Introduction
Reactive oxygen species (ROS), which mainly
encompass oxygen molecules containing one or more unpaired,
unstable electrons, are important cellular signalling molecules
involved in a variety of biological processes, including cell
proliferation, differentiation and apoptosis (1). Both endogenous sources (such as
mitochondria and peroxisomes) and exogenous sources (such as
environmental agents, pharmaceuticals and irradiation) can induce
ROS accumulation (2). ROS
homeostasis is critical for maintaining normal physiological and
biological processes. Accumulation of excess ROS, which is termed
oxidative stress, can lead to irreversible injury to cells via
damaged biomolecules, including DNA, resulting in genomic
instability and genetic mutations that contribute to diseases such
as tumourigenesis. In this case, the mechanisms for maintaining the
low levels of intracellular ROS in mammalian cells are extremely
important. The powerful scavenger antioxidant enzyme systems,
including superoxide dismutase (SOD), catalase and glutathione
peroxidase (GPX), have been well studied. Once formed, ROS are
rapidly converted by SODs to H2O2. Newly
formed H2O2 is converted to
H2O+O2 by GPX through coupling with reduced
glutathione (GSH) and its subsequent conversion to oxidized
glutathione (3). It has been
reported that changes in GPX levels, especially in 5 of the 8
sub-members of the GPX family, are tightly associated with
malignant phenotypes in several types of tumours (4). GPX1, GPX3 and GPX4 are reported to
function as tumour suppressors in breast (5), colorectal (6), endometrial (7), pancreatic (8) and prostate (9) cancer. However, the regulatory roles
that GPX family members play in tumours are still largely
unknown.
Recently, the existence of cancer stem-like cells
(CSCs) has been recognized (10).
As a small proportion of cancer cells, CSCs exert potential roles
in aggressive tumour phenotypes and malignant behaviours, such as
chemoresistance, recurrence, relapse and metastasis. It is well
established that, in stem cells, several signalling molecules are
involved in and play critical roles in maintaining low levels of
ROS, which may facilitate chemo/radiotherapy resistance and
increased self-renewal capacity in stem cells (11–15).
In cancer cells, accumulated intracellular ROS may function as a
tumour suppressor or enhancer depending on the cellular mechanisms
activated (3,15). In comparison with normal stem cells
or a heterogeneous population of cancer cells, the regulatory role
of intracellular ROS in CSCs is still largely unknown. It has been
reported that CSCs have lower intracellular ROS contents compared
with a heterogeneous population of cancer cells (16), which may be due to upregulated
expression of members of the free radical scavenging system,
including the SODs or GPXs (17).
Kim and colleagues showed that increased CD13, a CSC molecule,
negatively regulates ROS, resulting in increased stemness in liver
CSCs (18). This suggests an
association between low levels of ROS with CSC stemness. However,
the underlying mechanism for decreased ROS is not well understood
in CSCs.
The EMT programme has been recognized as a major
mechanism of tumour metastasis for 2 decades (19). Inactivation of E-cadherin,
activation of vimentin, Slug and Snail are considered hallmarks of
EMT programme activation (19).
Surprisingly, it was observed that experimental activation of EMT,
via either the overexpression of Twist1 or treatment with TGFβ,
confers many of the characteristics of CSCs on non-CSC epithelial
carcinoma cells (20,21). The association between EMT and CSCs
indicates that EMT programme activation in non-CSC cells may enable
their conversion into CSCs. In this case, both self-renewal
capacity and an EMT phenotype are considered as CSC characteristics
(22). However, little is known
about whether inactivation of the EMT programme or activation of
the MET programme affects biological processes in CSCs.
The established relationship between ROS and EMT
(23,24), and the regulatory mechanisms between
EMT and CSCs (25) suggests that
maintenance of oxidative homeostasis regulates the EMT process and
the stemness phenotype via balancing ROS in CSCs. Differential
expression of endogenous oxidative stress scavengers may play a
critical role in leading to the imbalance or rebalance of oxidative
stress and the ultimate effect on biological processes.
In the present study, we demonstrated the regulatory
role of GPX4 in maintaining oxidative homeostasis and thus
affecting the EMT programme and stemness phenotype of Panc-1 CSCs.
We describe a novel role of GPX4 in regulating biological processes
in Panc-1 CSCs, which indicates a promising further in CSCs induced
metastasis.
Materials and methods
Cell cultures
The human pancreatic cancer cell line, Panc-1, was
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA) and frozen in our laboratory in liquid nitrogen and
cultured in Dulbecco's modified Eagle's medium (DMEM; Life
Technologies; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Paisley, UK), 100 U/ml of penicillin (Gibco;
Thermo Fisher Scientific, Inc.), 100 µg/ml of streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) in a 5% CO2 incubator at
37°C. For enriching CSCs from parental Panc-1 cells, a suspension
culture supporting proliferation of undifferentiated cells was
adopted (26). Briefly, Panc-1
cells were maintained in DMEM/Ham's Nutrient Mixture F-12 (F-12)
(1:1) (Life Technologies; Thermo Fisher Scientific, Inc.) with the
addition of epidermal growth factor (EGF, 20 ng/ml; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), human fibroblast growth factor
basic (hFGFb, 10 ng/ml; Sigma-Aldrich; Merck KGaA) and 2% B27 (Life
Technologies; Thermo Fisher Scientific, Inc.) for 14–21 days.
Medium was half-refreshed every 3 days until the spheres were
observed.
Cell viability and apoptosis
assays
Cell viability was determined by the Cell Counting
Kit-8 (CCK-8; Dojindo Laboratories Co., Ltd., Kumamoto, Japan)
assay. Briefly, Panc-1 or Panc-1 cancer stem-like cells (Panc-1
CSCs) (5×104) were cultured in 96-well plates. A total
of 10 µl of freshly prepared CCK-8 solution was added to the cell
culture for a 2-h co-incubation. The absorbance was measured at 620
nm. The cell viability was expressed as OD (OD620).
The apoptosis of Panc-1 or Panc-1 CSCs was
determined by FACS analysis of Annexin V-FITC/propidium iodide (PI;
Roche, Basel, Switzerland)-double stained cells. Briefly, cells
were grown in a 6-well plate in serum-free medium. After treatment,
Panc-1 and Panc-1 CSCs were trypsinized, washed with
phosphate-buffered saline (PBS), resuspended in 200 µl PBS with 10
µl RNAase (10 mg/ml) and incubated at 37°C for 30 min. At the end
of incubation, 50 µl of Annexin V-FITC/PI labeling solution (BD
Biosciences, San Jose, CA, USA) was added and cells were analyzed
for apoptosis using a flow cytometry (BD FACSCanto II; BD
Biosciences).
Hypoxia exposure
For hypoxia induction, cells were cultured under a
hypoxic condition (1% O2, 5% CO2 and 94%
N2) in a multigas incubator (MCO-19; Sanyo Scientific,
Tokyo, Japan) for 6, 12 or 24 h. For normoxia incubation, cells
were incubated under the condition of 20% O2, 5%
CO2 and 75% N2.
Serial replating assay
For detecting self-renewal capacity of Panc-1 CSCs,
CSCs were grown in 6-well ultra-low attachment plates (Corning
Inc., Corning, NY, USA) at a density of 1,000 cells/ml in
well-defined serum-free medium at 37°C in a humidified atmosphere
of 95% air and 5% CO2. Fourteen days later, the spheres
with a diameter of >40 µm were counted under an Olympus X71
(U-RFL-T) fluorescence microscope (Olympus Corp., Melville, NY,
USA). Then, similarly, for secondary spheroids, the same number of
CSCs from spheroids were reseeded for another 14 days. All the
procedures were repeated 4 times.
siRNA transfection
Knockdown of the mRNA level of GPX4 was
achieved by transient transfection of cells with siRNA duplexes
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), specific to the
mRNA of GPX4. The relevant siRNA sequences were: GPX4 sense,
5′-UUCGAUAUGUUCAGCAAGAUU-3′ and antisense,
5′-UCUUGCUGAACAUAUCGAAUU-3′; negative control (NC) sense,
5′-GUUCAAUAUUAUCAAGCGGUU-3′ and antisense,
5′-CCGCUUGAUAAUAUUGAACUU-3′. According to the manufacturer's
instructions, 5×105 cells were grown in 2 ml of
serum-free medium. A siRNA/transfection reagent complex was formed
at room temperature by combining siRNA oligomer (50 nM) with 5 µl
(2 µg/ml) Lipofectamine™ 2000 transfection reagent (Thermo Fisher
Scientific, Inc.) in 0.5 ml Opti-MEM medium (Thermo Fisher
Scientific, Inc.) and this was applied to cells for 48 h until they
were harvested. Cells transfected with NC siRNA were considered as
the control cells. For erastin treatment, 48 h after transfection,
the cells were cultured in media supplemented with 2 µM erastin or
an equal amount of dimethyl sulfoxide (DMSO) (mock group) for 2, 4,
6 and 8 h, respectively. After 3 washes with PBS, the cells were
harvested for further analysis.
For H2O2 treatment, 48 h after
transfection, cells were cultured in media supplemented with 50,
100, 200 and 500 µM H2O2, respectively, for
24 h. After 3 washes with PBS, cells were harvested for further
analysis.
Construction of the expression plasmid
and transfection
The full-length complementary DNA (cDNA) of
GPX4 (human GPX4 GenBank accession no. NC_000019) was
obtained from RiboBio Co., Ltd. (Guangzhou, China) and ligated into
the NheI-XhoI site of the pcDNA3.1 vector (Life
Technologies; Thermo Fisher Scientific, Inc.). The NheI and
XhoI restriction enzymes and T4 ligase were purchased from
Takara Bio (Heidelberg, Germany). A total of 0.8 µg of the plasmid
or empty vector was mixed with 4 µl Lipofectamine™ 2000
transfection reagent (Thermo Fisher Scientific, Inc.) in 0.5 ml
Opti-MEM medium (Thermo Fisher Scientific, Inc.) and this was
applied to cells for 4 h followed by medium-refresh. After 24 h,
the culture medium was refreshed containing 500 µg/ml geneticin
sulfate 418 (G418; Sigma-Aldrich; Merck KGaA) for antibiotic
selection. Two weeks later, survival-transfected cells were
collected and maintained in 250 µg/ml G418.
For the apoptosis assay, cells were pretreated with
20 µM caspase inhibitor Z-VAD-FMK (Promega Corporation, Madison,
WI, USA), 1 µM Ferrostatin-1 (Ferr-1; Sigma-Aldrich; Merck KGaA),
or 0.3 µM Necrostatin-1 (Nec-1; Sigma-Aldrich; Merck KGaA) for 12 h
followed by co-incubation of H2O2 or erastin,
respectively. Twenty-four hours later, the cells were washed 3
times with PBS and harvested for further analysis.
RT-qPCR
The mRNA expression of GPX4, E-cadherin, vimentin,
Slug, Snail and the housekeeping gene β-actin was determined by
RT-qPCR using primer oligomers as followed: GPX sense primer,
5′-CGATACGCTGAGTGTGGTTTGC-3′ and antisense primer,
5′-CATTTCCCAGGATGCCCTTG-3′; E-cadherin sense primer,
5′-CGAGAGCTACACGTTCACGG-3′ and antisense primer,
5′-GGGTGTCGAGGGAAAAATAGG-3′; vimentin sense primer,
5′-GACGCCATCAACACCGAGTT-3′ and antisense primer,
5′-CTTTGTCGTTGGTTAGCTGGT-3′; Slug sense primer,
5′-CGAACTGGACACACATACAGTG-3′ and antisense primer,
5′-CTGAGGATCTCTGGTTGTGGT-3′; Snail sense primer,
5′-TCGGAAGCCTAACTACAGCGA-3′ and antisense primer,
5′-AGATGAGCATTGGCAGCGAG-3′; β-actin sense primer,
5′-CATGTACGTTGCTATCCAGGC-3′ and antisense primer,
5′-CTCCTTAATGTCACGCACGAT-3′. Reverse transcription was carried out
amplifying 1 µg total RNA using a reverse transcriptase kit
(Guangzhou Ribobio Co., Ltd.) according to the manufacturer's
instructions. PCR was then carried out using PowerUp SYBR™-Green
Master Mix (Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. Briefly, the PCR conditions were
started by denaturation for 5 min at 95°C and followed by 35 cycles
of 95°C for 10 sec and 60°C for 1 min. mRNA levels were normalized
against β-actin mRNA and for relative quantification,
2−ΔΔCq method was used (27).
ROS measurement
Total cellular ROS levels were determined using the
Image-iT™ LIVE Green Reactive Oxygen Species (ROS)
Detection kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. A total of
1×105 cells were used for analysis. Cells were washed
with ice-cold 1X PBS and incubated with 50 µl staining solution
containing 25 µM 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein
diacetate (carboxy-H2DCFDA) for 30 min in darkness.
Cells were washed twice with ice-cold 1X PBS and ROS levels were
measured at 495/529 nm (for carboxy-H2DCFDA) wavelengths
on a Multiskan spectrum microplate reader (Thermo Fisher
Scientific, Inc.).
Western blotting
Panc-1 or Panc-1 CSCs were washed twice with
ice-cold 1X PBS, and then resuspended in lysis buffer containing 25
mM HEPES buffer (pH 7.6), 3 mM MgCl2, 40 mM KCl, 2 mM
DTT, 5% glycerol and 0.5% NP-40, and 1X protease inhibitor, and
were maintained in an ice bath for 30 min. After a 15-min
centrifugation at 12,000 × g, at 4°C, the supernatant was collected
as total lysate. The protein concentration was determined using the
Bradford protein assay procedure (Sigma-Aldrich; Thermo Fisher
Scientific, Inc.). Twenty micrograms of protein was subjected to
10% SDS-PAGE, transferred to a polyvinylidene fluoride (PVDF)
membrane (Thermo Fisher Scientific, Inc.), and incubated at room
temperature overnight in PBS containing 5% dried milk, 0.05%
Tween-20 and primary antibodies: Anti-superoxide dismutase 1
antibody (cat. no. ab13498; diluted at 1:2,000), anti-superoxide
dismutase 2/MnSOD antibody (diluted at 1:2,000), anti-glutathione
peroxidase 1 antibody (cat. no. ab22604; diluted at 1:1,000),
anti-glutathione peroxidase 4 antibody (cat. no. ab125066; diluted
at 1:2,000) (all from Abcam, Cambridge, MA, USA); anti-Nanog
XP® rabbit monoclonal antibody (mAb) (cat. no. D73G4;
diluted at 1:2,500), anti-c-Myc rabbit mAb (cat. no. D3N8F; diluted
at 1:1,000), anti-Oct-4 antibody (cat. no. 2750; diluted at
1:1,000), anti-Sox2 antibody (cat. no. 3579; diluted at 1:2,000),
anti-E-cadherin rabbit mAb (cat. no. 24E10; diluted at 1:2,000),
anti-vimentin XP® rabbit mAb (cat. no. D21H3; diluted at
1:1,000), anti-Slug rabbit mAb (cat. no. C19G7; diluted at
1:1,000), anti-Snail rabbit mAb (cat. no. C15D3; diluted at
1:2,000), anti-β-actin mouse mAb (cat. no. 8H10D10; diluted at
1:5,000) (all from Cell Signaling Technology, Inc., Danvers, MA,
USA). After 4 washes in PBS containing 0.05% Tween-20, the membrane
was incubated with secondary antibody [anti-mouse IgG, HRP-linked
antibody (cat. no. 7076; diluted at 1:5,000), anti-rabbit IgG,
HRP-linked antibody (cat. no. 7074; diluted at 1:5,000) (both from
Cell Signaling Technology, Inc,)] for 1 h, washed 4 times and the
bound antibody was detected by chemiluminescence, and analyzed with
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All the data are expressed as means ± SD of 3
independent experiments. Unpaired Student's t-tests were used to
compare the means of 2 groups. Differences between groups were
analyzed by performing the ANOVA Kruskal-Wallis test, with Dunn's
multiple group comparison test. P-values <0.05 were considered
to indicate a statistically significant result.
Results
Characterization of CSCs derived from
Panc-1 cells
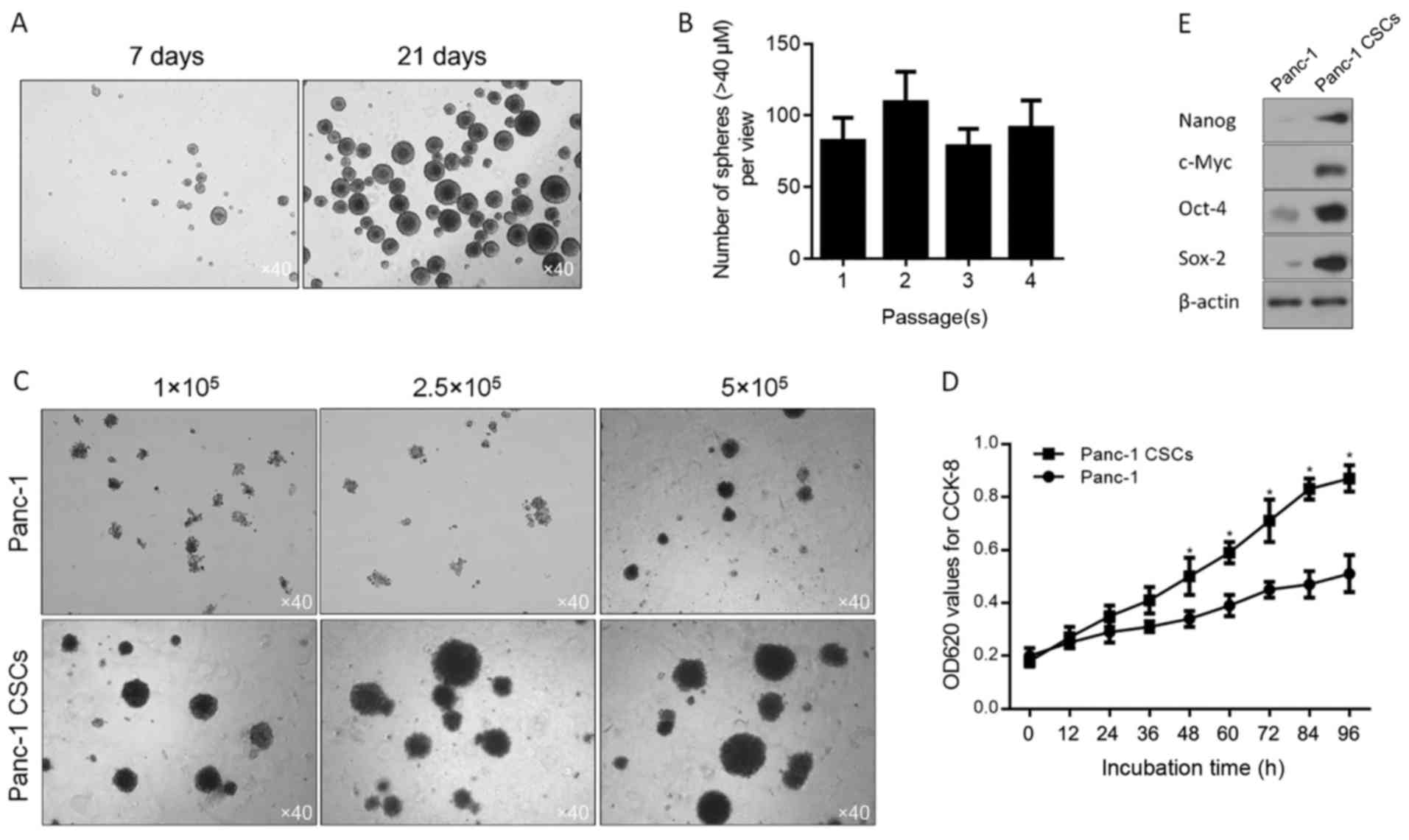
After enriched Panc-1 CSCs were cultured for 7 and
21 days, it was obvious that the morphology of the pancreatic CSCs
displayed sphere-like appearance (Fig.
1A). The ability of self-renew is the definition of a stem cell
that is thought to be functionally mimicked by CSCs (28–30)
and serial-replating assay which is widely employed (31), was performed to assess self-renewal
of derived cells. As shown in Fig.
1B, no obvious difference in sphere formation ability was found
between the cells at the 1st to 4th passage. We then determined
whether the spheroid cells have the capacity of high colony
formation using Soft agar colony formation assay. Sphere-forming
assay was performed to evaluate the stemness property of the Panc-1
cells. The results indicated that the stemness property of the
Panc-1 cells was higher than that of their parental counterpart
after a period of adaptation to 10% FBS containing medium, instead
of serum-free medium (Fig. 1C). And
as expected, the capacity of cell proliferation in the spheroid
cells was significantly higher than that of their parental
counterpart (Fig. 1D). Furthermore,
4 transcription factors which regulate the self-renewal capacity of
CSCs (32) were detected by western
blot analysis and the results showed that spheroid cells derived
from Panc-1 cells displayed obviously higher expression levels of
these 4 factors, compared to these levels in the parental
counterpart (Fig. 1E).
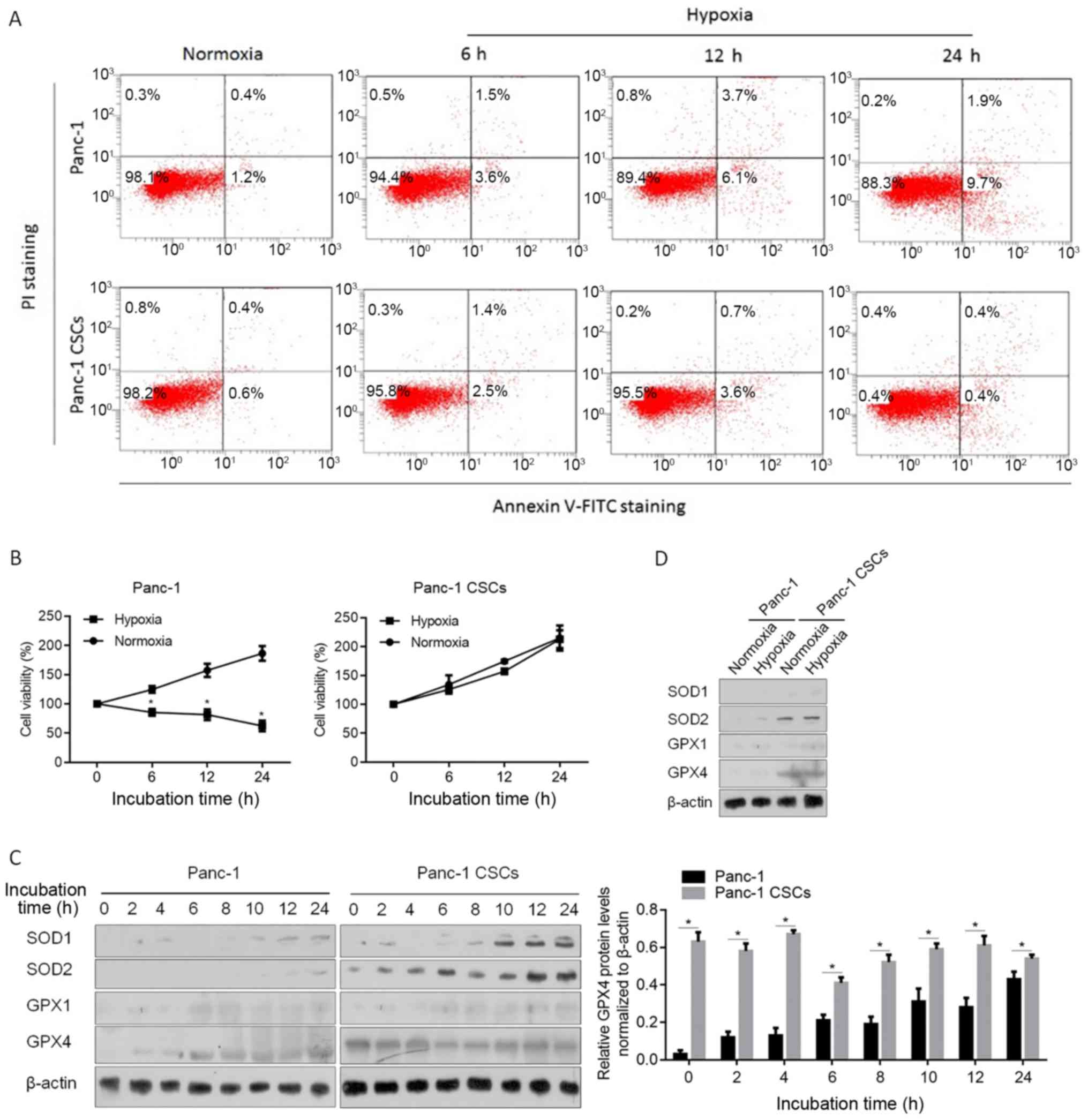
Panc-1 CSCs display more resistant to
hypoxic exposure and show induced expression levels of SOD1, SOD2,
GPX1 and GPX4
Hypoxia has been reported to be a promoter of
maintaining the stem-like phenotype of CSCs (33). To confirm whether CSCs derived from
Panc-1 cells are resistant to hypoxic exposure, Panc-1 or Panc-1
CSCs were exposed to hypoxia for 6, 12 or 24 h and stained using
Annexin V-FITC/PI double staining. As shown in Fig. 2A, in normoxia condition, no
detectable difference between Panc-1 and Panc-1 CSCs in apoptotic
cell death rate (Annexin V-FITC-positive/PI-negative and Annexin
V-FITC-positive/PI-positive subpopulation) was noted. After being
exposed to hypoxia for 12 and 24 h, the apoptotic cell death rate
in the Panc-1 CSCs (4.3±0.5% for 12 h; 0.8±0.6% for 24 h) was
obviously lower than that of the Panc-1 cells (9.8±0.9% for 12 h;
11.6±1.7% for 24 h). Then the effects of hypoxia on cell viability
were assessed. It was observed that, in Panc-1 CSCs, hypoxia
exposure failed to decrease cell viability after 24 h (Fig. 2B). By considering the roles of SODs
and GPXs in maintaining low intracellular ROS level in mammalian
cells (34), western blot analysis
was performed to detect the SOD and GPX protein levels in the
Panc-1 and Panc-1 CSCs after hypoxic exposure. Expectedly, SOD1,
SOD2 and GPX1 levels were induced by hypoxic exposure (Fig. 2C). However, GPX4 presented a
relative high expression level in the Panc-1 CSCs compared with
that in the Panc-1 cells, and GPX4 expressing levels were not
obviously affected by hypoxic exposure in the Panc-1 CSCs. To
confirm whether GPX4 presents a high endogenous level beyond
hypoxic exposure, SODs and GPXs were compared in the Panc-1 and
Panc-1 CSCs. In Fig. 2D, it was
observed that, in the Panc-1 CSCs, GPX4 presented a consistently
high endogenous level, which was obviously higher than that noted
in the Panc-1 cells and did not respond to hypoxic exposure. This
demonstrated that GPX4 potentially play critical roles in
regulating physiological processes in panc-1 CSCs, not only after
hypoxic exposure.
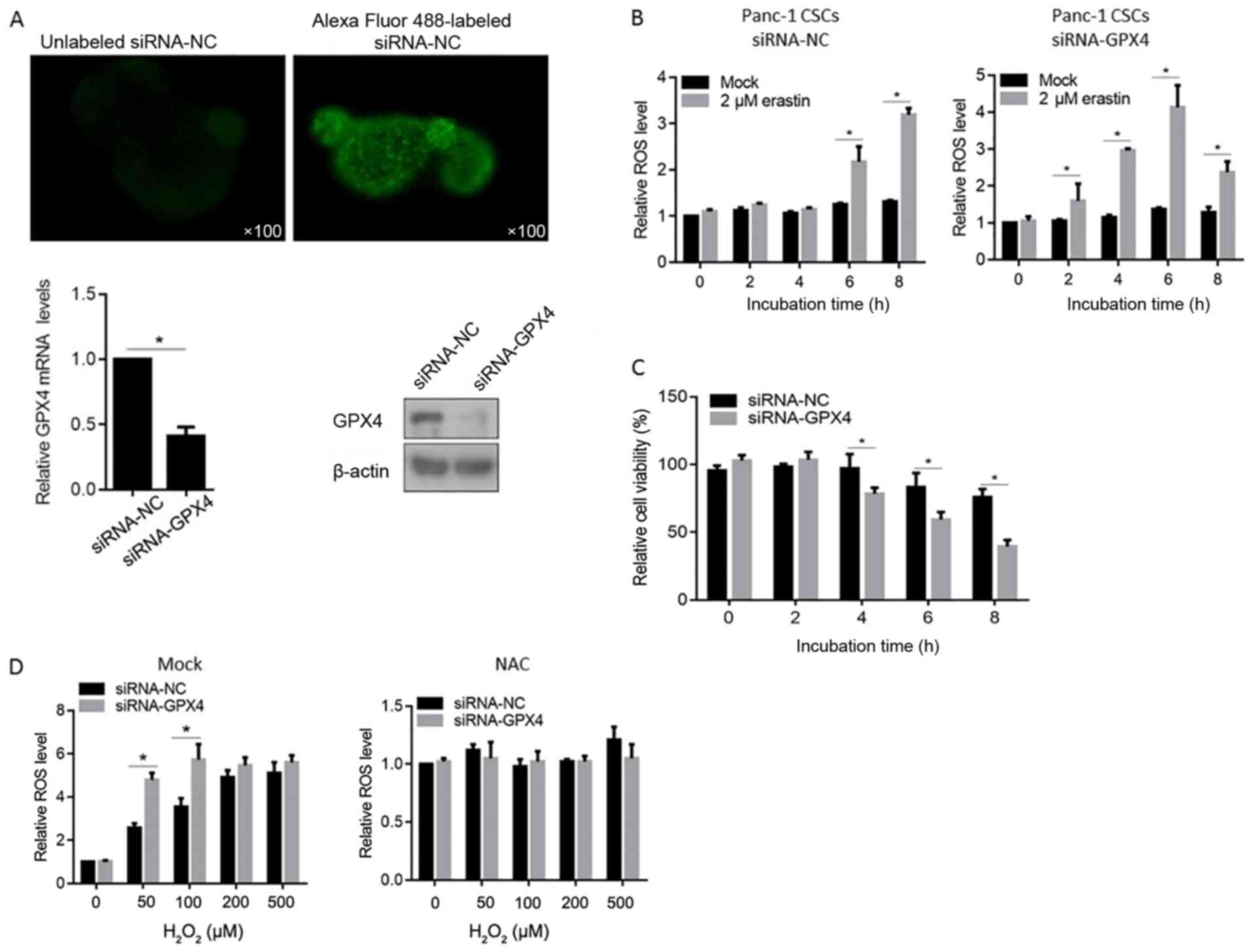
Endogenous GPX4 regulates oxidative
homeostasis and exerts a protective effect on cell viability
For identifying the transfecting efficiency of siRNA
by Lipofectamine™ 2000, Alexa Fluor 488-labeled siRNA-NC
was introduced into the Panc-1 CSCs and was detected immediately
after 3 PBS washes. As shown in Fig.
3A (upper panels), siRNA-NC was successfully introduced into
the Panc-1 CSCs. Then, the knockdown efficiency of the siRNA
targeted to GPX4 mRNA (siRNA-GPX4) was measured by RT-qPCR and
western blot analysis 2 days after transfection. The mRNA level of
GPX4 was downregulated by >50%. In addition, a detectable
downregulation in the protein level of GPX4 was also confirmed
(Fig. 3A, lower panels).
By considering the inactivating ability of erastin
to GPX enzymes, especially GPX4 (35), we examined the ROS accumulation of
Panc-1 CSCs treated with targeted siRNA 3 days after transfection
treated with 2 µM erastin (Fig.
3B). Cells transfected with siRNA-GPX4 presented significant
high ROS levels at 2- or 4-h of erastin treatment, while the
control cells presented no detectable ROS accumulation. We then
examined the effects of GPX4 knockdown on the cell growth of Panc-1
CSCs, which were exposed to hypoxia for 12 h previously, using
CCK-8 assay. There was no significant difference in cell viability
between cells transfected with GPX4 and control siRNA up to 0 and 2
h after erastin treatment (Fig.
3C). However, at 4 h after erastin treatment, the viability of
the siRNA-GPX4 transfected cells was significantly lower than that
of the siRNA-NC transfected cells, suggesting that GPX4 exerts
protective effect for cell viability against erastin treatment. We
further clarify the ROS eliminating effect of GPX4 under oxidative
stress condition induced by H2O2. As shown in
Fig. 3D right panel, NAC
pre-treatment scavenged ROS in cells. In Fig. 3D left panel, without NAC treatment
(mock groups), GPX4 knockdown promoted the ROS accumulation at 50
and 100 µM (Fig. 3D).
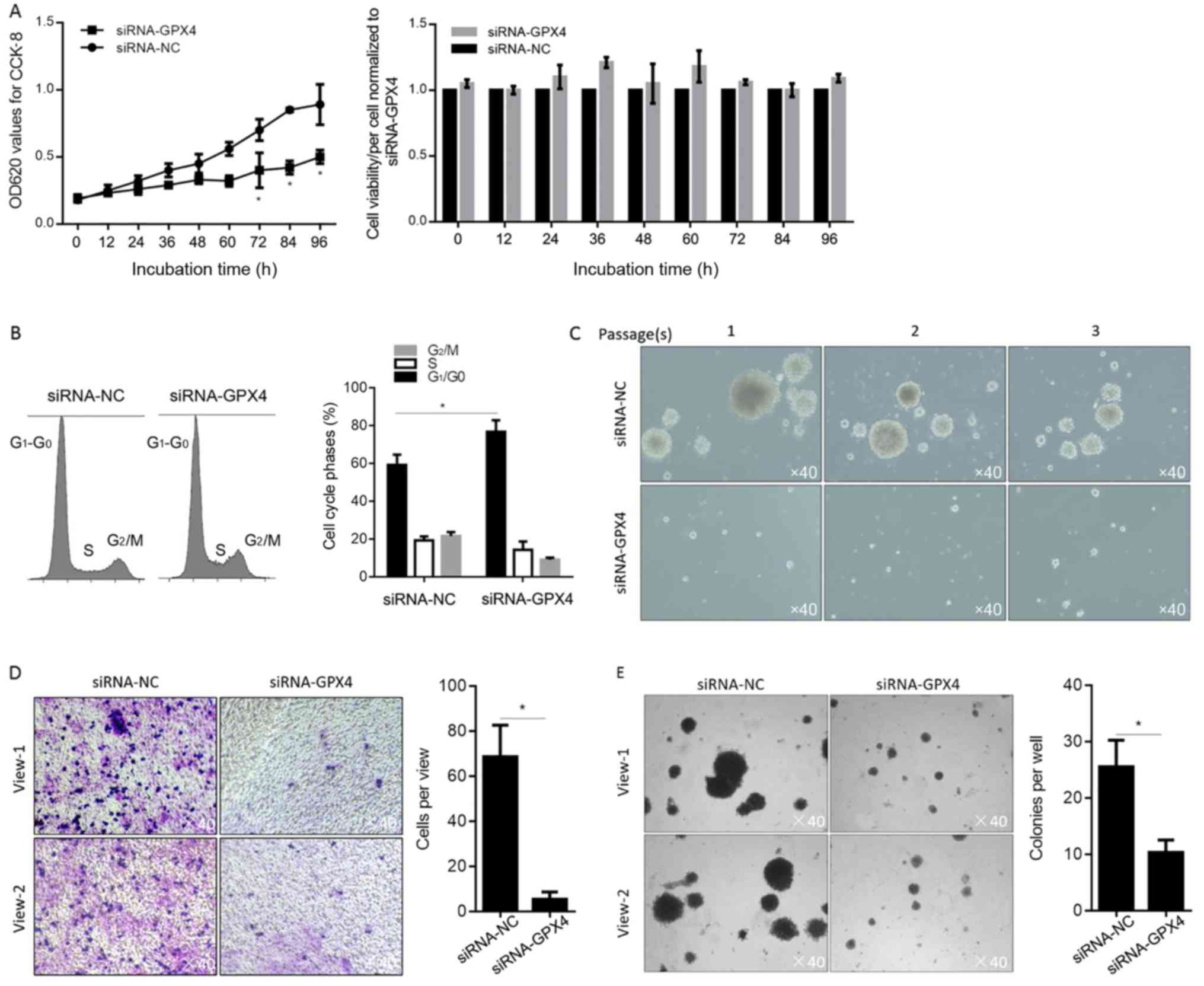
Knockdown of GPX4 tightly regulates
physiological processes and inhibits the stemness phenotype in the
Panc-1 CSCs
Previously, we showed that the presence of GPX4
exerted protective effects to Panc-1 CSCs under oxidative stresses
including H2O2 or erastin (Fig. 3). To further investigate whether
GPX4 plays certain roles without oxidative stress in Panc-1 CSCs,
its effects on cell proliferation was determined. By performing
CCK-8 assay and cell counting, it was observed that, without
disturbing cell viability, GPX4 knockdown decreased cell
proliferation compared to that of the siRNA-NC (Fig. 4A). Flow cytometric analysis after PI
staining further showed that GPX4 knockdown arrested the cell cycle
at the G1/G0 phase, indicating cell cycle
arrest at this phase (Fig. 4B). The
results of functional analysis showed that knockdown of GPX4
significantly suppressed sphere formation ability (Fig. 4C), migration and invasion capacity
(Fig. 4D and E) in Panc-1 CSCs, as
compared with siRNA-NC cells. Collectively, our results revealed
that knockdown of GPX4 suppressed the stemness phenotype of Panc-1
CSCs and inhibited in vitro cell functions.
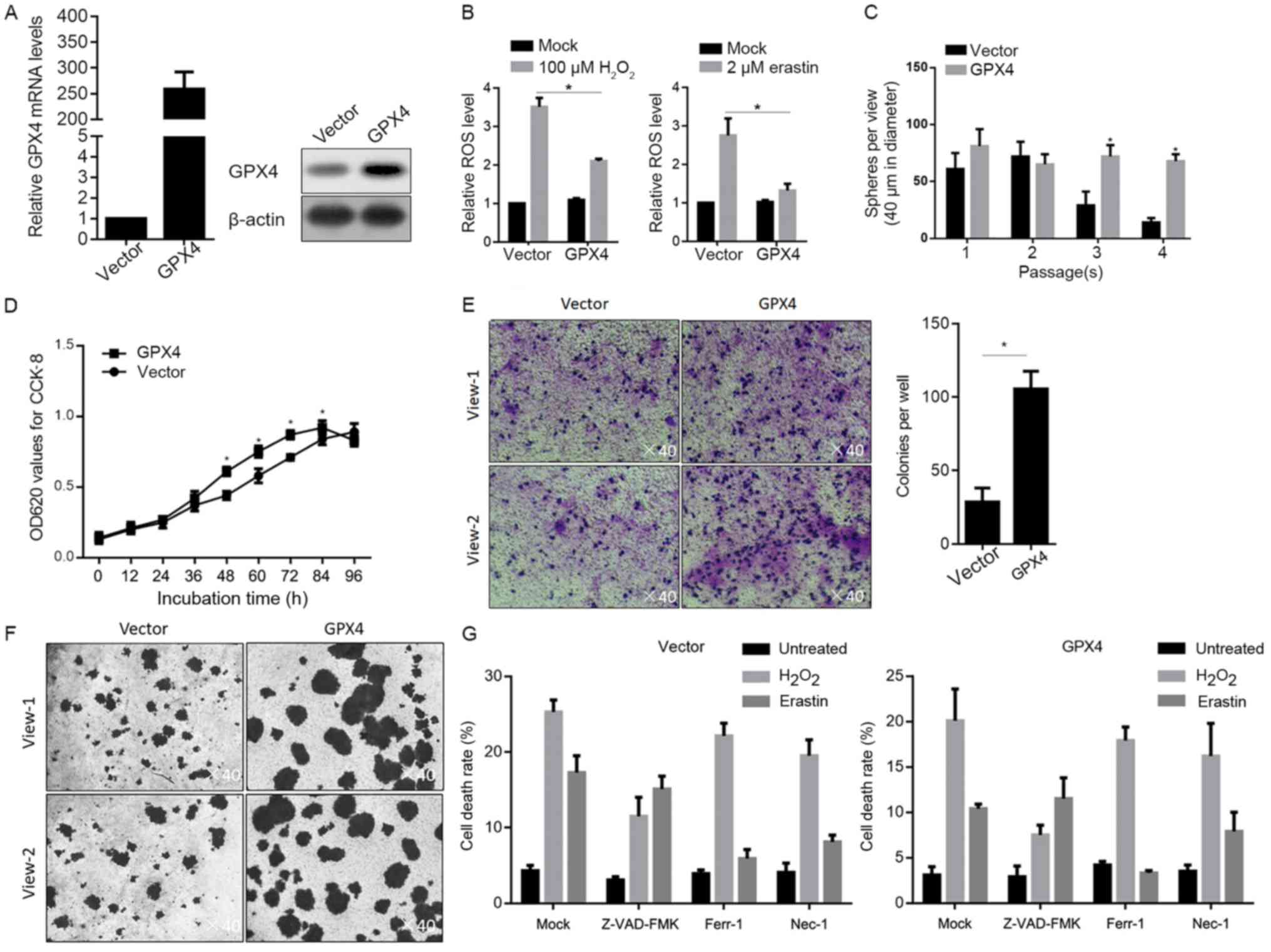
Overexpression of GPX4 suppressed the
stemness phenotype and exerts protective effects under oxidative
stress
We further evaluated the effects of the
overexpression of GPX4 on stemness characteristics and
chemosensitivity in Panc-1 CSCs. After stable transfection of GPX4,
its mRNA and protein levels were obviously overexpressed (Fig. 5A). Expectedly, after oxidative
stress exposure, overexpression of GPX4 inhibited the accumulation
of ROS after H2O2 or erastin treatment
(Fig. 5B). By performing
serial-replating assay with the existence of 100 µM
H2O2, it was found that overexpression of
GPX4 promoted sphere formation at passage 3 and 4, indicating that
the self-renewal capacity of the Panc-1 CSCs was promoted by
overexpression of GPX4 (Fig. 5C).
Followed by detection of physiological processes under oxidative
stress (100 µM H2O2), it was revealed that
overexpression of GPX4 promoted cell proliferation (Fig. 5D), migration (Fig. 5E) and invasion (Fig. 5F). In order to ascertain whether
overexpression of GPX4 inhibits oxidative stress-induced cell death
in Panc-1 CSCs, the cells pretreated with apoptotic death inhibitor
(Z-VAD-FMK), ferroptotic death inhibitor (Ferr-1) or necrotic death
inhibitor (Nec-1) were treated with oxidative stress (100 µM
H2O2 or 2 µM erastin) and stained using
CFSE/PI followed by flow cytometric analysis. As shown in Fig. 5G, overexpression of GPX4 inhibited
apoptotic cell death induced by H2O2,
ferroptotic and necrotic cell death induced by erastin. All these
data showed that overexpression of GPX4 exerts protective effects
under oxidative stress.
GPX4 plays a critical role in the
partial regulation of the EMT phenotype in Panc-1 CSCs
ROS level is reported to be directly and tightly
related to EMT-programme activation (35). This promoted us to ascertain whether
H2O2 or erastin-induced ROS accumulation
regulates EMT-programme processes. Firstly, we tested the ROS
eliminating effect of N-acetyl cysteine (NAC), a ROS scavenger,
after H2O2 or erastin induction. As expected,
NAC efficiently eliminated ROS after oxidative stress (Fig. 6A), thus, in further experiments, NAC
was employed as a ROS scavenger. RT-qPCR results for detection of
mRNA levels of EMT hallmark genes, including E-cadherin, vimentin,
Slug and Snail showed that both H2O2 and
erastin treatment upregulated E-cadherin and downregulated
vimentin, Slug and Snail mRNA levels (Fig. 6B), while elimination of ROS by a
scavenger inhibited these changes, indicating that the generated
ROS are attributed to the regulation of EMT. Consistently, protein
levels of E-cadherin, vimentin, Slug and Snail were consistent with
the tendency of mRNA levels (Fig.
6C).
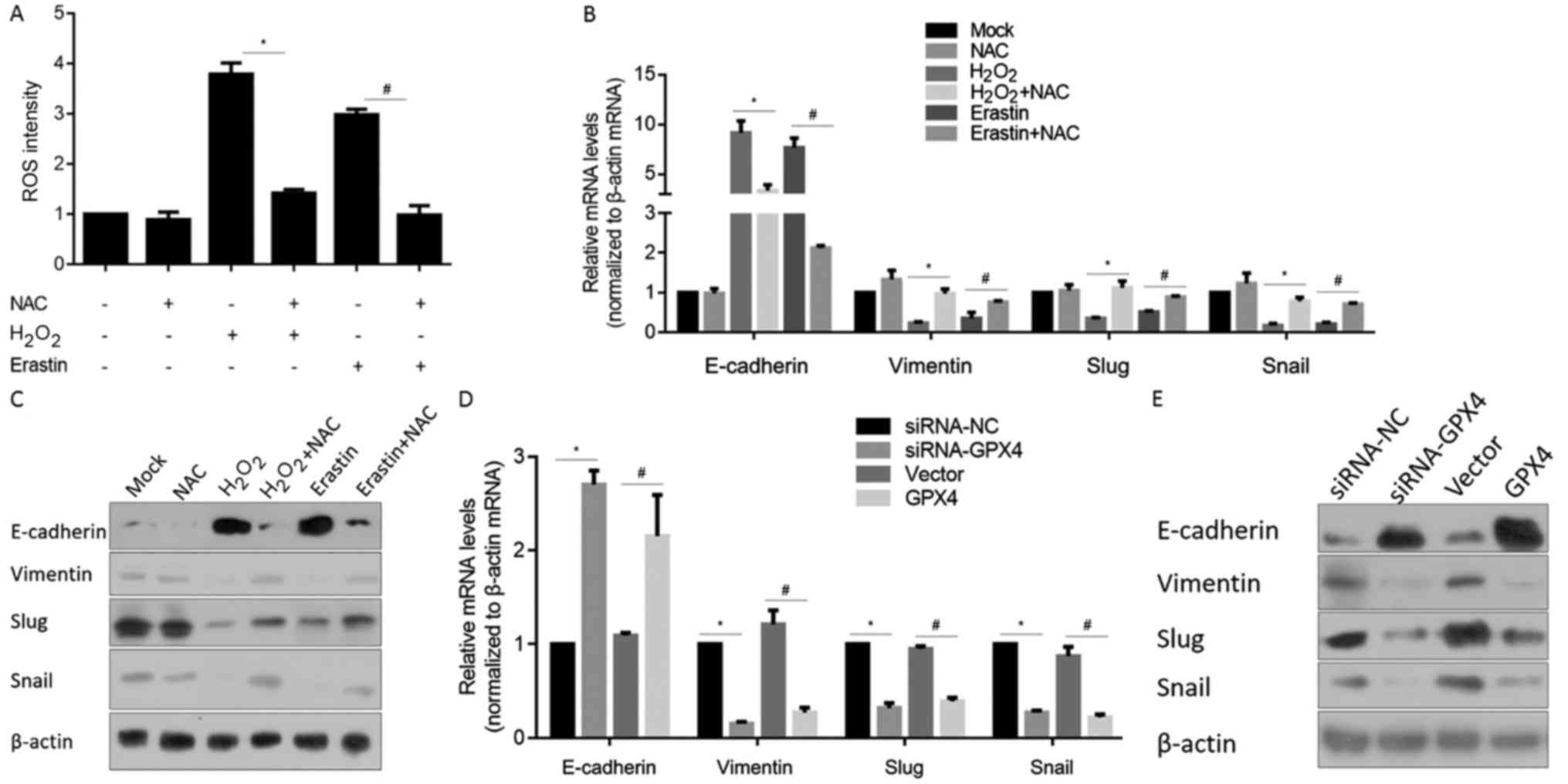 | Figure 6.GPX4 plays a partial role in
maintaining the EMT phenotype. (A) ROS levels were measured after
H2O2 or erastin treatment in Panc-1 CSCs.
*P<0.05 vs. H2O2-treated cells;
#P<0.05 vs. erastin-treated cells. (B) RT-qPCR and
(C) western blot analysis were performed to detect the mRNA and
protein levels of E-cadherin, vimentin, Slug and Snail. *P<0.05
vs. H2O2-treated cells; #P<0.05
vs. erastin-treated cells. (D) RT-qPCR and (E) western blot
analysis were performed to detect the mRNA and protein levels of
E-cadherin, vimentin, Slug and Snail in siRNA-NC, siRNA-GPX4,
vector- or GPX4-transfected Panc-1 CSCs. *P<0.05 vs. siRNA-NC;
#P<0.05 vs. vector-transfected cells. CSCs, cancer
stem-like cells; GPX4, glutathione peroxidase 4; EMT,
epithelial-to-mesenchymal transition; ROS, reactive oxygen
species. |
By considering that the difference between CSCs and
non-CSCs is likely to be attributable largely to the cell
biological programme termed EMT (37,38),
the effects of modified GPX4 on EMT hallmark genes were further
detected by RT-qPCR and western blot analysis. Both knockdown and
overexpression of GPX4 upregulated E-cadherin, downregulated
vimentin, Slug and Snail at the mRNA and protein levels, indicating
its inhibitory effect on the EMT programme (Fig. 6D and E), which is consistent with
the morphologic changes.
Discussion
ROS are recognized as critical cellular signalling
molecules. The maintenance of ROS is critical for a wide array of
biological processes, including cell viability, proliferation,
apoptosis and angiogenesis (1). In
tumours, emerging evidence suggests that accumulation of ROS is
associated with the generation of CSCs and the activation of the
EMT programme upon hypoxia exposure, and it is considered the most
common characteristic associated with tumourigenesis and tumour
progression (39). The antioxidant
system is intensively studied in mammalian cells (40), however, the importance of a specific
antioxidant enzyme has not been fully understood in Panc-1 CSCs. In
the present study, we enriched CSCs from Panc-1 cells using
serum-free medium. After comparison of several major antioxidant
enzymes, including SOD1, SOD2, GPX1 and GPX4 after hypoxic
exposure, we found that SOD1, SOD2 and GPX1 were upregulated in
both Panc-1 and Panc-1 CSCs. Surprisingly, GPX4 displayed a much
higher level of expression in Panc-1 CSCs compared with Panc-1
cells under normoxic conditions. This suggests the importance of
GPX4 as a potential key regulator of oxidative homeostasis under
normoxic conditions in Panc-1 CSCs. Knockdown and overexpression
studies of GPX4 were employed to discern the importance of GPX4 in
maintaining oxidative homeostasis in Panc-1 CSCs. According to our
results, both overexpression and knockdown of GPX4 increased
epithelial markers and decreased mesenchymal ones, and eliminated
the characteristics of Panc-1 CSCs without oxidative stress,
suggesting that GPX4 may be critical for maintaining oxidative
homeostasis. In our hypothesis, both upregulation and
downregulation of GPX4 inactivate the EMT programme in unknown
mechanisms, which requires further investigation.
Both SODs and GPXs are critical scavenger
antioxidative enzyme systems, which are responsible for eliminating
accumulated intracellular ROS induced by hypoxia or oxidant
exposure. Formed ROS are converted by SODs to
H2O2 and subsequently converted by GPXs to
H2O+O2 (3).
In both Panc-1 and Panc-1 CSCs, hypoxia exposure stimulated the
expression of SOD1, SOD2 and GPX1, which is consistent with a
previous report (4). To avoid the
involvement of SODs in ROS elimination, cells were treated with
H2O2 to generate ROS in Panc-1 CSCs. As
expected, without the involvement of SODs, GPX4 exerted a critical
role in eliminating ROS in CSCs (41). After hypoxic exposure, both SODs and
GPXs were upregulated in Panc-1 CSCs, which is consistent with a
previous report (42). Notably,
before hypoxia stimulation, endogenous GPX4 was relatively high in
CSCs and there was no obvious upregulation after hypoxic exposure,
suggesting a role for GPX4 under normal conditions. Erastin induces
ROS accumulation and ferroptotic cell death (43). We showed that expression of GPX4
exerted antioxidant effects in response to erastin-induced oxidant
injury, suggesting that GPX4 exerts protective effects mainly via
eliminating ROS induced in a different manner.
EMT has been reported as an important regulator of
cancer cells exhibiting stem cell-like properties (44), and EMT is closely linked to a stem
cell phenotype in CSCs. In the present study, we demonstrated that
changes in GPX4 expression led to decreases in the EMT phenotype
and reduction in the stemness phenotype in Panc-1 CSCs. These
results indicate that GPX4 potentially regulates the stemness
phenotype via regulation of ROS accumulation and the EMT programme.
There is still a limitation of this study that only a single cell
line was used. For generalizing the results obtained, we intend to
test the roles of GPX4 in another cellular system in vitro
and in other cancer stem-like cells derived from other organs, and
to confirm the roles of GPX4 in human pancreatic tumor tissues in
further studies.
Collectively, we demonstrated that GPX4 is
upregulated in Panc-1 CSCs compared with parental Panc-1 cells. The
elevated levels of GPX4 are responsible for regulating oxidative
homeostasis, maintaining the EMT programme and maintaining the CSC
phenotype. These data suggest that GPX4 is a possible therapeutic
target to prevent the development of resistance to oxidative
stress.
Acknowledgements
We would like to thank Professor Hummin Zhao
(Sichuan University) for the language editing.
Funding
The present study was funded by the Traditional
Chinese Medicine Scientific Support program of Health and Family
Planning Commision of Chongqing (no. ZY201703017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GP designed part of the experiments and performed
the gene expressing and the cell culture relative experiments. ZT
and YX performed the gene expression analysis and some cell culture
relative experiments. YX wrote the draft of the manuscript. WC
designed part of the experiments and supervised the whole
procedure. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bao B, Azmi AS, Li Y, Ahmad A, Ali S,
Banerjee S, Kong D and Sarkar FH: Targeting CSCs in tumor
microenvironment: The potential role of ROS-associated miRNAs in
tumor aggressiveness. Curr Stem Cell Res Ther. 9:22–35. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klaunig JE, Kamendulis LM and Hocevar BA:
Oxidative stress and oxidative damage in carcinogenesis. Toxicol
Pathol. 38:96–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiao Y, Wang Y, Guo S and Wang G:
Glutathione peroxidases as oncotargets. Oncotarget. 8:80093–80102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cejas P, García-Cabezas MA, Casado E,
Belda-Iniesta C, De Castro J, Fresno JA, Barriuso J, Espinosa E,
Zamora P, Feliu J, et al: Phospholipid hydroperoxide glutathione
peroxidase (PHGPx) expression is downregulated in poorly
differentiated breast invasive ductal carcinoma. Free Radic Res.
41:681–687. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Taie OH, Uceyler N, Eubner U, Jakob F,
Mörk H, Scheurlen M, Brigelius-Flohe R, Schöttker K, Abel J,
Thalheimer A, et al: Expression profiling and genetic alterations
of the selenoproteins GI-GPx and SePP in colorectal carcinogenesis.
Nutr Cancer. 48:6–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Falck E, Karlsson S, Carlsson J, Helenius
G, Karlsson M and Klinga-Levan K: Loss of glutathione peroxidase 3
expression is correlated with epigenetic mechanisms in endometrial
adenocarcinoma. Cancer Cell Int. 10:462010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Du J, Zhang Y, Sun W, Smith BJ,
Oberley LW and Cullen JJ: Suppression of the malignant phenotype in
pancreatic cancer by overexpression of phospholipid hydroperoxide
glutathione peroxidase. Hum Gene Ther. 17:105–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu YP, Yu G, Tseng G, Cieply K, Nelson J,
Defrances M, Zarnegar R, Michalopoulos G and Luo JH: Glutathione
peroxidase 3, deleted or methylated in prostate cancer, suppresses
prostate cancer growth and metastasis. Cancer Res. 67:8043–8050.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito K, Hirao A, Arai F, Takubo K, Matsuoka
S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, et al:
Reactive oxygen species act through p38 MAPK to limit the lifespan
of hematopoietic stem cells. Nat Med. 12:446–451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bell EL, Klimova TA, Eisenbart J, Moraes
CT, Murphy MP, Budinger GR and Chandel NS: The Qo site
of the mitochondrial complex III is required for the transduction
of hypoxic signaling via reactive oxygen species production. J Cell
Biol. 177:1029–1036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang J, Yang J, Maity B, Mayuzumi D and
Fisher RA: Regulator of G protein signaling 6 mediates
doxorubicin-induced ATM and p53 activation by a reactive oxygen
species-dependent mechanism. Cancer Res. 71:6310–6319. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Juntilla MM, Patil VD, Calamito M, Joshi
RP, Birnbaum MJ and Koretzky GA: AKT1 and AKT2 maintain
hematopoietic stem cell function by regulating reactive oxygen
species. Blood. 115:4030–4038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye XQ, Li Q, Wang GH, Sun FF, Huang GJ,
Bian XW, Yu SC and Qian GS: Mitochondrial and energy
metabolism-related properties as novel indicators of lung cancer
stem cells. Int J Cancer. 129:820–831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HM, Haraguchi N, Ishii H, Ohkuma M,
Okano M, Mimori K, Eguchi H, Yamamoto H, Nagano H, Sekimoto M, et
al: Increased CD13 expression reduces reactive oxygen species,
promoting survival of liver cancer stem cells via an
epithelial-mesenchymal transition-like phenomenon. Ann Surg Oncol.
19 Suppl 3:S539–S548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thompson EW, Newgreen DF and Tarin D:
Carcinoma invasion and metastasis: A role for
epithelial-mesenchymal transition? Cancer Res. 65:5991–5995. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chi HC, Tsai CY, Tsai MM, Yeh CT and Lin
KH: Roles of long noncoding RNAs in recurrence and metastasis of
radiotherapy-resistant cancer. Int J Mol Sci. 18(pii): E19032017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cannito S, Novo E, di Bonzo LV, Busletta
C, Colombatto S and Parola M: Epithelial-mesenchymal transition:
From molecular mechanisms, redox regulation to implications in
human health and disease. Antioxid Redox Signal. 12:1383–1430.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giannoni E, Parri M and Chiarugi P: EMT
and oxidative stress: A bidirectional interplay affecting tumor
malignancy. Antioxid Redox Signal. 16:1248–1263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bao B, Azmi AS, Ali S, Ahmad A, Li Y,
Banerjee S, Kong D and Sarkar FH: The biological kinship of hypoxia
with CSC and EMT and their relationship with deregulated expression
of miRNAs and tumor aggressiveness. Biochim Biophys Acta.
1826:272–296. 2012.PubMed/NCBI
|
|
26
|
Fu Z, Li G, Li Z, Wang Y, Zhao Y, Zheng S,
Ye H, Luo Y, Zhao X, Wei L, et al: Endogenous miRNA sponge
lincRNA-ROR promotes proliferation, invasion and stem cell-like
phenytype of pancreatic cancer cells. Cell Death Discov.
3:170042017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pradal R, Clarke MF and Morrison SJ:
Applying the principle of stem-cell biology to cancer. Nat Rev
Cancer. 3:895–902. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Somervaille TC and Cleary ML:
Identification and characterization of leukemia stem cells in
murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 10:257–268.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sharma N, Nanta R, Sharma J, Gunewardena
S, Singh KP, Shankar S and Srivastava RK: PI3K/AKT/mTOR and sonic
hedgehog pathways cooperate together to inhibit human pancreatic
cancer stem cell characteristics and tumor growth. Oncotarget.
6:32039–32060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yeung TM, Gandhi SC, Wilding JL, Muschel R
and Bodmer WF: Cancer stem cells from colorectal cancer-derived
cell lines. Proc Natl Acad Sci USA. 107:3722–3727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galadari S, Rahman A, Pallichankandy S and
Thayyullathil F: Reactive oxygen species and cancer paradox: To
promote or to suppress? Free Radic Biol Med. 104:144–164. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Medema JP: Cancer stem cells: The
challenges ahead. Nat Cell Biol. 15:338–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ischenko I, Seeliger H, Kleespies A,
Angele MK, Eichhorn ME, Jauch KW and Bruns CJ: Pancreatic cancer
stem cells: New understanding of tumorigenesis, clinical
implications. Langenbecks Arch Surg. 395:1–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hayashi R, Himori N, Taguchi K, Ishikawa
Y, Uesugi K, Ito M, Duncan T, Tsujikawa M, Nakazawa T, Yamamoto M,
et al: The role of the Nrf2-mediated defense system in corneal
epithelial wound healing. Free Radic Biol Med. 61:333–342. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu L, Oveson BC, Jo YJ, Lauer TW, Usui S,
Komeima K, Xie B and Campochiaro PA: Increased expression of
glutathione peroxidase 4 strongly protects retina from oxidative
damage. Antioxid Redox Signal. 11:715–724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Perrella MA and Yet SF: Role of heme
oxygenase-1 in cardiovascular function. Curr Pharm Des.
9:2479–2487. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maldonado EN, Sheldon KL, DeHart DN,
Patnaik J, Manevich Y, Townsend DM, Bezrukov SM, Rostovtseva TK and
Lemasters JJ: Voltage-dependent anion channels modulate
mitochondrial metabolism in cancer cells: Regulation by free
tubulin and erastin. J Biol Chem. 288:11920–11929. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Scheel C and Weinberg RA: Phenotypic
plasticity and epithelial-mesenchymal transitions in cancer and
normal stem cells? Int J Cancer. 129:2310–2314. 2011. View Article : Google Scholar : PubMed/NCBI
|