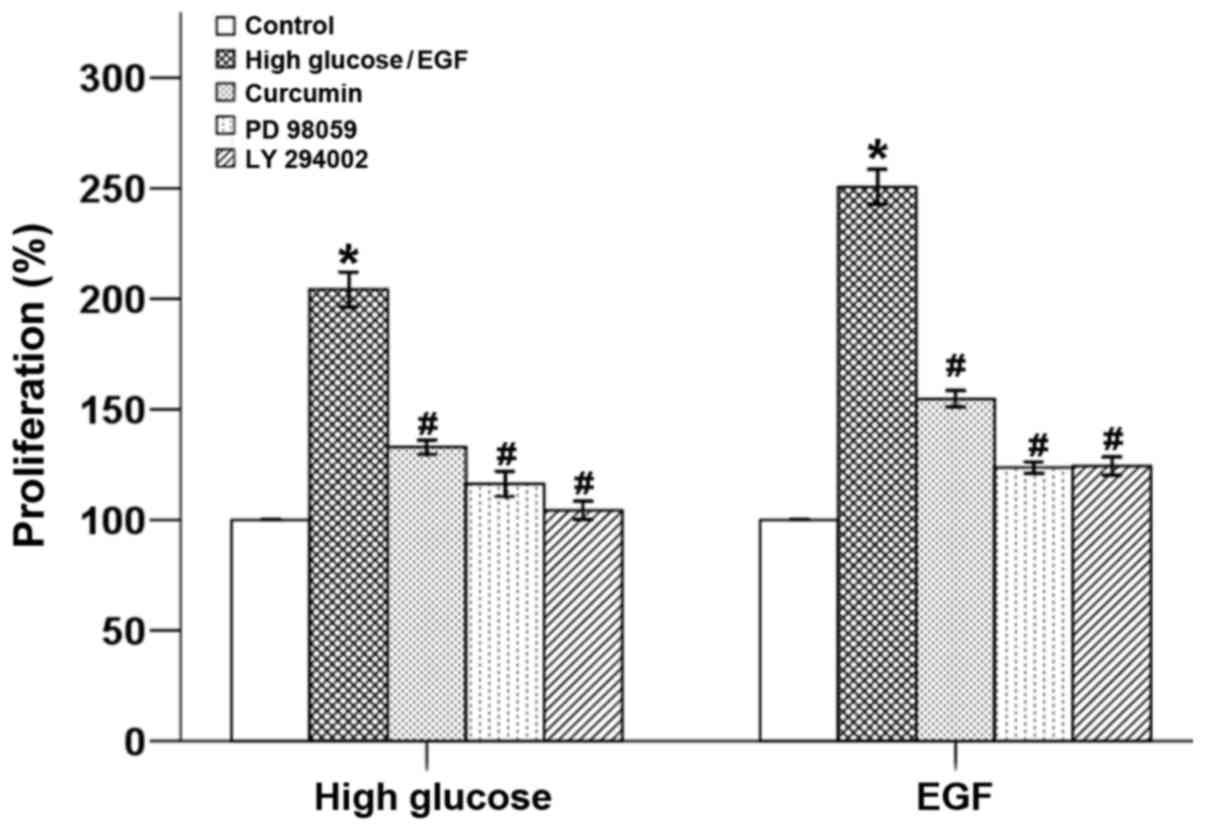
Curcumin inhibits high glucose and
EGF-induced proliferation in pancreatic cancer cells
Our previous study demonstrated that a hyperglycemic
condition promotes the proliferation of pancreatic cancer cells via
the induction of EGF expression as well as the transactivation of
EGFR (11). As our previous studies
found that curcumin shows a 50% inhibitory concentration
(IC50) of ~20 µM and this concentration exhibited no
cytotoxic effects in BxPC-3 cells, the treatment concentration of
20 µM of curcumin was used in the present experiments (17). Here, it was shown that both a high
glucose condition and EGF stimulation accelerated the growth of
BxPC-3 cells, which was able to be counter -balanced by curcumin.
In addition both PD 98059 and LY 294002 inhibited the high glucose-
and EGF-induced cellular proliferation, which indicated that the
growth of tumor cells was mediated via the ERK and Akt pathways
(Fig. 1).
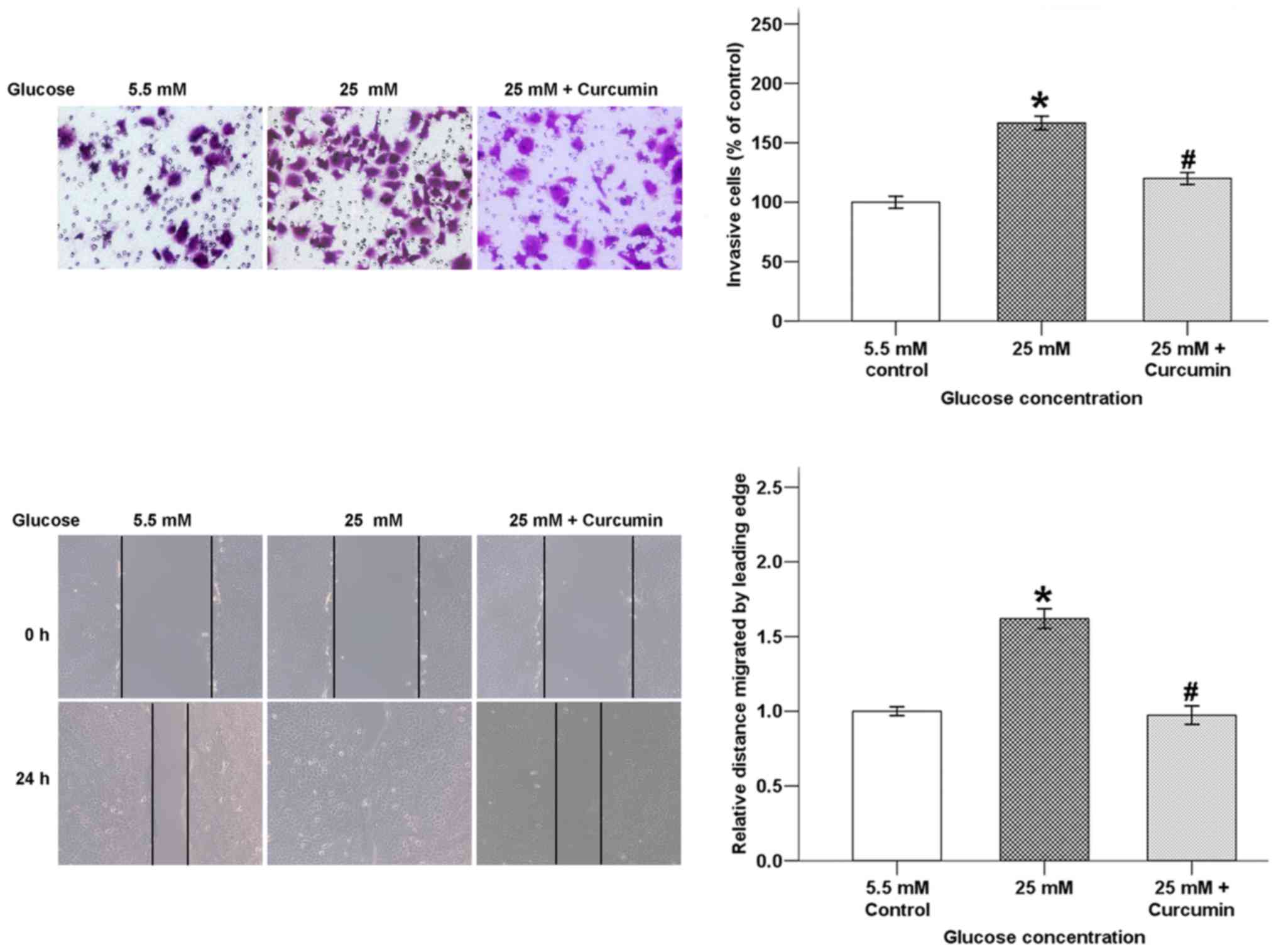
Curcumin inhibits high glucose-induced
invasive ability and wound closure of pancreatic cancer cells
Invasion and migration of cancer cells are
considered detrimental aspects in the development of tumor
metastasis. In order to confirm whether curcumin influences high
glucose-induced pancreatic cancer cell invasion and migration,
Transwell invasion and wound-healing assays were conducted. As
shown in Fig. 2, the average cell
numbers that invaded into the lower chamber were significantly
increased in the high glucose condition after incubation for 48 h
and this increase was reversed by co-treatment with curcumin. The
migratory ability of BxPC-3 cells after incubation for 24 h was
also suppressed by curcumin.
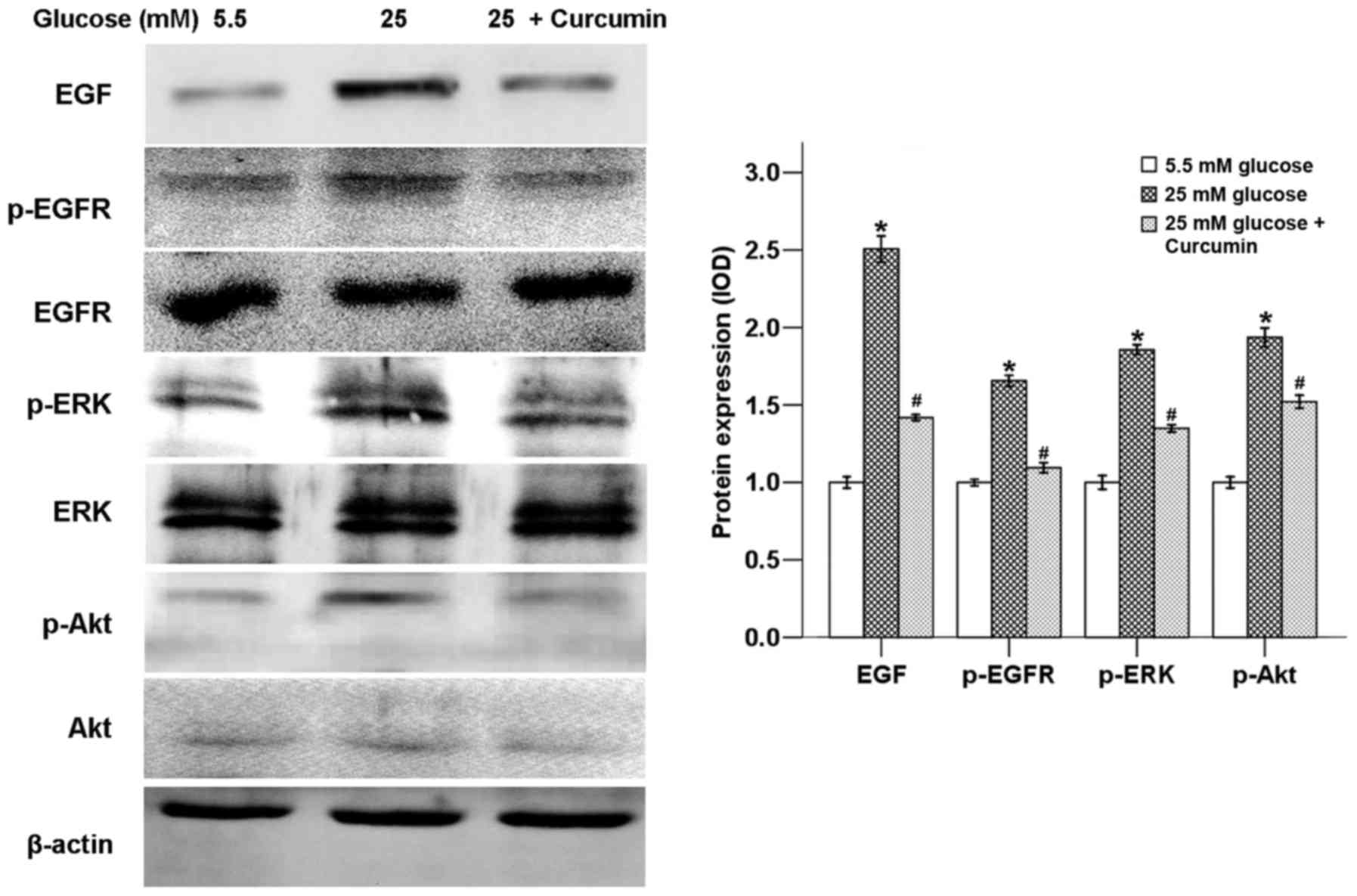
Curcumin downregulates high
glucose-induced activation of EGF/ERK and EGF/Akt pathways
In order to form metastases, cancer cells undergo
various steps, including local invasion, intravasation, survival in
the circulation, arrest at distant organ site, micrometastasis
formation and metastatic colonization (18). Different molecular pathways are
activated to pass these important steps, such as the ERK pathway
and PI-3K/Akt pathway (18). The
ERK pathway, which belongs to the mitogen-activated protein kinase
(MAPK) signaling pathway, is involved in tumor proliferation,
differentiation, migration and invasion (19). The PI3K/Akt pathway promotes cancer
development, angiogenesis as well as metastasis (20).
Our previous study confirmed that high glucose could
promote the expression of EGF and transactivation of EGFR (11). Hyperglycemic conditions could also
activate the ERK and p38 MAPK signaling pathways via the production
of H2O2 in pancreatic cancer (21). In this study, it was shown that a
high glucose condition activated ERK and Akt signaling pathways, as
both ERK and Akt phosphorylation were strongly increased. Curcumin
was able to suppress the expression of EGF and activation of EGFR,
ERK and Akt in the high glucose condition (Fig. 3).
In order to assess whether the high glucose-induced
activation of ERK and Akt signaling pathways was
EGF/EGFR-dependent, BxPC-3 cells were treated with EGF. As shown in
Fig. 4, EGF significantly promoted
the phosphorylation of EGFR, ERK and Akt, while curcumin was able
to counter-balance these effects of EGF.
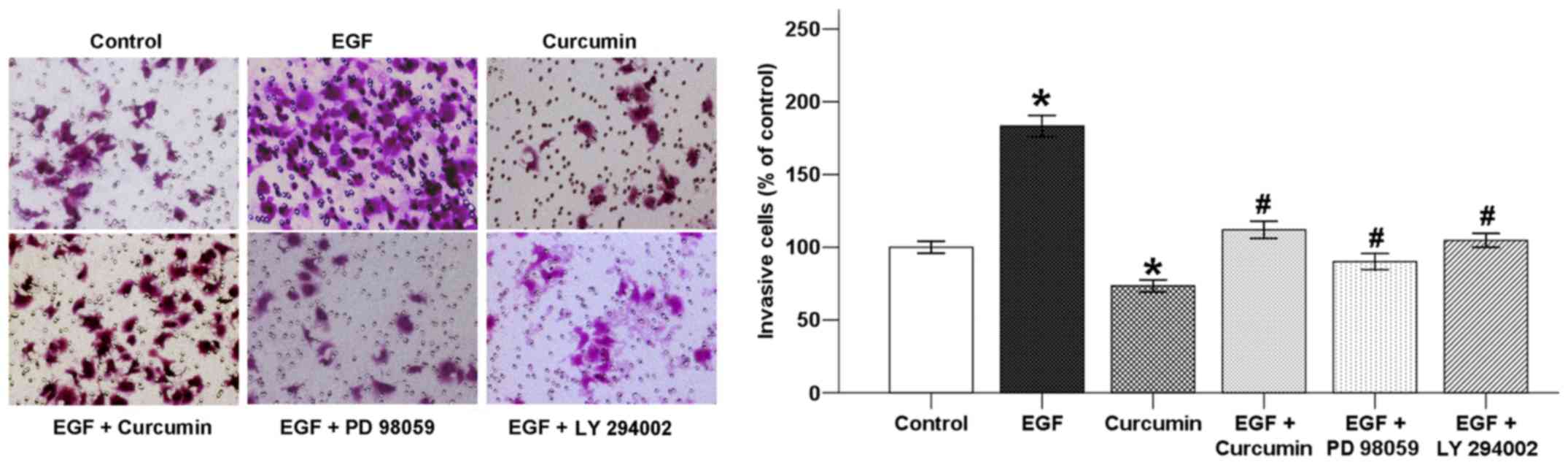
Curcumin inhibits EGF-induced invasive
ability of pancreatic cancer cells
Growth factors, such as EGF and vascular endothelial
growth factor (VEGF), are intimately related with cancer cell
migration, angiogenesis, regulation of cell adhesion and EMT
(22). Activation of ErbB ligands
and overexpression of EGFR significantly promote tumor metastasis
via chemotaxis and intravasation (23).
In order to confirm whether curcumin inhibits
EGF-induced BxPC-3 cell invasion, a Transwell invasion assay was
utilized. As shown in Fig. 5, the
number of cells that invaded into the lower chamber was increased
with the addition of EGF after incubation for 48 h. This increase
was reversed by co-treatment with curcumin. In addition, both PD
98059 and LY 294002 also suppressed the effect of EGF which
indicated that EGF-induced invasion was related to the ERK and Akt
pathways.
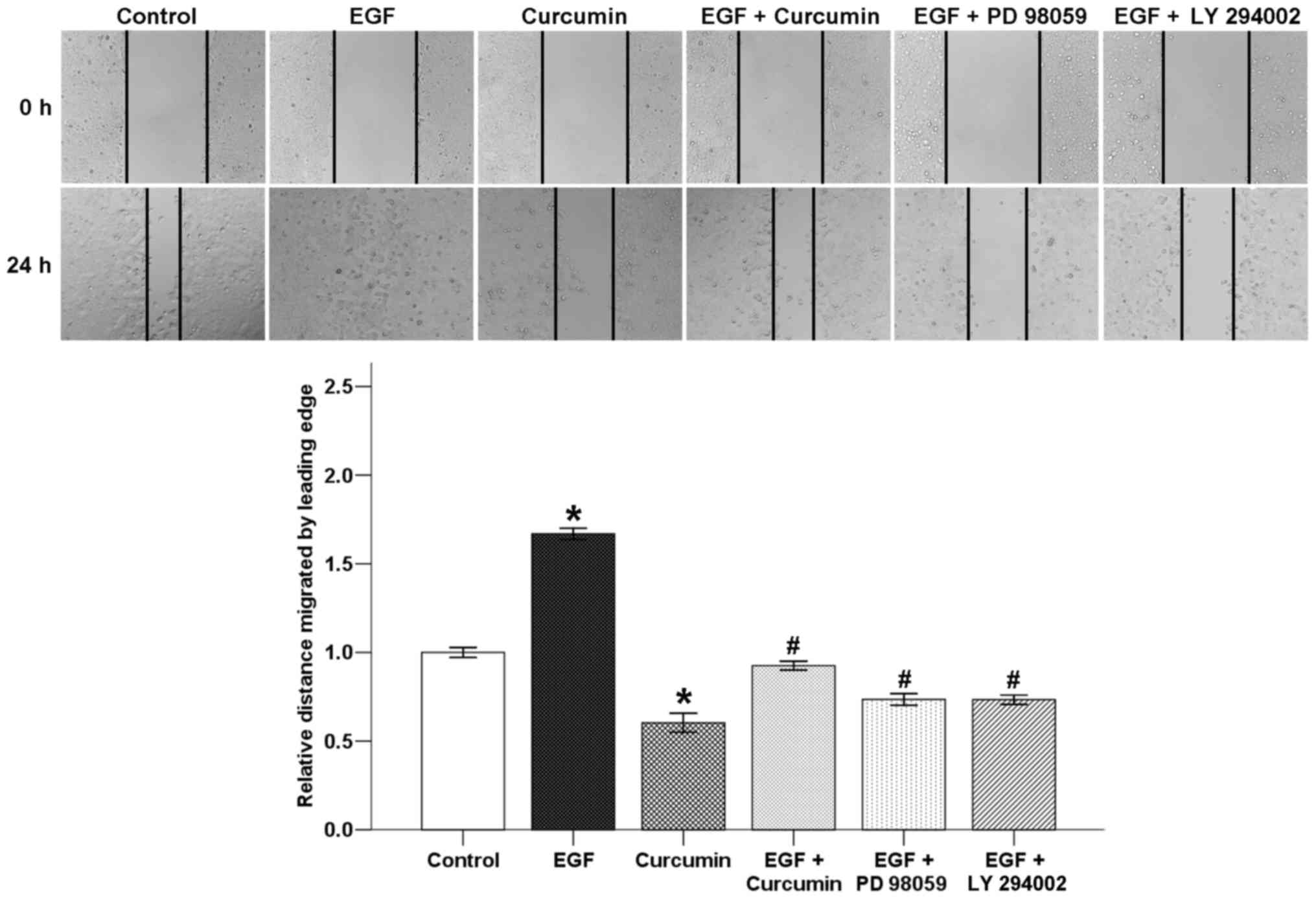
Curcumin suppresses EGF-induced wound
closure of pancreatic cancer cells
A classic wound healing assay was used to evaluate
the effect of curcumin on EGF-induced BxPC-3 cell motility. The
results showed that EGF significantly increased the migratory
ability of the BxPC-3 cells after incubation for 24 h. Curcumin
counter-balanced these effects of EGF. Both PD 98059 and LY 294002
inhibited EGF-induced wound closure of pancreatic cancer cells.
Curcumin exerts its inhibitory effect on cellular motility, which
might be attributed to the suppression of the EGF/ERK and EGF/Akt
pathways (Fig. 6).
Curcumin inhibits EGF-modulated
expression of metastatic-related factors
Our previous study demonstrated that a high glucose
condition could induce the invasive and migratory abilities of
pancreatic cancer cells by regulating metastatic-related factors,
including uPA and E-cadherin (24).
In the present study, it was shown that EGF direct stimulation also
promoted the mRNA expression of uPA and down-regulated the mRNA
level of E-cadherin. Curcumin significantly reversed these
EGF-induced effects (Fig. 7). In
addition, curcumin also reversed the EGF-modulated
metastatic-related factors at the protein level, and the trend was
consistent with the mRNA results. Taken together, these results
indicate that curcumin inhibits invasion and migration of
pancreatic cancer cells under high glucose conditions, which maybe
attributed to the EGF/ERK and EGF/Akt signaling pathways.
Discussion
Due to local recurrence, lymphnode and liver
metastases as well as peritoneal dissemination, pancreatic cancer
is one of the most aggressive malignant diseases, the hallmarks of
which includes poor outcome, short survival duration and resistance
to therapy (25). In China,
pancreatic cancer is the seventh deadliest disease with annual
mortality rates almost equal to incidence rates, of which the
5-year relative survival rate is 4.1% and the median survival time
is 3.9 months (6). The exploration
of risk factors and newer effective therapeutic options are
important to improve the treatment outcome for pancreatic cancer
patients. Diabetes mellitus can be both a risk factor and a
consequence of pancreatic cancer. Our previous study confirmed that
high glucose could promote pancreatic cancer cell proliferation via
the induction of epidermal growth factor (EGF) expression and
transactivation of the epidermal growth factor receptor (EGFR)
(11). Hyperglycemic conditions
could worsen the prognosis of pancreatic cancer by enhancing
invasive ability and promoting epithelial-mesenchymal transition
(EMT) through the production of hydrogen peroxide (8). In addition, diabetes mellitus was also
found to enhance perineural invasion in pancreatic cancer patients
and to aggravate a poor prognosis (7). It has been verified that the addition
of 5.5 and 25 mM glucose resulted in balanced osmotic pressure
inside and outside the cell membrane (21), thus we used 5.5 and 25 mM of glucose
as it has been applied worldwide in the related field as a
classical high glucose model in the cell culture. In the present
study, we focused on whether curcumin is able to suppress high
glucose-induced cancer proliferation, migratory and invasive
abilities and the underlying mechanism.
Our data showed that a high glucose condition could
promote the proliferation, migration and invasion of pancreatic
cancer cells. High glucose was not only able to increase the
expression of EGF and transactivation of EGFR, but also activate
the ERK and Akt signaling pathways. Curcumin was able to abrogate
these effects of a high glucose condition. In addition, in order to
ascertain whether the effect of a high glucose condition on
pancreatic cancer cells was EGF-dependent, we also treated BxPC-3
cells with EGF directly. Data showed that EGF treatment
significantly promote the proliferative, migratory and invasive
abilities of the BxPC-3 cells. EGF treatment also modulated the
expression levels of p-EGFR, p-ERK, p-Akt, uPA and E-cadherinin
pancreatic cancer cells. Curcumin was also able to suppress the
effects of EGF treatment. The addition of PD 98059 and LY 294002 to
the cell culture resulted in the inhibition of cellular growth and
invasion. Our results indicate that curcumin inhibits high
glucose-induced invasion and migration of pancreatic cancer cells,
which might be attributed to the EGF/ERK and EGF/Akt signaling
pathways.
EGF belongs to the EGF family, which includes EGF,
transforming growth factor α(TGFA), amphiregulin, betacellulin,
cripto, heparin-binding EGF (HB-EGF), epigen, epiregulin and
neuregulin. EGF exertsits biological effects by binding to the
receptors in an autocrine or paracrine manner. The ErbB family
consists of four receptors: EGFR (ErbB-1), ErbB-2, ErbB-3 and
ErbB-4. After binding of EGF, the four members of the family are
able to form various heterodimers, which lead to
autophosphorylation and further activatedownstream signaling
pathways, including the PI3K/Akt pathway and MAPKs, such as the ERK
pathway (9). The signaling cascades
activated by EGF/EGFR could further regulate different cellular
responses such as proliferation, survival, migration, invasion,
intravasation and metastasis (26).
The ERK MAPK signaling pathway is involved in the
regulation of several cancer cell processes, including
proliferation, differentiation, survival, cellular motility and
metastasis. Aberrant signaling drives tumor initiation and
progression. The PI3K/Akt/mTOR signaling pathway also promotes
cancer development, migration, invasion, angiogenesis and
metastasis (18). Overexpression or
mutational activation of EGFR has been found in cancer cells
leading to activation of the ERK and PI3K/Akt signaling pathways
(27). Various EGFR inhibitors have
been developed for cancer treatment, including EGFR monoclonal
antibodies (cetuximab, panitumumab) and tyrosine kinase inhibitors,
including erlotinib, gefitinib and lapatinib. These drugs have been
successfully approved for the treatment of metastatic colorectal
cancer (cetuximab and panitumumab), locally advanced, unresectable,
or metastatic pancreatic cancer (erlotinib), breast cancer
(lapatinib) and non-small cell lung cancer (gefitinib and
erlotinib) (28). In the present
study, it was found that high glucose and EGF stimulation could
activate EGFR, ERK and Akt, which further induce cancer
proliferation, migration and invasion. As a type of natural
polyphenol compound derived from turmeric, curcumin was able to
abrogate these effects leading to cancer prevention.
Accumulating evidence suggests that curcumin
inhibits cancer initiation promotion and progression through
regulation of multiple signaling pathways including EGF/EGFR,
Akt/mTOR, NF-κB, Notch as well as MAPK pathways (29). Soung and Chung (29) showed that curcumin was able to
inhibit cancer cell functions by disrupting the interaction between
integrin α6β4 and EGFR. Cheng et al (12) demonstrated that curcumin could
exhibit inhibitory effects on the proliferation, invasion and
metastasis of prostate cancer via inhibition of MMP-9 activity,
downregulation of cellular matriptase, suppression of the effects
of androgens and EGFR ligands on the protease and promotion of
protease shedding. Le et al (30) found that targeting EGFR with
EGF-conjugated curcumin liposomes increased the antitumor activity
of curcumin against pancreatic cancer cells. Treatment with
curcumin effectively attenuated tobacco smoke-induced activation of
the ERK and JNK MAPK pathways as well as EMT alterations in the
mouse liver (31). Curcumin was
also found to weaken pancreatic cancer cell growth, clonogenic
potential, migration and invasion abilities by downregulation of
the expression of two homologous transcriptional co-activators, YAP
(Yes-associated protein) and TAZ (transcriptional coactivator with
PDZ-binding motif) via the Notch signaling pathway (32). As an activator of the ligase
anaphase-promoting complex/C (APC/C), cell division cycle 20
(Cdc20) plays an oncogenic role in tumorigenesis.
A recent study found that curcumin could suppress
cell growth, induce apoptosis and trigger cell cycle arrest via
inhibition of Cdc20 expression in pancreatic cancer cells (33). S-phase kinase associated protein 2
(Skp2) plays an oncogenic role in pancreatic cancer development and
progression. Su et al (34)
demonstrated that curcumin-induced cellular proliferation
inhibition, cell cycle arrest, apoptosis and invasion suppression
in pancreatic cancer cells was partly attributed to the
downregulation of Skp2. Curcumin exerts its anticancer effects both
alone and in combination with other anticancer drugs, including
gemcitabine and 5-fluorouracil, by modulating multiple
therapeutical molecular targets and signaling pathways. For
instance, curcumin sensitized gemcitabine-resistant cancer cells by
inhibiting the expression of the PRC2 subunit EZH2 and its related
lncRNA PVT1 (35). We demonstrated
that curcumin suppressed hydrogen peroxide-inducedcell migration
and invasion through the inhibition of the ROS/ERK/NF-κB signaling
pathway (17). Curcumin was also
found to inhibit superoxide dismutase-induced cancer EMT via the
suppression of the PI3K/Akt/NF-κB signaling pathway in pancreatic
cancer cells (14). In the present
study, our data showed that curcumin inhibited the invasive and
migratory abilities of pancreatic cancer cells under high glucose
conditions, which maybe attributed to the EGF/ERK and EGF/Akt
signaling pathways. Our future experiments may include more in
vivo experiments to further validate the findings.
In conclusion, the present study demonstrated that
curcumin plays an important role in suppressing high
glucose-induced proliferative, invasive and migratory abilities of
pancreatic cancer cells via inhibiting the EGF/ERK and EGF/Akt
signaling pathways. Curcumin might be a potential anticancer agent
for the treatment of pancreatic cancer patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Natural Science Foundation of Shaanxi Province (grant serial no.
2017JM8037) and the Fundamental Research Funds for the Central
Universities.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
QM, ZWa and LC conceived and designed the study; WL,
LH and XX conducted the experiments; and ZWu and LC performed the
data analysis. WL wrote the paper. LC and QM reviewed and edited
the manuscript. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michl P and Gress TM: Current concepts and
novel targets in advanced pancreatic cancer. Gut. 62:317–326. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bosetti C, Rosato V, Li D, Silverman D,
Petersen GM, Bracci PM, Neale RE, Muscat J, Anderson K, Gallinger
S, et al: Diabetes, antidiabetic medications, and pancreatic cancer
risk: An analysis from the International Pancreatic Cancer
Case-Control Consortium. Ann Oncol. 25:2065–2072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang PH, Chen ZW, Lv D, Xu YY, Gu WL,
Zhang XH, Le YL, Zhu HH and Zhu YM: Increased risk of cancer in
patients with type 2 diabetes mellitus: A retrospective cohort
study in China. BMC Public Health. 12:5672012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin QJ, Yang F, Jin C and Fu DL: Current
status and progress of pancreatic cancer in China. World J
Gastroenterol. 21:7988–8003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Ma J, Han L, Xu Q, Lei J, Duan W, Li
W, Wang F, Wu E, Ma Q, et al: Hyperglycemic tumor microenvironment
induces perineural invasion in pancreatic cancer. Cancer Biol Ther.
16:912–921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li W, Zhang L, Chen X, Jiang Z, Zong L and
Ma Q: Hyperglycemia promotes the epithelial-mesenchymal transition
of pancreatic cancer via hydrogen peroxide. Oxid Med Cell Longev.
2016:51903142016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Appert-Collin A, Hubert P, Crémel G and
Bennasroune A: Role of ErbB receptors in cancer cell migration and
invasion. Front Pharmacol. 6:2832015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ardito CM, Grüner BM, Takeuchi KK,
Lubeseder-Martellato C, Teichmann N, Mazur PK, Delgiorno KE,
Carpenter ES, Halbrook CJ, Hall JC, et al: EGF receptor is required
for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 22:304–317.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han L, Ma Q, Li J, Liu H, Li W, Ma G, Xu
Q, Zhou S and Wu E: High glucose promotes pancreatic cancer cell
proliferation via the induction of EGF expression and
transactivation of EGFR. PLoS One. 6:e270742011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng TS, Chen WC, Lin YY, Tsai CH, Liao
CI, Shyu HY, Ko CJ, Tzeng SF, Huang CY, Yang PC, et al:
Curcumin-targeting pericellular serine protease matriptase role in
suppression of prostate cancer cell invasion, tumor growth, and
metastasis. Cancer Prev Res (Phila). 6:495–505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allegra A, Innao V, Russo S, Gerace D,
Alonci A and Musolino C: Anticancer activity of curcumin and its
analogues: Preclinical and clinical studies. Cancer Invest.
35:1–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Jiang Z, Xiao X, Wang Z, Wu Z, Ma Q
and Cao L: Curcumin inhibits superoxide dismutase-induced
epithelial-to-mesenchymal transition via the PI3K/Akt/NF-κB pathway
in pancreatic cancer cells. Int J Oncol. 52:1593–1602. 2018.
|
|
15
|
Cao L, Xiao X, Lei J, Duan W, Ma Q and Li
W: Curcumin inhibits hypoxia-induced epithelial-mesenchymal
transition in pancreatic cancer cells via suppression of the
hedgehog signaling pathway. Oncol Rep. 35:3728–3734. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao L, Liu J, Zhang L, Xiao X and Li W:
Curcumin inhibits H2O2-induced invasion and
migration of human pancreatic cancer via suppression of the
ERK/NF-κB pathway. Oncol Rep. 36:2245–2251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pachmayr E, Treese C and Stein U:
Underlying mechanisms for distant metastasis - Molecular biology.
Visc Med. 33:11–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu WS, Wu JR and Hu CT: Signal cross talks
for sustained MAPK activation and cell migration: The potential
role of reactive oxygen species. Cancer Metastasis Rev. 27:303–314.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayer IA and Arteaga CL: The PI3K/AKT
pathway as a target for cancer treatment. Annu Rev Med. 67:11–28.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li W, Ma Z, Ma J, Li X, Xu Q, Duan W, Chen
X, Lv Y, Zhou S, Wu E, et al: Hydrogen peroxide mediates
hyperglycemia-induced invasive activity via ERK and p38 MAPK in
human pancreatic cancer. Oncotarget. 6:31119–31133. 2015.PubMed/NCBI
|
|
22
|
Jiang WG and Ablin RJ: Cancer metastasis,
challenges, progress and the opportunities. Front Biosci (Elite
Ed). 3:391–394. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xue C, Wyckoff J, Liang F, Sidani M,
Violini S, Tsai KL, Zhang ZY, Sahai E, Condeelis J and Segall JE:
Epidermal growth factor receptor overexpression results in
increased tumor cell motility in vivo coordinately with enhanced
intravasation and metastasis. Cancer Res. 66:192–197. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao L, Chen X, Xiao X, Ma Q and Li W:
Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and
migration of pancreatic cancer cells via suppression of the ERK and
p38 MAPK signaling pathways. Int J Oncol. 49:735–743. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Q, Zong L, Chen X, Jiang Z, Nan L, Li
J, Duan W, Lei J, Zhang L, Ma J, et al: Resveratrol in the
treatment of pancreatic cancer. Ann NY Acad Sci. 1348:10–19. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiang SP, Cabrera RM and Segall JE: Tumor
cell intravasation. Am J Physiol Cell Physiol. 311:C1–C14. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers (Basel).
9:92017.
|
|
28
|
Modjtahedi H and Essapen S: Epidermal
growth factor receptor inhibitors in cancer treatment: Advances,
challenges and opportunities. Anticancer Drugs. 20:851–855. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Soung YH and Chung J: Curcumin inhibition
of the functional interaction between integrin α6β4 and the
epidermal growth factor receptor. Mol Cancer Ther. 10:883–891.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Le UM, Hartman A and Pillai G: Enhanced
selective cellular uptake and cytotoxicity of epidermal growth
factor-conjugated liposomes containing curcumin on
EGFR-overexpressed pancreatic cancer cells. J Drug Target.
26:676–683. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang Z, Wu R, Xie W, Xie C, Wu J, Geng S,
Li X, Zhu M, Zhu W, Zhu J, et al: Effects of curcumin on tobacco
smoke-induced hepatic MAPK pathway activation and
epithelial-mesenchymal transition In Vivo. Phytother Res.
31:1230–1239. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou X, Su J, Feng S, Wang L, Yin X, Yan J
and Wang Z: Antitumor activity of curcumin is involved in
down-regulation of YAP/TAZ expression in pancreatic cancer cells.
Oncotarget. 7:79076–79088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Xue YB, Li H, Qiu D, Wang ZW and
Tan SS: Inhibition of cell survival by curcumin is associated with
downregulation of cell division cycle 20 (Cdc20) in pancreatic
cancer cells. Nutrients. 9:92017. View Article : Google Scholar :
|
|
34
|
Su J, Zhou X, Wang L, Yin X and Wang Z:
Curcumin inhibits cell growth and invasion and induces apoptosis
through down-regulation of Skp2 in pancreatic cancer cells. Am J
Cancer Res. 6:1949–1962. 2016.PubMed/NCBI
|
|
35
|
Yoshida K, Toden S, Ravindranathan P, Han
H and Goel A: Curcumin sensitizes pancreatic cancer cells to
gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1
expression. Carcinogenesis. 38:1036–1046. 2017. View Article : Google Scholar : PubMed/NCBI
|