Introduction
Despite the advances in therapeutic strategies in
hepatocellular carcinoma (HCC) treatment, HCC is still one of the
most common cancers worldwide (1).
Recent studies have demonstrated that HCC stem cells that exist
within the tumor mass have the ability to propagate and are
considered to play an important role in liver tumor initiation,
progression and metastasis (2). In
addition, growing evidence has revealed that HCC stem cells are
responsible for the resistance of chemotherapy and radiotherapy,
and the recurrence of HCC (3).
Thus, HCC stem cells are becoming crucial for evaluating new
therapeutic strategies and monitoring the progress of HCC
therapy.
CD133, also known as prominin-1, was first
identified as a potential subpopulation of cancer stem cells
(4,5). It is now widely described as a marker
of cancer stem cells in the brain (6), esophagus (7), lung (8), colon (9), prostate (10) and ovaries (11). Notably, CD133 is also a marker
highly recognized in HCC stem cells (12), which is reported to be an important
target for improving chemotherapeutic efficacy of recurrent HCC
cells (13). CD133-expressing HCC
stem cells have also been demonstrated to be involved in liver
tumorigenicity in HCC, conferring radiotherapy resistance due to
their high activation of the AKT/Protein kinase B (PKB), B-cell
lymphoma 2 (Bcl-2) (14) and
mitogen-activated protein kinase (MAPK)/PI3K (15) signaling pathways. In the present
study, CD133-expressing HCC stem cells were chosen as an in
vitro model.
Docetaxel belonging to the taxane family is a
promising anticancer agent which is a semi-synthetic derivative
from the needles of European yew (Taxus baccata) (16). Docetaxel has been widely used to
treat breast (17), prostate
(18), bladder (19), gastric (20), ovarian (21), head and neck (22) and non-small cell lung (23) cancers. Furthermore, the role of
docetaxel in HCC treatment has been recognized due to its low
toxicity and high therapeutic efficacy. Presently, docetaxel has
demonstrated its ability to reduce the hepatocellular tumor size in
nude mice and to inhibit the proliferation of the HepG2 cell line
(24). After intravenously treating
mice with 20 mg/kg silica nanorattle-encapsulated docetaxel, the
hepatocellular tumor size of the mice was significantly decreased
(25). However, the mechanism by
which docetaxel maintains its antitumor capabilities in human
CD133-expressing HCC stem cells remains to be explored.
The aim of the present study was to elucidate the
mechanism by which docetaxel regulated SOX2 expression and cell
apoptosis in CD133-expressing HCC stem cells. We revealed that
docetaxel inhibited SOX2 accumulation and induced cancer cell death
through the suppression of the PI3K/AKT signaling pathway.
Collectively, these findings revealed a novel mechanism that
mediates the regulation of SOX2 and the anticancer effects of
docetaxel in human HCC stem cells.
Materials and methods
Patients and tissue samples
Normal liver tissues and HCC tissues used in this
study were obtained between June 2016 and July 2017 from 48 HCC
patients (aged 47.36±4.57; 36 male and 12 female patients) who had
been treated with 20 mg/kg docetaxel (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) intravenously for a week at our hospital
(Department of Oncology, Jingjiang People's Hospital, Jingjiang
China). Informed consent was obtained from all patients. All
experimental protocols were approved by the Institutional Ethics
Committee of Jingjiang People's Hospital, Jiangsu, China (no.
2018-122).
Cell isolation and culture
Human normal liver stem cells and HCC stem cells
were isolated from liver tissues of HCC patients at our hospital
according to a previous study (26). Briefly, cell suspensions were
centrifuged at 300 × g for 10 min and cell pellets were resuspended
in 300 µl of buffer/108 total cells after aspirating the
supernatant completely. Then, 100 µl of FcR Blocking
Reagent/108 total cells and 100 µl of CD133/CD44/CD24
MicroBeads/108 total cells were added, mixed well and
incubated for 30 min in the refrigerator. Cells were washed by
adding 1–2 ml of buffer/108 cells and centrifuged at 300
× g for 10 min. An appropriate MACS Column and MACS Separator was
chosen according to the number of total cells and the number of
CD133+/CD44+/CD24+ cells. The
column was placed in the magnetic field of a suitable MACS
Separator, and prepared by rinsing with 500 µl buffer MS. The cell
suspension was applied onto the column and washed with the
appropriate amount of buffer. Unlabeled cells that passed through
and combined with the effluent from the MS step were collected.
Cells were maintained in Dulbecco's modified Eagle's medium (DMEM;
Gibco-BRL Life Technologies; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS),
100 IU/ml penicillin, and 10 mg/ml streptomycin (Sigma-Aldrich;
Merck KGaA) in an incubator with a humidified atmosphere of 5%
CO2 at 37°C.
Cell viability assay
Cell viability was assessed using an MTT assay
(Bestbio Biotechnology, Shanghai, China). Briefly, human HCC stem
cells at a concentration of 2×103 cells/well were seeded
in 96-well flat-bottomed tissue culture plates (Corning Inc.,
Corning, NY, USA) for 24 h. Following two washes with
phosphate-buffered saline (PBS), cells were incubated in 100 µl
culture medium containing 50 nM docetaxel for 12, 24 and 48 h at
37°C prior to the MTT assay. Then, a total of 10 µl MTT and 100 µl
culture medium was added to each well, and following incubation for
1 h at 37°C, the optical densities of the samples were measured
directly using a spectrophotometric microplate reader (Beyotime
Institute of Biotechnology, Haimen, China) at a wavelength of 490
nm.
Cell apoptosis assay
The apoptotic cells were identified by the
terminal-deoxynucleotidyl transferase mediated nick end-labeling
(TUNEL) apoptosis assay kit (KeyGen Biotech Co., Ltd., Nanjing,
China) according to manufacturer's instructions. Cells at a density
of 2×104/ml were cultured in 10% FBS-containing DMEM
with 50 nM docetaxel for 24 h and harvested and washed twice with
cold PBS by gentle shaking. Resuspended cells were added to 1X
binding buffer and the cell density was adjusted to
200,000-500,000/ml. Cell apoptosis was analyzed using a FACScan
flow cytometric apparatus (BD Biosciences, San Jose, CA, USA) and
the percentage of apoptotic cells was analyzed using FlowJo 7.6.1
software (TreeStar, Inc., Ashland, OR, USA).
RT-PCR
The expression of CD133 and SOX2 in human HCC stem
cells was assessed by RNA preparation and quantitative reverse
transcription-polymerase chain reaction (RT-PCR). Total cellular
RNA isolation using TRIzol reagent and cDNA synthesis using Takara
PrimeScript II First Strand cDNA Synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.) was conducted according to the
manufacturers' instructions. Specific primer sequences were
synthesized in BioSune Biological Technology Corp. (Shanghai,
China), and the sequences of the primers were as follows: CD133
forward, 5′-CCATACCTAGGTCCCCGTCC-3′ and reverse,
5′-TTCACTCAAGGCACCATCCC-3′; SOX2 forward,
5′-AACCAGCGCATGGACAGTTA-3′ and reverse, 5′-GACTTGACCACCGAACCCAT-3′;
GAPDH forward, 5′-AATGGGCAGCCGTTAGGAAA-3′ and reverse,
5′-GCGCCCAATACGACCAAATC-3′. Data was analyzed using Bio-Rad CFX
Manager 1.6 Software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA.
Western blot analysis
Following the treatment with 50 nM docetaxel for 6
h, the cells were incubated for an additional 24 h prior to the
collection of cells for protein extraction. The examination of the
expression levels of CD133, SOX2, PI3K, AKT and p-AKT was then
performed separately. Total protein was extracted using Total
Protein Extraction kit (Sigma-Aldrich; Merck KGaA). Total protein
was quantified using a bicinchoninic acid assay kit (Beyotime
Institute of Biotechnology) and 30 µg protein/lane was separated
via SDS-PAGE on a 6% gel and run on a 10% gel. The separated
proteins were subsequently transferred to nitrocellulose (NC)
filter membrane and blocked with Tris-buffered saline (TBS)
containing 5% milk powder without fat and 0.05% Tween-20.
Antibodies of CD133 (dilution 1:1,000; cat. no. sc-33182), PI3K
(dilution 1:1,000; cat. no. 4922), AKT (dilution 1:1,000; cat. no.
sc-9272) and p-AKT (dilution 1:1,000; cat. no. sc-33437) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Antibodies of SOX2 (dilution 1:3,000; cat. no. 3579) and
GAPDH (dilution 1:6,000; cat. no. 5174) were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA) and incubated at
overnight at 4°C. Secondary antibodies were conserved in our
laboratory and incubated at 37°C for 1 h. The protein levels were
detected using the chemiluminescence reader, ImageQuant™ LAS4000
(GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Relative band
ratio was analyzed using ImageJ software (version 1.48; National
Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
Paraffin-embedded normal liver and cancer liver
tissues (3-µm in thickness) were prepared and immunohistochemistry
was performed as previously described (27). Primary antibodies against CD133
(dilution 1:500; Santa Cruz Biotechnology, Inc.) and SOX2 (dilution
1:200; Abcam, Cambridge, UK) were used. Data of
immunohistochemistry were analyzed using Image-Pro Plus (version
4.1; Media Cybernetics, Rockville, MD, USA).
Block of PI3K/AKT signal using an
inhibitor
Human HCC stem cells were treated with PI3K/AKT
inhibitor LY294002 at a concentration of 25 µM for 48 h, followed
by the addition of 50 nM docetaxel and further incubation for 48 h.
Then, cell proliferation, apoptosis, and SOX2 expression were
evaluated.
Statistical analysis
All results were analyzed by one-way analysis of
variance (ANOVA) using the SPSS 17.0 statistical software (SPSS,
Inc., Chicago, IL, USA). Data are presented as the mean ± standard
deviation (SD). Boferroni's post hoc test was used to determine the
statistical differences between the treatment and control groups.
P-values were based on the two-sided statistical analysis, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Docetaxel downregulates the expression
of CD133 and SOX2 in patients with HCC
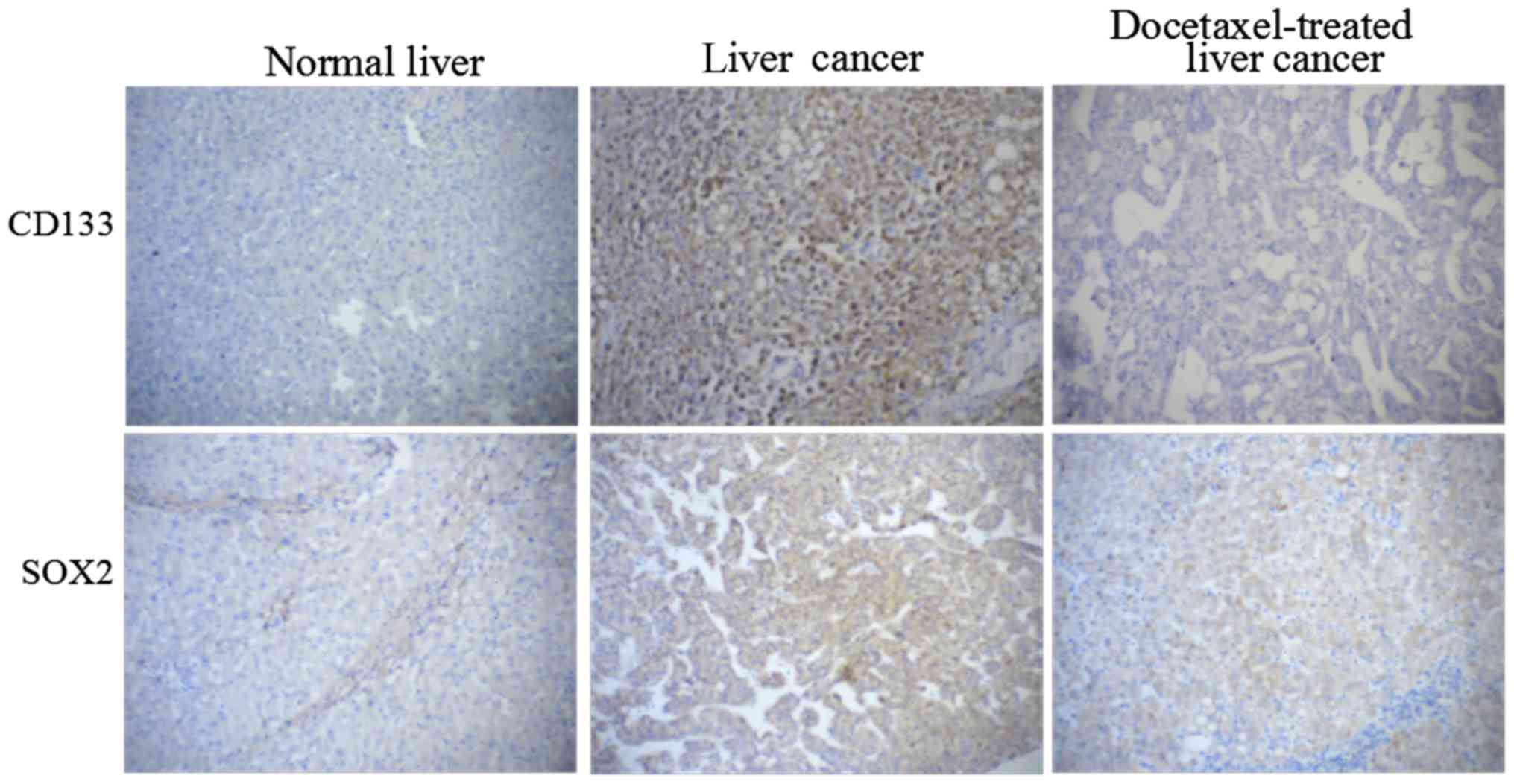
As shown in Fig. 1,
higher expression of CD133 and SOX2 was detected by
immunohistochemistry in HCC samples compared with normal liver
tissues, indicating that CD133 and SOX2 were two important factors
which may be involved in HCC. Moreover, the expression of CD133 and
SOX2 in human HCC tissues was significantly downregulated by
docetaxel compared to cells without docetaxel stimulation.
Identification of CD133-expressing HCC
stem cells
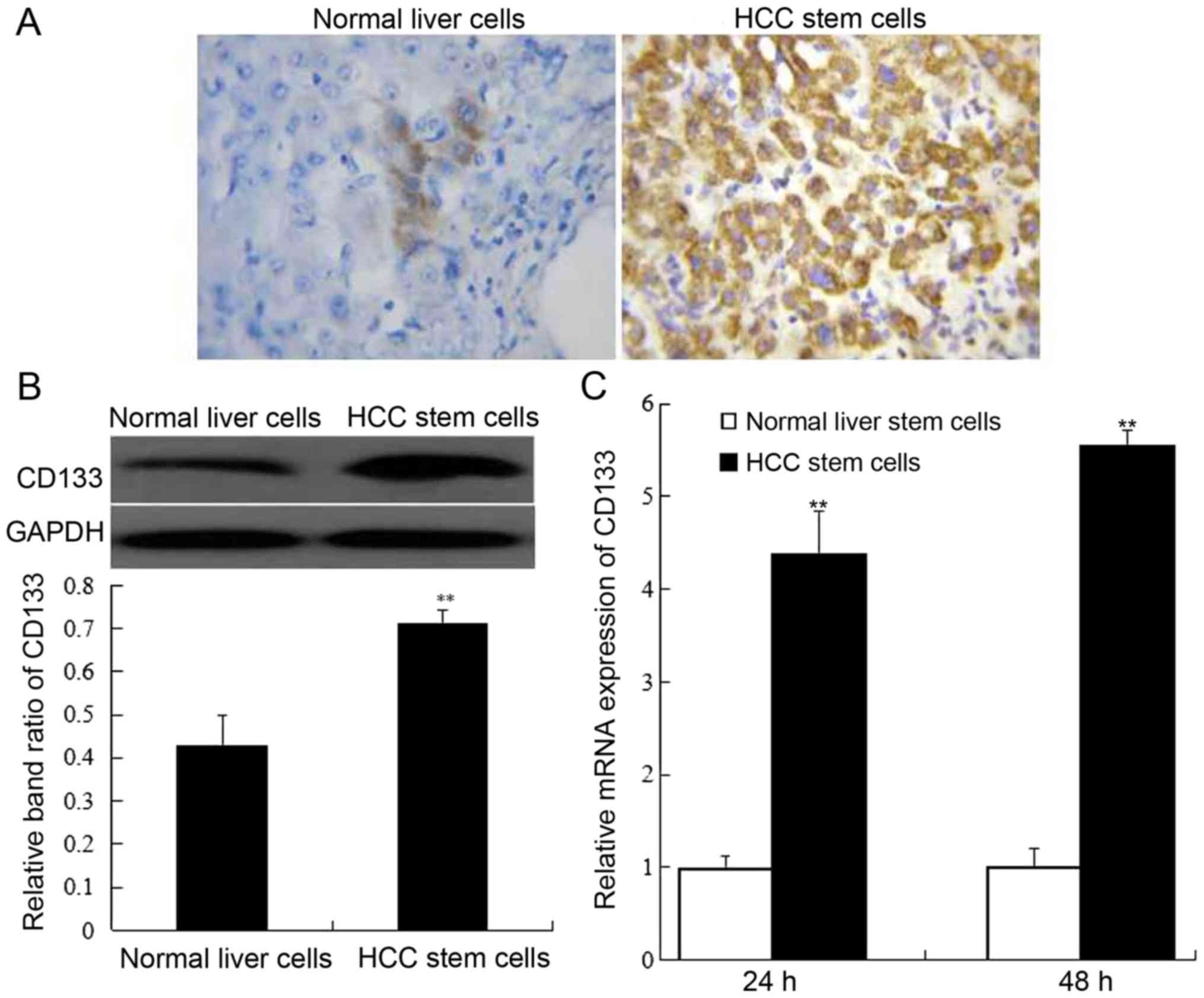
Following 24 and 48 h of cell culture, CD133
expression in normal liver stem cells and HCC stem cells was
determined by immunohistochemistry, western blotting and RT-PCR.
The results revealed that the percentage of CD133-positive cells
was obviously higher in HCC stem cells than that of normal liver
stem cells (Fig. 2A). Additionally,
compared to normal liver stem cells, CD133 protein expression was
highly promoted in HCC stem cells (P<0.01). (Fig. 2B). The assessment of CD133 mRNA
expression also supported the conclusion that CD133 expression was
increased in HCC stem cells (Fig.
2C).
Docetaxel inhibits the proliferation
while promoting the apoptotic rate of human CD133-expressing HCC
stem cells
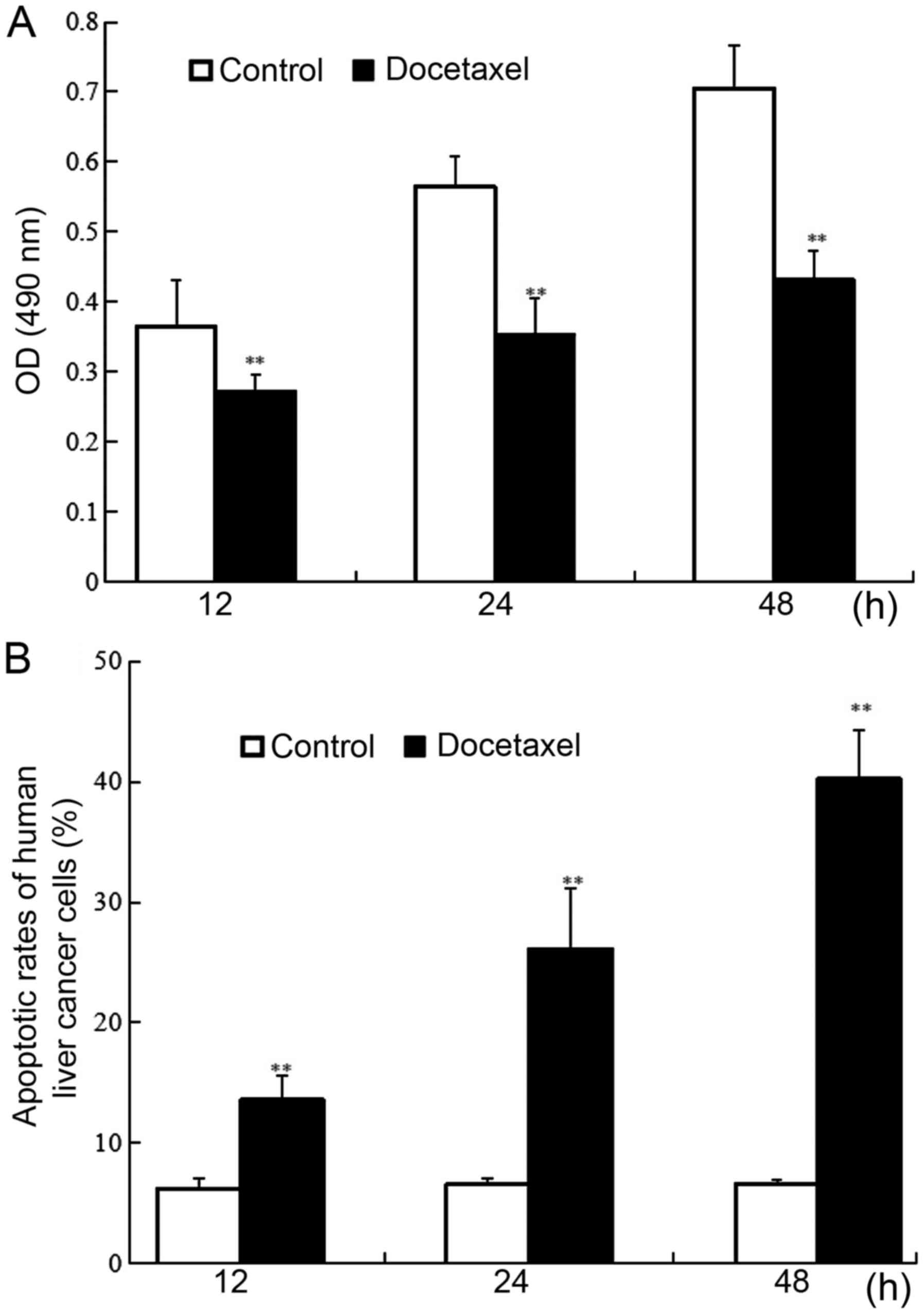
Human CD133-expressing HCC stem cells were
respectively exposed to docetaxel for 12, 24 and 48 h, and the role
of docetaxel in human CD133-expressing HCC stem cells was assessed
through MTT assay and flow cytometry. The results revealed that
compared to the control without docetaxel treatment, docetaxel
significantly downregulated cell viability in CD133-expressing HCC
stem cells in a time-dependent manner (P<0.01) (Fig. 3A). Conversely, as shown in Fig. 3B, docetaxel significantly promoted
apoptosis in CD133-expressing HCC stem cells in a time-dependent
manner. Approximately 13.58, 26.13 and 40.24% of cells underwent
apoptosis after exposure to docetaxel for 12, 24 and 48 h,
respectively, and there was a significant difference between
docetaxel-treated groups and the control without docetaxel
treatment (P<0.01). The findings above indicated that docetaxel
significantly increased the apoptosis in CD133-expressing HCC stem
cells.
Docetaxel suppresses the expression of
SOX2 in human CD133-expressing HCC stem cells
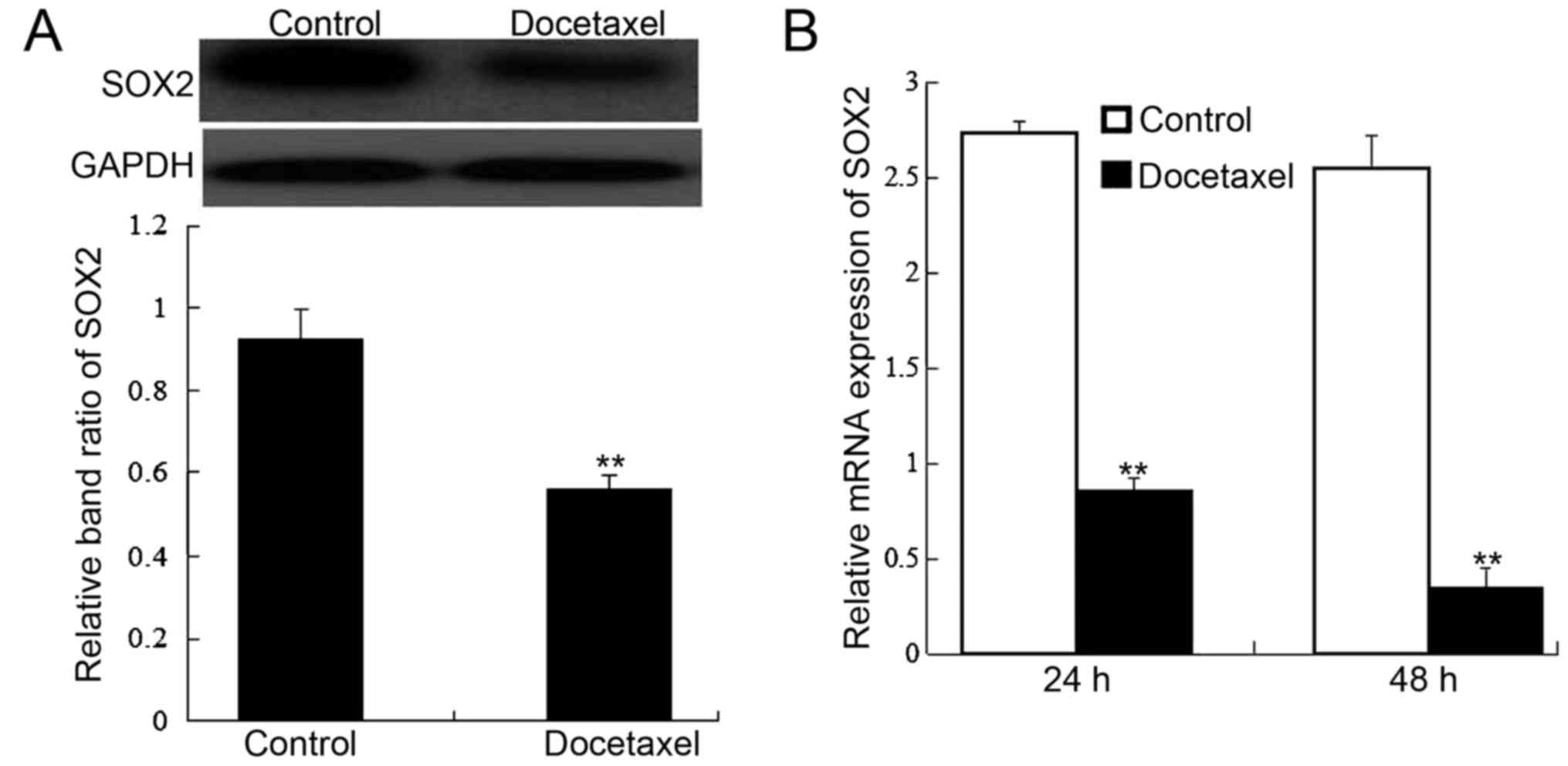
Since SOX2 expression is associated with HCC, we
further detected SOX2 protein and mRNA expression using western
blotting and RT-PCR at indicated time-points after docetaxel
stimulation. The results demonstrated that SOX2 protein (Fig. 4A) and mRNA (Fig. 4B) expression was significantly
inhibited by docetaxel in human HCC stem cells (P<0.05) in
comparison with the control.
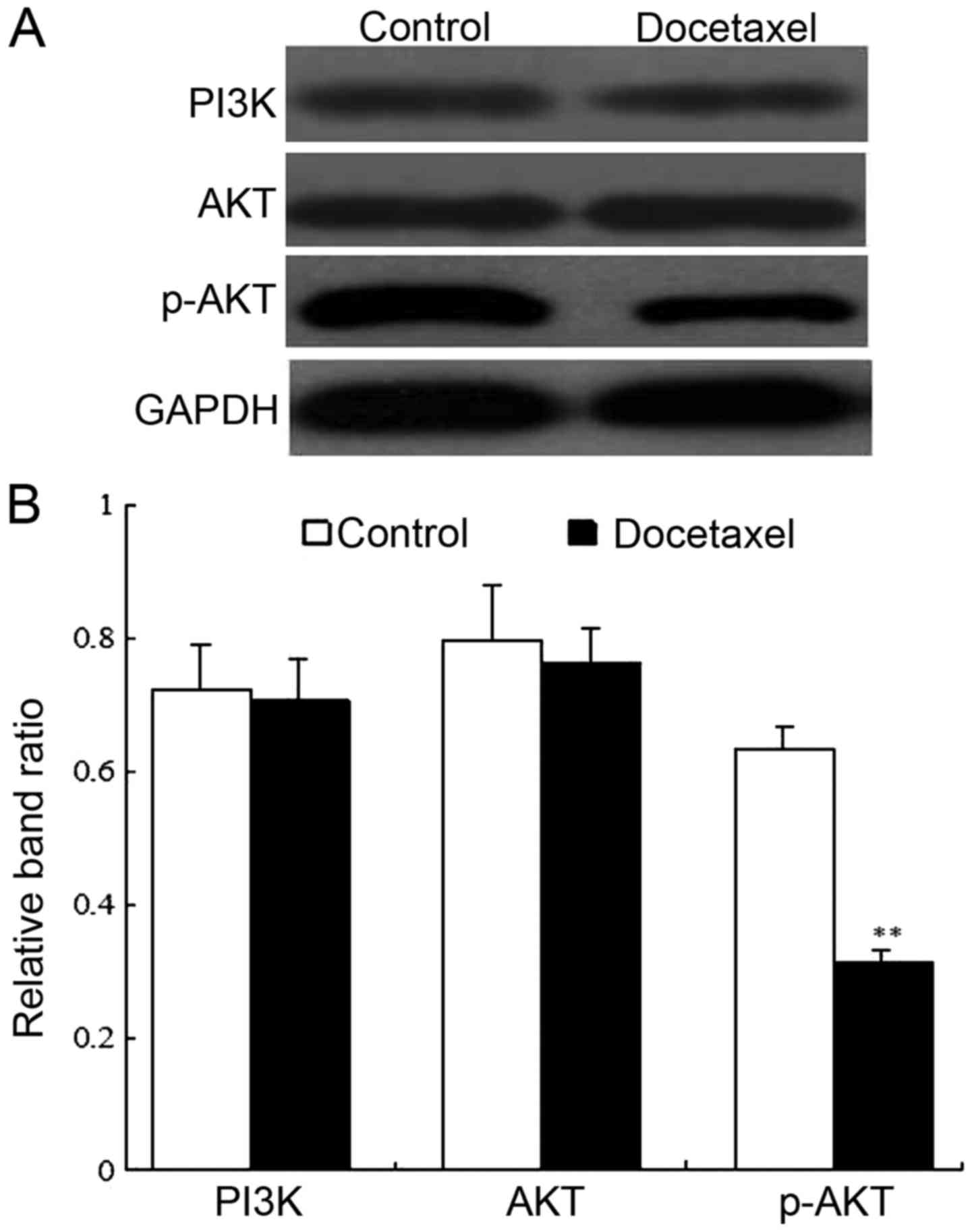
Docetaxel inhibits the PI3K/AKT
signaling pathway in human CD133-expressing HCC stem cells
To further verify the underlying mechanisms of
docetaxel in human CD133-expressing HCC stem cells, we explored the
expression of PI3K, AKT and p-AKT by western blotting (Fig. 5A). Band analysis indicated that the
protein level of PI3K and AKT exhibited no difference between the
docetaxel-treated group and non-docetaxel-treated group
(P>0.05). Moreover, the p-AKT protein level was comparatively
low in docetaxel-treated cells in comparison with the control
(P<0.01) (Fig. 5B). It was thus
suggested that docetaxel could suppress the PI3K/AKT signaling
pathway in human CD133-expressing HCC stem cells.
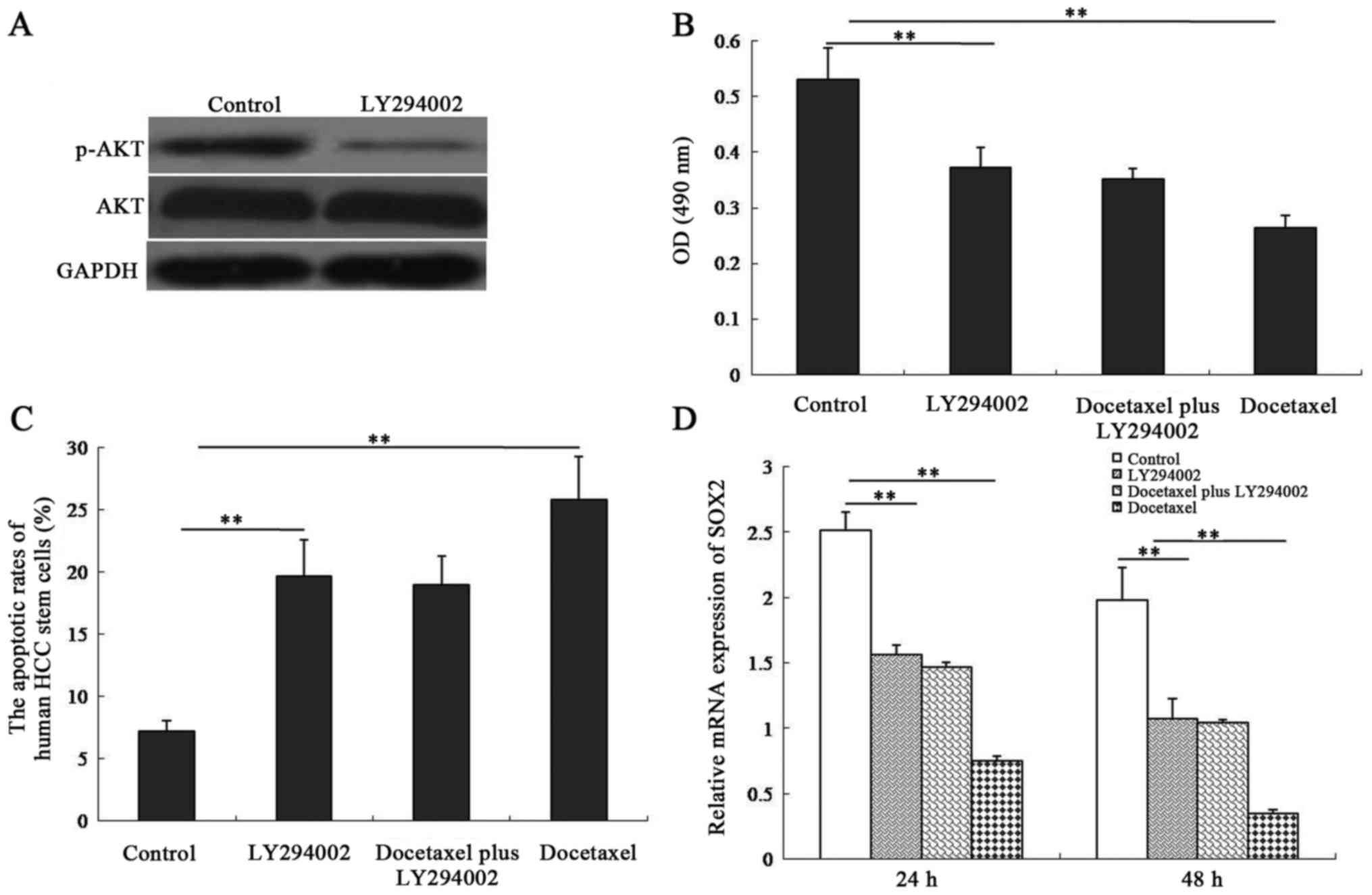
Inhibition of the PI3K/AKT signaling
pathway in human CD133-expressing HCC stem cells is required for
docetaxel-induced cell apoptosis and decreased SOX2 expression
We next examined the influence of the PI3K/AKT
signaling on docetaxel-induced cell apoptosis and -decreased SOX2
expression in human CD133-expressing HCC stem cells. The results
revealed that HCC stem cells treated with the PI3K/AKT inhibitor
LY294002 exhibited a significant decrease in p-AKT expression
compared with untreated cells (Fig.
6A). Cell viability (Fig. 6B)
and SOX2 expression (Fig. 6D) were
inhibited in both LY294002-treated cells and docetaxel-treated
cells. However, there was no difference in cell viability and SOX2
expression between LY294002 plus docetaxel-treated cells and
LY294002-treated cells (P>0.05). As demonstrated in Fig. 6C, LY294002 or docetaxel
significantly promoted the apoptotic rate in CD133-expressing HCC
stem cells (P<0.01). Moreover, the apoptotic rate exhibited no
difference between the LY294002-treated group and LY294002 plus
docetaxel-treated group (P>0.05). These results indicated that
the PI3K/AKT signaling pathway was involved in docetaxel-exerted
biological functions in human CD133-expressing HCC stem cells.
Discussion
In the present study, we investigated the mechanisms
underlying docetaxel-induced cell apoptosis in CD133-expressing HCC
stem cells. We determined that docetaxel induced the suppression of
the PI3K/AKT signaling pathway, thereby causing HCC stem cell death
and decreased SOX2 expression.
Docetaxel has been used to treat various types of
cancers. In vitro, docetaxel suppresses proliferation and
induces apoptosis by suppressing the mitogen-activated protein
kinase (MAPK) signaling in renal cell carcinoma cells (28). Docetaxel induces cell apoptosis and
suppresses cell proliferation in non-small cell lung cancer cells
by upregulating microRNA-7 expression (29). In a study on HCC treatment with
docetaxel, Geng et al demonstrated that docetaxel enhanced
radiation sensitivity of human HCC cells (30). Additionally, another study from the
same authors provided the evidence that docetaxel reduced the
proliferation of SMMC-7721 HCC cells in vitro, kept their
morphology, and induced cell death by apoptosis (31). Docetaxel was revealed to inhibit the
growth of hepatoma cells by arresting the G2/M-phase, activating
caspases, and fragmenting DNA (32). A recent investigation confirmed that
docetaxel inhibited the progression of cultured human hepatoma
cells in advanced HCC (33). In the
present study, we also revealed that docetaxel inhibited
proliferation while promoting apoptosis in CD133-expressing HCC
stem cells through the inactivation of the PI3K/AKT signaling
pathway.
According to research, SOX2, a major transcription
factor is regarded as a stemness-related factor. It has been
demonstrated that SOX2 is associated with various types of cancers
and has been used as a marker to identify cancer stem cells
(34). Furthermore, SOX2
suppression is mandatory for cellular differentiation. For these
reasons, SOX2 has been investigated in cancer stem cells in several
cancer types. Notably, predictive value of SOX2 in cancers is
associated with the prognosis of patients and is regarded as a
possible therapeutic target. In a review study, the role of SOX2 as
a prognostic marker, indicator of metastasis, or biomarker in
cancer pathogenesis was highlighted (35). Recently, numerous studies have
provided evidence that SOX2 mRNA expression was significantly
higher in patients with small-cell lung (36), gastric (37), breast (38), cervical (39) and ovarian epithelial cancer
(40) compared to the healthy
controls. A high level of SOX2 expression was revealed to be
correlated with metastasis and a low survival rate in HCC (41). In the present study, we attempted to
reveal the relationship between docetaxel and SOX2, and
demonstrated that protein and mRNA expression of SOX2 exhibited a
significant decrease in docetaxel-treated HCC stem cells.
In order to investigate the mechanisms underlying
the antitumor effects of docetaxel in CD133-expressing HCC stem
cells, the activation of the PI3K/AKT signaling pathway was
determined after cells were treated with docetaxel. Our results
demonstrated that p-AKT expression was significantly decreased in
the drug-treated CD133-expressing HCC stem cells compared with that
of the control group, indicating that the suppression of the
PI3K/AKT signaling pathway occured in the presence of docetaxel,
and this signal suppression was involved in the changes induced by
docetaxel in CD133-expressing HCC stem cells. Previous studies have
indicated that PI3K is a type of lipid kinase, and its cascades
play an essential role in regulating growth, migration, and
survival of different types of tumor cells (42). Additionally, accumulating evidence
has revealed that PI3K/AKT was associated with tumorigenesis,
cancer progression and drug resistance (43). Several drugs targeting PI3K/ATK are
currently used in clinical trials to treat cancers (44–46).
Previous research has revealed that docetaxel induced the apoptosis
in human prostate cancer cells by modulating the PI3K/AKT pathway
(47). Thus, docetaxel and its
target PI3K/AKT may offer a new direction for cancer therapy.
In summary, we reported for the first time, to the
best of our knowledge, that docetaxel inhibited the growth of human
CD133-expressing HCC stem cells by causing cell apoptosis. Further
investigation revealed that the PI3K/AKT signaling pathway played a
key role in docetaxel-induced apoptosis of human CD133-expressing
HCC stem cells. These findings may add new insights to HCC stem
cells and help to explore their functions in tumor therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XZ and ZT were involved in the manuscript
preparation and the conception of the study; XZ, JS and ZT were
responsible for the manuscript editing, reviewing and study design,
carried out the experimental studies and were involved in the data
acquisition; XZ, XL and ZT were responsible for revising the
manuscript critically for important intellectual content; XZ, XL,
LC and ZT performed the literature research; XL and LC were
involved in the clinical studies; XZ, XL and LC were responsible
for the data analysis; XZ and LC performed the statistical
analysis. All authors read and approved the manuscript and agree to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Institutional Ethics Committee of Jingjiang People's Hospital,
Jiangsu, China (no. 2018-122). Informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
SD
|
standard deviation
|
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun JH, Luo Q, Liu LL and Song GB: Liver
cancer stem cell markers: Progression and therapeutic implications.
World J Gastroenterol. 22:3547–3557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karakasiliotis I and Mavromara P:
Hepatocellular carcinoma: From hepatocyte to liver cancer stem
cell. Front Physiol. 6:1542015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grosse-Gehling P, Fargeas CA, Dittfeld C,
Garbe Y, Alison MR, Corbeil D and Kunz-Schughart LA: CD133 as a
biomarker for putative cancer stem cells in solid tumours:
Limitations, problems and challenges. J Pathol. 229:355–378. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suetsugu A, Nagaki M, Aoki H, Motohashi T,
Kunisada T and Moriwaki H: Characterization of CD133+
hepatocellular carcinoma cells as cancer stem/progenitor cells.
Biochem Biophys Res Commun. 351:820–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li B, McCrudden CM, Yuen HF, Xi X, Lyu P,
Chan KW, Zhang SD and Kwok HF: CD133 in brain tumor: The prognostic
factor. Oncotarget. 8:11144–11159. 2017.PubMed/NCBI
|
|
7
|
Okamoto K, Ninomiya I, Ohbatake Y, Hirose
A, Tsukada T, Nakanuma S, Sakai S, Kinoshita J, Makino I, Nakamura
K, et al: Expression status of CD44 and CD133 as a prognostic
marker in esophageal squamous cell carcinoma treated with
neoadjuvant chemotherapy followed by radical esophagectomy. Oncol
Rep. 36:3333–3342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tu Z, Xie S, Xiong M, Liu Y, Yang X, Tembo
KM, Huang J, Hu W, Huang X, Pan S, et al: CXCR4 is involved in
CD133-induced EMT in non-small cell lung cancer. Int J Oncol.
50:505–514. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shmelkov SV, Butler JM, Hooper AT, Hormigo
A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, et
al: CD133 expression is not restricted to stem cells, and both
CD133+ and CD133 metastatic colon cancer cells initiate
tumors. J Clin Invest. 118:2111–2120. 2008.PubMed/NCBI
|
|
10
|
Vander Griend DJ, Karthaus WL, Dalrymple
S, Meeker A, DeMarzo AM and Isaacs JT: The role of CD133 in normal
human prostate stem cells and malignant cancer-initiating cells.
Cancer Res. 68:9703–9711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin Q, Sun Y, Fei M, Zhang J, Jia Y, Gu M,
Xia R, Chen S and Deng A: Expression of putative stem marker nestin
and CD133 in advanced serous ovarian cancer. Neoplasma. 59:310–315.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jang JW, Song Y, Kim SH, Kim JS, Kim KM,
Choi EK, Kim J and Seo HR: CD133 confers cancer stem-like cell
properties by stabilizing EGFR-AKT signaling in hepatocellular
carcinoma. Cancer Lett. 389:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xi G, Li YD, Grahovac G, Rajaram V,
Wadhwani N, Pundy T, Mania-Farnell B, James CD and Tomita T:
Targeting CD133 improves chemotherapeutic efficacy of recurrent
pediatric pilocytic astrocytoma following prolonged chemotherapy.
Mol Cancer. 16:212017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma S, Lee TK, Zheng BJ, Chan KW and Guan
XY: CD133+ HCC cancer stem cells confer chemoresistance
by preferential expression of the Akt/PKB survival pathway.
Oncogene. 27:1749–1758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Li H, Ge C, Li M, Zhao FY, Hou
HL, Zhu MX, Tian H, Zhang LX, Chen TY, et al: Inhibitory effects of
transcription factor Ikaros on the expression of liver cancer stem
cell marker CD133 in hepatocellular carcinoma. Oncotarget.
5:10621–10635. 2014.PubMed/NCBI
|
|
16
|
Guéritte-Voegelein F, Guénard D, Dubois J,
Wahl A and Potier P: Chemical and biological studies on Taxol
(Paclitaxel) and Taxotere (Docetaxel), new antineoplastic agents. J
Pharm Belg. 49:193–205. 1994.(In French). PubMed/NCBI
|
|
17
|
Seguin C, Kovacevich N and Voutsadakis IA:
Docetaxel-associated myalgia-arthralgia syndrome in patients with
breast cancer. Breast Cancer. 9:39–44. 2017.PubMed/NCBI
|
|
18
|
Belz J, Castilla-Ojo N, Sridhar S and
Kumar R: Radiosensitizing silica nanoparticles encapsulating
docetaxel for treatment of prostate cancer. Methods Mol Biol.
1530:403–409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albany C and Sonpavde G: Docetaxel for the
treatment of bladder cancer. Expert Opin Investig Drugs.
24:1657–1664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dassen AE, Bernards N, Lemmens VE, van de
Wouw YA, Bosscha K, Creemers GJ and Pruijt HJ: Phase II study of
docetaxel, cisplatin and capecitabine as preoperative chemotherapy
in resectable gastric cancer. World J Gastrointest Surg. 8:706–712.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hami Z, Rezayat SM, Gilani K, Amini M and
Ghazi-Khansari M: In-vitro cytotoxicity and combination effects of
the docetaxel-conjugated and doxorubicin-conjugated poly(lactic
acid)-poly(ethylene glycol)-folate-based polymeric micelles in
human ovarian cancer cells. J Pharm Pharmacol. 69:151–160. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Posch D, Fuchs H, Kornek G, Grah A, Pammer
J, Aretin MB and Fuereder T: Docetaxel plus cetuximab biweekly is
an active regimen for the first-line treatment of patients with
recurrent/metastatic head and neck cancer. Sci Rep. 6:329462016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukae M, Shiraishi Y, Hirota T, Sasaki Y,
Yamahashi M, Takayama K, Nakanishi Y and Ieiri I: Population
pharmacokinetic-pharmacodynamic modeling and model-based prediction
of docetaxel-induced neutropenia in Japanese patients with
non-small cell lung cancer. Cancer Chemother Pharmacol.
78:1013–1023. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu D, Tao W, Zhang H, Liu G, Wang T,
Zhang L, Zeng X and Mei L: Docetaxel (DTX)-loaded
polydopamine-modified TPGS-PLA nanoparticles as a targeted drug
delivery system for the treatment of liver cancer. Acta Biomater.
30:144–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li L, Tang F, Liu H, Liu T, Hao N, Chen D,
Teng X and He J: In vivo delivery of silica nanorattle encapsulated
docetaxel for liver cancer therapy with low toxicity and high
efficacy. ACS Nano. 4:6874–6882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pu H, Zheng Q, Li H, Wu M, An J, Gui X, Li
T and Lu D: CUDR promotes liver cancer stem cell growth through
upregulating TERT and C-Myc. Oncotarget. 6:40775–40798. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng X, Zhu K, Liu J, Chen J, Tang J,
Liang Y, Jin R, Liang X and Cai X: The evaluative value of Sema3C
and MFN2 co-expression detected by immunohistochemistry for
prognosis in hepatocellular carcinoma patients after hepatectomy.
Onco Targets Ther. 9:3213–3221. 2016.PubMed/NCBI
|
|
28
|
Han TD, Shang DH and Tian Y: Docetaxel
enhances apoptosis and G2/M cell cycle arrest by suppressing
mitogen-activated protein kinase signaling in human renal clear
cell carcinoma. Genet Mol Res. 15:2016. View Article : Google Scholar :
|
|
29
|
He X, Li C, Wu X and Yang G: Docetaxel
inhibits the proliferation of non-small-cell lung cancer cells via
upregulation of microRNA-7 expression. Int J Clin Exp Pathol.
8:9072–9080. 2015.PubMed/NCBI
|
|
30
|
Geng CX, Zeng ZC, Wang JY, Xuan SY and Lin
CM: Docetaxel shows radiosensitization in human hepatocellular
carcinoma cells. World J Gastroenterol. 11:2990–2993. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geng CX, Zeng ZC and Wang JY: Docetaxel
inhibits SMMC-7721 human hepatocellular carcinoma cells growth and
induces apoptosis. World J Gastroenterol. 9:696–700. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin HL, Liu TY, Chau GY, Lui WY and Chi
CW: Comparison of 2-methoxyestradiol-induced, docetaxel-induced,
and paclitaxel-induced apoptosis in hepatoma cells and its
correlation with reactive oxygen species. Cancer. 89:983–994. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yata Y, Xue F, Takahara T, Kudo H, Hirano
K, Yasumura S, Minemura M, Scanga AE and Sugiyama T: Docetaxel
inhibits progression of human hepatoma cell line in vitro and is
effective in advanced hepatocellular carcinoma. Hepatol Res.
40:304–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang L, Xu JF, Kang Q, Li AQ, Jin P, Wang
X, He YQ, Li N, Cheng T and Sheng JQ: Predictive value of stemness
factor Sox2 in gastric cancer is associated with tumor location and
stage. PLoS One. 12:e01691242017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weina K and Utikal J: SOX2 and cancer:
Current research and its implications in the clinic. Clin Transl
Med. 3:192014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sodja E, Rijavec M, Koren A, Sadikov A,
Korošec P and Cufer T: The prognostic value of whole blood SOX2,
NANOG and OCT4 mRNA expression in advanced small-cell lung cancer.
Radiol Oncol. 50:188–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carrasco-Garcia E, Santos JC, Garcia I,
Brianti M, García-Puga M, Pedrazzoli J Jr, Matheu A and Ribeiro ML:
Paradoxical role of SOX2 in gastric cancer. Am J Cancer Res.
6:701–713. 2016.PubMed/NCBI
|
|
38
|
Zheng Y, Qin B, Li F, Xu S, Wang S and Li
L: Clinicopathological significance of Sox2 expression in patients
with breast cancer: A meta-analysis. Int J Clin Exp Med.
8:22382–22392. 2015.PubMed/NCBI
|
|
39
|
Kim BW, Cho H, Choi CH, Ylaya K, Chung JY,
Kim JH and Hewitt SM: Clinical significance of OCT4 and SOX2
protein expression in cervical cancer. BMC Cancer. 15:10152015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Du J, Li B, Fang Y, Liu Y, Wang Y, Li J,
Zhou W and Wang X: Overexpression of Class III β-tubulin, Sox2, and
nuclear Survivin is predictive of taxane resistance in patients
with stage III ovarian epithelial cancer. BMC Cancer. 15:5362015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun C, Sun L, Li Y, Kang X, Zhang S and
Liu Y: Sox2 expression predicts poor survival of hepatocellular
carcinoma patients and it promotes liver cancer cell invasion by
activating Slug. Med Oncol. 30:5032013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guerrero-Zotano A, Mayer IA and Arteaga
CL: PI3K/AKT/mTOR: Role in breast cancer progression, drug
resistance, and treatment. Cancer Metastasis Rev. 35:515–524. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sharma VR, Gupta GK and Sharma AK, Batra
N, Sharma DK, Joshi A and Sharma AK: PI3K/Akt/mTOR Intracellular
Pathway and Breast Cancer: Factors, Mechanism and Regulation. Curr
Pharm Des. 23:1633–1638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang
Z, Li Z, Feng X, Hao J, Zhang K, et al: Melatonin synergizes the
chemotherapeutic effect of 5-fluorouracil in colon cancer by
suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J Pineal
Res. 62:2017. View Article : Google Scholar
|
|
46
|
Luo Y, Wu JY, Lu MH, Shi Z, Na N and Di
JM: Carvacrol alleviates prostate cancer cell proliferation,
migration, and invasion through regulation of PI3K/Akt and MAPK
signaling pathways. Oxid Med Cell Longev. 2016:14696932016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dirican A, Atmaca H, Bozkurt E, Erten C,
Karaca B and Uslu R: Novel combination of docetaxel and
thymoquinone induces synergistic cytotoxicity and apoptosis in
DU-145 human prostate cancer cells by modulating PI3K-AKT pathway.
Clin Transl Oncol. 17:145–151. 2015. View Article : Google Scholar : PubMed/NCBI
|