Introduction
Renal cell carcinoma (RCC) is the leading cause of
cancer-related death among all urological malignancies (1). Early-stage RCC is usually curable by
surgical resection, but a large number of early-stage RCC cases are
asymptomatic, with approximately one-third of all patients
presenting with metastatic cancer at the time of diagnosis
(2,3). Therefore, a better understanding of
the molecular mechanisms of RCC progression is crucial for the
discovery of novel prognostic markers and targeted therapies.
Extracellular vesicles (EVs), including exosomes,
microvesicles and apoptotic bodies, are released by almost all cell
types, including tumor cells. Recently, EVs have been shown to play
an important role in the development of cancer, facilitating
cell-cell communication between cancer cells and the surrounding
microenvironment (4). However,
previous EV-related studies have mainly focused on exosomes derived
from cultured cells. Therefore, to understand cancer-associated EV
functions in the human body, we obtained EVs from freshly resected
RCC tissue (tumor and adjacent normal renal tissue) by means of a
brief incubation in serum-free medium, yielding what we term
tissue-exudative extracellular vesicles (Te-EVs) (5). Quantitative LC/MS analysis revealed
106 RCC-specific Te-EV proteins. Among the 106 upregulated
proteins, we focused on leukocyte-associated immunoglobulin-like
receptor 1 (LAIR1) as a novel cancer-associated EV protein.
LAIR1 is a type I transmembrane glycoprotein of 287
amino acids containing a single extracellular C2-type Ig-like
domain and two immunoreceptor tyrosine-based inhibitory motif
(ITIMs) in its cytoplasmic tail (6). LAIR1 is expressed on almost all cells
of the immune system including natural killer (NK) cells, T cells,
B cells and monocytes, monocyte-derived dendritic cells,
eosinophils, and basophils and mast cells (6–10).
LAIR1 can inhibit T-cell receptor complex (TCR) mediated signals,
possibly via the recruitment of Csk, the SH2 domain-containing
protein tyrosine phosphatases SHP-1 or SHP-2, and to a certain
extent on signaling through p38 and Erk (11). Unlike other immune-inhibitory
receptors such as PD-1 or CTLA4, the functional ligands of LAIR1
are collagens, which upon binding to LAIR1 inhibit immune cell
activation (12). However, to the
best of our knowledge, no reports have investigated the expression
and function of LAIR1 in RCC.
In the present study, we found that LAIR1 was
significantly upregulated in RCC tissues compared to that noted in
the normal renal tissues. LAIR1 overexpression promoted cell
proliferation by upregulating Akt phosphorylation in RCC cells
in vitro. Moreover, LAIR1 overexpression accelerated in
vivo tumor growth in a mouse xenograft model. To the best of
our knowledge, this is the first report showing that overexpression
of LAIR1 contributes to RCC progression.
Materials and methods
Chemicals and antibodies
Monoclonal anti-CD9 antibody (clone 12A12),
monoclonal anti-CD63 antibody (clone 8A12) and monoclonal anti-CD81
antibody (clone 12C4), which were previously confirmed to have high
specificity for their targets (13,14),
were purchased from Cosmo Bio Co., Ltd. (Tokyo, Japan). Monoclonal
anti-LAIR1 antibody (cat. no. ab14826) and polyclonal anti-mouse
IgG (20 nm gold) preadsorbed antibody (cat. no. ab27242) were
purchased from Abcam (Burlingame, CA, USA). Polyclonal anti-LAIR1
antibody (cat. no. HPA011155), monoclonal anti-β-actin antibody
(cat. no. A2228) and LY294002 were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Monoclonal anti-pErk1/2 (cat. no.
4370), anti-Erk1/2 (cat. no. 4695), anti-pAkt (cat. no. 4060),
anti-Akt (cat. no. 4691), anti-pS6 kinase (cat. no. 9234) and
anti-S6 kinase (cat. no. 9202) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The C terminal FLAG-tagged
LAIR1 inserted into pcDNA3.1 (pcDNA3.1/LAIR1-FLAG) was purchased
from GenScript Biotech Corp. (Township, NJ, USA).
Clinical specimens
The RCC specimens (total 95 paired samples) were
obtained from patients undergoing primary resection at the Osaka
University Medical Hospital (Osaka, Japan) between 2002 and 2011.
Tumor-associated normal renal tissue was also obtained from a
subset of these patients when possible. There were no cases with
multiple tumors in the present study. Histological diagnosis was
established with standard hematoxylin and eosin-stained sections by
two senior pathologists experienced in RCC diagnosis. Tumors were
staged according to the 6th AJCC TNM staging system (https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspx)
and graded according to Fuhrman's nuclear grading system (15). Written informed consent was obtained
from each patient, and the study was approved by the ethics review
board of the Osaka University Medical Hospital. The pathological
information for the clinical tissue is documented in Table I.
 | Table I.Features of the ccRCC clinical
samples used in the different analyses. |
Table I.
Features of the ccRCC clinical
samples used in the different analyses.
| A, LAIR1 mRNA
expression as examined in 30 matched-pair ccRCC clinical samples by
qPCR (Fig. 1D) |
|---|
| Age (years) |
|
Mean | 61.5 |
|
Range | 27-86 |
| Sex |
|
Male | 22 |
|
Female | 8 |
| Maximum tumor
diameter (mm) |
|
Median | 41 (21–160) |
| TNM
classification |
| I | 18 |
|
≥II | 12 |
| Pathological
grade |
|
≤G2 | 17 |
|
≥G3 | 13 |
| Pathological
stage |
|
≤pT1b | 19 |
|
≥pT2a | 11 |
|
| B, ccRCC
clinical samples used for overall survival analysis (Fig. 5A) |
|
| Age (years) |
|
Mean | 65 |
|
Range | 34-82 |
| Sex |
|
Male | 44 |
|
Female | 21 |
| LAIRI mRNA
expression |
|
High | 21 |
|
Low | 44 |
| Maximum tumor
diameter (mm) |
|
Median | 70 (40–130) |
| TNM
classification |
| I | 20 |
| II | 10 |
|
III | 20 |
| IV | 15 |
| Pathological
grade |
| G1 | 5 |
| G2 | 43 |
| G3 | 17 |
| Pathological
stage |
|
pT1 | 21 |
|
pT2 | 15 |
|
pT3 | 28 |
|
pT4 | 1 |
|
| C, ccRCC samples
used for progression-free survival analysis (Fig. 5B) |
|
| Age (years) |
|
Mean | 68 |
|
Range | 44-82 |
| Sex |
|
Male | 36 |
|
Female | 16 |
| LAIRI mRNA
expression |
|
High | 17 |
|
Low | 35 |
| Maximum tumor
diameter (mm) |
|
Median | 68 (40–130) |
| TNM
classification |
| I | 20 |
| II | 9 |
|
III | 20 |
| IV | 3 |
| Pathological
grade |
| G1 | 5 |
| G2 | 36 |
| G3 | 11 |
| Pathological
stage |
|
pT1 | 21 |
|
pT2 | 10 |
|
pT3 | 21 |
Purification of extracellular
vesicles
Purification of Te-EVs was performed according to
the method of Jingushi et al (5). In brief, following excision, the
tissue samples were immediately immersed in 4 ml Dulbecco's
modified Eagle's medium (DMEM) (Wako Pure Chemical Industries,
Ltd., Tokyo, Japan) without fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and stored at 4°C for 1 h.
Tissue-immersed medium was then centrifuged at 2,000 × g for 30
min, and the collected supernatants were subjected to the
ultracentrifuge method for recovery of Te-EVs. The protein
concentration of the obtained tissue-exudative EVs was measured
using Micro BCA Protein Assay kit (Thermo Fisher Scientific,
Inc.).
For purification of EVs from cell cultured
conditioned medium (CM-EVs), RCC cell lines (renal cell carcinoma:
786-O; clear cell renal cell carcinoma: Caki-1; papillary renal
cell carcinoma: Caki-2 and ACHN) were seeded into three 10-cm
dishes (5.0×105 cells/dish) and incubated for 48 h.
Cells in cultured medium (RPMI-1640) were then centrifuged at 2,000
× g for 30 min, then at 16,000 × g for 30 min, and the collected
supernatants were subjected to the ultracentrifuge method for
recovery of EVs. The protein concentration of the obtained CM-EVs
was measured using the Micro BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.).
Liquid chromatography-tandem mass
spectrometry (LC/MS) analysis
The EV-containing eluates of EVSecond columns (GL
Sciences, Tokyo, Japan) were dried and resolved in 20 mM HEPES-NaOH
(pH 8.0), 12 mM sodium deoxycholate and 12 mM sodium N-lauroyl
sarcosinate. Following reduction with 20 mM dithiothreitol (DTT),
at 100°C for 10 min and alkylation with 50 mM iodoacetamide at
ambient temperature for 45 min, proteins were digested with 5 µl of
immobilized trypsin (Thermo Fisher Scientific, Inc.) with shaking
at 1,000 rpm at 37°C for 6 h. After removal of sodium deoxycholate
and sodium N-lauroyl sarcosinate by ethyl acetate extraction, the
resulting peptides were desalted by Oasis HLB µElution plate
(Waters Corp., Milford, MA, USA) and subjected to mass
spectrometric analysis. Peptides were analyzed by
LTQ-Orbitrap-Velos mass spectrometer (Thermo Fisher Scientific,
Inc.) combined with UltiMate™ 3000 RSLCnano-flow HPLC system
(Thermo Fisher Scientific, Inc.). Protein identification and
quantification analysis were performed with MaxQuant software
(https://www.biochem.mpg.de/5111795/maxquant). The
MS/MS spectra were searched against the Homo sapiens protein
database in SwissProt, with a false discovery rate set to 1% for
both peptide and protein identification filters. Only ‘Razor +
unique peptides’ were used for the calculation of relative protein
concentration. For Te-EV protein cargo analysis, all detected peaks
were standardized by adjusting the median value to
1.0×104.
Western blot analysis
Cells were lysed with Laemmli SDS sample buffer
containing 5% 2-mercaptoethanol. EV samples were lysed with Laemmli
SDS sample buffer with or without 2-mercaptoethanol. Protein
samples were separated on a 7.5–15% sodium dodecyl sulphate
(SDS)-polyacrylamide gel electrophoresis (PAGE) gel and then
transferred to a polyvinylidene difluoride (PVDF) membrane using
the Bio-Rad semi-dry transfer system (Bio-Rad-Laboratories,
Hercules, CA, USA) (1 h, 12 V). Immunoreactive proteins made to
react with the antibodies previously described (1:1,000 dilution)
were visualized by treatment with a detection reagent (ECL Prime
Western Blotting Retection reagent; GE Healthcare, Chicago, IL,
USA). Densitometric analysis was performed using the NIH ImageJ
software (version 1.51v; NIH; National Institutes of Health,
Bethesda, MD, USA).
Transmission electron microscope (TEM)
analysis
TEM analysis was performed according to the method
of Lässer et al (16). EV
samples (1 µg) were placed on a formvar carbon-coated nickel grid
for 1 h. EVs were fixed with 2% paraformaldehyde and then incubated
with the primary antibodies (1:500 dilution) at room temperature
for 1 h. Immunoreactive EVs were labeled with the secondary
(anti-mouse IgG) antibody preadsorbed onto 20 nm gold nanoparticles
and visualized (magnification, ×25,000) with the Hitachi H-7650
transmission electron microscope (Hitachi Ltd., Tokyo, Japan).
qPCR for LAIR1 mRNA expression
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). PrimeScript RT
Reagent kit (Takara Bio, Inc., Otsu, Japan) was used to prepare
cDNA from 500 ng total RNA. The LightCycler® 96 System
(Roche, Rotkreuz, Switzerland) was used for qPCR analysis. Thermal
cycling conditions for LAIR1 included an initial step at 95°C for
30 sec, followed by 40 cycles of 95°C for 15 sec, 60°C for 15 sec,
and 72°C for 15 sec. Thermal cycling conditions for GAPDH included
an initial step at 95°C for 30 sec, followed by 40 cycles of 95°C
for 15 sec, 59°C for 30 sec, and 72°C for 15 sec. Primer sequences
for gene amplification were as follows: LAIR1 forward,
5′-CCTGACCTGGCTGTTGATGTTCT-3′ and reverse,
5′-GCCCGGGCTGTCCTCTGT-3′; GAPDH forward, 5′-CCATCACCATCTTCCAGGAG-3′
and reverse, 5′-AATGAGCCCCAGCCTTCTCC-3′.
Immunohistochemistry
The expression of LAIR1 was determined by
immunohistochemical staining of paraffin-embedded tissues of normal
kidney or RCC. Formalin-fixed paraffin-embedded sections (5-µm in
thickness) were deparaffinized and rehydrated. After the slides
were steamed for 20 min in 10 mmol/l citrate buffer (pH 6.0) for
antigen retrieval, endogenous peroxidase was blocked using 3%
H2O2. Immunohistochemical staining for LAIR1
was performed using anti-LAIR1 (dilution 1:500; cat. no. HPA011155;
Atlas Antibodies, Romma, Sweden) and the EnVision+ Detection System
(Dako; Agilent Technologies, Inc., Santa Clara, CA, USA), according
to the manufacturer's instructions. Primary antibodies were
incubated overnight at 4°C and counter-stained with
hematoxylin.
Cell culture
Four human RCC cell lines (786-O, Caki-1, Caki-2 and
ACHN), obtained from the American Type Culture Collection (ATCC;
Manassas, VI, USA), were cultured in RPMI-1640 medium (Wako Pure
Chemical Industries, Ltd.) supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin G and 0.1 µg/ml
streptomycin.
siRNA and DNA transfection
siRNA duplexes used to downregulate LAIR1 expression
(LAIR1 stealth siRNA #1: HSS142792, LAIR1 stealth siRNA #2:
HSS142794, LAIR1 stealth siRNA #3: HSS180495) and a negative
control stealth siRNA duplex (#12935110) were purchased from Thermo
Fisher Scientific, Inc. For all siRNA transfection studies,
5×104 Caki-2 cells were seeded in a 12-well plate and 50
nM siRNA was transfected using Lipofectamine RNAiMAX reagent (Life
Technologies; Thermo Fisher, Scientific, Inc.). For DNA
transfection, 8×104 ACHN cells were seeded in a 12-well
plate 24 h before transfection. DNA transfection (1 µg) was
performed using Lipofectamine® 2000 transfection reagent
(Life Technologies; Thermo Fisher, Scientific, Inc.).
Establishment of cell lines with
stable LAIR1-FLAG expression
The vector expressing LAIR1-FLAG
(pcDNA3.1/LAIR1-FLAG) or empty vector (pcDNA3.1) was transfected
into ACHN cells and cultured in a medium containing 2 mg/ml G418
(Roche, Basel, Switzerland) for selection.
Water-soluble tetrazolium salt-8
(WST-8) cell proliferation assay
Cell proliferation was examined by WST-8 assay. ACHN
cells stably expressing LAIR1 or empty vector, or Caki-2 cells
transfected with the LAIR1 siRNA or a negative control siRNA, were
seeded in a 96-well plate (0.05×104 cells/well) and
incubated for the indicated time. After incubation for 2 h with the
WST-8 reagent (Dojindo Laboratories, Osaka, Japan) at 37°C and 5%
CO2, the optical density was read at a wavelength of
450/630 nm (Ex/Em).
Anchorage-independent cell
proliferation assay
Single cells were seeded into Nunclon Sphera
96F-well plates (Corning Inc., Corning, NY, USA) (ACHN cells:
0.4×104 cells; Caki-2 cells: 0.8×104 cells)
in RPMI-1640 supplemented with 0.03% SphereMAX (Nissan Chemical
Industries, Osaka, JAPAN), 10% FBS, 100 U/ml penicillin G and 0.1
µg/ml streptomycin. After 7 days, the spheres were stained with 10
µg/ml of calcein AM (BD Biosciences, Franklin Lakes, NJ, USA) and
incubated for 30 min at 37°C, 5% CO2. Total sphere
number was measured using the BZ-X800 fluorescence microscope at
×20 magnification (Keyence Corp., Osaka, Japan).
Wound healing assay
Cells were seeded into a 24-well plate
(4.0×104 cells/well) and incubated for 72 h at 37°C, in
5% CO2. A wound was created in a monolayer of ~90%
confluent cells using a sterile 1-ml pipette tip. Cell images were
recorded at 0 and 24 h for ACHN cells after wound creation using an
Olympus IX71 fluorescence microscope at ×40 magnification (Olympus
Corp., Tokyo, Japan).
Cell invasion assay
The BioCoat Tumor Invasion system with the 8.0-µm
pore size FluoroBlok membrane (Corning Inc.) was used to perform
the cell invasion assay. Cells were seeded in the insert of 96-well
plate (2×104 cells/well) in serum-free conditions, and
medium supplemented with 10% FBS was used as a chemoattractant in
the base plate. Following incubation for 12 h at 37°C, in 5%
CO2, the cells were labeled with calcein AM (4 µg/ml),
and the fluorescence of the invaded cells was read at a wavelength
of 494/517 nm (Ex/Em).
Establishment of LAIR1-FLAG stable
cell-xenograft mice
Nine female BALB/c nude mice were obtained from
Oriental Yeast Co., Ltd. (Tokyo, Japan). Six-week-old mice were
used for LAIR1-FLAG stable cell-xenograft mouse experiments.
Animals were kept under a 12-h light/dark cycle at 22–24°C in a
pathogen-free mouse facility. Food and water were given ad
libitum. ACHN-LAIR1-FLAG (LAIR1 #2) and ACHN-empty vector
(mock) cells were both adjusted to a concentration of
1.0×107 cells suspended in 50 µl serum-free RPMI-1640.
The cell suspensions with 50 µl Μatrigel (Corning Inc.) were then
injected subcutaneously into the right flanks of the BALB/c nude
mice (LAIR1 #2, N=4; mock #1, N=5). The tumor volume (V) was
calculated as follows: V = (tumor length × tumor
width2)/2. The weight of the mice was 20.68±1.4 g on day
32 after xenografts. All procedures were performed under a protocol
approved by the Animal Experimentation Committee at Osaka
University. Developed tumors were resected 32 days after
xenografts. Mice were euthanized by overdose of isoflurane (2 times
the anesthetic dose) for 5 min.
Nanoparticle tracking analysis
(NTA)
EV samples diluted with phosphate-buffered saline
(PBS) (1:1,000 dilution) were analyzed with the NanoSight LM10
instrument (Quantum Design Japan, Tokyo, Japan) equipped with the
NTA 2.0 analytical software (NanoSight Ltd., Malvern, UK). All
measurements were performed under identical processing conditions
(NTA 2.3 build 0034, Detection Threshold: 4 Multi, min Track
Length: Auto, min Expected Size: Auto).
Evaluation of RCC EVs on
anchorage-dependent and anchorage-independent cell
proliferation
The EVs obtained from ACHN-LAIR1-FLAG (LAIR1 #2) or
ACHN-empty vector (mock #1) cells (2 µg) were incubated with
ACHN-empty vector (mock #1) cells and subjected to
anchorage-dependent or anchorage-independent cell proliferation
assays.
Association of LAIR1 expression with
patient prognosis
Patients with no prognostic information were
excluded and then the LAIR1 expression profiles were combined with
the corresponding survival prognostic information. The prognostic
value of each LAIR1 level was evaluated using a Kaplan-Meier curve
and the log-rank method by GraphPad Prism 6.0 (GraphPad Software,
Inc., La Jolla, CA, USA). Patients were divided into the high
expression and low expression groups for each of the LAIR1
according to the mean value of the expression. Follow-up time was
as follows. Overall survival: Median, 2,110 days (8-4,721 days);
Progression-free survival: Median, 1,443.5 days (28-4,721
days).
Statistical analysis
Results are expressed as the mean ± standard
deviation of the mean (SD). Differences between the values were
statistically analyzed using the Student's t-test, paired t-test or
one-way analysis of variance (ANOVA) with Tukey's post hoc tests
(GraphPad Prism 6.0; GraphPad Software, Inc.). Association of
clinical parameters with the LAIR1 mRNA level was tested by
Mann-Whitney test. Overall survival and progression-free survival
were calculated using the Kaplan-Meier method, and differences
between groups were assessed by log-rank tests. A P-value of
<0.05 was considered to indicate a statistically significant
result.
Results
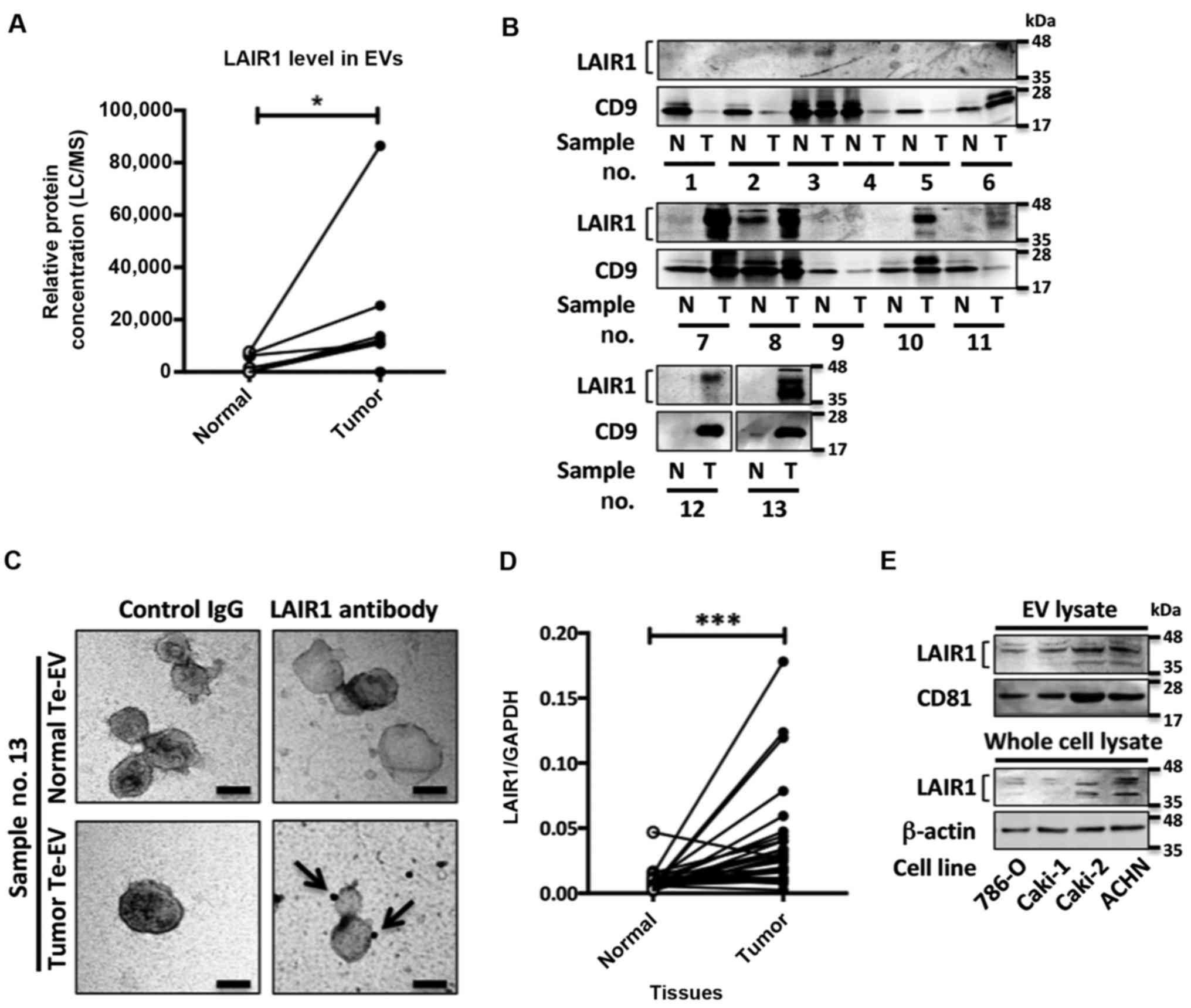
LAIR1 is enriched in RCC Te-EVs
LC/MS analysis identified LAIR1 as aberrantly
enriched in RCC Te-EVs compared to Te-EVs obtained from adjacent
normal renal tissues (Fig. 1A). The
mass spectrometric quantification results were further confirmed by
western blot analysis (Fig. 1B).
Transmission electron microscope (TEM) analysis illustrated that
the isolated Te-EVs expressed LAIR1 on their surfaces (Fig. 1C). LAIR1 mRNA levels were
significantly elevated in RCC tissues compared with the adjacent
normal renal tissues (Fig. 1D and
Table II). To investigate the
LAIR1 function in RCC cells, we evaluated the LAIR1 protein
expression in four RCC cell lines. Although the LAIR1 expression
differed among the four cell lines, all cell lines expressed LAIR1
in whole cell lysates as well as in the EVs isolated from the
cultured media (Fig. 1E). These
results suggest that LAIR1 expression is elevated in RCC tissue,
and that LAIR1 is secreted in extracellular vesicles (EVs) from RCC
cells.
 | Table II.Statistical association of LAIR1 mRNA
level with clinical parameters of the RCC patient tissues. |
Table II.
Statistical association of LAIR1 mRNA
level with clinical parameters of the RCC patient tissues.
| Clinical
parameters | n | LAIRI mRNA
expression (median) | P-value
(Mann-Whitney test) |
|---|
| TNM
classification |
|
| 0.458 |
| I | 18 | 0.030 |
|
|
≥II | 12 | 0.023 |
|
| Pathological
grade |
|
| 1.000 |
|
≤G2 | 17 | 0.026 |
|
|
≥G3 | 13 | 0.030 |
|
| Pathological
stage |
|
| 0.796 |
|
≤pT1b | 19 | 0.028 |
|
|
≥pT2a | 11 | 0.026 |
|
LAIR1 promotes cell proliferation in
RCC cells
To investigate the biological functions of LAIR1 in
RCC cells, we first constructed ACHN cells stably overexpressing
LAIR1 (Fig. 2A). LAIR1
overexpression upregulated both anchorage-dependent (Fig. 2B) and anchorage-independent
(Fig. 2C) cell proliferation in
ACHN cells. Moreover, although it did not significantly impact
invasion ability (Fig. 2E), LAIR1
overexpression upregulated cell migration (Fig. 2D) in ACHN cells. Since LAIR1 siRNA
#1 had no knockdown effect on LAIR1 protein levels, LAIR1 siRNA #2
and #3 were used for subsequent experiments (Fig. 2F). LAIR1 knockdown suppressed
anchorage-dependent cell proliferation in Caki-2 cells (Fig. 2G).
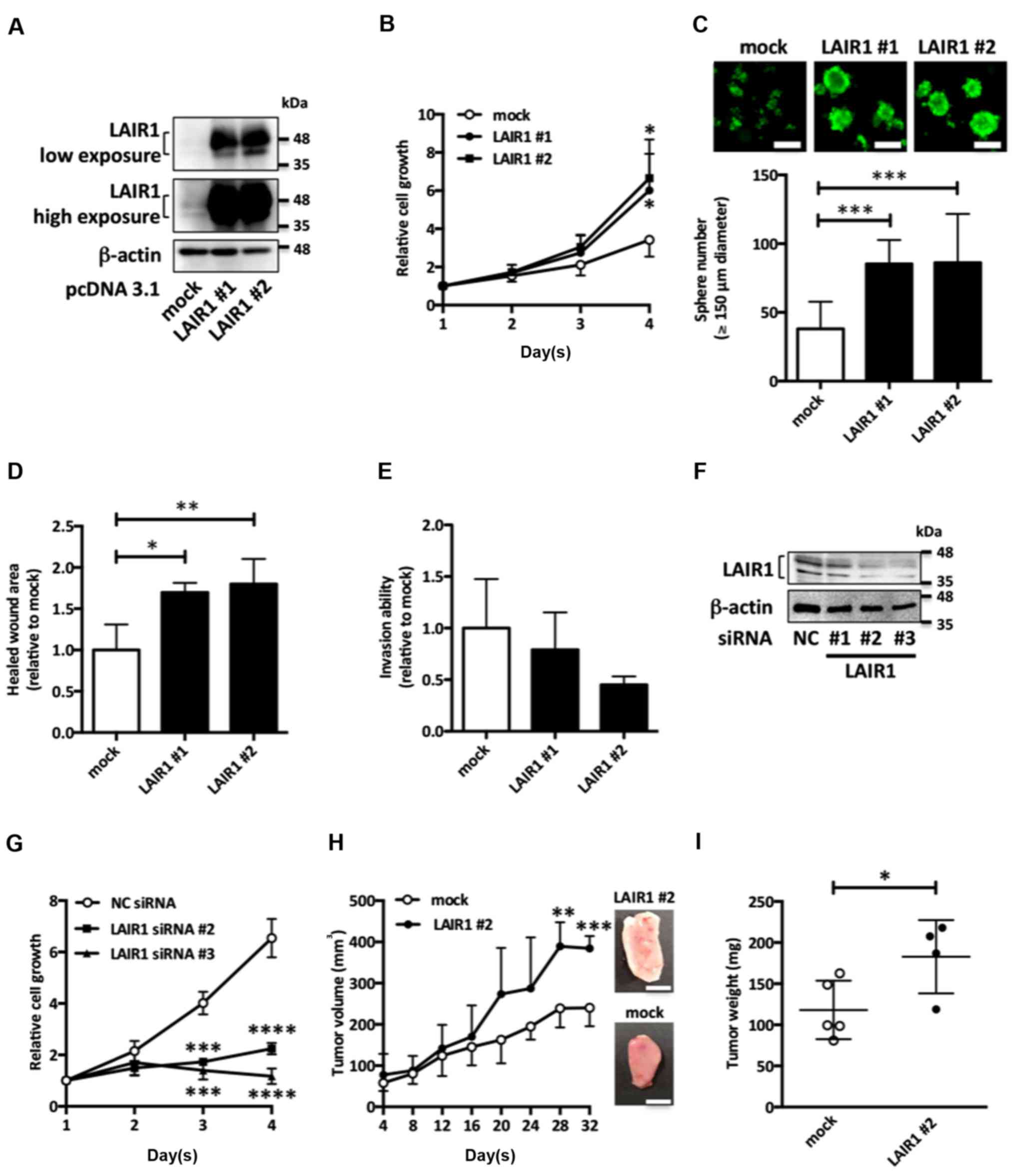 | Figure 2.LAIR1 overexpression promotes
tumorigenesis in RCC. (A) ACHN cells stably overexpressing
LAIR1-FLAG were subjected to western blot analysis. Representative
results of three independent experiments are shown. ACHN cells
stably overexpressing LAIR1-FLAG (LAIR1 #1, LAIR1 #2) or control
cells (mock) were examined in anchorage-dependent (B) and
anchorage-independent (C) cell proliferation assays. Values are the
mean ± SD of six independent experiments. *P<0.05, ***P<0.001
vs. mock. One-way ANOVA. White scale bars, 150 µm. (D) Cell
motility was measured 24 h after wound formation by scraping in
serum-free conditions. The results are expressed as mean ± SD of
four independent experiments. *P<0.05, **P<0.01 vs. mock.
One-way ANOVA. (E) The cell suspension was added to the upper
chamber of Μatrigel-coated Τranswell membrane inserts, and the
lower chamber was filled with the medium and then cultured for 12
h. Fluorescence derived from invasive cells was measured. Values
are mean ± SD of four independent experiments. (F) Caki-2 cells
transfected with negative control (NC) siRNA or LAIR1 siRNA were
subjected to western blot analysis. Representative results of three
independent experiments are shown. (G) Caki-2 cells transfected
with LAIR1 siRNA were examined in anchorage-dependent cell
proliferation assays. Values are the mean ± SD of three independent
experiments. ***P<0.001, ****P<0.0001 vs. NC siRNA. One-way
ANOVA. (H) ACHN cells stably overexpressing LAIR1-FLAG (LAIR1 #2,
N=4) and control ACHN cells (mock, N=5) were injected into nude
mice. Tumor size was measured and calculated every four days. The
values are presented as the mean ± SD for each group. **P<0.01,
***P<0.001 vs. control tumor. t-test. Representative xenograft
tumor images (upper position: LAIR1 #2, lower position: mock) are
shown. Maximum tumor volume and diameter of resected tumors was as
follows. Mock: 304.2 mm3, 10.5 mm; LAIR1 #2: 416.3
mm3, 15.1 mm. There were no multiple tumors on
xenografted mouse. White scale bar, 5 mm. (I) Tumor weight for mock
and LAIR1 #2 ×enografted mice. The values are presented as the mean
± SD for each group. *P<0.05 vs. control tumor. t-test. RCC,
renal cell carcinoma; LAIR1, leukocyte-associated
immunoglobulin-like receptor 1. |
To clarify the tumor-promoting potential of LAIR1
expression in vivo, ACHN cells stably expressing LAIR1 were
xenografted into nude mice. These cells showed accelerated growth
of tumor volume and increased tumor weight compared with empty
vector-transfected ACHN cells (Fig. 2H
and I), suggesting that increased LAIR1 expression upregulates
RCC cell proliferation, leading to accelerated tumor growth.
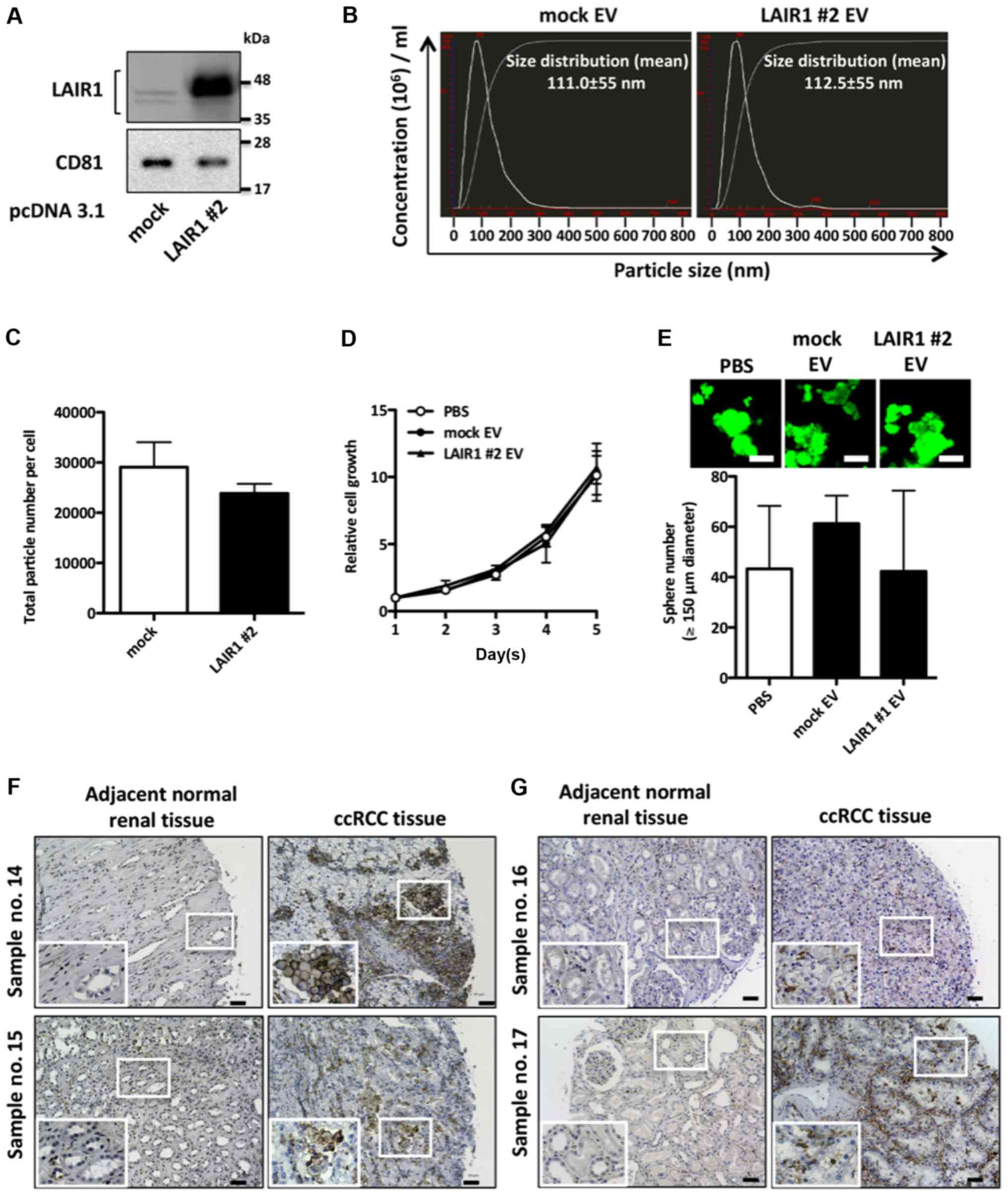
EV-LAIR1 has no significant effect on
cell proliferation in RCC cells
To elucidate whether RCC-derived EV-LAIR1 has the
potential to upregulate RCC cell proliferation, we examined the
biological effects of LAIR1-overexpressed EVs (LAIR1 #2 EVs) on RCC
cells. EVs isolated from ACHN cells stably overexpressing LAIR1
were confirmed by western blot analysis using the well-defined EV
marker CD81 (Fig. 3A). Nanoparticle
tracking analysis showed that the isolated particles were under 200
nm in size and that there was no significant difference in secreted
particle number between the mock vector-transfected ACHN cells and
LAIR1-overexpressing ACHN cells (Fig.
3B and C). As shown in Fig. 3D and
E, LAIR1 #2 EVs did not significantly impact either
anchorage-dependent or anchorage-independent cell proliferation in
empty vector-transfected ACHN cells (mock cells), suggesting that
LAIR1 upregulates RCC cell proliferation irrespective of secreted
EV-LAIR1. To examine whether LAIR1 was expressed not only in RCC
cells but also in surrounding stromal cells, immunohistochemical
staining was carried out on RCC tissues. Immunohistochemical
staining revealed that RCC cells (Fig.
3F) and stromal cells (Fig. 3G)
expressed LAIR1 in RCC tissues. Notably, stromal cells adjacent to
RCC cells also expressed LAIR1 in RCC tissues (Fig. 3F, sample no. 15), suggesting that
EV-LAIR1 secreted from RCC cells may be taken up by surrounding
stromal cells.
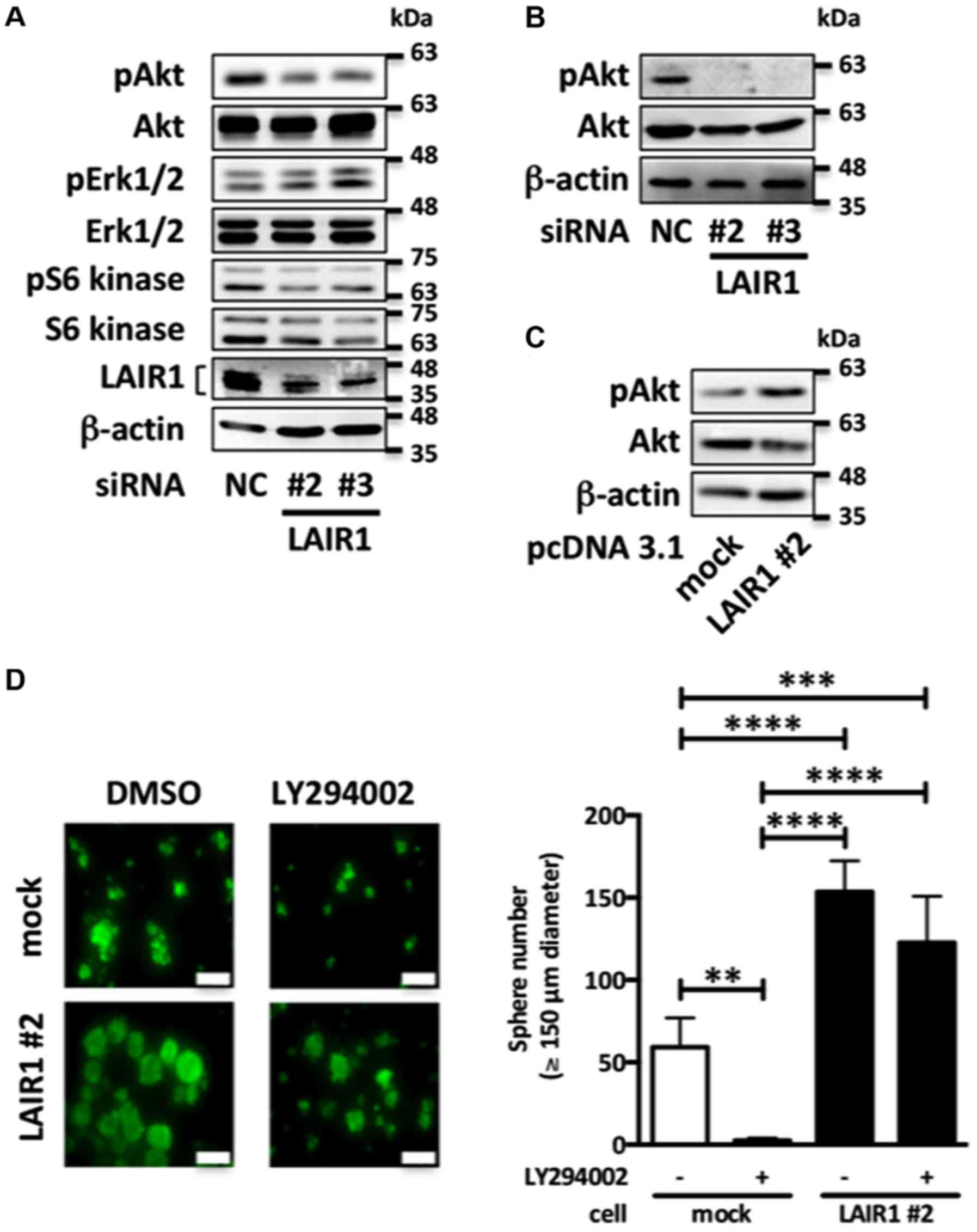
LAIR1 promotes cell proliferation via
Akt in RCC cells
Since the PI3K/Akt/mTOR pathway is a key hub for
oncogenic processes including cell proliferation in RCC (17), we next examined the effect of LAIR1
on the PI3K/Akt/mTOR pathway. As shown in Fig. 4A, LAIR1 knockdown in Caki-2 cells
decreased phosphorylation of Akt, whereas Erk1/2 and S6 kinase were
not significantly affected. Moreover, in anchorage-independent
growth systems, LAIR1 knockdown decreased the level of Akt
phosphorylation in Caki-2 cells (Fig.
4B). On the other hand, LAIR1 overexpression upregulated the
level of Akt phosphorylation in ACHN cells (Fig. 4C). To clarify the relationship
between LAIR1 and Akt phosphorylation status, we evaluated the
effect of the PI3K inhibitor (LY294002) on LAIR1-upregulated
anchorage-independent cell proliferation. Although LY294002
significantly decreased the cell proliferation in empty
vector-transfected ACHN cells, LAIR1 overexpression attenuated the
effect of LY294002 on ACHN cells, suggesting that LAIR1 promotes
cell proliferation via upregulation of Akt phosphorylation in RCC
cells (Fig. 4D).
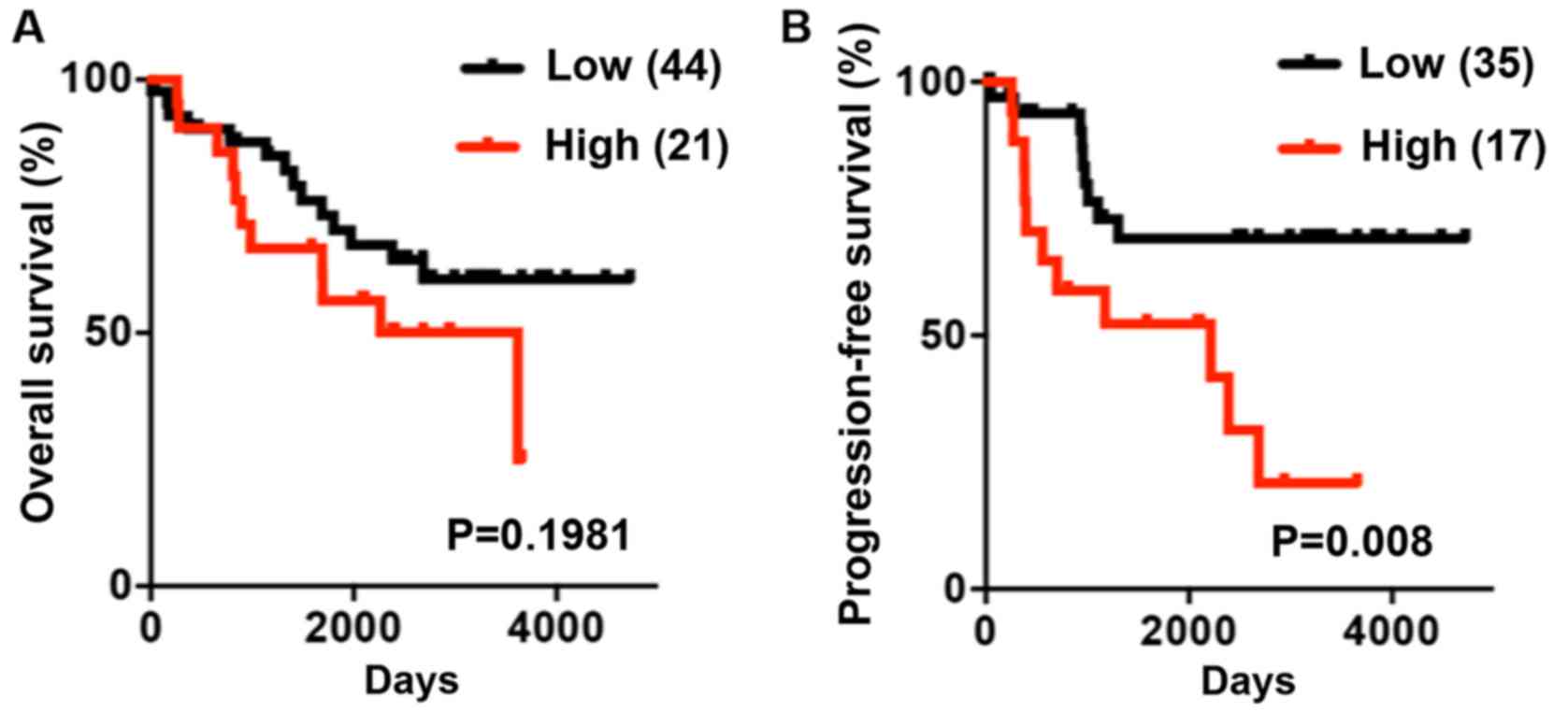
High LAIR1 expression correlates with
poor progression-free survival in RCC
Finally, Kaplan-Meier survival curve analyses were
performed in RCC patients. These curves indicated that high
expression of LAIR1 mRNA in RCC tissue was associated with poor
progression-free survival (Fig.
5).
Discussion
In the present study, we identified aberrant LAIR1
overexpression as a consequential factor in the development of
renal cell carcinoma (RCC). A receptor widely expressed on immune
cells, leukocyte-associated immunoglobulin-like receptor 1 (LAIR1)
has previously been reported to play a role in leukemia, with
multiple studies indicating that blocking LAIR1 activation in
leukemia cells decreases cell proliferation. Poggi et al
demonstrated that in B-cell chronic lymphocyte leukemia cells,
antibody engagement with LAIR1 blocks the activation of Akt and
NF-κB leading to decreased cell proliferation (18) and Kang et al showed that
LAIR1 knockdown significantly inhibited in vitro and in
vivo cell growth in human leukemia cell lines (19). These findings are consistent with
our current study, which shows that siRNA knockdown of LAIR1
reduced cell proliferation via suppression of Akt phosphorylation
in RCC cells. Moreover, Kang et al revealed that LAIR1
deficiency exhausted tumor-initiating cells via apoptosis in mouse
acute myeloid leukemia cells. Our data showed that LAIR1 promotes
anchorage-independent cell proliferation activity, which represents
one of the key features of cancer stem cells. LAIR1 upregulated Akt
phosphorylation, which leads to cancer stem cell activation and
promotes tumor development in RCC (20,21).
Therefore, LAIR1 may regulate cell proliferation by activating
cancer stem cells via upregulation of Akt phosphorylation in
RCC.
In addition to leukocyte Ig-like receptor subfamily
B (LILRB)2, LILRB4 (22,23), which contains identical domain
organization to LAIR1, LAIR1 has been shown to have tumor-promoting
roles in leukemia (19) and RCC
cells. SHP-1, which directly binds to the ITIM region of LAIR1 and
transfers the signal downstream, is known to act as either an
oncogene or as a tumor suppressor depending on the type of cancer
(24). In RCC, inhibition of SHP-1
significantly decreased the proliferation of RCC cell lines
(25). Moreover, SHP-1 inhibition
downregulated the phosphorylation status of Akt in RCC cells,
suggesting that downstream of LAIR1, SHP-1 acts as an oncogene in
RCC cells leading to upregulation of Akt phosphorylation.
Although we performed flow cytometric analysis, we
could not detect LAIR1 on the cell surface in RCC cell lines (data
not shown). Our IHC data (Fig. 3F)
and the IHC data from the public database (The Human Protein Atlas)
show both membranous and cytoplasmic staining of LAIR1 in RCC
cells. In ovarian cancer cells, the localization of LAIR1 was
dependent on cell lines. LAIR-1 proteins were localized on both the
plasma membrane and in the cytoplasm of COC1 cells, while
predominately localized in the cytoplasm of HO8910 cells (26). Moreover, LAIR1 knockdown affected
the cell proliferation in HO8910 cells, where LAIR1 was localized
in the cytoplasm (26). Therefore,
although the underlying mechanism is unclear, we think that LAIR1
may localize in the cytosol and promote tumorigenesis in RCC
cells.
Some of the tissue-exudative extracellular vesicles
(Te-EVs) obtained from normal renal tissue showed high CD9
expression compared to those in RCC Te-extracellular vesicles
(EVs). In our previous study (5),
we found that most of tetraspanins including CD9 were often
downregulated in tumor-derived EVs compared to those in
normal-derived EVs. Moreover, Kwon et al showed that 54% of
RCC patients had low CD9 expression (27). Therefore, the low CD9 expression in
RCC Te-EVs may be due to the CD9 expression pattern in RCC
tissue.
We initially identified LAIR1 as a protein enriched
in RCC-derived Te-EVs. Since overexpression of LAIR1 accelerated
tumor growth in vivo, we investigated whether treatment of
RCC cells with EV-LAIR1 isolated from RCC EVs were sufficient to
affect cell proliferation. Our data indicated that incubation of
RCC cells with EV-LAIR1 had little direct effect on proliferation.
EVs are one of the tools by which cancer cells communicate with
themselves, other cell types (e.g., vascular endothelial cells,
immune cells and fibroblast), and the surrounding supportive
structures constituting the tumor microenvironment (28). It is becoming increasingly apparent
that the contents of EVs may endow them with potent reprogramming
capacity for manipulating recipient cells (29–32).
In renal cancer, Grange et al (33) reported that renal cancer cells
secrete EVs, which activate vascular endothelial cells to organize
capillary-like structures and induce enhanced chemoresistance. Our
data showed that not only RCC cells but also stromal cells adjacent
to RCC were LAIR1-positive (Fig.
3F), suggesting that EV-LAIR1 secreted from RCC cells may
influence the tumor microenvironment and lead to accelerated tumor
growth. Although our in vivo xenograft model could not
address the effect of EV-LAIR1 on tumor microenvironment including
immune cells, further studies using mouse allograft model may solve
this issue.
In conclusion, the present study showed that LAIR1
was secreted in EVs from RCC cells, and that LAIR1 intracellular
signaling enhanced Akt phosphorylation, leading to accelerated
tumor growth in RCC cells.
Acknowledgements
We thank Dr Eiji Oiki (Graduate School of Medicine,
Osaka University) for the technical assistance for the TEM
analysis.
Funding
The present study was supported by the Project for
Cancer Research and Therapeutic Evolution from the Japan Agency for
Medical Research and Development (AMED) to KU under grant no.
18cm0106405h0003.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author contributions
KJ, MU and KU were involved in the conception and
design of the study; KJ, MU, KT, KU and NN were involved in the
data collection; KJ, MU, KN, YH, CW, YI, YY, TH, ToK, KM, TaK, AK,
TU, AN, KF and KU were involved in the data analysis; KJ, MU and KU
conducted the investigative experiments; MU, KU and NN were
involved in the project administration; MU, KT and NN supervised
the study; KJ, MU and KF wrote and edited the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient, and the study was approved by the ethics review board of
the Osaka University Medical Hospital.
Patient consent for publication
Informed consent was obtained from all participants
included in the study.
Competing interests
NN reports receiving commercial research grants from
Takeda Pharmaceutical, Novartis Pharma, and Astra Zeneca. No
potential conflicts of interest were disclosed by the other
authors.
Glossary
Abbreviations
Abbreviations:
|
EVs
|
extracellular vesicles
|
|
Te-EVs
|
tissue-exudative extracellular
vesicles
|
|
RCC
|
renal cell carcinoma
|
|
LAIR1
|
leukocyte-associated
immunoglobulin-like receptor 1
|
References
|
1
|
Jonasch E, Futreal PA, Davis IJ, Bailey
ST, Kim WY, Brugarolas J, Giaccia AJ, Kurban G, Pause A, Frydman J,
et al: State of the science: An update on renal cell carcinoma. Mol
Cancer Res. 10:859–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nerich V, Hugues M, Paillard MJ, Borowski
L, Nai T, Stein U, Nguyen Tan Hon T, Montcuquet P, Maurina T,
Mouillet G, et al: Clinical impact of targeted therapies in
patients with metastatic clear-cell renal cell carcinoma. Onco
Targets Ther. 7:365–374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mickisch GH: Principles of nephrectomy for
malignant disease. BJU Int. 89:488–495. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalluri R: The biology and function of
exosomes in cancer. J Clin Invest. 126:1208–1215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jingushi K, Uemura M, Ohnishi N, Nakata W,
Fujita K, Naito T, Fujii R, Saichi N, Nonomura N, Tsujikawa K, et
al: Extracellular vesicles isolated from human renal cell carcinoma
tissues disrupt vascular endothelial cell morphology via
azurocidin. Int J Cancer. 124:607–617. 2018. View Article : Google Scholar
|
|
6
|
Meyaard L, Adema GJ, Chang C, Woollatt E,
Sutherland GR, Lanier LL and Phillips JH: LAIR-1, a novel
inhibitory receptor expressed on human mononuclear leukocytes.
Immunity. 7:283–290. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poggi A, Pella N, Morelli L, Spada F,
Revello V, Sivori S, Augugliaro R, Moretta L and Moretta A: p40, a
novel surface molecule involved in the regulation of the non-major
histocompatibility complex- restricted cytolytic activity in
humans. Eur J Immunol. 25:369–376. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poggi A, Tomasello E, Ferrero E, Zocchi MR
and Moretta L: p40/LAIR-1 regulates the differentiation of
peripheral blood precursors to dendritic cells induced by
granulocyte-monocyte colony-stimulating factor. Eur J Immunol.
28:2086–2091. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Florian S, Sonneck K, Czerny M,
Hennersdorf F, Hauswirth AW, Bühring HJ and Valent P: Detection of
novel leukocyte differentiation antigens on basophils and mast
cells by HLDA8 antibodies. Allergy. 61:1054–1062. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verbrugge A, De Ruiter T, Geest C, Coffer
PJ and Meyaard L: Differential expression of leukocyte-associated
Ig-like receptor-1 during neutrophil differentiation and
activation. J Leukoc Biol. 79:828–836. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maasho K, Masilamani M, Valas R, Basu S,
Coligan JE and Borrego F: The inhibitory leukocyte-associated
Ig-like receptor-1 (LAIR-1) is expressed at high levels by human
naive T cells and inhibits TCR mediated activation. Mol Immunol.
42:1521–1530. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lebbink RJ, De Ruiter T, Kaptijn GJ, Bihan
DG, Jansen CA, Lenting PJ and Meyaard L: Mouse leukocyte-associated
Ig-like receptor-1 (mLAIR-1) functions as an inhibitory
collagen-binding receptor on immune cells. Int Immunol.
19:1011–1019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishida-Aoki N, Tominaga N, Takeshita F,
Sonoda H, Yoshioka Y and Ochiya T: Disruption of circulating
extracellular vesicles as a novel therapeutic strategy against
cancer metastasis. Mol Ther. 25:181–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshioka Y, Kosaka N, Konishi Y, Ohta H,
Okamoto H, Sonoda H, Nonaka R, Yamamoto H, Ishii H, Mori M, et al:
Ultra-sensitive liquid biopsy of circulating extracellular vesicles
using ExoScreen. Nat Commun. 5:35912014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lässer C, Eldh M and Lötvall J: Isolation
and characterization of RNA-containing exosomes. J Vis Exp.
9:e30372012.
|
|
17
|
Guo H, German P, Bai S, Barnes S, Guo W,
Qi X, Lou H, Liang J, Jonasch E, Mills GB and Ding Z: The PI3K/AKT
pathway and renal cell carcinoma. J Genet Genomics. 42:343–353.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poggi A, Catellani S, Bruzzone A,
Caligaris-Cappio F, Gobbi M and Zocchi MR: Lack of the
leukocyte-associated Ig-like receptor-1 expression in high-risk
chronic lymphocytic leukaemia results in the absence of a negative
signal regulating kinase activation and cell division. Leukemia.
22:980–988. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang X, Lu Z, Cui C, Deng M, Fan Y, Dong
B, Han X, Xie F, Tyner JW, Coligan JE, et al: The ITIM-containing
receptor LAIR1 is essential for acute myeloid leukaemia
development. Nat Cell Biol. 17:665–677. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan MI, Czarnecka AM, Lewicki S,
Helbrecht I, Brodaczewska K, Koch I, Zdanowski R, Król M and
Szczylik C: Comparative gene expression profiling of primary and
metastatic renal cell carcinoma stem cell-like cancer cells. PLoS
One. 11:e01657182016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia P and Xu XY: PI3K/Akt/mTOR signaling
pathway in cancer stem cells: From basic research to clinical
application. Am J Cancer Res. 5:1602–1609. 2015.PubMed/NCBI
|
|
22
|
Khan MF, Bahr JM, Yellapa A, Bitterman P,
Abramowicz JS, Edassery SL, Basu S, Rotmensch J and Barua A:
Expression of leukocyte inhibitory immunoglobulin-like transcript 3
receptors by ovarian tumors in laying hen model of spontaneous
ovarian cancer. Transl Oncol. 5:85–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Yu X, Xie J, Zhan M, Yu Z, Xie L,
Zeng H, Zhang F, Chen G, Yi X and Zheng J: ANGPTL2/LILRB2 signaling
promotes the propagation of lung cancer cells. Oncotarget.
6:21004–21015. 2015.PubMed/NCBI
|
|
24
|
Kang X, Kim J, Deng M, John S, Chen H, Wu
G, Phan H and Zhang CC: Inhibitory leukocyte immunoglobulin-like
receptors: Immune checkpoint proteins and tumor sustaining factors.
Cell Cycle. 15:25–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tao T, Yang X, Zheng J, Feng D, Qin Q, Shi
X, Wang Q, Zhao C, Peng Z, Liu H, et al: PDZK1 inhibits the
development and progression of renal cell carcinoma by suppression
of SHP-1 phosphorylation. Oncogene. 36:6119–6131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao Q, Fu A, Yang S, He X, Wang Y, Zhang
X, Zhou J, Luan X, Yu W and Xue J: Leukocyte-associated
immunoglobulin-like receptor-1 expressed in epithelial ovarian
cancer cells and involved in cell proliferation and invasion.
Biochem Biophys Res Commun. 458:399–404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kwon HJ, Min SY, Park MJ, Lee C, Park JH,
Chae JY and Moon KC: Expression of CD9 and CD82 in clear cell renal
cell carcinoma and its clinical significance. Pathol Res Pract.
210:285–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kosaka N, Yoshioka Y, Fujita Y and Ochiya
T: Versatile roles of extracellular vesicles in cancer. J Clin
Invest. 126:1163–1172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Al-Nedawi K, Meehan B, Micallef J, Lhotak
V, May L, Guha A and Rak J: Intercellular transfer of the oncogenic
receptor EGFRvIII by microvesicles derived from tumour cells. Nat
Cell Biol. 10:619–624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yokoi A, Yoshioka Y, Yamamoto Y, Ishikawa
M, Ikeda SI, Kato T, Kiyono T, Takeshita F, Kajiyama H, Kikkawa F,
et al: Malignant extracellular vesicles carrying MMP1 mRNA
facilitate peritoneal dissemination in ovarian cancer. Nat Commun.
8:144702017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song X, Ding Y, Liu G, Yang X, Zhao R,
Zhang Y, Zhao X, Anderson GJ and Nie G: Cancer cell-derived
exosomes induce mitogen-activated protein kinase-dependent monocyte
survival by transport of functional receptor tyrosine kinases. J
Biol Chem. 291:8453–8464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Webber J, Steadman R, Mason MD, Tabi Z and
Clayton A: Cancer exosomes trigger fibroblast to myofibroblast
differentiation. Cancer Res. 70:9621–9630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grange C, Tapparo M, Collino F, Vitillo L,
Damasco C, Deregibus MC, Tetta C, Bussolati B and Camussi G:
Microvesicles released from human renal cancer stem cells stimulate
angiogenesis and formation of lung premetastatic niche. Cancer Res.
71:5346–5356. 2011. View Article : Google Scholar : PubMed/NCBI
|