Introduction
As a common cancer of men, prostate cancer (PC)
gradually becomes an androgen-independent phenotype following its
dependence on androgens for proliferation. PC is resistant to
chemotherapy and secondary endocrine therapy in the
androgen-independent phenotype (1,2). PC is
accompanied by various molecular changes, particularly the
elevation of serum interleukin-6 (IL-6) (3).
IL-6 is a multifunctional cytokine and is related to
the differentiation and growth of all types of malignant tumors
including prostate carcinomas (4).
IL-6 has been demonstrated to be associated with the transformation
of PC to an androgen-independent state from an androgen-dependent
state. IL-6 uses many cellular transducers and mediators through
the receptor, such as phospho-Akt (S473), phosphorylated ERK1/2
(T202/Y204) and Janus kinase (JAK)/signal transducer and activator
of transcription (STAT). p-STAT3 is the key component of the
JAK/STAT pathway. Consequently, the JAK/STAT pathway is regarded as
a target of novel therapies (5,6).
Recent research results have indicated that androgen
receptor (AR) transactivation can be activated through IL-6 in
human prostate cancer (LNCaP) cells in an androgen-independent
manner. Although the PI3K pathway is considered to be the main
contributor to IL-6 signaling, its role in activation of AR by IL-6
is debated. PI3K can activate AR through IL-6. However, activation
of AR-mediated by IL-6 does not depend on the PI3K pathway
(7,8).
Sch B, extracted from the fruit of Schisandra
chinensis, has been applied to treat diseases, including
myocardial disorders and hepatitis (9,10). Sch
B has antitumor activities in various types of cancers including
cholangiocarcinoma, gastric, breast and glioma (11–13).
Recent studies have demonstrated that, due to its low substantial
antioxidant and anti-inflammatory activity, Sch B may attenuate
metastasis invasion in cancer (14,15).
However, to the best of our knowledge, the
influences of Sch B on prostate cancer cells and the corresponding
mechanism have not been previously reported. The present study
investigated the anticancer effect of Sch B on prostate cell lines
and its molecular mechanism, and demonstrated that Sch B can be
used as a novel and natural anti-prostate cancer drug.
Materials and methods
Chemicals and reagents
Sch B was purchased from Baoji Herbest Bio-tech Co.,
Ltd., (Beijing, China). Fetal bovine serum (FBS) was purchased from
Hangzhou Sijiqing Biological Engineering Material, Co., Ltd.
(Beijing, China). Dulbecco's modified Eagle's medium (DMEM) was
obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). 3-4-5-Dimethylthiazol-2-yl-25-diphenyltetrazolium bromide
(MTT) and dimethyl sulfoxide (DMSO) were purchased from
Sigma-Aldrich (Darmstadt, Germany). An apoptosis kit, a Cell Cycle
kit, a Reactive Oxygen Species (ROS) kit, and Rhodamine 123 and
DAPI staining kits as well as RIPA were purchased from Beyotime
Institute of Biotechnology (Beijing, China). Antibodies against
phospho-PI3 kinase (1:1,000; cat. no. 4249), phospho-anti-Akt
antibody (1:1,000; cat. no. 9272), JAK2 (1:1,000, cat. no. 3230),
p-Jak2 (1:1,000; cat. no. 3771), p-ERK (1:2,000; cat. no. 4370),
p38 (1:500; cat. no. 8690), STAT3 (1:1,000; cat. no. 12640),
p-STAT3 (1:500; cat. no. 9145), Bcl-2 (1:1,000; cat. no. 2872) and
Bax (1:1,000; cat. no. 2774) antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Cyclin D1 (1:500;
cat. no. Sc-8396), cyclin A (1:1,000; cat. no. 271682), cyclin E
(1:1,000; cat. no. Sc-48420), CDK2 (1:500; cat. no. Sc-70829),
mouse (1:500; cat. no. Sc-2791), anti-rabbit (1:1,000; cat. no.
Sc-2774) and p53 (1:1,000; cat. no. Sc-47698), antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA).
Ethics
All ethical and laboratory guidelines were followed
and no misidentified cell lines were used. The LNCaP, DU145,
SGC-7901 and SW480 cell lines were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA).
Cell culture
The LNCaP, DU145, SGC-7901 and SW480 cell lines were
cultured and maintained in DMEM containing 10% FBS. Cells were
incubated at 37°C in a humidified atmosphere with 5% CO2
and allowed to grow to 70–80% confluence.
Cell proliferation assay
Cells were cultured and maintained in DMEM,
containing 1% FBS then incubated at 37°C in a humidified atmosphere
of 5% CO2 and allowed to grow to 70–80% confluence.
Then, the cells were harvested and seeded in a 96-well plate to a
final concentration of 5×103 cells/well and incubated in
DMEM containing 1% FBS for 24 h. Next, the cells were treated with
0, 50, 100, 150 and 200 µM of Sch B, and incubated for 24, 48 or 72
h. Subsequently, 20 µl of MTT solution (5 mg/ml) was added to each
well and then incubated for 4 h. Finally, the medium was discarded
and 150 µl of DMSO was added to each well. The plates were read at
a wavelength of 570 nm using Varioskan Flash Multimode Reader
(Thermo Fisher Scientific, Inc.). A total of 6 reduplicate wells
were used for each treatment, and experiments were repeated 3
times. Their inhibition ratio (I %) was based on the equation
(18).
I%=[A570(control)-A570(treated)]/A570(control)x100
Where, I is the inhibition rate and A is the
absorbance at 570 nm.
Annexin V/PI assay for apoptosis
Apoptotic cells were investigated by Annexin V/PI
staining followed by flow cytometry according to the manufacturer's
protocol. LNCaP and DU145 cells (5×103 cells/well) were
cultured in 6-wells plates and treated with different
concentrations (12.5, 25 and 50 µM) of Sch B for 48 h. Then, the
cells were washed twice with PBS and then stained with 5 µl of
Annexin V-FITC and 10 µl of PI in 500 µl binding buffer for 15 min
at room temperature in the dark. The apoptotic cells were
determined by flow cytometry (Cytomics FC 500; Beckman Coulter
Inc., Miami, FL, USA).
Determination of LNCaP cell cycle
distribution
The cell cycle distribution in different phases
following exposure to Sch B was analyzed by flow cytometry. In
brief, LNCaP cells (5×103 cells/well plates) were seeded
into 6-well plates, and exposed to different concentrations (12.5,
25 and 50 µM) of Sch B for 48 h. The cells were harvested, washed
with PBS twice and subsequently fixed with 70% ethanol for 2 h. The
cells were centrifuged at 67 × g for 4 min and washed with PBS,
resuspended in 500 µl of buffer containing 10 µl of RNase and 25 µl
of PI, and incubated at room temperature in the dark for 15 min.
The distributions of the cell cycle were determined by flow
cytometry (Cytomics FC 500; Beckman Coulter Inc.).
Detection of ROS
The generation of ROS was determined with
2,7-dichlorofluorescein diacetate (DCFH-DA) according to the
manufacturer's instructions. In brief, LNCaP cells were cultured
(5×103 cells/well), incubated with or without NAC for 1
h and then treated with Sch B (0, 12.5, 25 and 50 µM) for 48 h. The
cells were collected, centrifuged at 67 × g for 4 min and washed
with PBS, then resuspended in PBS containing 10 µM of DCFH-DA and
incubated at room temperature in the dark for 15 min. The cells
were then washed with PBS and measured immediately using a flow
cytometer (Cytomics FC 500; Beckman Coulter Inc.) to monitor the
formation of the fluorescent-oxidized derivative of DCFH-DA at an
emission wavelength of 525 nm and an excitation wavelength of 488
nm.
Flow cytometry of Rhodamine 123
The changes induced by Sch B in the mitochondrial
membrane were determined by Rhodamine 123 staining according to the
manufacturer's instructions. Briefly, LNCaP cells (5×103
cells/well) were seeded in 6-well plates and then treated with or
without NAC and incubated at 37°C for 1 h. Subsequently, the cells
were treated with or without 12.5, 25 or 50 µM Sch B for 48 h and
stained with Rhodamine 123 for 15 min at 37°C. Mitochondrial
membrane potential (MMP) was detected by flow cytometry (Cytomics
FC 500; Beckman Coulter Inc.).
Western blot analysis
The protein expression regulated by Sch B was
analyzed by western blotting and followed the protocol as
previously described (16) with
small modifications. In brief, LNCaP and DU145 cells were treated
with 12.5, 25 or 50 µM of Sch B for 48 h and then harvested and
lysed with RIPA buffer. Subsequently, the insoluble protein lysate
was removed by centrifugation at 12,225 × g for 15 min at 4°C. The
protein concentrations were determined using a NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE,
USA). An equal amount of protein was loaded on SDS-PAGE
electrophoresis gel (10 or 12% according to the protein size) and
the gel was transferred onto polyvinylidene fluoride (PVDF)
membranes. The membranes were blocked in 5% (w/v) non-fat milk and
incubated for 2 h. The membranes were thereafter incubated with
appropriate primary antibodies at 4°C overnight and washed three
times with a Tris-buffered saline-Tween solution (TBST). Finally,
the blots were incubated with appropriate secondary antibodies
(anti-rabbit or anti-mouse horseradish peroxidase-conjugated) for 1
h at room temperature, then washed with TBST for 30 min. Signals
were detected using ECL plus chemiluminescence kit on X-ray film
(EMD Millipore, Billerica, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol (Life
Technologies; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. Briefly, first-stand cDNA was
reverse-transcribed from 1 µg total RNA using the Super-Script
First-Strand cDNA System (Invitrogen; Thermo Fisher Scientific,
Inc.) and amplified by Platinum SYBR-Green qPCR Super Mix-UDG
(Invitrogen; Thermo Fisher Scientific, Inc.). A Master Mix was
prepared for each PCR reaction, which included Platinum SYBR Green
qPCR Super Mix-UDG, forward primer, reverse primer and 10 ng of
template cDNA. PCR conditions were 10 min at 95°C, followed by 50
cycles at 95°C for 30 sec and 60°C for 1 min and 72°C for 30 sec.
The forward and reverse primer sequences were as follows: Akt
forward, 5′-TCTATGGCGCTGAGATTGTG-3′ and reverse,
5′-CTTAATGTGCCCGTCCTTGT-3′; and β-actin forward,
5′-CACGATGGAGGGGCCGGACTCATC-3 and reverse,
5′-TAAAGACCTCTATGCCAACACAGT-3′. Relative gene expression was
obtained after normalization with β-actin and determination of the
difference in cycle quantification (Cq) between treated and
untreated cells was performed using the 2−∆∆Cq method
(17).
Transient transfection and luciferase
assay
Luciferase assays were performed as previously
described (18). Briefly, transient
transfections were performed using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) following the manufacturer's
protocol. Cells were seeded into 48-well plates for 16 h and
transected with STAT3-Luc promoter 100 ng in the presence of
Renilla Luciferase control pREP7 vector 25 ng, and then
treated with or without Sch B (12.5, 25 and 50 µM). Firefly
luciferase activities were calculated using the Dual-Luciferase
reporter assay system (Promega Corp., Madison, WI, USA) and the
ratio of firefly luciferase activity to Renilla luciferase
activity was measured as the relative luciferase activity.
Statistical analysis
All statistical analyses were performed using Origin
Lab software version 8.0 (Origin Lab, Northampton, MA, USA), and
statistically significant differences between groups were
determined by one-way ANOVA followed by Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cytotoxicity of Sch B in LNCaP
cells
An MTT assay was used to determine the cell
viability of human PC, gastric and colon cancer cells in presence
of various concentrations of Sch B ranging 0–200 µM and incubated
for 24, 48 and 72 h. As observed in Fig. 1 the results revealed a steady
increase in growth inhibition in a concentration-dependent manner
over the course of the incubation. Particularly, the estimated
half-maximal inhibitory concentration (IC50) value was
25, 48, 84 and 93 µM for LNCaP, DU145, SW480 and SGC-7901 cells,
respectively. Regarding these results it was evident that Sch B
induced more cytotoxicity in PC cancer cells and particularly in
the LNCaP cell line. We therefore performed the cytotoxicity of Sch
B on LNCaP cells. The results revealed a steady increase in growth
inhibition in a time dependent-manner respectively for 24, 48 and
72 h. The estimated half-maximal inhibitory concentration
(IC50) was 56, 33 and 25 µM, respectively for 24, 48 and
72 h. In the present study, the concentrations of 12.5, 25 and 50
µM were used for 48 h of incubation in subsequent experiments.
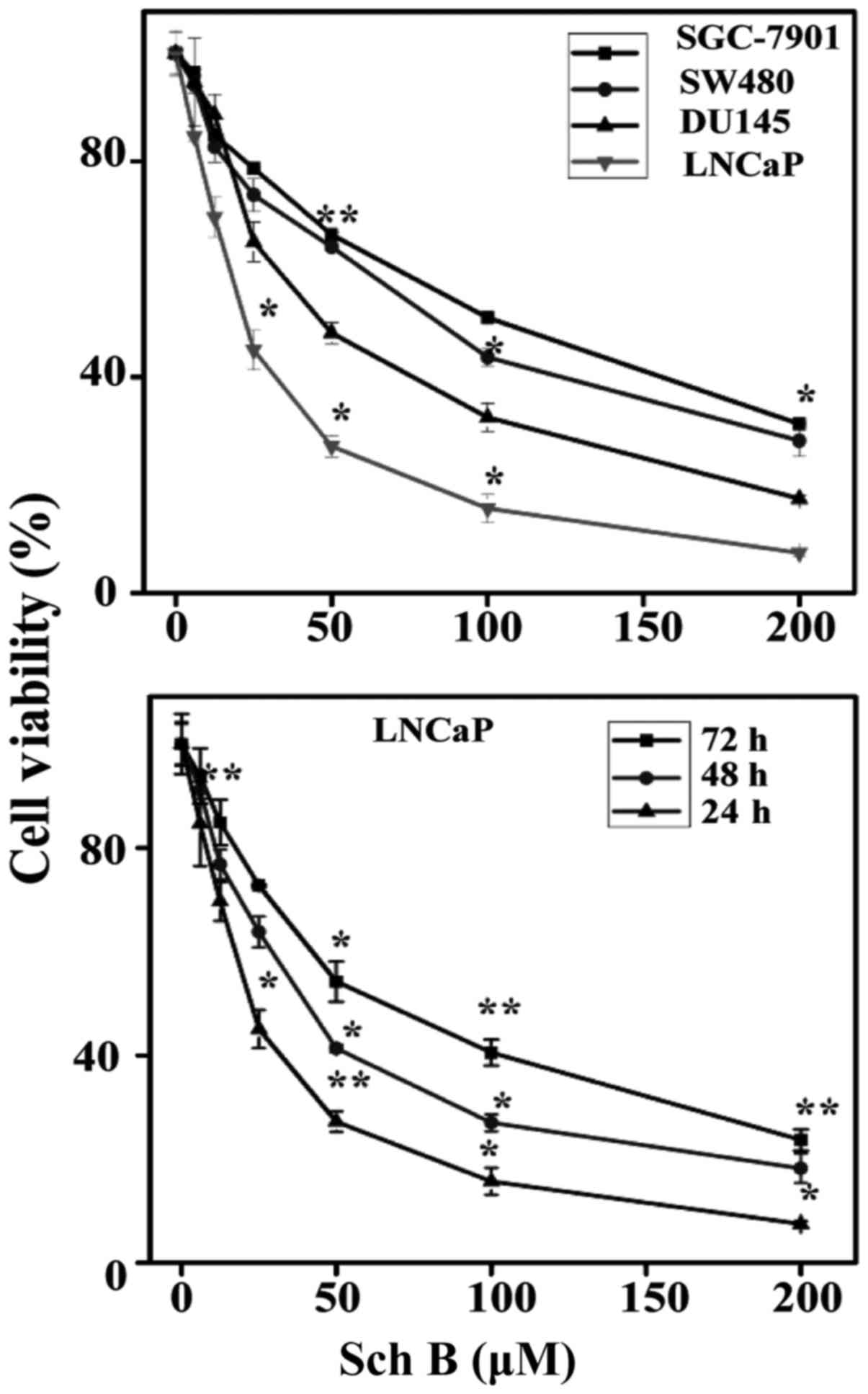 | Figure 1.Cytotoxicity of Sch B on cancer cell
lines. LNCaP, DU145, SGC-7901 and SW480 cell lines were treated
with 0, 6, 12.5, 25, 50, 100, and 200 µM of Sch B. Proliferation
was assessed after 72 h, as described in Materials and methods.
*P<0.05 and **P<0.01 compared with the control. (B) LNCaP
cells were treated with 0, 6, 12.5, 25, 50, 100 and 200 µM of Sch B
for 24, 48 and 72 h. Each bar represents the mean ± standard
deviation of three experiments. *P<0.05 and **P<0.01 compared
with the control. Sch B, Schisandrin B. |
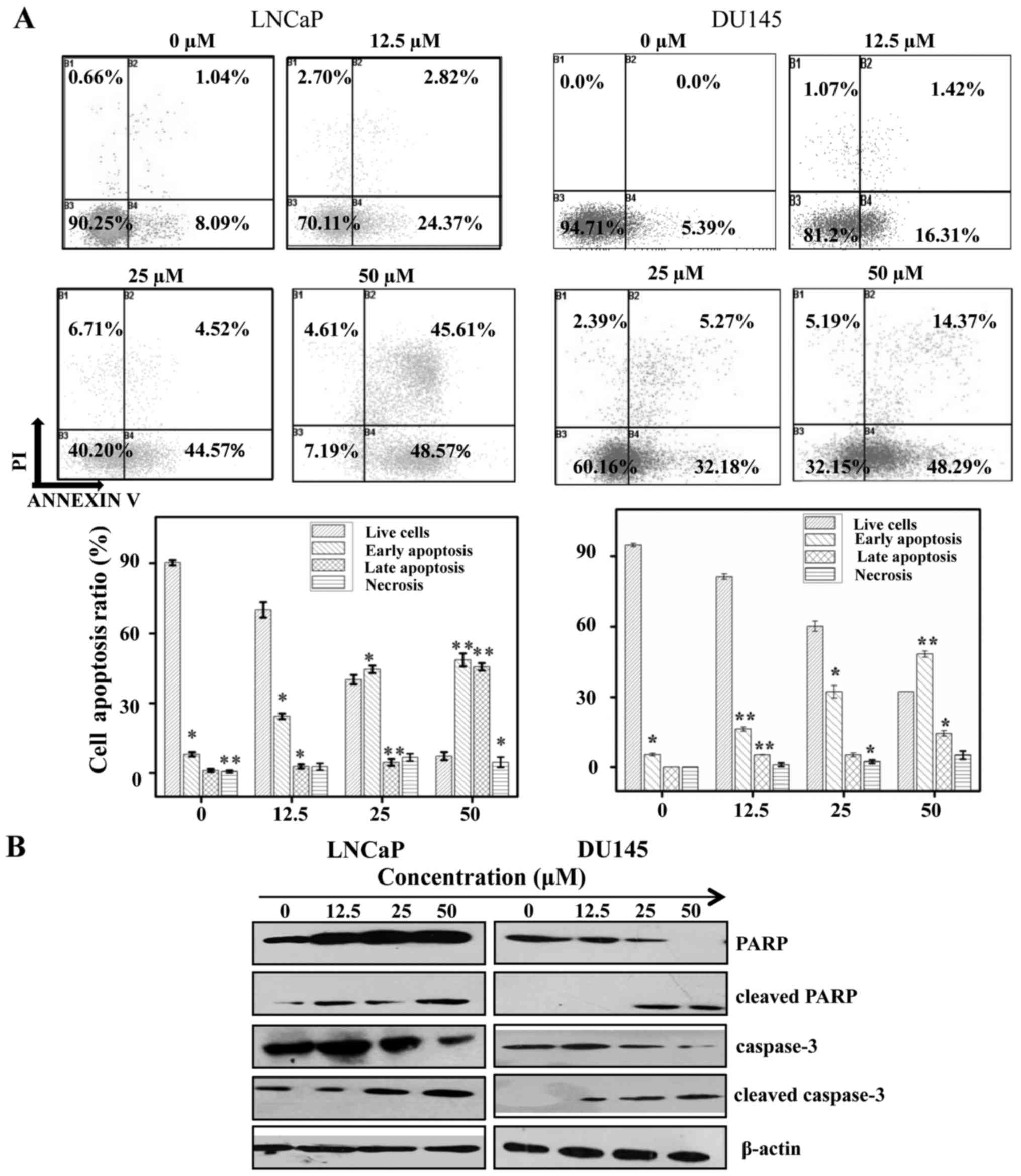
Sch B induces apoptosis in LNCaP and
DU145 cells
To determine whether the cell cytotoxicity induced
by Sch B affected apoptosis, the cells were labeled with Annexin V
and PI and then subjected to flow cytometry to determine the early
apoptosis (B4), late apoptosis (B2) and necrosis (B1) of LNCaP and
DU145 cells. As observed in Fig.
2A, Sch B induced apoptosis in both LNCaP and DU145 cells. The
cell apoptosis rate of B1+B2+B3 was 27.19, 49.09 and 94.18% for the
LNCaP cells after exposure to Sch B (2.5, 25 and 50 µM) for 48 h
and 17.73, 37.45 and 62.66% in the DU145 cells after respective
exposure to Sch B (2.5, 25 and 50 µM) for 48 h. These data
confirmed the results obtained from our MTT assay which revealed
that following exposure to Sch B, LNCaP cells (IC50=25)
were more sensitive than DU145 cells (IC50=48).
Collectively, we therefore hypothesized that the cytotoxicity of
Sch B was in time- and dose-dependent manner in PC cells.
To further assess the mechanism by which Sch B
induced LNCaP and DU145 cell apoptosis, apoptosis-related proteins
protein poly ADP-ribose (PARP), caspase-3 were analyzed. The
results revealed that Sch B cleaved PARP and caspase-3 proteins
expression (Fig. 2B).
Sch B generates ROS, and causes
collapse in MMP
The generation of ROS serves a crucial role in the
activation of mitochondrial-mediated apoptosis in PC (19). The present study evaluated whether
Sch B can induce ROS in human prostate cancer LNCaP cells by
treating cells with or without N-acetyl-L-cysteine (NAC), an
inhibitor of ROS, in presence or absence of Sch B (12.5, 25 and 50
µM) and then analyzing the cells by flow cytometry. The results
demonstrated that in the absence of NAC, Sch B increased the
generation of ROS by 18.73±1.876%, 39.1±2.437%, 66.1±1.854 and
73.36±1.629 for 0, 12.5, 25 and 50 µM, respectively, of Sch B,
while the generation of ROS remained almost unchanged in presence
of NAC for the same concentrations (Fig. 3A). This was due to the oxidative
stress generated by Sch B in LNCaP cells resulting in an increased
rate of ROS production.
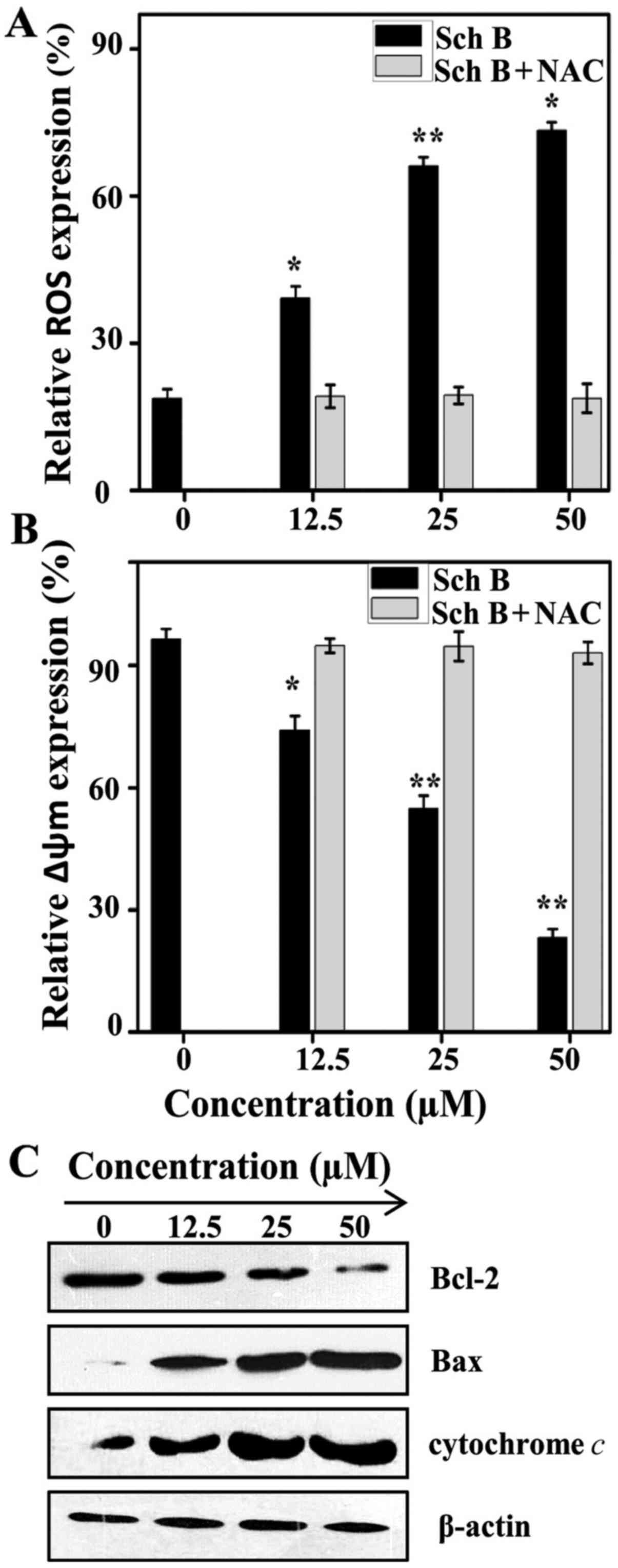 | Figure 3.Sch B collapses mitochondrial
membrane potential, while generating oxidative stress in LNCaP
cells. (A) LNCaP cells were pre-incubated in the absence or
presence of NAC 3 mM for 30 min, then treated with or without Sch B
(0, 12.5, 25 and 50 µM) for 48 h, and finally stained with DCFH-DA
and analyzed by flow cytometry. *P<0.05 and **P<0.01 compared
with the control. (B) LNCaP cells were pre-incubated in the absence
or presence of NAC 3 mM for 30 min, then treated with or without
Sch B (0, 12.5, 25 and 50 µM) for 48 h, and finally stained with
Rhodamine 123. The data shown are representative of three
independent experiments with similar results. *P<0.05 and
**P<0.01 compared with the control. (C) LNCaP cells were treated
with or without Sch B (0, 12.5, 25 and 50 µM) for 48 h and then
cellular proteins were extracted to detect the levels of Bax,
Bcl-2, cytochrome c, as well as β-actin (control) protein.
Sch B, Schisandrin B; NAC, N-acetyl-L-cysteine. |
The generation of ROS is mainly associated with the
collapse of MMP in many cancer cells and induces cells apoptosis
including in PC (20). To
understand the role of Sch B in LNCaP cell apoptosis, the cells
were stained with Rhodamine 123. Fig.
3B shows that, in the absence of NAC, the MMP expression was
decreased 96.53±2.496, 74.19±3.479, 54.82±3.2017 and 23.21±2.1649%
respectively for 0, 12.5, 25, and 50 µM of Sch B, while the MMP
expression remained unchanged for the same concentrations in
presence of NAC. To further understand the mechanism by which Sch B
decreased MMP, western blot analysis was conducted to confirm the
level of cytochrome c, Bax, and Bcl-2 to obtain more insight
into cell apoptosis. The results demonstrated that Sch B led to the
release of cytochrome c from the mitochondrial membrane.
This consequently drove the activation of Bax and deactivated Bcl-2
(Fig. 3C). This release of
cytochrome c is the key factor in the formation of
apoptosomes and is due to the role of the Bcl-2 protein family in
the regulation of the apoptotic pathway of the mitochondrial
membrane.
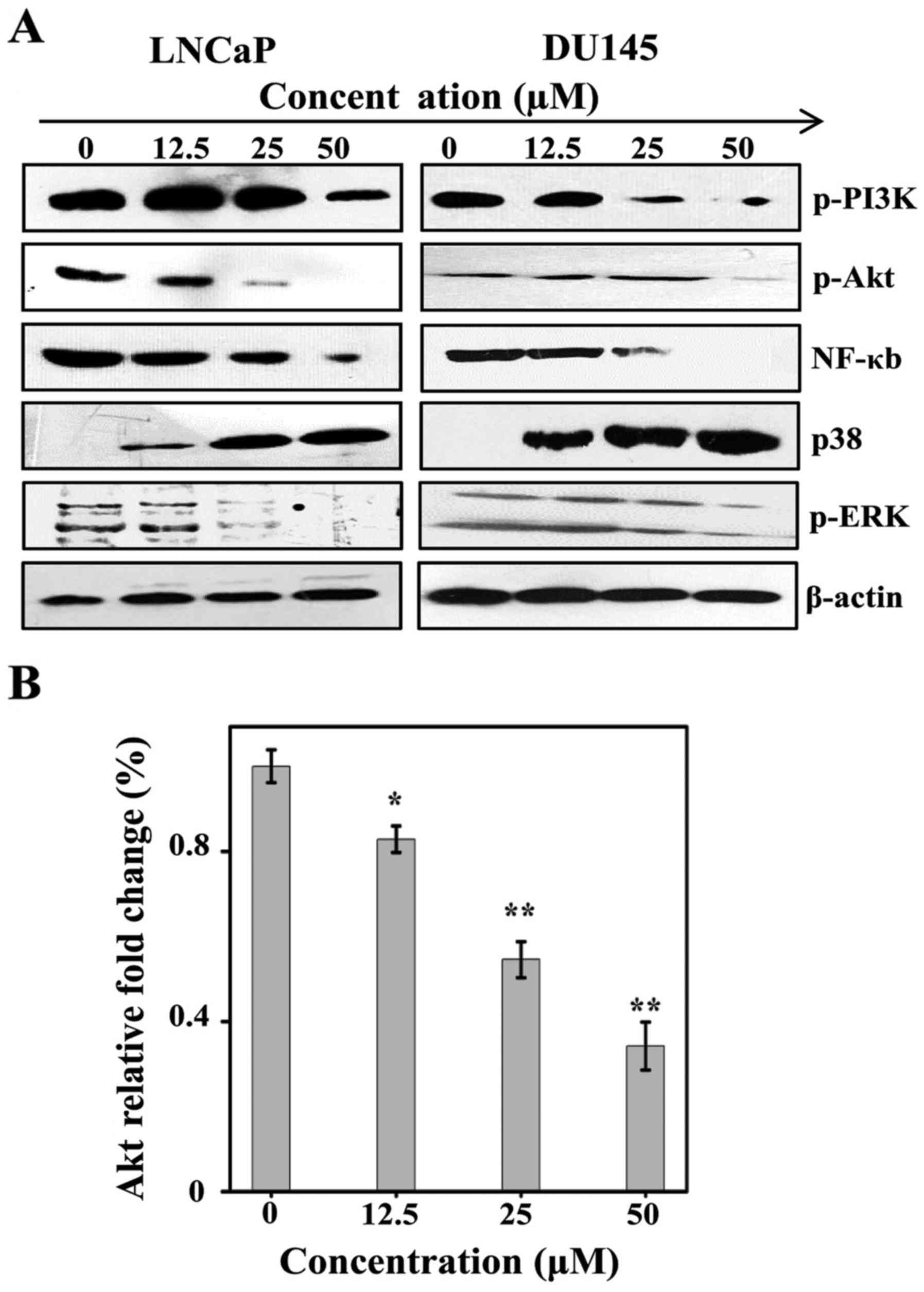
Sch B induces apoptosis via
phosphorylated PI3K/Akt as well as NF-κB and overexpression of
p38
It has been demonstrated that NF-κB can regulate
cell apoptosis and growth. Studies have analyzed the functional
mechanism of PI3K/Akt, ERK and p38 by investigating NF-κB (21,22).
The present study hypothesized that the pathway can be adjusted by
Sch B. The functions of Sch B in Akt proteins, ERK and p38 in LNCaP
and DU145 at different concentrations are discussed in the present
study. Sch B induced overexpression of p38 while it inhibited
phosphorylation of p-PI3K, p-Akt and p-ERK (Fig. 4A). To this end, the present study
assumed that Sch B exercises its function through the PI3K/Akt, ERK
and p38 pathways.
To further validate these findings, RT-qPCR was
performed to evaluate the expression of Akt mRNA in LNCaP cells
following exposure to Sch B. As shown in Fig. 4B, Sch B induced the inhibition of
Akt expression in the LNCaP cell line.
Sch B induces inhibition of AR, IL6-
and STAT3 induced STAT3 transcription and STAT3 phosphorylation in
LNCaP cells
It has been demonstrated that inhibition of the
phosphorylation of STAT3 and JAK2 can lead to the apoptosis of
cancer cells (23). The expression
levels of JAK2 and STAT3 were assessed by western blot analysis. As
demonstrated in Fig. 5A, Sch B
induced inhibition of phosphorylation of JAK2 and downstream STAT3;
however, it was identified that total JAK2 and STAT-3 remain
unchanged in LNCaP and DU145 cells. To further validate these
findings, RT-qPCR was performed to evaluate the expression of STAT3
mRNA in LNCaP cells following exposure to Sch B (12.5, 25 and 50
µM). As observed in Fig. 5B, Sch B
induced inhibition of STAT3 mRNA levels. Next, transient luciferase
and transfection tests in LNCaP cells were conducted and results
confirmed our findings. As shown in Fig. 5C, Sch B influenced STAT3
transcription and this was increased in association with IL-6.
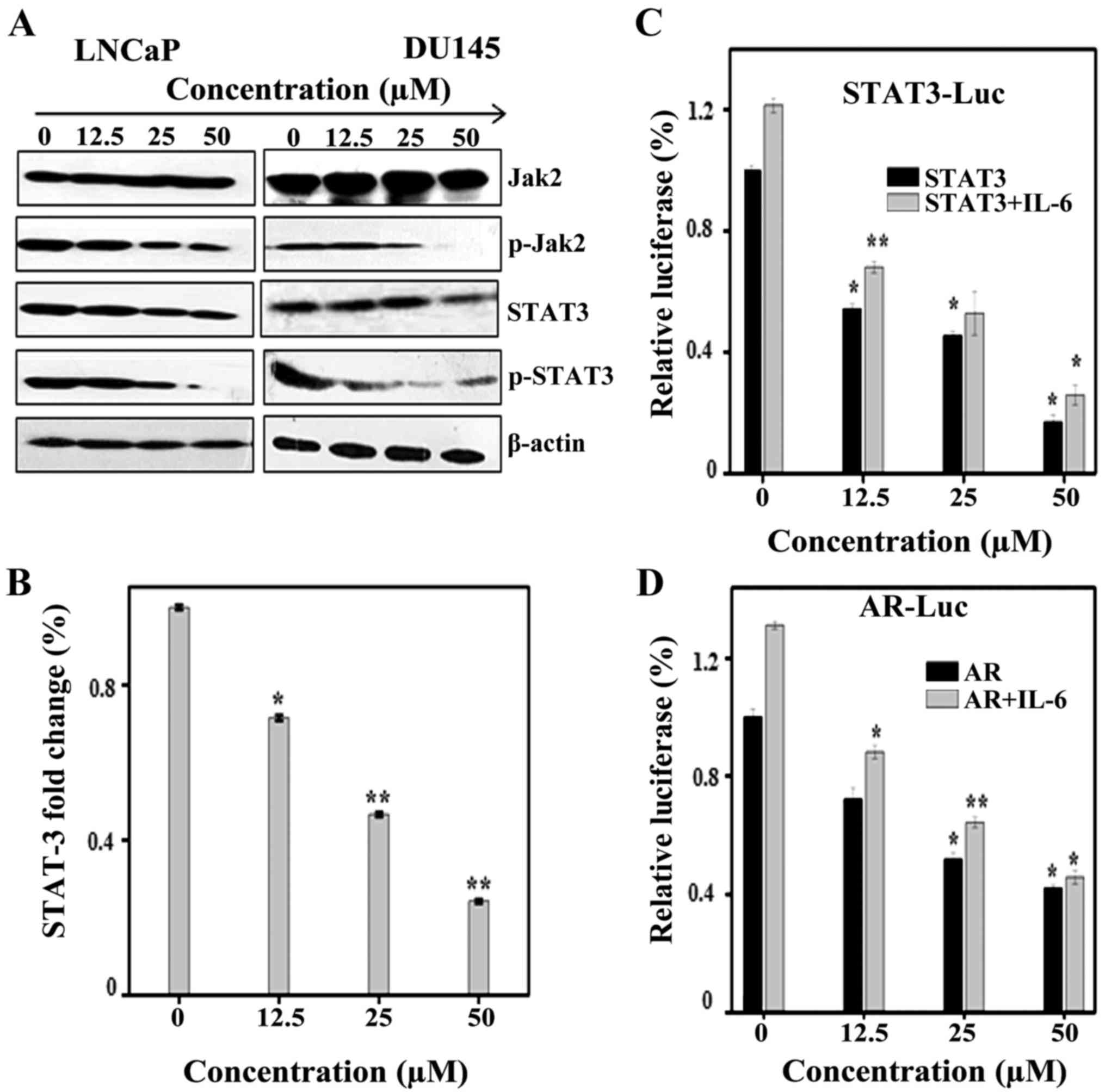 | Figure 5.Sch B induces apoptosis in LNCaP
cells through phosphorylation of JAK2 and STAT3 or AR independently
of IL6 (A) LNCaP cells were treated with or without Sch B (0, 12.5,
25 and 50 µM) for 48 h. The cellular proteins were extracted to
detect the levels of JAK2, p-JAK2, STAT3, p-STAT3 as well as
β-actin (control) by western blotting. (B) Real-time RT-PCR was
used to quantify the STAT3 mRNA levels in LNCaP cells after
treatment with Sch B (0, 12.5, 25 and 50 µM) for 24 h. Fold change
of gene expression was calculated by dividing the normalized gene
expression activity by that of the untreated control. The data
shown are representative of three independent experiments with
similar results. *P<0.05; and **P<0.01 compared with the
control (C) LNCaP cells were transiently transfected with
M67-Luciferase plasmid. After 16 h of transfections, the cells were
treated with Sch B (0, 12.5, 25 and 50 µM) for 48 h and then
harvested for luciferase assays. Cells were treated in the presence
or absence of IL-6 10 ng/ml for stimulation. *P<0.05 and
**P<0.01 compared with the control. (D) LNCaP cells were
transiently transfected with 0.5 mg of AR-Luc plasmids. After 16 h
of transfections, the cells were treated with Sch B (0, 12.5, 25
and 50 µM) for 48 h and then harvested for luciferase assays. Cells
were treated in the presence or absence of IL-6 10 ng/ml for
stimulation. *P<0.05 and **P<0.01 compared with the control.
Sch B, Schisandrin B. |
It has been reported that IL-6 can activate AR
(24). Therefore, transient
luciferase and transfection tests in LNCaP cells were performed to
evaluate whether Sch B inhibited AR. As shown in Fig. 5D, treatment of LNCaP cells with IL-6
increased AR expression whereas in the presence of Sch B (12.5, 25
and 50 µM) AR expression was inhibited.
Sch B induces LNCaP cell arrest at the
S phase
The regulation of the cell cycle is a key point of
cell proliferation and survival, and a disturbance during cell
cycle regulation results in cell death by apoptosis. Flow cytometry
was performed to determine the stage at which Sch B induced cell
cycle arrest. The results demonstrated an increased in S phase
percentage of 5.1730±1.875, 6.9490±1.683, 51.033±2.0165 and
54.966±2.3561%, respectively for 0, 12.5, 25 and 50 µM of Sch B,
while the percentage of G0/G1 53.751±2.54852,
52.598±1.3794, 35.102±2.13712 and 27.31±2.141 as well as the
percentage of G2/M 41.076±2.276, 39.454±1.947,
13.865±1.539, 13.725±1.9853 percentages decreased, respectively for
the same concentrations. These results indicated that Sch B induced
cell arrest of LNCaP cells at the S phase (Fig. 6A).
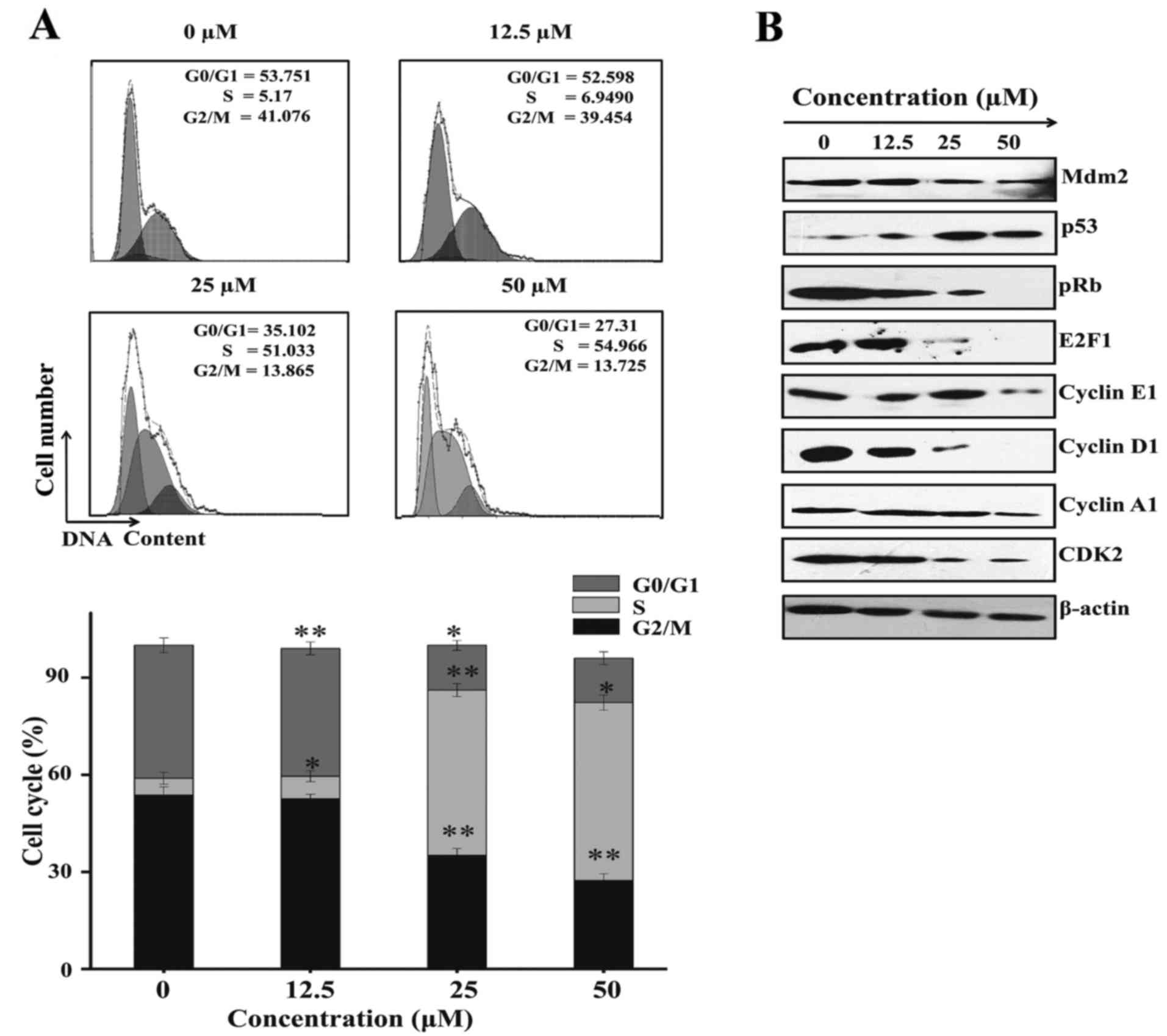 | Figure 6.Sch B induces LNCaP cell cycle arrest
at the S phase. (A) LNCaP cells were treated with or without Sch B
(0, 12.5, 25 and 50 µM) for 48 h, and then they were stained with
PI for flow cytometric analysis. Histograms show the number of
cells/channel (y-axis) vs. DNA content (x-axis). The values
indicate the percentage of cells in the indicated phases of the
cell cycle. The data shown are representative of three independent
experiments with the similar results. *P<0.05; and **P<0.01
compared with the control. (B) LNCaP cells were treated with or
without Sch B (0, 12.5, 25 and 50 µM) for 48 h. The cellular
proteins were extracted to detect the levels of p53, pRb, cyclin
A1, cyclin D1, cyclin E1, CDK2, E2F1, Mdm2 as well as β-actin
(control) by western blotting. Sch B, Schisandrin B. |
To further elucidate the molecular mechanism
underlying S phase arrest western blotting was performed to
characterize the protein-regulated S phase. As shown in Fig. 6B, Sch B suppressed the expression of
pRb, cyclin E1, cyclin A1, cyclin D1, E2F1 and Mdm2 while
increasing p53. These results confirmed S-phase arrest.
Discussion
Previous studies have suggested a positive
transactivation loop between STAT3 and Akt-NF-κB (25). Cells with anti-apoptotic ability
usually have higher expression levels of STAT3 and Akt. Akt was
inhibited through PI3K, which can reduce the regulation level of
STAT3 (26). Similarly, STAT3 was
inhibited if the regulation level of Akt was lowered by JAK2
(27). The PI3K/Akt pathway
influences STAT tyrosine phosphorylation by Src tyrosine kinase,
which is related to the cytokine receptors with PI3K by JAK2. JAK2
inhibited the transcription function of STAT, which was verified by
the results of the present study. Sch B inhibited PI3K/Akt and the
activity of JAK2 and STAT3. This negative effect eliminates the
positive regulation effect of the feedback mechanism of maintaining
equilibrium between STAT3 and Akt
The progression of cell apoptosis is impeded by
NF-κB, but STAT3 expression is accelerated by NF-κB. STAT3 induced
by NF-κB and JAK2 can prevent cell apoptosis and accelerate the
expression of Akt, which has positive effects on apoptosis
prevention. To sum up, JAK2 regulation can be reduced by the
increase of Akt, which further decreases the expression of Akt and
STAT3, and which leads to the negative feedback mechanism between
STAT3 and Akt (28,29). Akt can activate ERK in tumor cells
(30).
These two feedback signals indicate that molecules
serve an important role in cell proliferation, escape and
apoptosis. Sometimes these two signal paths compensate for each
other. Due to the limited efficacy of a single reagent, clinical
trials are performed using the combined application of inhibitors
that target pathways like ERK and PI3K. As a powerful inhibitor of
ERK and Akt, Sch B can improve the antiproliferative effects of
LNCaP cells compared with a single therapy. Therefore, Sch B can be
considered as a potential natural antineoplastic alternative to ERK
and Akt inhibitors (31).
According to a previous study, Sch B maintains
antioxidant properties (15). To
verify whether the production of H2O2 can
mediate cell apoptosis stimulated by Sch B in LNCaP cells, a flow
cytometric analysis of ROS was performed.
H2O2 was generated through Sch B, which was
inhibited in the presence of NAC. Furthermore, the generation of
ROS was associated with enhanced cell apoptosis. This demonstrated
that Sch B has an antioxidant effect on prostate cancer.
The proliferation of cells is auto-regulated by the
cell cycle, which is regulated through a myriad of complex CDK and
cyclin-CDK proteins. The cell channel in
G0/G1 to S-phase is synchronized through the
composite cyclinE-CDK2 (32). The
introduction of E2F1 is prompted, via phosphorylated pRb, to enter
G2/M transition (33).
LNCaP cells were treated with Sch B, which reduced the levels of
both cyclin E and CDK2. The expression of p21 was enhanced by
phosphorylated pRb. Therefore, the S phase arrest in LNCaP cells
may be caused by the decrease of the complex CyclinE/CDK2 and
enhanced regulation of p21.
In summary, the present study predominantly
explained the mitochondrial anti-proliferation role and
pro-apoptosis role of Sch B in PC (LNCaP and DU145) cells. Sch B
was identified to inhibit phosphorylation of JAK2 and itsdownstream
STAT3. The inhibition of the phosphorylation of JAK2 by Sch B
resulted in the inhibition of PI3K/Akt. Furthermore, JAK2
inhibition by Sch B led to the inhibition of Bcl-2, promoted
mitochondrial membrane potential expression collapse with induced
oxidative stress in LNCaP cells therefore leading cells to
apoptosis. In addition, we found that Sch B induced LNCaP cell
inhibition of STAT3 and AR, which was dependent of IL-6. Finally,
Sch B-induced LNCaP toxicity was associated with S-phase cell cycle
arrest.
Acknowledgements
The authors gratefully acknowledge the supports from
Liaoning Provincial Post Graduate Launching foundation
201601300.
Funding
The present study was supported by the Liaoning
Provincial Post Graduate Launching foundation 201601300.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MIN and NJ conceived and designed the study. MIN,
TH, SA and YT performed the experiments, collected and analyzed the
data. MIN, SA, YT and NJ wrote, reviewed and edited the manuscript.
All authors contributed equally in this study. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ho Y and Dehm SM: Androgen receptor
rearrangement and splicing variants in resistance to endocrine
therapies in prostate cancer. Endocrinology. 158:1533–1542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pedraza-Arevalo S, Hormaechea-Agulla D,
Gomez-Gomez E, Requena MJ, Selth LA, Gahete MD, Castaño JP and
Luque RM: Somatostatin receptor subtype 1 as a potential diagnostic
marker and therapeutic target in prostate cancer. Prostate.
77:1499–1511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Culig Z: Androgen receptor coactivators in
regulation of growth and differentiation in prostate cancer. J Cell
Physiol. 231:270–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo C and Zhang H: The role of
proinflammatory pathways in the pathogenesis of colitis-associated
colorectal cancer. Mediators Inflamm. 2017:51260482017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Debes JD, Schmidt LJ, Huang H and Tindall
DJ: p300 mediates androgen-independent transactivation of the
androgen receptor by interleukin 6. Cancer Res. 62:5632–5636.
2002.PubMed/NCBI
|
|
6
|
Han IH, Kim JH, Kim SS, Ahn MH and Ryu JS:
Signalling pathways associated with IL-6 production and
epithelial-mesenchymal transition induction in prostate epithelial
cells stimulated with Trichomonas vaginalis. Parasite Immunol.
38:678–687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie S, Lin HK, Ni J, Yang L, Wang L, di
Sant'Agnese PA and Chang C: Regulation of interleukin-6-mediated
PI3K activation and neuroendocrine differentiation by androgen
signaling in prostate cancer LNCaP cells. Prostate. 60:61–67. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Wang L, Lin HK, Kan PY, Xie S,
Tsai MY, Wang PH, Chen YT and Chang C: Interleukin-6 differentially
regulates androgen receptor transactivation via PI3K-Akt, STAT3,
and MAPK, three distinct signal pathways in prostate cancer cells.
Biochem Biophys Res Commun. 305:462–469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu N, Zheng JX, Zhuang YS, Zhou ZK, Zhao
JH and Yang L: Anti-inflammatory effects of Schisandrin B on
LPS-stimulated BV2 microglia via activating PPAR-γ. Inflammation.
40:1006–1011. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Checker R, Patwardhan RS, Sharma D, Menon
J, Thoh M, Bhilwade HN, Konishi T and Sandur SK: Schisandrin B
exhibits anti-inflammatory activity through modulation of the
redox-sensitive transcription factors Nrf2 and NF-κB. Free Radic
Biol Med. 53:1421–1430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang X, Wang S, Mu Y and Zheng Y:
Schisandrin B inhibits cell proliferation and induces apoptosis in
human cholangiocarcinoma cells. Oncol Rep. 36:1799–1806. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu XN, Zhang CY, Jin XD, Li YZ, Zheng XZ
and Li L: Inhibitory effect of Schisandrin B on gastric cancer
cells in vitro. World J Gastroenterol. 13:6506–6511. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Y, Zhang Q, Bao J, Du C, Wang J,
Tong Q and Liu C: Schisandrin B suppresses glioma cell metastasis
mediated by inhibition of mTOR/MMP-9 signal pathway. Biomed
Pharmacother. 74:77–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xin DQ, Hu ZM, Huo HJ, Yang XJ, Han D,
Xing WH, Zhao Y and Qiu QH: Schisandrin B attenuates the
inflammatory response, oxidative stress and apoptosis induced by
traumatic spinal cord injury via inhibition of p53 signaling in
adult rats. Mol Med Rep. 16:533–538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inoue H, Waiwut P, Saiki I, Shimada Y and
Sakurai H: Gomisin N enhances TRAIL-induced apoptosis via reactive
oxygen species-mediated up-regulation of death receptors 4 and 5.
Int J Oncol. 40:1058–1065. 2012.PubMed/NCBI
|
|
16
|
Nasser MI, Masood M, Wei W and Li X, Zhou
Y, Liu B, Li J and Li X: Cordycepin induces apoptosis in SGC7901
cells through mitochondrial extrinsic phosphorylation of PI3K/Akt
by generating ROS. Int J Oncol. 50:911–919. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mao J, Hu X, Pang P, Zhou B, Li D and Shan
H: miR-30e acts as a tumor suppressor in hepatocellular carcinoma
partly via JAK1/STAT3 pathway. Oncol Rep. 38:393–401. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim KY, Park KI, Kim SH, Yu SN, Park SG,
Kim YW, Seo YK, Ma JY and Ahn SC: Inhibition of autophagy promotes
salinomycin-induced apoptosis via reactive oxygen species-mediated
PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human
prostate cancer cells. Int J Mol Sci. 18:E10882017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Changou CA, Chen YR, Xing L, Yen Y, Chuang
FY, Cheng RH, Bold RJ, Ann DK and Kung HJ: Arginine
starvation-associated atypical cellular death involves
mitochondrial dysfunction, nuclear DNA leakage, and chromatin
autophagy. Proc Natl Acad Sci USA. 111:14147–14152. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dilly AK, Ekambaram P, Guo Y, Cai Y,
Tucker SC, Fridman R, Kandouz M and Honn KV: Platelet-type
12-lipoxygenase induces MMP9 expression and cellular invasion via
activation of PI3K/Akt/NF-κB. Int J Cancer. 133:1784–1791. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He Y, Huang H, Farischon C, Li D, Du Z,
Zhang K, Zheng X and Goodin S: Combined effects of atorvastatin and
aspirin on growth and apoptosis in human prostate cancer cells.
Oncol Rep. 37:953–960. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abedinpour P, Baron VT, Chrastina A,
Rondeau G, Pelayo J, Welsh J and Borgström P: Plumbagin improves
the efficacy of androgen deprivation therapy in prostate cancer: A
pre-clinical study. Prostate. 77:1550–1562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Culig Z and Puhr M: Interleukin-6: A
multifunctional targetable cytokine in human prostate cancer. Mol
Cell Endocrinol. 360:52–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang WL, Yeh HH, Lin CC, Lai WW, Chang
JY, Chang WT and Su WC: Signal transducer and activator of
transcription 3 activation up-regulates interleukin-6 autocrine
production: A biochemical and genetic study of established cancer
cell lines and clinical isolated human cancer cells. Mol Cancer.
9:3092010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Zhu W, Tao J, Xin P, Liu M, Li J and
Wei M: Fasudil protects the heart against ischemia-reperfusion
injury by attenuating endoplasmic reticulum stress and modulating
SERCA activity: The differential role for PI3K/Akt and JAK2/STAT3
signaling pathways. PLoS One. 7:e481152012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shanmugam MK, Rajendran P, Li F, Nema T,
Vali S, Abbasi T, Kapoor S, Sharma A, Kumar AP, Ho PC, et al:
Ursolic acid inhibits multiple cell survival pathways leading to
suppression of growth of prostate cancer xenograft in nude mice. J
Mol Med. 89:713–727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma M, Ma Y, Zhang GJ, Liao R, Jiang XF,
Yan XX, Bie FJ, Li XB and Lv YH: Eugenol alleviated breast
precancerous lesions through HER2/PI3K-AKT pathway-induced cell
apoptosis and S-phase arrest. Oncotarget. 8:56296–56310.
2017.PubMed/NCBI
|
|
29
|
Cho SH, Park MH, Lee HP, Back MK, Sung HC,
Chang HW, Kim JH, Jeong HS, Han SB and Hong JT:
(E)-2,4-Bis(p-hydroxyphenyl)-2-butenal enhanced TRAIL-induced
apoptosis in ovarian cancer cells through downregulation of
NF-κB/STAT3 pathway. Arch Pharm Res. 37:652–661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Selvaraj N, Budka JA, Ferris MW, Jerde TJ
and Hollenhorst PC: Prostate cancer ETS rearrangements switch a
cell migration gene expression program from RAS/ERK to PI3K/AKT
regulation. Mol Cancer. 13:612014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chae JK, Subedi L, Jeong M, Park YU, Kim
CY, Kim H and Kim SY: Gomisin N inhibits melanogenesis through
regulating the PI3K/Akt and MAPK/ERK signaling pathways in
melanocytes. Int J Mol Sci. 18:E4712017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye D, Luo H, Lai Z, Zou L, Zhu L, Mao J,
Jacob T, Ye W, Wang L and Chen L: ClC-3 chloride channel proteins
regulate the cell cycle by up-regulating cyclin D1-CDK4/6 through
suppressing p21/p27 expression in nasopharyngeal carcinoma Cells.
Sci Rep. 6:302762016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hamid SM, Cicek S, Karamil S, Ozturk MB,
Debelec-Butuner B, Erbaykent-Tepedelen B, Varisli L, Gonen-Korkmaz
C, Yorukoglu K and Korkmaz KS: HOXB13 contributes to G1/S and G2/M
checkpoint controls in prostate. Mol Cell Endocrinol. 383:38–47.
2014. View Article : Google Scholar : PubMed/NCBI
|