Introduction
Prostatic carcinoma is one of the most common
malignancies worldwide, affecting 1 in 9 men >65 years of age
(1–3). According to the statistical analysis,
the percentage of patients with prostatic carcinoma among men aged
65 years or older will rise to 19.6% (4). Currently, there are no effective
treatments for advanced-stage prostatic carcinoma, and it is the
second leading cause of cancer-associated mortality in men
(5,6). Therefore, the identification of novel
endogenous factors responsible for proliferation, migration and
invasion will facilitate understanding of the progression of
prostatic carcinoma, as well as the development of novel approaches
for its diagnosis and therapy.
There are four mitogen-activated protein kinase
(MAPK) pathways in mammalian cells: The extracellular
signal-regulated kinase (ERK) 1/2, Janus kinase, p38 and ERK5
pathways. Activation of the MAPK pathway results in the
phosphorylation of the downstream mediators, including substrates,
transcription factors, protein kinases, and enzymes, to regulate
cell proliferation, differentiation, apoptosis, and migration, as
well as tumourigenesis, tumour invasion and migration and
chemoresistance (7,8). The ERK5 pathway was the most recently
identified and is the least studied mammalian MAPK cascade. The
ERK5 pathway is usually activated by mitogens and oncogenic signals
and has been demonstrated to be involved in tumourigenesis.
Deregulated ERK5 signalling has been associated with properties of
human malignancies, including the chemoresistance of breast tumour
cells (9), the uncontrolled
proliferation of erb-b2 receptor tyrosine kinase 2-overexpressing
carcinomas (10), the metastatic
potential of prostatic carcinoma cells (11) and tumour-associated angiogenesis
(12). Additionally, our previous
study indicated that ERK5 pathway activation induces cell
proliferation and regulates the cell cycle in prostatic carcinoma
cells (13).
Ras-like oestrogen-regulated growth inhibitor (RERG)
was initially identified as a potential tumour suppressor gene and
is regulated by oestrogen in breast tumours (14,15).
RERG is widely expressed in multiple normal tissues, whereas RERG
expression is inhibited in tumours of the breast, kidney, ovary and
colon (15,16). Previous studies have indicated that
RERG expression may be associated with cell proliferation and
distant metastasis in breast cancer and nasopharyngeal carcinoma
(17,18), suggesting that it has potential as a
prognostic biomarker in malignant tumours. Similarly, several
reports have revealed the presence of significantly hypermethylated
RERG in breast cancer and colorectal adenocarcinoma carcinoma
(19,20). Although this evidence has been
demonstrated to be associated with cell proliferation and tumour
metastasis, its biological significance for the progression of
prostatic carcinoma remains elusive.
In the present study, mRNA microarray analysis
demonstrated RERG expression inhibition in EGF-treated prostatic
carcinoma cells. Further investigations revealed that the
expression level of RERG protein was associated with malignancy in
patients with prostatic carcinoma. In addition, the results
indicated that the inhibition of RERG promoted cell proliferation
by inhibiting NF-κB-mediated apoptosis. Furthermore, the
overexpression of RERG protein suppressed tumour metastasis in
prostatic carcinoma cells. It was additionally demonstrated that
matrix metallopeptidase (MMP)-2 and MMP-9 were associated with
RERG-induced tumour metastasis in this process. Taken together, the
findings highlighted the importance of ERK5-regulated RERG
expression in prostatic carcinoma progression.
Materials and methods
Cell culture, antibodies and
reagents
PC-3 prostatic carcinoma cells were obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA) and
cultured at 37°C in a humidified environment containing 5%
CO2. The cells were grown in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) and 1:100 penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.).
Anti-mouse ERK5 antibody (dilution 1:2,000; cat. no.
sc-398015) was obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Anti-rabbit RERG antibody (1:2,000 for western
blotting; 1:200 for immunohistochemistry; cat. no. 10687-1-AP) was
purchased from ProteinTech Group, Inc. (Rosemone, IL, USA).
Anti-MMP-2 antibody (dilution 1:2,000; cat. no. ab92536),
anti-MMP-9 antibody (dilution 1:2,000; cat. no. ab137867), anti-B
cell lymphoma (Bcl)-2 antibody (dilution 1:2,000; cat. no.
ab196495), and anti-c-Myc antibody (dilution 1:2,000; cat. no.
ab39688) were purchased from Abcam (Cambridge, UK; dilution
1:2,000). Anti-GAPDH antibody (dilution 1:4,000; cat. no.
100242-MM05) was purchased from Sino Biological, Inc. (Beijing,
China). All of the secondary antibodies, including goat anti-mouse
IgG antibody-(cat. no. 715-035-003) and goat anti-rabbit IgG
antibody (cat. no. 111-035-045)-conjugated horseradish peroxidase
(HRP), were purchased from Jackson ImmunoResearch Laboratories,
Inc. (West Grove, PA, USA; dilution 1:4,000). XMD8-92, an ERK5
inhibitor that specifically inhibits the phosphorylation of ERK5,
but not the phosphorylation of ERK1/2, was purchased from Selleck
Chemicals (Houston, TX, USA). EGF was obtained from Sino
Biological, Inc. The final concentration of the XMD8-92 and EGF
treatment was 5 µM and 0.5 ng/ml, respectively. The cells were
serum starved overnight followed by treatment with XMD8-92 as
indicated for 1 h, and/or stimulated with EGF for 20 min, as
previously described (21).
Plasmid construction
The human RERG open-reading frame was amplified by
RT-polymerase chain reaction (PCR) from the total RNA of PC-3
cells. The primer sequences of RERG are presented in Table I. The RERG cDNA were amplified by
PCR and inserted into the EcoRI/XbaI sites of the
pCDNA3.1(+) eukaryotic expression vector (Invitrogen; Thermo Fisher
Scientific, Inc.) with T4 DNA ligase (New England Biolabs, Ipswich,
MA, USA). To construct pSpCas9-RERGtarget-2A-GFP, the RERG sgRNA
oligo was designed in http://crispr.mit.edu, phosphorylated, ligated, and
inserted in the pSpCas9-2A-GFP vector (Addgene, Cambridge, MA, USA)
after BbsI digestion (Thermo Scientific, Inc.).
 | Table I.Primers for recombinant plasmid
construction and RT-qPCR assay. |
Table I.
Primers for recombinant plasmid
construction and RT-qPCR assay.
| A, Plasmid
construction |
|---|
|
|---|
| Primer name | Sequence
(5′-3′) |
|---|
| RERG |
| F | CCCGAATTCATGGCTAAAAGT |
| R | CCCTCTAGACTAAGTACTGATTTT |
| RERG gRNA |
| F |
CACCTAGTGAGATTTCTGACCAAA |
| R |
GTGGTCGATGATGAAGTTGTTTCC |
|
| B,
RT-qPCR |
|
| Primer
name | Sequence
(5′-3′) |
|
| RERG |
|
| F |
CAACCATCGATGATGAAGTTG |
| R |
TCAGCTTTGTTTCCAACCAAG |
| MMP-2 |
| F |
AGAAGTTCTTTGGACTGCCCC |
| R |
CAGGTGTGTAGCCAATGATCC |
| MMP-9 |
| F |
TGTACCGCTATGGTTACACTC |
| R |
GGCAGGGACAGTTGCTTCT |
| GAPDH |
| F |
TGCACCACCAACTGCTTAGC |
| R |
GGCATGGACTGTGGTCATGAG |
Overexpression and knockdown of RERG
expression
To establish PC-3 cells with stable RERG protein
overexpression or inhibition, PC-3 cells were transfected with
pCDNA3.1(+)-RERG, pSpCas9-RERGtarget-2A-GFP recombinant plasmid, or
pCDNA3.1(+) using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. Briefly, for transfection, 1×106 cells were
seeded in a 6-well plate. A total of 5 µl Lipofectamine®
3000, and 5 µg pCDNA3.1(+)-RERG or pSpCas9-RERGtarget-2A-GFP were
diluted in 200 µl Opti-MEM medium (Invitrogen; Thermo Fisher
Scientific, Inc.) and incubated for 30 min, respectively. The
mixture was then added and incubated in the cell culture medium for
48 h. For the selection of RERG-overexpressing PC-3 cells, cells
were transfected with pCDNA3.1(+)-RERG and selected by G-418
treatment for three weeks. For the selection of cells with silenced
RERG expression, pSpCas9-RERGtarget-2A-GFP-transfected PC-3 cells
were sorted by flow cytometry on a FACSAria II system (BD
Biosciences, San Jose, CA, USA), and the selected fluorescently
labelled cells were then cultured in 6-well plates. PC-3 cells were
also transfected with pCDNA3.1(+) and selected using G-418, as
vector control cells.
Western blot analysis
To prepare protein extracts, the cells were washed
with PBS three times. Following centrifugation, the harvested cells
were resuspended and protein extracted using lysis buffer (Pierce;
Thermo Fisher Scientific, Inc.) for 30 min on ice. The lysates were
centrifuged at 16,000 × g for 10 min at 4°C. The supernatants of
the lysates were mixed with SDS sample buffer and boiled for 10
min. The samples (~100 µg of each lane) were separated on a 10% SDS
polyacrylamide gel and then transferred to a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA). The
membrane was blocked with 5% (w/v) dry skim milk for 1 h at room
temperature, and then blotted with the corresponding antibody in
PBST buffer (0.1% Tween-20 in PBS) under gentle shaking at room
temperature for 2 h. After being washed with PBST three times, the
membranes were incubated with the indicated secondary antibody for
2 h at room temperature. The signals were detected using a
SuperSignal West Pico Substrate kit (Pierce; Thermo Fisher
Scientific, Inc.). The signals were measured by fluorescence
intensity with ImageJ software (version no. 1.8.0; National
Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RT-qPCR was performed using a SYBR PrimeScript RT-PCR kit
(Takara Bio, Inc., Otsu, Japan) on a Rotor-Gene 6000 Real-Time
Genetic Analyser (Qiagen, Valencia, CA, USA), and quantified
according to the previously described method (22). The primer sequences of RERG, MMP-2,
MMP-9, and GAPDH are presented in Table
I. The PCR protocol included a denaturation programme (95°C for
2 min), followed by 40 cycles of an amplification and
quantification programme (95°C for 5 sec and 55–57°C for 30 sec)
and a melting curve programme (55–95°C, with a 0.5°C increment each
cycle). Each sample was replicated three times.
Immunohistochemistry (IHC)
A paraffin-embedded tissue microarray (Alenabio,
Xi'an, China), which contains 58 prostatic carcinoma specimens and
6 normal prostate tissues, was dewaxed with xylene and rehydrated
in descending concentrations of ethanol. The slide was blocked with
Immuol Staining blocking buffer (cat. no. P0102; Beyotime Institute
of Biotechnology, Shanghai, China) for 60 min at room temperature,
and then was incubated with anti-RERG antibody (dilution 1:200;
Santa Cruz Biotechnology, Inc.) at 4°C overnight. After washing
three times in PBST buffer for 10 min, the slide was processed
according to the instructions of the Super Sensitive Polymer HRP
Detection System/DAB kit (Thermo Fisher Scientific, Inc.) and
counterstained with haematoxylin for 15 min at room temperature.
The intensity of the immunostaining (1, weak; 2, moderate, and 3,
intense) and the percentage of positive cells (0, <0.5%; 1,
5–35%; 2, 26–50%; 3, 51–75%; 4, >75%) were assessed in at least
5 high-power fields under a light microscope (Nanjing Jiangnan
Inc., Nanjing, China). All values for each sample were multiplied
to obtain a final score, and the tissues with scores <4 and ≥4
were determined to have low and high expression, respectively.
Cell proliferation assay
Cell counting kit (CCK)-8 assay was performed to
examine PC-3 cell proliferation. The cells were seeded at
2×103 cells/well in phenol red-free DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS in a 96-well
plate. The cell growth rate was determined by CCK-8 assay (Beyotime
Institute of Biotechnology). A total of 10 µl of CCK-8 working
solution was added to each well on days 1–5, followed by incubation
for 2 h at 37°C, and the absorbance at a wavelength of 450 nm was
measured using a model 3550 microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Colony formation assay
A total of 5×102 cells were added to a
24-well plate. All of the cells were then cultured for 2 weeks with
fresh medium every three days. Cells were washed with PBS for three
times, and stained with 0.1% crystal violet for 10 min at room
temperature, and destained with PBS three times. Colonies were
counted using ImageJ software for each well, and each condition was
tested in triplicate.
Transwell assay
A 24-well micropore polycarbonate membrane filter
with 8-µm pores (Corning Inc., Corning, NY, USA) was coated with 10
µl of Matrigel (BD Biosciences). Then, 200 µl of cell suspension
(1×104/ml) was seeded into the top chamber in serum-free
DMEM medium. The bottom chamber was filled with DMEM supplemented
with 10% FBS. After 24 h of incubation, the membranes were fixed
and stained by 0.1% crystal violet for 15 min at room temperature,
and the cells on the upper surface were then removed with a cotton
swab. The invasive cells on the lower surface were observed and
counted under a phase-contrast microscope. Each condition was
tested with five replicates.
Wound healing assay
Cell monolayers in all groups were wounded using a
200-µl pipette tip in 6-well plates and then incubated in DMEM
supplemented with 10% FBS. The wound width was imaged at 0, 12, 24,
or 36 h post-scratching under a phase-contrast microscope.
Apoptosis assay
Hoechst 33342 staining (Beyotime Institute of
Biotechnology) was performed to examine apoptosis. In brief, cells
were seeded on coverslips in 24-well plates and cultured for 24 h;
then, the DMEM was removed, and the cells were washed three times.
Cells in all of the groups were incubated in Hoechst labelling
solution (2 µg/ml) for 20 min at room temperature before being
washed with PBS three times. The cells were then observed under a
ZEISS Observer A1 fluorescence microscope at 350 nm.
Nuclear factor (NF)-κB luciferase
activity assay
In total, 5×104 cells were seeded into
24-well plates and cultured for 24 h. Cells in the different groups
were transfected with 0.3 µg of pNF-κB-Luc plasmid (Agilent
Technologies, Inc., Santa Clara, CA, USA) with
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 48 h after transfection, the cells
were lysed in lysis buffer. The luciferase activity was determined
using a luciferase assay kit (Promega Corp., Madison, WI, USA).
Each experiment was repeated at least three times.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. The data were analysed using SPSS software, version 14.0
(SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5.0. (GraphPad
Software, Inc., La Jolla, CA, USA). Statistical analyses were
performed by analysis of variance using Dunnett's multiple
comparison test, or Students t-test. P<0.05 was considered to
indicate a statistically significant difference. Each experiment
was replicated at least three times.
Results
Activation of the ERK5 pathway induces
the inhibition of RERG expression in prostate tumour cells
Our previous study indicated that activation of the
ERK5 pathway induces prostatic carcinoma cell proliferation
(13). To further investigate the
function of ERK5 in the progression of prostatic carcinoma, the
present study activated or inhibited the ERK5 pathway with
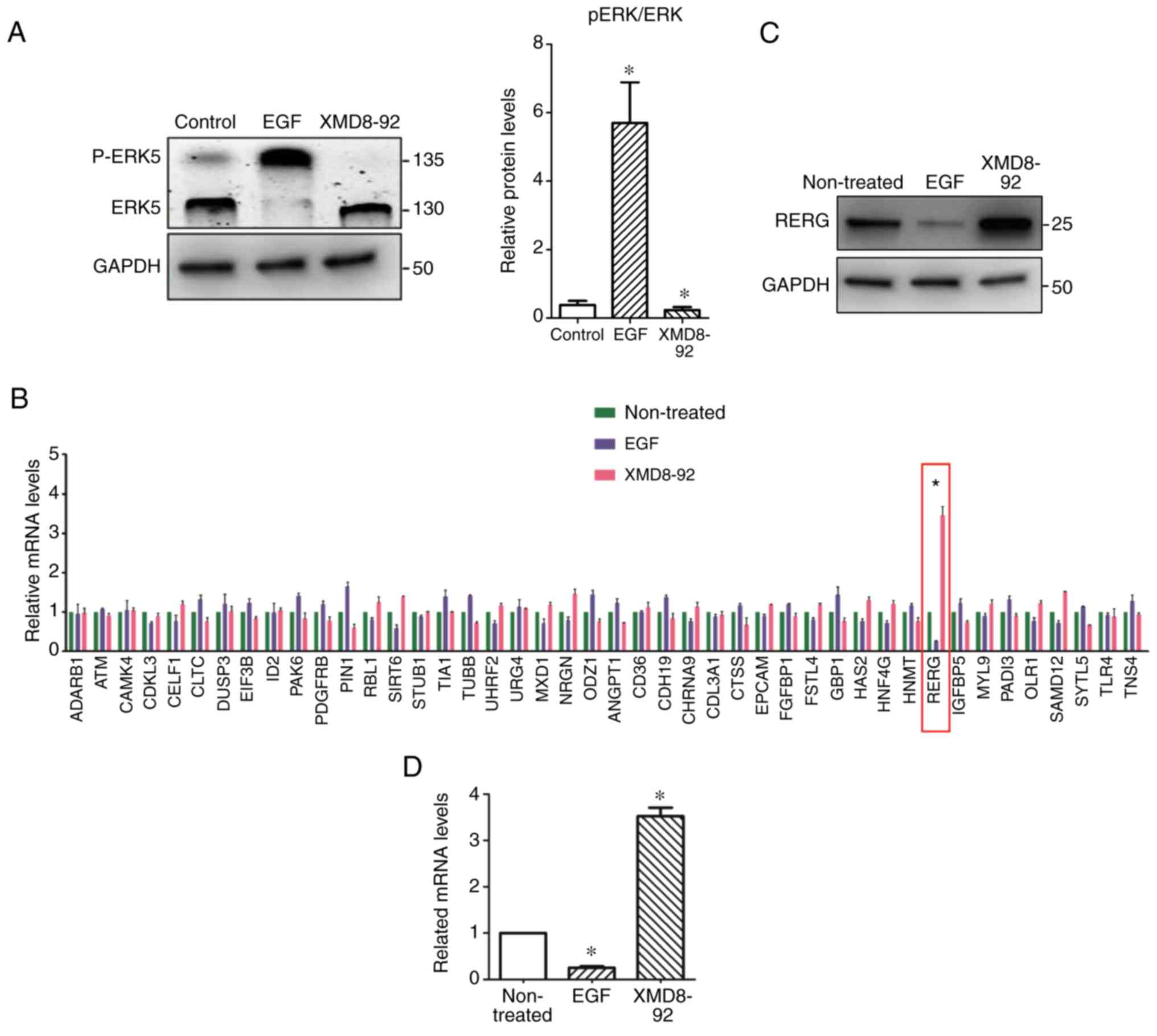
treatments with EGF or XMD8-92, respectively. Fig. 1 revealed that phosphorylated ERK5
was markedly upregulated in EGF-treated PC-3 cells, indicating that
the ERK5 pathway can be activated by EGF treatment (21). The results indicated that EGF
treatment can activate the ERK5 pathway, which induces more
phosphorylated ERK5 in total ERK5 protein. Thereafter, EGF- and
XMD8-92-treated PC-3 cells were harvested and examined by
microarray analysis (Sangon Biotech, Shanghai, China). Fig. 1B revealed the regulation of various
genes in EGF- and XMD8-92-treated PC-3 cells. Notably, it was noted
that RERG expression was significantly upregulated in
XMD8-92-treated PC-3 cells, but inhibited in EGF-treated PC-3
cells, compared with control, which was also confirmed by western
blot analysis (Fig. 1C). Similarly,
RT-PCR also demonstrated that RERG was regulated by ERK5 signalling
at the transcriptional level (Fig.
1D). Therefore, the results suggested that RERG may be
associated with ERK5-mediated cell proliferation in prostate tumour
cells.
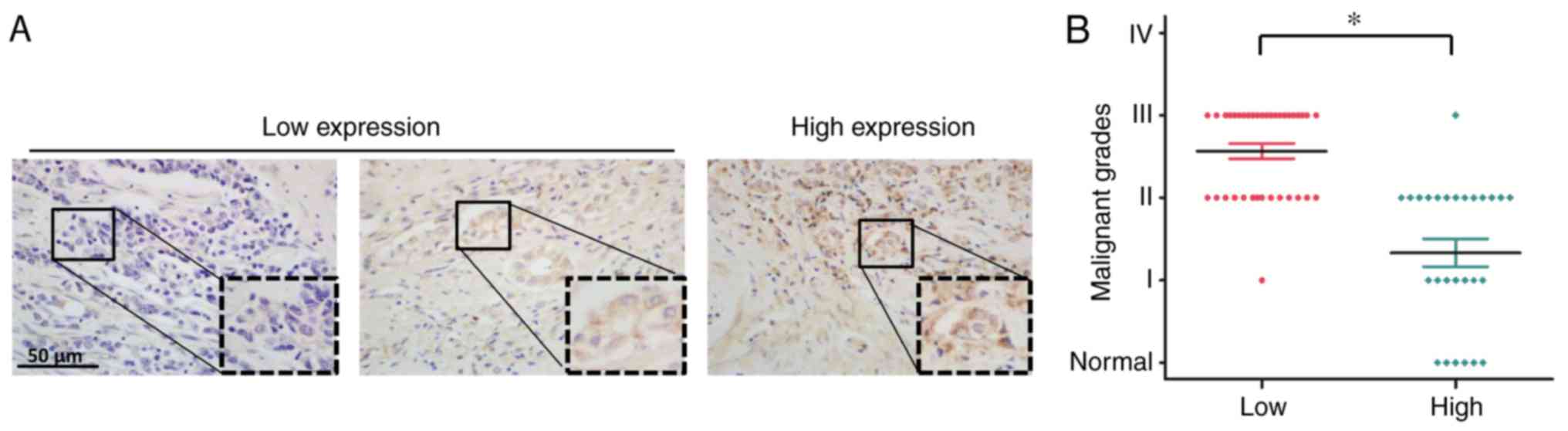
RERG expression is associated with
malignancy in prostatic carcinoma
To investigate the role of RERG expression in
prostatic carcinoma, the present study then analysed the expression
level of RERG in the specimens of tissue microarray, which
contained 58 prostatic carcinoma specimens and 6 normal prostate
tissues. The characteristics of the patients with prostatic
carcinoma are presented in Table
II. Immunohistochemistry revealed that RERG expression was
lower in advanced prostatic carcinoma tissues, whereas RERG protein
expression was higher in normal prostate tissues and less malignant
tumour tissues (Fig. 2A),
suggesting that the loss of RERG expression may result in cancer
progression. In addition, the results indicated that the expression
level of RERG was negatively associated with malignancy in
prostatic carcinoma (Fig. 2B),
which is consistent with previous reports regarding other tumours
(16–18).
 | Table II.Characteristics of patients with
prostatic carcinoma. |
Table II.
Characteristics of patients with
prostatic carcinoma.
| Characteristic | Adenocarcinoma
(n=58) (%) | Normal tissues
(n=6) |
|---|
| Age, years |
|
≥60 | 52 (90) | 0 |
|
<60 | 6 (10) | 6 |
| Grades |
|
1-2 | 35 (60) | – |
|
3-4 | 23 (40) | – |
| Stages |
|
I+II | 33 (57) | – |
|
III+IV | 25 (43) | – |
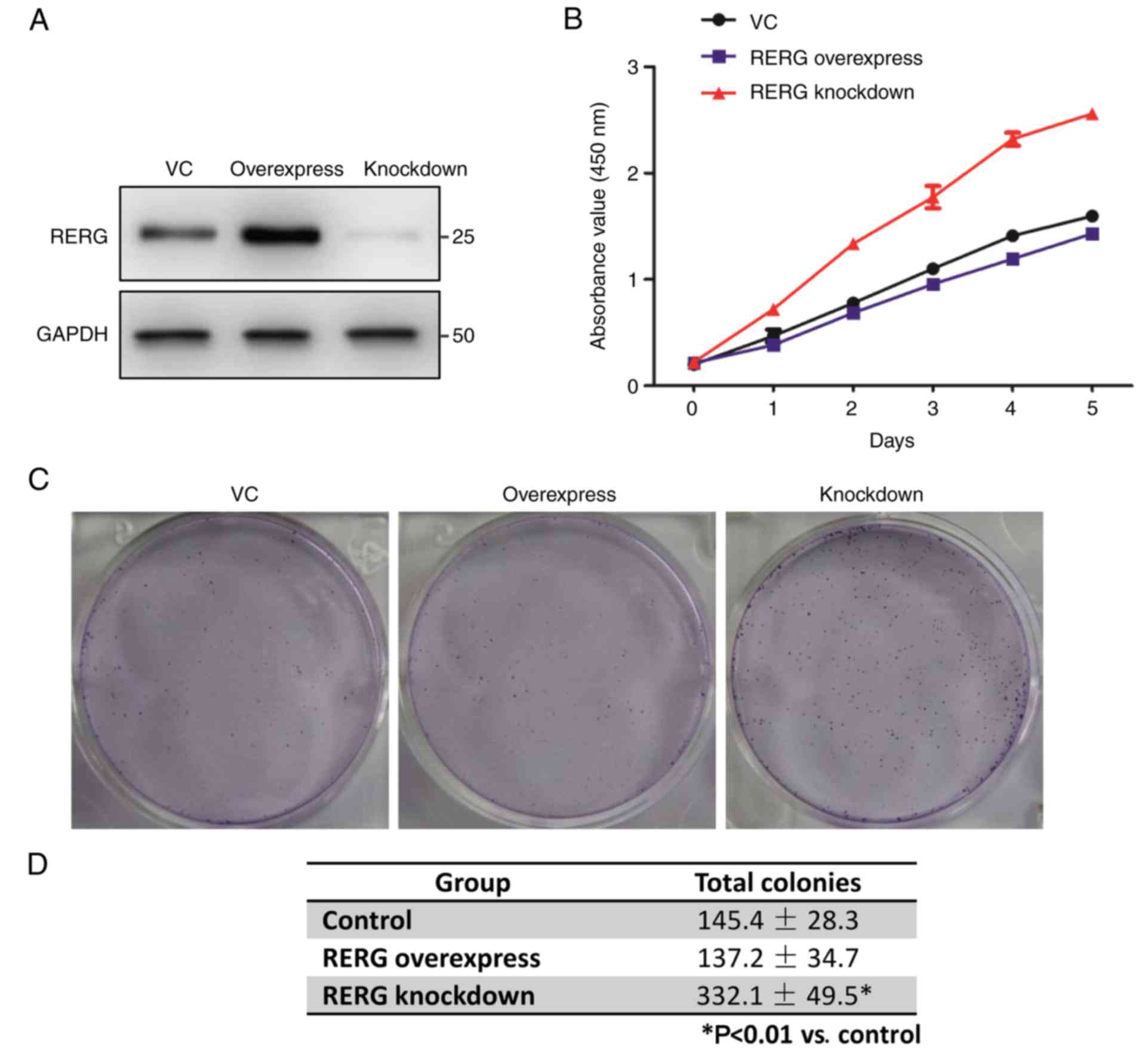
Inhibition of RERG expression promotes
prostate cancer cell proliferation
The aforementioned results indicated that RERG
expression may be involved in the malignancy of prostatic
carcinoma. It was expected that RERG expression may play a role in
tumourigenesis in prostatic carcinoma. To confirm our hypothesis,
the present study stably overexpressed and knocked down RERG
expression in PC-3 cells. RT-qPCR and western blot analysis
demonstrated the overexpression and knockdown of RERG mRNA and
protein in PC-3 cells (data not shown and Fig. 3A, respectively). Additionally, cell
proliferation assays demonstrated that the inhibition of RERG
expression promoted cell proliferation compared with RERG
overexpression and the control (empty vector; Fig. 3B). Moreover, the colony formation
assay indicated that the colony number was much greater in
RERG-inhibited PC-3 cells compared with RERG-overexpressing or
empty-vector-transfected PC-3 cells (Fig. 3C and D), suggesting that RERG
expression is associated with prostate cancer cell
proliferation.
RERG expression regulates tumour
invasion and migration by inhibiting MMP-2 and MMP-9 expression in
prostate cancer cells
As RERG has been reported to be associated with
tumour metastasis in breast cancer, the present study then examined
whether the regulation of RERG expression was associated with
tumour invasion and metastasis in prostatic carcinoma. The invasion
and migration of RERG-overexpressing, RERG-inhibited, and
empty-vector-transfected PC-3 cells was examined. The in
vitro wound healing assay demonstrated that RERG-inhibited PC-3
cells migrated into the scratched area more rapidly than
RERG-overexpressing or empty-vector-transfected PC-3 cells at 12,
24, and 36 h (Fig. 4A and B).
Further transwell studies demonstrated that RERG-inhibited PC-3
cells displayed an increased invasive capacity compared with
RERG-overexpressing and empty-vector-transfected PC-3 cells
(Fig. 4C and D).
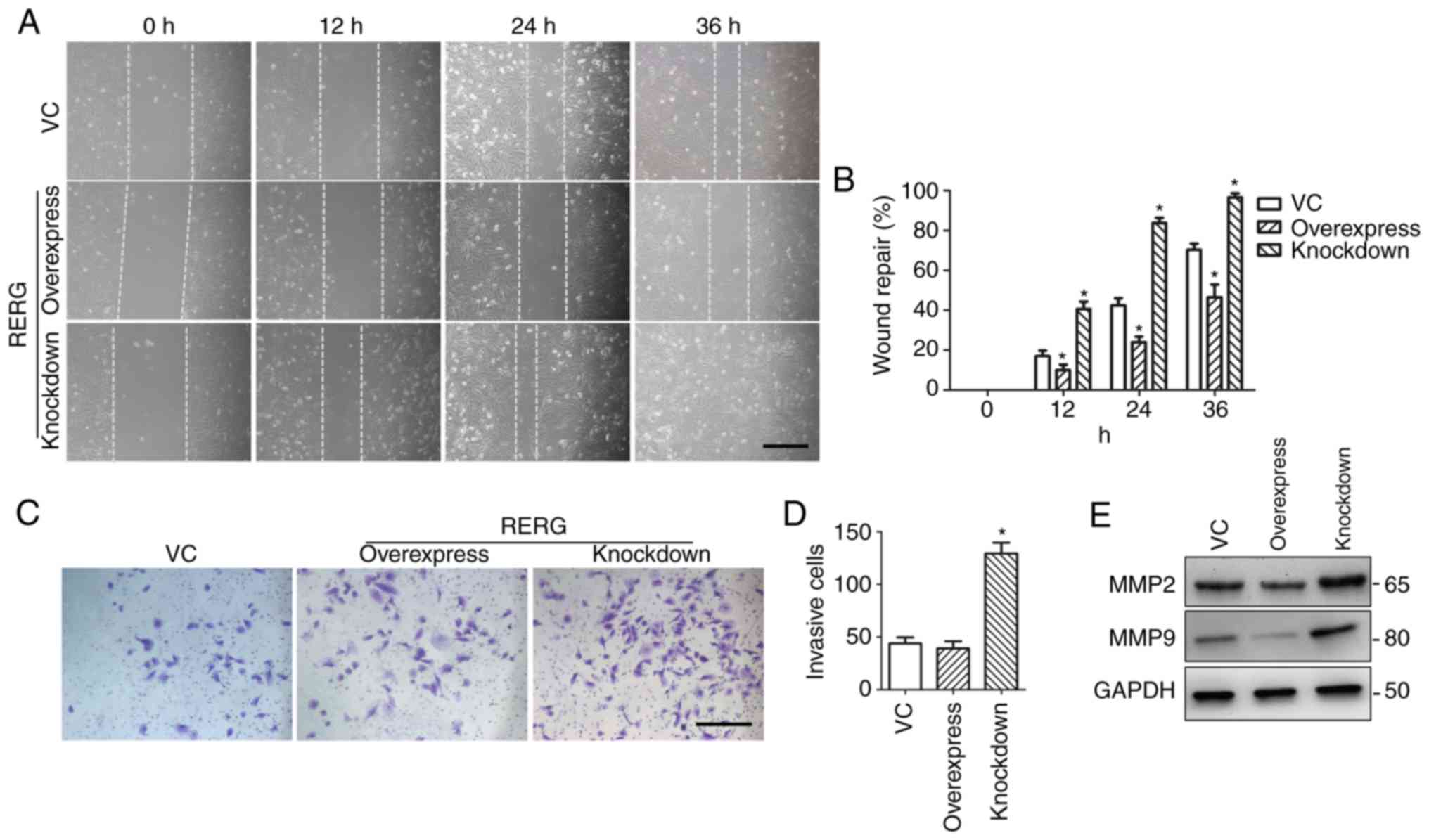 | Figure 4.Effects of RERG on tumour invasion
and migration in RERG-overexpressing, RERG-inhibited, and
empty-vector-transfected PC-3 cells. (A and B) The migration of
RERG-overexpressing, RERG-inhibited, and empty-vector-transfected
(VC group) PC-3 cells was measured by wound-healing assay at 0, 12,
24, and 36 h. (C and D) Cell invasion was determined by transwell
assay in RERG-overexpressing, RERG-inhibited, and
empty-vector-transfected (VC group) PC-3 cells at 24 h. (E) MMP-2
and MMP-9 protein expression levels in RERG-overexpressing,
RERG-inhibited, and empty-vector-transfected (VC group) PC-3 cells.
*P<0.05 vs. VC. VC, pcDNA3.1-transfected PC-3 cells; RERG,
Ras-like oestrogen-regulated growth inhibitor; MMP, MMP, matrix
metallopeptidase. Scale bar, 200 µm. |
MMP-2 and MMP-9 have been reported to be associated
with the invasion and migration of many malignant tumours (23). Therefore, the present study examined
the function of MMP-2 and MMP-9 in RERG-associated invasion and
migration in prostatic carcinoma. The expression levels of MMP-2
and MMP-9 in RERG-overexpressing, RERG-inhibited, and
empty-vector-transfected PC-3 cells were determined. Notably,
western blot analysis revealed that the expression levels of MMP-2
and MMP-9 were inhibited in RERG-overexpressing PC-3 cells, but
upregulated in RERG-inhibited PC-3 cells (Fig. 4E). Therefore, the results indicated
that MMP-2 and MMP-9 play important roles in RERG-associated
invasion and migration in prostatic carcinoma.
NF-κB-mediated apoptosis is involved
in RERG-regulated prostate cancer cell proliferation
As apoptosis plays important roles in the regulation
of cell proliferation in various malignant tumours, it was expected
that RERG may promote cell proliferation by inhibiting apoptosis in
prostatic carcinoma cells. To clarify the mechanism of RERG
function in prostatic carcinoma cell proliferation, Hoechst
staining was performed to confirm whether the regulation of RERG
expression was associated with the induction of cell apoptosis in
RERG-overexpressing or RERG-inhibited PC-3 cells. The results
indicated that cell apoptosis was increased in RERG-overexpressing
PC-3 cells, suggesting that the regulation of RERG expression was
involved in the apoptosis of prostatic carcinoma cells (Fig. 5A and B).
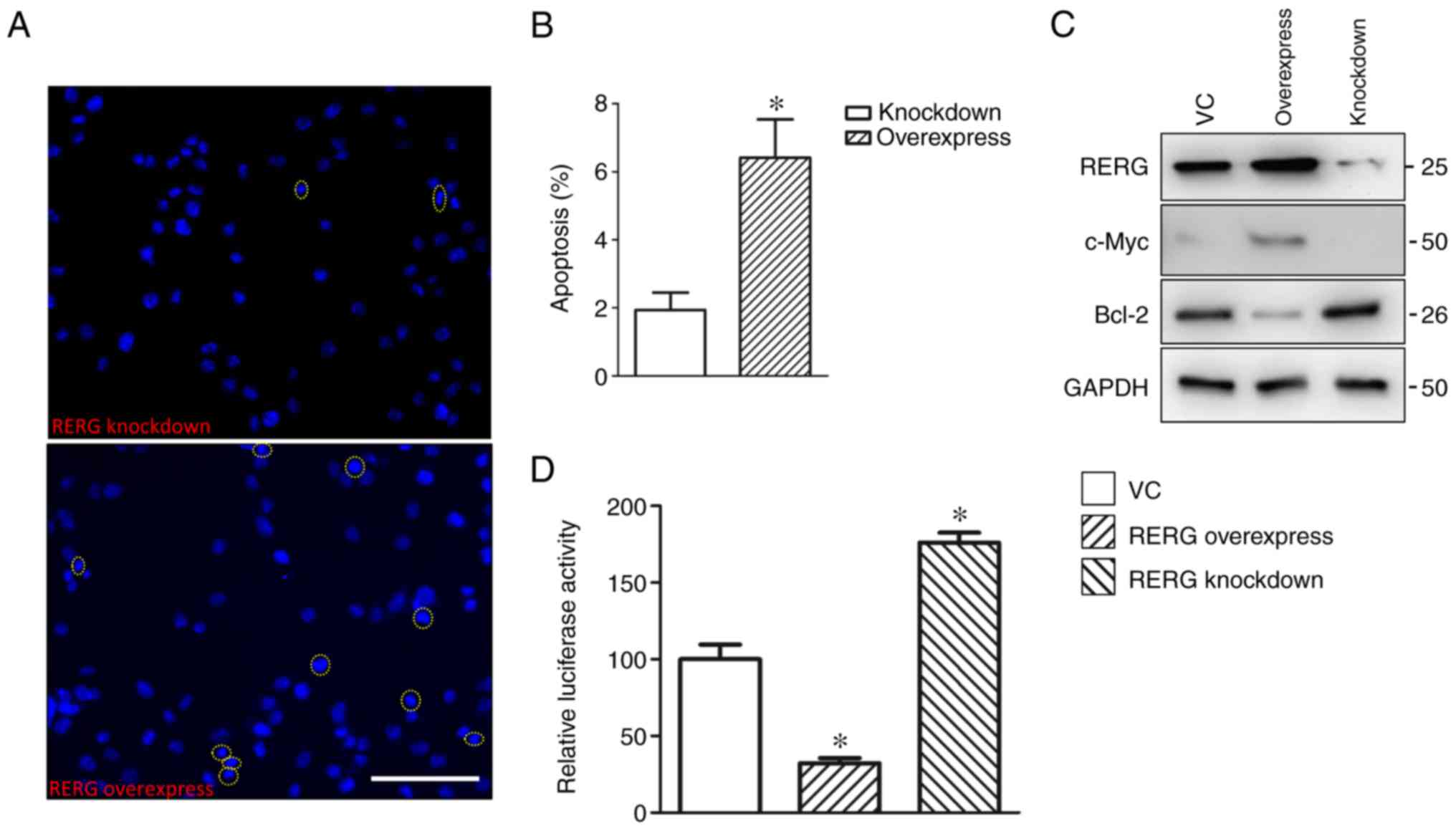 | Figure 5.NF-κB-mediated apoptosis and
RERG-regulated proliferation of RERG-overexpressing,
RERG-inhibited, and empty-vector-transfected (VC group) PC-3 cells.
(A) Representative images of Hoechst-stained RERG-overexpressing
and RERG-inhibited PC-3 cells. (B) Percentage of apoptotic cells
among RERG-overexpressing and RERG-inhibited PC-3 cells. (C)
Expression levels of RERG, c-Myc, and Bcl-2 in RERG-overexpressing,
RERG-inhibited, and empty-vector-transfected (VC group) PC-3 cells.
(D) NF-κB activity in RERG-overexpressing, RERG-inhibited, and
empty-vector-transfected (VC group) PC-3 cells. *P<0.05 vs. VC.
VC, pcDNA3.1-transfected PC-3 cells; RERG, Ras-like
oestrogen-regulated growth inhibitor; Bcl-2, B cell lymphoma-2.
Scale bar, 200 µm. |
To further illustrate the possible mechanism of
RERG-induced apoptosis in prostatic carcinoma cells, the present
study then determined the protein expression levels of c-Myc and
Bcl-2, which are key factors in apoptosis (24,25),
in RERG-overexpressing, RERG-inhibited, and
empty-vector-transfected PC-3 cells. Notably, it was revealed that
the expression level of Bcl-2 was significantly inhibited, whereas
that of c-Myc was upregulated in RERG-overexpressing PC-3 cells
compared with empty-vector-transfected PC-3 cells (Fig. 5C). Bcl-2 is an anti-apoptotic factor
that is also a regulator of the NF-κB signalling pathway (26). Inactivation of the NF-κB pathway can
also induce apoptosis in malignant tumours (27,28).
Thus, NF-κB activation in RERG-overexpressing, RERG-inhibited, and
empty-vector-transfected PC-3 cells was investigated. As expected,
the results indicated that RERG overexpression blocked NF-κB
signalling pathway activation (Fig.
5D), which suggested that NF-κB-mediated apoptosis may be
involved in RERG-regulated cell proliferation in prostatic
carcinoma.
Discussion
As an MAPK family member, ERK5 is essential for cell
proliferation and differentiation (29). Kato et al (30) observed that ERK5 can promote cell
survival and inhibit apoptosis (30). In addition, the ERK5 pathway is
essential for vascular development and proliferation (31) and plays a key role in the
maintenance of the functions and integrity of endothelial cells
(29). Activation of the ERK5
signalling pathway can cause disordered cell cycle regulation,
which subsequently results in cell proliferation and tumourigenesis
in malignant cancers (13,32). Furthermore, several studies have
found high ERK5 expression in breast cancer, squamous cell
carcinoma, and prostatic carcinoma, and its expression is
associated with malignancy and prognosis in these tumours (33–36),
suggesting that the ERK5 signalling pathway may be a potential
target for the treatment of patients with malignant cancer.
RERG is a member of the Ras GTPase superfamily,
which plays important roles in cell growth, proliferation, survival
and differentiation. Unlike the functions of most Ras superfamily
members, RERG is an inhibitor of tumourigenesis. Recent studies
have demonstrated that RERG expression is associated with the ERK
signalling pathway (18,37). In the present study, it was
demonstrated that the EGF-activated ERK5 pathway induced the
inhibition of RERG expression, whereas treatment with XMD8-92,
which is a specific inhibitor of the ERK5 pathway, promoted the
expression of RERG protein in prostatic carcinoma cells.
Additionally, RERG expression has been reported to be involved in
activation of the Ras/MEK/ERK pathway (14). Therefore, it was expected that ERK5
activation may be a key factor in the Ras/MEK/ERK signalling axis
in this process.
ERK5 can be activated by a series of stimulators,
including mitogens and growth factors. Phosphorylated ERK5 can
translocate from the cytoplasm to the nucleus and subsequently
regulate the activity of transcriptional factors to regulate cell
proliferation and differentiation. Previous studies have indicated
that ERK5 can promote cell proliferation by regulating cell cycle
progression in breast cancer and prostatic carcinoma cells
(13,38). Herein, the results indicated that
activation of the ERK5 pathway inhibited apoptosis to promote cell
proliferation by regulating RERG expression in prostatic carcinoma
cells. As a tumour suppressor gene, high RERG expression is
associated with the expression of a series of genes that define an
oestrogen receptor-positive breast tumour subtype and are
associated with not only a slow rate of tumour cell proliferation
but also a favourable prognosis in cancer patients (14). Moreover, it was demonstrated that
the loss of RERG expression regulated the expression of Bcl-2 and
c-Myc expression in PC-3 cells, which may be associated with
RERG-mediated cell apoptosis. The results also indicated that NF-κB
activation may be associated with RERG-induced apoptosis in
prostatic carcinoma cells. A previous study demonstrated that the
NF-κB/miRNA21/Bcl-2 signalling axis inhibits apoptosis in
macrophages (26). Yuan et
al (39) found that ANXA1
inhibits miRNA-196a in a negative feedback loop through NF-κB and
c-Myc to reduce breast cancer cell proliferation (39). Therefore, it was expected that the
ERK5/RERG/Bcl-2/NF-κB axis inhibited cell proliferation by inducing
apoptosis in prostatic carcinoma cells.
The wound-healing assay indicated that the
inhibition of RERG expression induced PC-3 cell migration. To
further confirm the possibility that increased/decreased wound
healing did not result from increased/decreased cell proliferation
but from an increased invasive capability induced by the inhibition
of RERG expression in PC-3 cells, a transwell assay, which is
commonly used to study the migratory response of endothelial cells,
was performed to examine the effects of RERG on PC-3 cell invasion.
It was noticed that the number of invasive cells among
RERG-inhibited PC-3 cells was much greater than that among
RERG-overexpressing PC-3 cells in the transwell assay within only
12 h, which could hardly have been affected by cell proliferation,
suggesting that the increased invasive capability of RERG-inhibited
PC-3 cells was not a result of RERG-induced proliferation. Notably,
the migration and invasion assay indicated that the regulation of
RERG expression was also associated with PC-3 cell invasion and
migration, and either MMP-2 or MMP-9 was downregulated in
RERG-overexpressing PC-3 cells. A previous report indicated that
the inhibition of NF-κB activity results in the downregulation of
downstream target genes, such as MMPs (40). Therefore, it was hypothesized that
XMD8-92 inhibited ERK5 phosphorylation, which inactivated the ERK5
signalling pathway and upregulated RERG expression in prostatic
carcinoma cells. Increasing RERG expression subsequently
significantly inhibited NF-κB transcriptional activity, which
suppressed the expression of MMPs and finally inhibited tumour cell
invasion and migration in prostatic carcinoma. However, further
investigation, especially into the association underlying RERG,
NF-κB and MMPs, will be required to fully clarify the mechanisms
underlying RERG-regulated tumour cell invasion and migration in
prostatic carcinoma.
In conclusion, the present data provided0. evidence
indicating involvement of the ERK5/RERG/NF-κB axis in prostatic
carcinoma cell proliferation, invasion and migration. Our findings
reveal that the ERK5/RERG/NF-κB axis might be a potential for the
targeted treatment of patients with prostatic carcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hubei
Province Health and Family Planning Scientific Research Projects
(grant nos. WJ2017Q41 and WJ2016-Y-25).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
LZ conceived and designed the experiments and
revised the paper; YX performed the experiments, analysed the data
and wrote the paper; HH, SC and HD performed the experiments. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Sauer AM, Chen MS Jr,
Kagawa-Singer M, Jemal A and Siegel RL: Cancer statistics for Asian
Americans, Native Hawaiians, and Pacific Islanders, 2016:
Converging incidence in males and females. CA Cancer J Clin.
66:182–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stangelberger A, Waldert M and Djavan B:
Prostate cancer in elderly men. Rev Urol. 10:111–119.
2008.PubMed/NCBI
|
|
5
|
Debes JD and Tindall DJ: Mechanisms of
androgen-refractory prostate cancer. N Engl J Med. 351:1488–1490.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schröder FH: Progress in understanding
androgen-independent prostate cancer (AIPC): A review of potential
endocrine-mediated mechanisms. Eur Urol. 53:1129–1137. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Engelberg D: Stress-activated protein
kinases-tumor suppressors or tumor initiators? Semin Cancer Biol.
14:271–282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dent P, Yacoub A, Fisher PB, Hagan MP and
Grant S: MAPK pathways in radiation responses. Oncogene.
22:5885–5896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weldon CB, Scandurro AB, Rolfe KW, Clayton
JL, Elliott S, Butler NN, Melnik LI, Alam J, McLachlan JA, Jaffe
BM, et al: Identification of mitogen-activated protein kinase
kinase as a chemoresistant pathway in MCF-7 cells by using gene
expression microarray. Surgery. 132:293–301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Esparis-Ogando A, Diaz-Rodriguez E,
Montero JC, Yuste L, Crespo P and Pandiella A: Erk5 participates in
neuregulin signal transduction and is constitutively active in
breast cancer cells overexpressing ErbB2. Mol Cell Biol.
22:270–285. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mehta PB, Jenkins BL, McCarthy L, Thilak
L, Robson CN, Neal DE and Leung HY: MEK5 overexpression is
associated with metastatic prostate cancer, and stimulates
proliferation, MMP-9 expression and invasion. Oncogene.
22:1381–1389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hayashi M and Lee JD: Role of the
BMK1/ERK5 signaling pathway: Lessons from knockout mice. J Mol Med.
82:800–808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiong Y, Zhang L and Wang T:
Phosphorylation of BMK1 induces prostatic carcinoma cell
proliferation by promoting entry into the S phase of the cell
cycle. Oncol Lett. 11:99–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Finlin BS, Gau CL, Murphy GA, Shao H,
Kimel T, Seitz RS, Chiu YF, Botstein D, Brown PO, Der CJ, et al:
RERG is a novel ras-related, estrogen-regulated and
growth-inhibitory gene in breast cancer. J Biol Chem.
276:42259–42267. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Key MD, Andres DA, Der CJ and Repasky GA:
Characterization of RERG An estrogen-regulated tumor
suppressor gene. Methods Enzymol. 407:513–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Habashy HO, Powe DG, Glaab E, Ball G,
Spiteri I, Krasnogor N, Garibaldi JM, Rakha EA, Green AR, Caldas C,
et al: RERG (Ras-like, oestrogen-regulated, growth-inhibitor)
expression in breast cancer: A marker of ER-positive luminal-like
subtype. Breast Cancer Res Treat. 128:315–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao W, Ma N, Wang S, Mo Y, Zhang Z, Huang
G, Midorikawa K, Hiraku Y, Oikawa S, Murata M and Takeuchi K:
RERG suppresses cell proliferation, migration and
angiogenesis through ERK/NF-κB signaling pathway in nasopharyngeal
carcinoma. J Exp Clin Cancer Res. 36:882017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo X, Huang R, Sun H, Liu Y, Bi H, Li J,
Yu H, Sun J, Lin S, Cui B, et al: Methylation of a panel of genes
in peripheral blood leukocytes is associated with colorectal
cancer. Sci Rep. 6:299222016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oster B, Thorsen K, Lamy P, Wojdacz TK,
Hansen LL, Birkenkamp-Demtröder K, Sørensen KD, Laurberg S, Orntoft
TF and Andersen CL: Identification and validation of highly
frequent CpG island hypermethylation in colorectal adenomas and
carcinomas. Int J Cancer. 129:2855–2866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Q, Deng X, Lu B, Cameron M, Fearns C,
Patricelli MP, Yates JR III, Gray NS and Lee JD: Pharmacological
inhibition of BMK1 suppresses tumor growth through promyelocytic
leukemia protein. Cancer Cell. 18:258–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Annabi B, Lachambre MP, Plouffe K,
Sartelet H and Béliveau R: Modulation of invasive properties of
CD133+ glioblastoma stem cells: A role for MT1-MMP in
bioactive lysophospholipid signaling. Mol Carcinog. 48:910–919.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang YB, Zhao XH, Li G, Zheng JH and Qiu
W: MicroRNA-184 inhibits proliferation and promotes apoptosis of
human colon cancer SW480 and HCT116 cells by downregulating C-MYC
and BCL-2. J Cell Biochem. 119:1702–1715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng Z, Wang Y, Fan J, Lin X, Liu C, Xu Y,
Ji W, Yan C and Su C: Costunolide and dehydrocostuslactone
combination treatment inhibit breast cancer by inducing cell cycle
arrest and apoptosis through c-Myc/p53 and AKT/14-3-3 pathway. Sci
Rep. 7:412542017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Q, Liu S, Tang Y, Liu Q and Yao Y:
MPT64 protein from Mycobacterium tuberculosis inhibits apoptosis of
macrophages through NF-κB-miRNA21-Bcl-2 pathway. PLoS One.
9:e1009492014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yen TH, Hsieh CL, Liu TT, Huang CS, Chen
YC, Chuang YC, Lin SS and Hsu FT: Amentoflavone induces apoptosis
and inhibits NF-ĸB-modulated anti-apoptotic signaling in
glioblastoma cells. In vivo. 32:279–285. 2018.PubMed/NCBI
|
|
28
|
Pan L, Li Y, Zhang HY, Zheng Y, Liu XL, Hu
Z, Wang Y, Wang J, Cai YH, Liu Q, et al: DHX15 is associated with
poor prognosis in acute myeloid leukemia (AML) and regulates cell
apoptosis via the NF-κB signaling pathway. Oncotarget.
8:89643–89654. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Spiering D, Schmolke M, Ohnesorge N,
Schmidt M, Goebeler M, Wegener J, Wixler V and Ludwig S: MEK5/ERK5
signaling modulates endothelial cell migration and focal contact
turnover. J Biol Chem. 284:24972–24980. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kato Y, Tapping RI, Huang S, Watson MH,
Ulevitch RJ and Lee JD: Bmk1/Erk5 is required for cell
proliferation induced by epidermal growth factor. Nature.
395:713–716. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Z, Geng J, Ge Z, Wang W, Zhang Y and
Kang W: Activation of ERK5 in angiotensin II-induced hypertrophy of
human aortic smooth muscle cells. Mol Cell Biochem. 322:171–178.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lochhead PA, Gilley R and Cook SJ: ERK5
and its role in tumour development. Biochem Soc Trans. 40:251–256.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Antoon JW, Martin EC, Lai R, Salvo VA,
Tang Y, Nitzchke AM, Elliott S, Nam SY, Xiong W, Rhodes LV, et al:
MEK5/ERK5 signaling suppresses estrogen receptor expression and
promotes hormone-independent tumorigenesis. PLoS One. 8:e692912013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sticht C, Freier K, Knöpfle K,
Flechtenmacher C, Pungs S, Hofele C, Hahn M, Joos S and Lichter P:
Activation of MAP kinase signaling through ERK5 but not ERK1
expression is associated with lymph node metastases in oral
squamous cell carcinoma (OSCC). Neoplasia. 10:462–470. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McCracken SR, Ramsay A, Heer R, Mathers
ME, Jenkins BL, Edwards J, Robson CN, Marquez R, Cohen P and Leung
HY: Aberrant expression of extracellular signal-regulated kinase 5
in human prostate cancer. Oncogene. 27:2978–2988. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ramsay AK, McCracken SR, Soofi M, Fleming
J, Yu AX, Ahmad I, Morland R, Machesky L, Nixon C, Edwards DR, et
al: ERK5 signalling in prostate cancer promotes an invasive
phenotype. Br J Cancer. 104:664–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ho JY, Hsu RJ, Liu JM, Chen SC, Liao GS,
Gao HW and Yu CP: MicroRNA-382-5p aggravates breast cancer
progression by regulating the RERG/Ras/ERK signaling axis.
Oncotarget. 8:22443–22459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cude K, Wang Y, Choi HJ, Hsuan SL, Zhang
H, Wang CY and Xia Z: Regulation of the G2-M cell cycle progression
by the ERK5-NFkappaB signaling pathway. J Cell Biol. 177:253–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yuan Y, Anbalagan D, Lee LH, Samy RP,
Shanmugam MK, Kumar AP, Sethi G, Lobie PE and Lim LH: ANXA1
inhibits miRNA-196a in a negative feedback loop through NF-κB and
c-Myc to reduce breast cancer proliferation. Oncotarget.
7:27007–27020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin ML, Lu YC, Chung JG, Wang SG, Lin HT,
Kang SE, Tang CH, Ko JL and Chen SS: Down-regulation of MMP-2
through the p38 MAPK-NF-kappaB-dependent pathway by aloe-emodin
leads to inhibition of nasopharyngeal carcinoma cell invasion. Mol
Carcinog. 49:783–797. 2010.PubMed/NCBI
|