Introduction
Osteosarcoma (OS) is the most common type of bone
cancer in children and adolescence. OS accounts for 2.4% of all
cancer-related mortality in children and 20% of all primary bone
cancers (1,2). Due to the lack of effective treatment
options to date, 50–70% of osteosarcoma patients survive no >5
years (3). Osteosarcoma, which is
derived from malignant mesenchymal stem cells, is believed to be a
primary tumor of the bone (4). The
tumor usually develops in the metaphyses of the long bones; thus,
the proximal tibia, the proximal humerus and the distal femur are
high risk areas for tumor development (5,6). At
present, chemotherapeutics is the first choice of treatment along
with surgical treatment. Yet, there are numerous side-effects
associated with chemotherapeutic drugs, such as cisplatin,
ifosfamide and high-dose methotrexate. Acquirement of drug
resistance is the most serious problem in osteosarcoma patients
following treatment with chemotherapeutic drugs (7). Therefore, the development of novel
effective therapeutic agents with moderate side-effects for the
treatment of osteosarcoma is urgent (8,9).
Natural or herbal medicines are often used as
alternative forms of chemotherapy, due to low mortality and side
effects (10). Currently, novel
anticancer agents from natural products have become increasingly
popular (11). In the history of
Traditional Chinese Medicine (TCM), medicinal plants and their
extracts have been used to treat various diseases. Accumulative
data concerning TCM have shown remarkable activity in influencing
the tumor cell death pathway, which can guide tumor treatment
decisions and clinical management (12). Natural products from TCM, with
unique and diverse chemical entities, are a considerable resource
for developing novel medications. Sodium cantharidinate (SC) has
powerful antitumor activity that has been confirmed in clinical
practice in recent years (13).
This compound directly inhibits multiple malignant tumors, and has
low toxic/adverse effects to date (14). In recent years, researchers have
confirmed through in vitro experiments that (SC) and its
derivatives directly kill liver cancer cells (15). SC induces HepG2 cell apoptosis
through the LC3 autophagy pathway, which has potential for the
treatment of human hepatocellular carcinoma (HCC) (16). Yet, no study exists concerning SC
activity in OS to date.
Cell cycle control is a major regulatory mechanism
of cell proliferation. Therefore, inhibition of cancer cell growth
is the most effective method for cancer treatment in the clinic
(17). Cytotoxic agents and/or DNA
damaging agents, which arrest the cell cycle at the G0/G1, S or
G2/M phase, induce cancer cell apoptosis (18). The cyclin-dependent kinases (Cdks),
highly conserved protein kinases, closely mediate the cell cycle
(19). Cyclins form complexes with
Cdks to activate Cdks to regulate the cell cycle. Cyclin D1, CDK4
and CDK6 are related closely to the G0/G1 phase, cyclin B1 and CDK1
are closely related to the G2/M phase, while cyclin A and CDK2 are
closely related to the S phase (20). Mitogen-activated protein kinase
(MAPK) and Akt pathways play an important role in the
antiproliferative actions in certain cells (21). Extracellular signal-regulated kinase
(ERK)1/2, p38 and c-Jun N-terminal kinase (JNK) are main MAPK
family members. Akt (also known as Akt1) may promote cell
proliferation via phosphorylation, which acts as a mediator of
growth factors (22). The present
study aimed to investigate the antiproliferation effect of SC on
the cell growth and cell cycle arrest of human OS MG-63 cells, to
evaluate whether SC may be a potential antitumor agent for the
treatment of this disease.
Materials and methods
Reagents
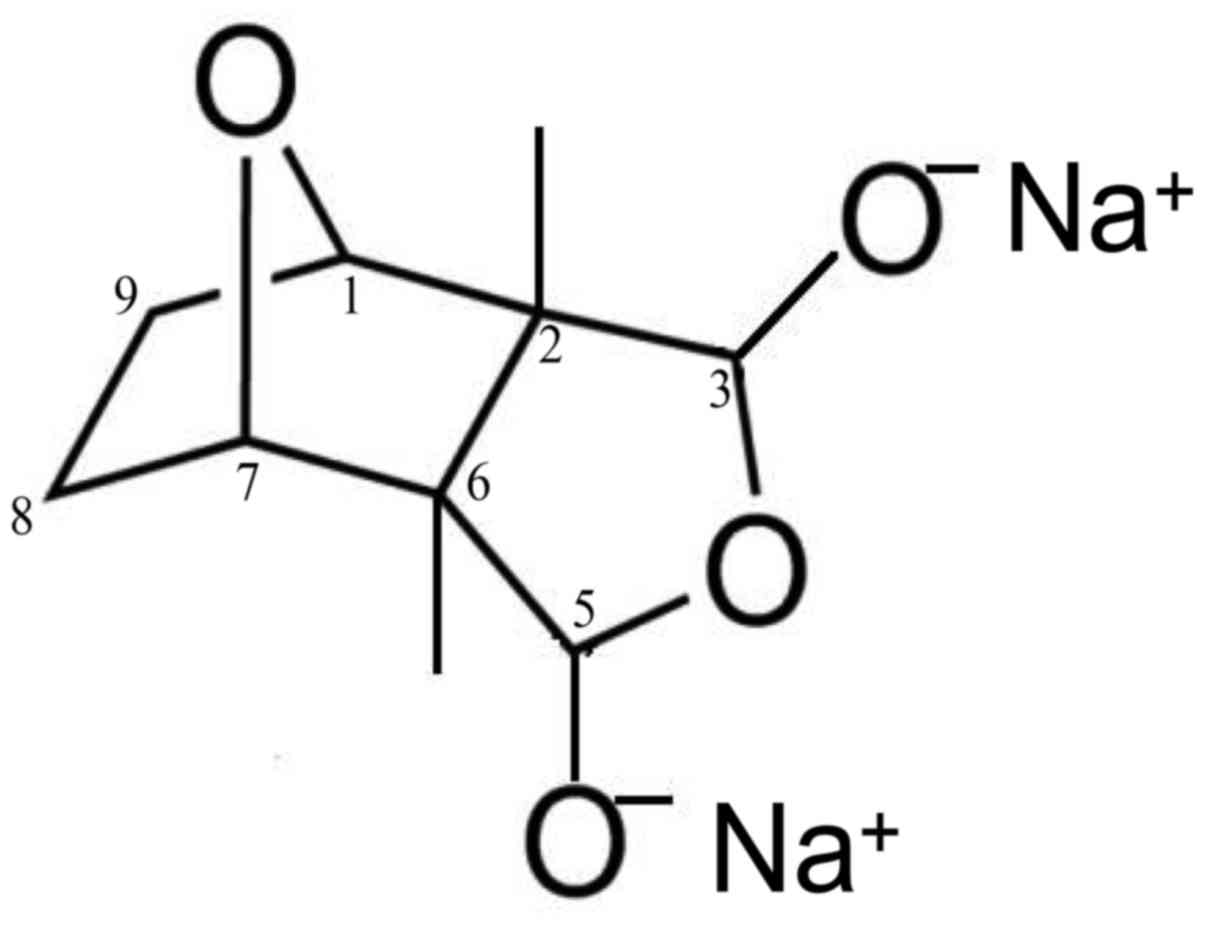
Sodium cantharidinate [(1R,2S,3R,4S)-rel-2,
3-dimethyl-7-oxabicyclo [2.2.1] heptane-2,3-dicarboxylic acid,
disodium salt, SC] was purchased from Cayman Chemical Company (Ann
Arbor, MI, USA) (cat. no. 1465-77-6) (Fig. 1). Water soluble tetrazolium (WST-1)
cell proliferation reagent was purchased from Roche (Shanghai,
China). Antibodies used in FACS were all purchased from BD
Biosciences (Franklin Lakes, NJ, USA). Antibodies used in western
blotting were all purchased from [Cell Signaling Technology (CST),
Inc., Danvers, MA, USA]. Dulbecco's modified Eagle's medium (DMEM),
fetal bovine serum (FBS), phosphate-buffered saline (PBS), trypsin,
penicillin and streptomycin were obtained from Gibco-BRL Life
Sciences/Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Preparation of SC
SC was dissolved in PBS (pH 7.2) to prepare a stock
solution (1.0 mM) and was stored at −20°C. Appropriate
concentrations of SC were prepared by dilution with DMEM complete
medium prior to use.
Cell cultivation and treatments
MG-63 cells were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA) and maintained in
DMEM. DMEM full cell culture medium was prepared and supplemented
with 1% antibiotics (penicillin-streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.) and 10% FBS (HyClone; GE Healthcare Life
Sciences, Beijing, China). MG-63 cells were cultured at 37°C with a
constant air flow of 5% CO2 in a humidified
incubator.
Cell proliferation assay
MG-63 cells (5×104) were seeded in a
96-well plate and then treated without or with SC (0.2, 1.0 and 5.0
µM) for 24 h in the dose-dependent experiment. MG-63 cells were
treated with SC (5.0 µM) for 12, 24, 48 or 72 h in the
time-dependent experiment. WST-1 cell proliferation reagent was
applied to determine the cell proliferation. Under the
manufacturer's instructions, 20 µl WST-1 was initially added to 200
µl of MG-63 cell, and then incubated in the dark for 2 h in the
original incubator. Subsequently, the absorbance at 450 and 630 nm
were measured using a microplate reader (Bioteck, Beijing, China).
Final the optical density (OD) was designated as OD450 -
OD630 - ODblank.
Cell cycle assay
MG-63 cells (4×103) treated with or
without SC were collected after the appropriate time, and then
washed with 1 ml cold PBS twice to remove the residual trypsin and
serum. Cells were pelleted and re-suspended in 1 ml fixation
solution (PBS:ethanol = 3:7). After incubation at 4°C for 4 h, the
cells were centrifuged at 300 × g for 5 min and fixation solution
was removed. After washing twice with 1 ml PBS, the cells were
pelleted and suspended in 0.5 ml propidium iodide (PI;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) staining solution
(50 µg/ml PI, 20 µg/ml RNase A and 0.2% Triton X-100) and incubated
in the dark at 37°C for 30 min. Cell suspensions were filtered
through a 400-mesh sieve before being analyzed by a BD FACSCalibur
flow cytometer (BD Biosciences, Sparks, MD, USA).
Western blotting
MG-63 cells treated without or with SC lysates were
separated by SDS-PAGE under nonreducing conditions on a 10%
polyacrylamide gel. The proteins were then transferred onto PVDF
membranes by electroblotting. The membranes were blocked with
blocking buffer overnight at 4°C and then incubated with the cyclin
A (dilution 1:2,000; cat. no. 4656), cyclin B (dilution 1:1,000;
cat. no. 4138), cyclin D1 (dilution 1:1,000; cat. no. 2922), CDK1
(dilution 1:1,000; cat. no. 9868), CDK2 (dilution 1:1,000; cat. no.
78B2), CDK4 (dilution 1:1,000; cat. no. D9G3E), CDK6 (dilution
1:1,000; cat. no. D4S8S), AKT (dilution 1:1,000; cat. no. 2966),
p-AKT (Ser-473, dilution 1:1,000; cat. no. 4060), mTOR (dilution
1:1,000; cat. no. 2972), p-mTOR (Ser-2448, dilution 1:1,000; cat.
no. 5536), JNK (dilution 1:1,000; cat. no. 9252), p-JNK (Tyr-185,
dilution 1:1,000; cat. no. 9251), P38 (dilution 1:1,000; cat. no.
8690), p-P38 (Thr180/Tyr182, dilution 1:1,000; cat. no. 9211) and
β-actin (dilution 1:1,000; cat. no. 3700) antibodies for 1.5 h at
room temperature. The membranes were then washed with TBS washing
buffer [Tris-buffered saline with Tween-20 (0.1%)] six times and
incubated with HRP-conjugated secondary antibodies for another 1 h.
After washing, protein bands were visualized using an enhanced
chemiluminescent system (Thermo Fisher Scientific, Inc., Shanghai,
China). The primary antibodies used were all obtained from Cell
Signalling Technology, Inc., (Danvers, MA, USA). Western blot
images were quantified by optical density analysis. Protein
expression levels were determined semi-quantitatively by
densitometric analysis with Quantity One software (v4.62; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
FCM for cell signal detection
Approximately 2×106 MG-63 cells treated
without or with SC were collected and washed with 1 ml cold PBS
twice to remove the residual trypsin and serum. Cells were pelleted
and resuspended in FACS tubes. Broken membrane buffer solution was
used initially, and incubation was carried out for 30 min, followed
by the addition of 1 ml washing buffer. The samples were then
incubated with p-AKT (PE), p-JNK (APC), p-P38 (FITC) and p-mTOR
(Percp) for another 15 min and immediately analyzed using a flow
cytometer (FACScan; BD Biosciences) with Flowjo 7.6 FACS analysis
software (FlowJo LLC, Ashland, OR, USA).
Statistical analysis
All data and results were calculated from at least
three replicate measurements and are presented as the mean ± SD.
Mean values were compared using paired t-tests (two groups)
followed by the Bonferroni correction for multiple comparison
tests. P-values <0.05 were considered significant. All
statistical tests were performed with GraphPad Prism software
(v5.0; GraphPad Software Inc., San Diego, CA, USA).
Results
SC inhibits cell growth in MG-63
cells
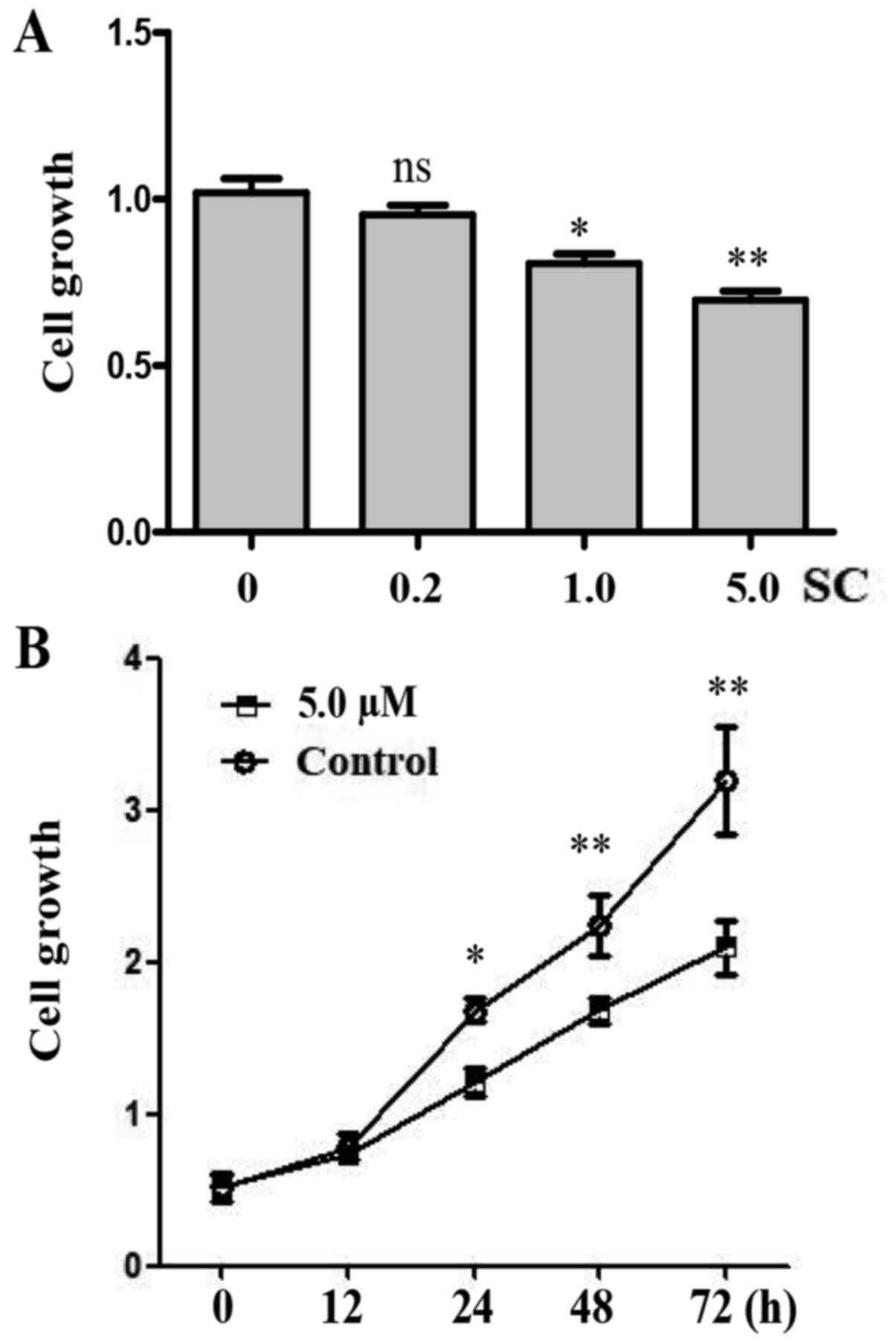
In order to investigate the function of SC on human
OS MG-63 cells, we established three different doses of SC
including 0.2, 1.0 and 5.0 µM. Cell proliferation was determined by
WST-1 assay 24 h post-treatment. We found that 1.0 and 5.0 µM doses
of SC significantly inhibited the growth of MG-63 cells when
compared to the control group (Fig.
2A, P<0.05 and P<0.01, respectively). The 5.0 µM dose of
SC had the most significant effect. We compared the cell growth in
MG-63 cells at different time points following treatment with 5.0
µM of SC. Cell proliferation was determined by WST-1 assay at 0,
12, 24, 48 and 72 h post-treatment. Apparently, MG-63 cells showed
a decelerated proliferation between 24 and 72 h after treatment
with 5.0 µM of SC (Fig. 2B,
P<0.05).
SC arrests the cell cycle at G0/G1 in
MG-63 cells
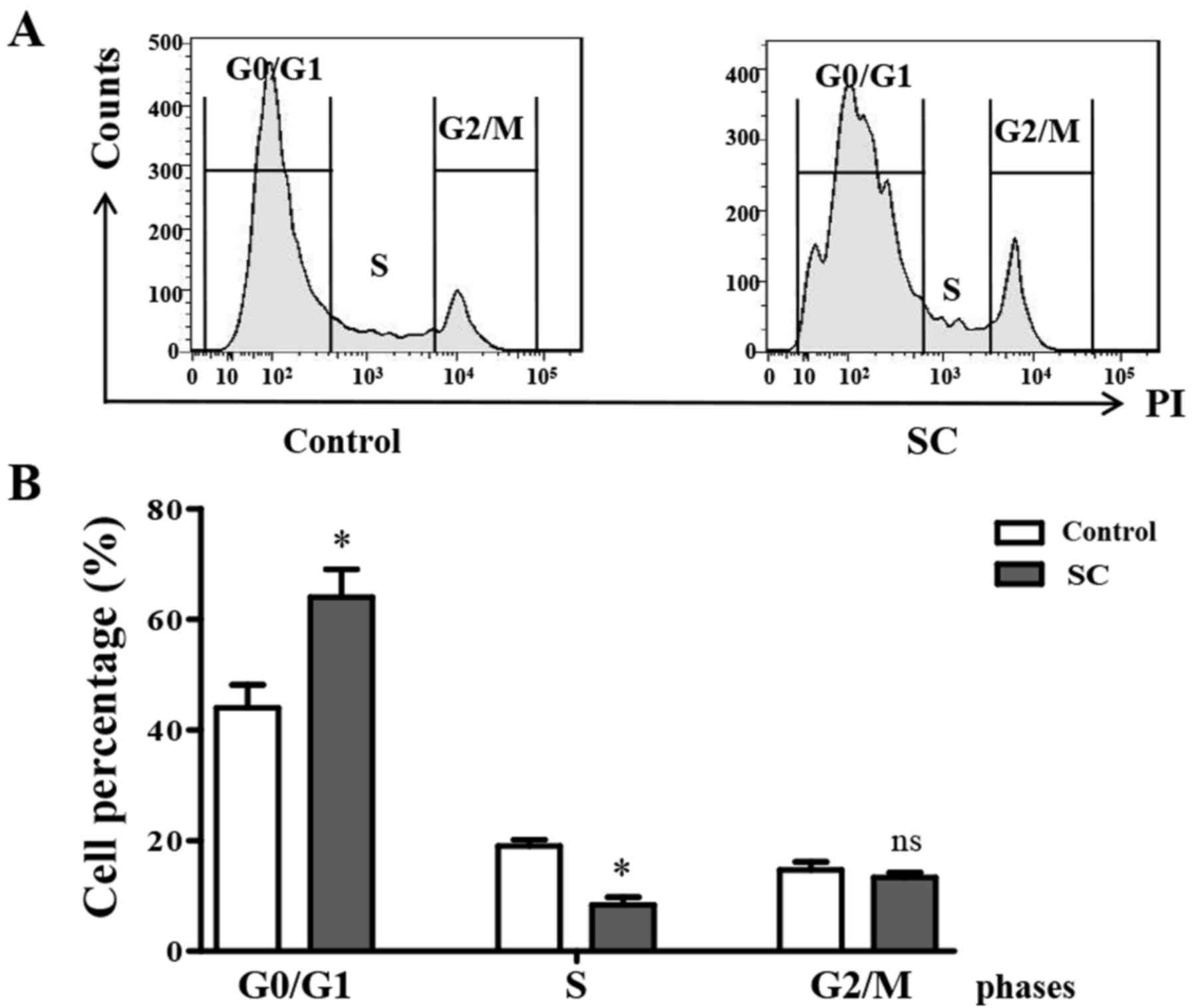
Flow cytometry was utilized to analyze cell cycle
distribution at 24 h post SC treatment. We found that after
treatment with 5.0 µM SC, MG-63 cells showed a significant G0/G1
phase arrest compared to the control group (Fig. 3A). Cell percentages in the different
phase analysis showed that the percentage of cells in the G0/G1
phase was increased from 42±2.5 to 63±3.5% in the SC treatment
groups comparing to the control (Fig.
3B, P<0.05). At the same time, the percentage of cells in
the S phase was significantly decreased (P<0.05), while the G2/M
phase cell percentage did not change. This finding indicated that
SC significantly induced MG-63 cell cycle arrest at the G0/G1
phase.
SC inhibits cyclin D1 expression in
MG-63 cells
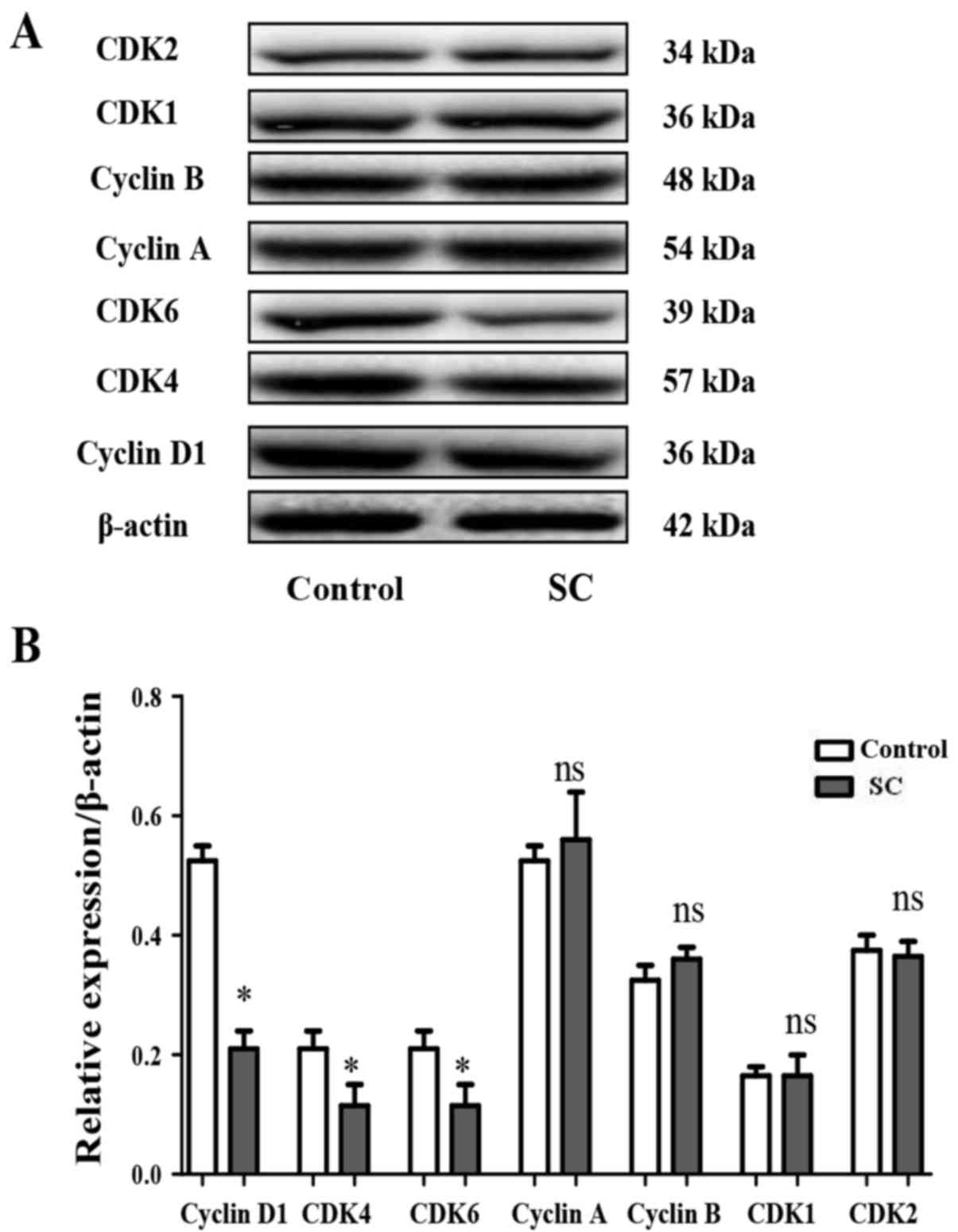
The cell cycle is precisely regulated by cyclins and
kinases. Cyclin D1, CDK4 and CDK6 are closely related to the G0/G1
phase, cyclin B1 and CDK1 are closely related to the G2/M phase,
while Cyclin A and CDK2 are closely related to the S phase. To
examine whether SC exhibits functions on cyclins and kinases in the
MG-63 cells, western blotting was performed to assess the levels of
cyclin A, cyclin B, cyclin D1, CDK1, CDK2, CDK4 and CDK6. It was
found that SC significantly inhibited cyclin D1 (Fig. 3A and B, P<0.05), CDK4 (Fig. 4A and B, P<0.05) and CDK6
(Fig. 4A and B, P<0.05)
expression, consistent with the cell cycle distribution assay. In
contrast, the expression of cyclin A, cyclin B, CDK1 and CDK2 did
not show a significant difference between the SC treatment group
and the control group (Fig. 4A and
B, P>0.05).
PI3K/AKT pathway participates in the
inhibition of MG-63 cell proliferation by SC
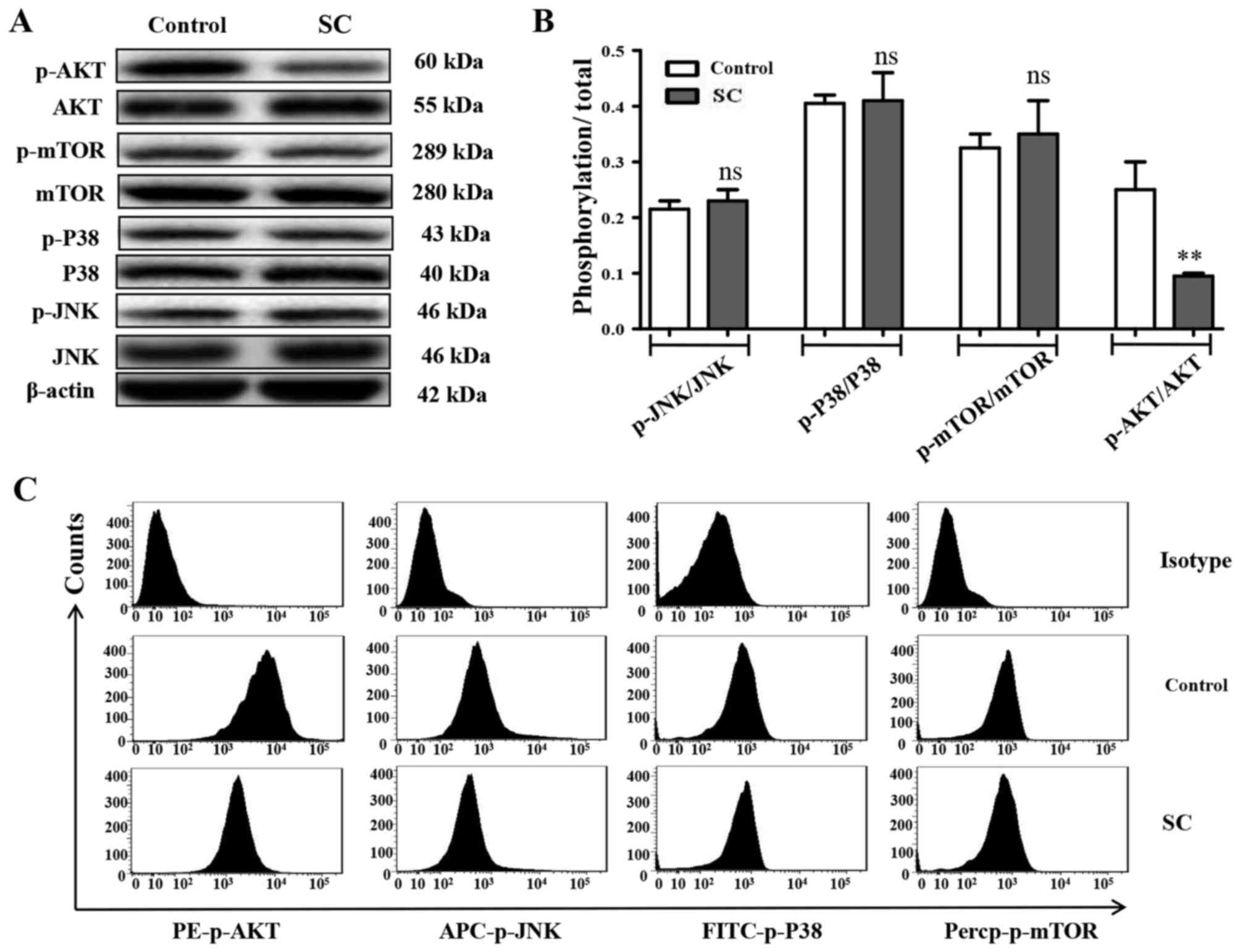
The expression of MAPK components and AKT was
evaluated using western blotting and FACS experiments. As shown in
Fig. 5A and B, SC significantly
inhibited the phosphorylation of AKT (P<0.05), but not mTOR
(P>0.05), JNK (P>0.05) or P38 (P>0.05).
Fluorescent-labeled flow cytometry was also applied to test the
MG-63 cell signaling pathway activation. The phosphorylation of AKT
fluorescence intensity was significantly inhibited by SC (Fig. 5C, P<0.05), consistent with the
western blot results.
Stimulation of the PI3K/AKT signaling
pathway reverses SC-induced cell cycle arrest
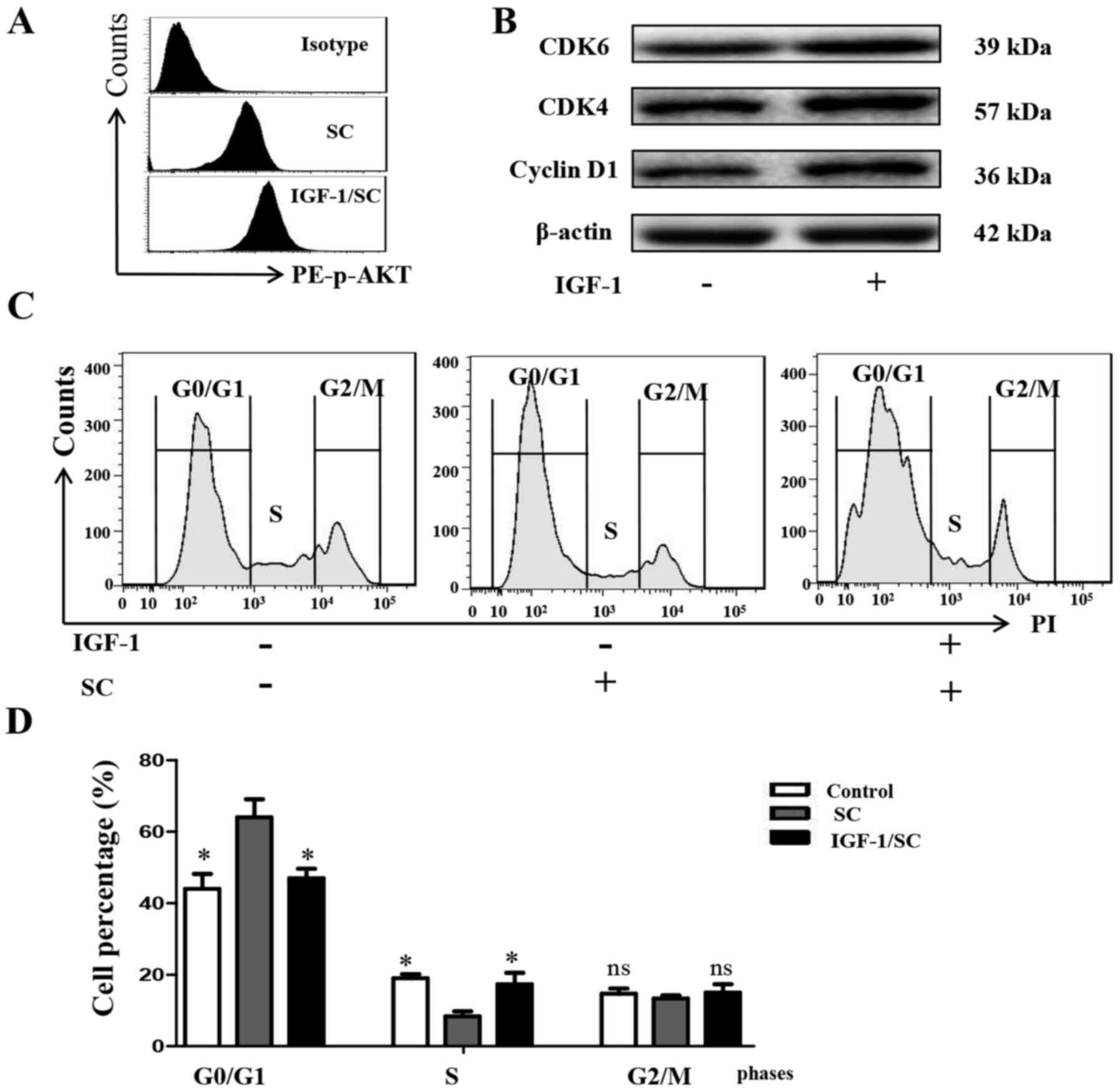
To further test whether stimulation of the PI3K/AKT
signaling pathway reverses SC-induced cell cycle arrest in MG-63
cells, the cells were treated with IGF-1 (50 µM), or SC for 24 h
alone, and in combination, to effectively stimulate the PI3K/AKT
signaling pathway in MG-63 cells, compared with the control cells.
IGF-1 significantly increased the phosphorylation level of AKT,
comparing with the SC treated alone group (Fig. 6A, P<0.05). Cyclin D1 (P<0.05),
CDK4 (P<0.05) and CDK6 (P<0.05) expression was also
observably reversed, consistent with the phosphorylation level of
AKT (Fig. 6B). Cell cycle detection
by FACS showed that IGF-1 reversed the SC-induced cell cycle
arrest, decreased the G0/G1 phase percentage and increased the S
phase percentage (Fig. 6C and D,
P<0.05). These findings indicated that the PI3K/AKT pathway
participates in the inhibition of MG-63 cell proliferation by SC.
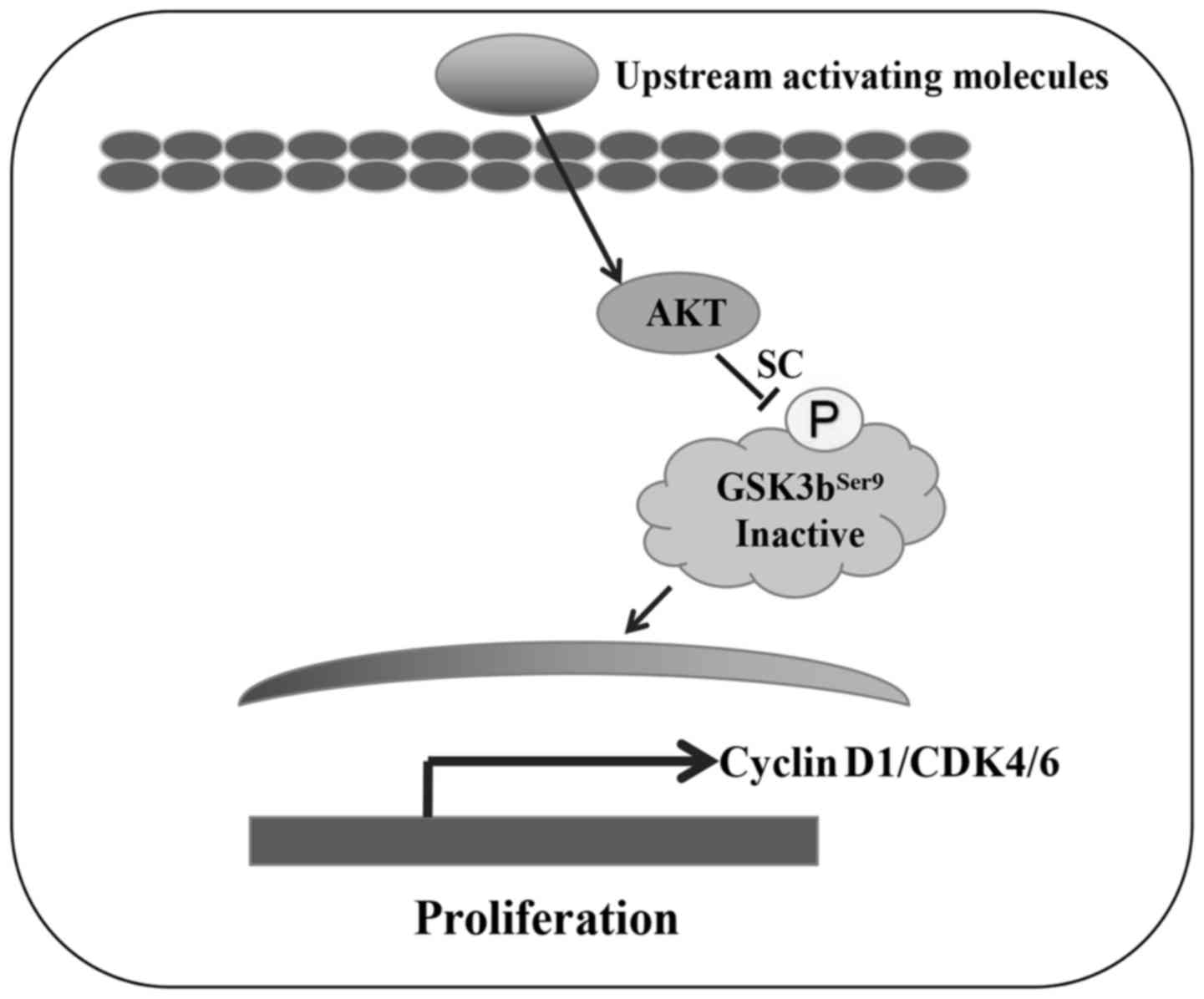
Together, these data suggest that SC inhibits the phosphorylation
of AKT, consequently decreasing the expression of cyclin D1, CDK4
and CDK6, and inducing MG-63 cell G0/G1 phase arrest (illustrated
in Fig. 7).
Discussion
Osteosarcoma is a common malignant cancer that has
threatened the health of children worldwide over the last few
decades. At present, a number of adverse side-effects are
associated with chemotherapeutic drugs, such as cisplatin,
ifosfamide and high-dose methotrexate. Drug resistance may be
acquired by osteosarcoma cells after treatment for an extended
period with such chemotherapeutic drugs. Therefore, development of
novel effective therapeutic drugs with moderate side-effects for
the treatment of osteosarcoma is urgent (23). There are many dangerous factors
affecting the biology of tumor cells during the occurrence and
progression of osteosarcoma (24).
Cell cycle control is a major regulatory mechanism of cell
proliferation. Thus, reprogramming of the cell cycle is the most
effective method for cancer treatment in the clinic (25). There are numerous natural compounds
that display significant inhibitory effects on osteosarcoma.
Shangguan et al demonstrated that a natural product from
ginseng, ginsenoside Rf, displays powerful cytotoxicity to human
osteosarcoma MG-63 cells, in a dose-dependent manner. Additionally,
ginsenoside Rf induced MG-63 cell cycle arrest at the G2/M phase
and then apoptosis (26). Liu et
al found that melatonin inhibits the ERK1/2 signaling pathway
to display antiproliferative action, but did not affect the p38,
JNK, or Akt pathways (27).
Although some studies have focused on the antitumor
activity of sodium cantharidinate (SC) in clinical practice in
recent years, there is no study concerning the activity of SC in
osteosarcoma to date. There are several human osteosarcoma cell
lines, such as MG-63, U2OS and 143B. SC was assessed using the 3
different cell lines in a pre-experiment, but no difference was
found. Thus, the MG-63 cell line was used in the following
mechanistic experiment. The present study investigated the
antiproliferation and cell cycle arrest effects of SC on MG-63
cells for the first time. We found that 1.0 and 5.0 µM doses of SC
significantly inhibited the growth of MG-63 cells. We then compared
the cell growth in MG-63 cells at different time points following
treatment with 5.0 µM of SC. Apparently, MG-63 cells showed a
decelerated proliferation between 24 and 72 h after 5.0 µM of SC
treatment. Cell cycle arrest is closely related to inhibition of
cell proliferation (28). Thus,
FACS experiment was established to analyze cell cycle distribution
after SC treatment. We found that MG-63 cells showed a significant
G0/G1 phase arrest compared to the control group after 5.0 µM SC
treatment. Analysis of the percentages of cells in the different
cell cycle phases showed that the G0/G1 phase percentage increased
from 42±2.5 to 63±3.5% in the SC treatment group comparing to the
control. At the same time, the S phase percentage decreased
significantly, while the G2/M phase percentage did not change. This
finding indicated that SC significantly induced MG-63 cell cycle
arrest at the G0/G1 phase. Cytotoxic agents and/or DNA damaging
agents, which arrest the cell cycle at the G0/G1, S or G2/M phase,
induce cancer cell apoptosis (18).
PI staining is a classical methods for the cell cycle, the peak
width represents the different phases, although BrdU staining is an
efficient methods for assessment of the cell cycle. Cyclins form
complexes with Cdks to activate Cdks to regulate the cell cycle. To
examine whether SC has functions on cyclins and kinases in MG-63
cells, western blotting was performed. SC significantly inhibited
cyclin D1, CDK4 and CDK6 expression, consistent with the cell cycle
detection assay. In contrast, expression of cyclin A, cyclin B,
CDK1 and CDK2 did not show a significant difference between the SC
treatment group and control group, which showed similar mechanisms
as in a previous study (29).
Akt (also known as Akt1) may promote cell
proliferation via phosphorylation, which acts as a mediator of
growth factors (21). In order to
further verify which signaling molecules is related to the MG-63
cell cycle arrest, we detected the expression of MAPK components
and AKT using western blotting and FACS experiments. SC
significantly inhibited phosphorylation of AKT, but not mTOR, JNK,
or P38. To confirm the importance of the AKT phosphorylation in the
SC-induced cell cycle arrest, we applied PI3K/AKT stimulator IGF-1
to pre-incubate MG-63 cells before SC treatment. IGF-1
significantly increased the phosphorylation level of AKT, compared
with the SC treated alone group. Cyclin D1, CDK4 and CDK6
expression were also significantly reversed. Cell cycle detection
by FACS showed that IGF-1 reversed SC-induced cell cycle arrest,
decreased G0/G1 phase percentage and increased S phase percentage.
These findings indicated that the PI3K/AKT pathway participated in
the inhibition of MG-63 cells by SC. In a previous study, Liu et
al found that that melatonin display antiproliferative action,
which is mediated by inhibition of the ERK1/2 signaling pathway
rather than the p38, JNK or Akt pathways (27). When cells were stimulated by
upstream activating molecules, PI3K/AKT were phosphorylated, and
then activated GSK3bSer9 phosphorylation for migration
into the nucleus and regulation of the cell cycle (30). In the present study, we revealed a
different mechanism: SC inhibited the phosphorylation of AKT, then
decreased the expression of cyclin D1, CDK4 and CDK6, and induced
MG-63 cell G0/G1 phase arrest. To the best of our knowledge, this
is the first study to reveal the exact mechanism of SC in the
induction of MG-63 cell inhibition. SC has potential for
development as a new drug for the treatment of human osteosarcoma,
although the results were only verified in vitro.
Experiments in vivo in mice or rats will potentially be
utilized for further investigation of the efficacy of SC for
osteosarcoma treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
DLK and YL designed and performed the experiments,
analyzed the data and wrote the manuscript. JYW and GL performed
the experiments. MLZ designed, interpreted and funded the study,
and wrote the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torres K and Horwitz SB: Mechanisms of
Taxol-induced cell death are concentration dependent. Cancer Res.
58:3620–3626. 1998.PubMed/NCBI
|
|
3
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrari S and Serra M: An update on
chemotherapy for osteosarcoma. Expert Opin Pharmacother.
16:2727–2736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jaffe N: Osteosarcoma. Review of the past,
impact on the future. The American experience. Cancer Treat Res.
152:239–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferguson JL and Turner SP: Bone cancer:
Diagnosis and treatment principles. Am Fam Physician. 98:205–213.
2018.PubMed/NCBI
|
|
7
|
Liu Q, Xu B and Zhou W: Correlation
between chemotherapy resistance in osteosarcoma patients and PAK5
and Ezrin gene expression. Oncol Lett. 15:879–884. 2018.PubMed/NCBI
|
|
8
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin J, Dong Q, Zheng M, Xu X, Zou G, Ma G
and Li K: Antitumor activity of dobutamine on human osteosarcoma
cells. Oncol Lett. 11:3676–3680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shah U, Shah R, Acharya S and Acharya N:
Novel anticancer agents from plant sources. Chin J Nat Med.
11:16–23. 2013. View Article : Google Scholar
|
|
11
|
Khan F, Ahmed F, Pushparaj PN, Abuzenadah
A, Kumosani T, Barbour E, AlQahtani M and Gauthaman K: Ajwa date
(Phoenix dactylifera L.) extract inhibits human breast
adenocarcinoma (MCF7) cells in vitro by inducing apoptosis and cell
cycle arrest. PLoS One. 11:e01589632016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerber DE: Targeted therapies: A new
generation of cancer treatments. Am Fam Physician. 77:311–319.
2008.PubMed/NCBI
|
|
13
|
Tsauer W, Lin JG, Lin PY, Hsu FL and
Chiang HC: The effects of cantharidin analogues on xanthine oxides.
Anticancer Res. 17:2095–2098. 1997.PubMed/NCBI
|
|
14
|
Lin LH, Huang HS, Lin CC, Lee LW and Lin
PY: Effects of cantharidinimides on human carcinoma cells. Chem
Pharm Bull. 52:855–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yeh CB, Su CJ, Hwang JM and Chou MC:
Therapeutic effects of cantharidin analogues without bridging ether
oxygen on human hepatocellular carcinoma cells. Eur J Med Chem.
45:3981–3985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tao R, Sun WY, Yu DH, Qiu W, Yan WQ, Ding
YH, Wang GY and Li HJ: Sodium cantharidinate induces HepG2 cell
apoptosis through LC3 autophagy pathway. Oncol Rep. 38:1233–1239.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang I, Beus M, Stochaj U, Le PU, Zorc B,
Rajić Z, Petrecca K and Maysinger D: Inhibition of glioblastoma
cell proliferation, invasion, and mechanism of action of a novel
hydroxamic acid hybrid molecule. Cell Death Discov. 5:412018.
View Article : Google Scholar
|
|
18
|
Gamet-Payrastre L, Li P, Lumeau S, Cassar
G, Dupont MA, Chevolleau S, Gasc N, Tulliez J and Tercé F:
Sulforaphane, a naturally occurring isothiocyanate, induces cell
cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer
Res. 60:1426–1433. 2000.PubMed/NCBI
|
|
19
|
Stewart ZA, Westfall MD and Pietenpol JA:
Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol
Sci. 24:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hindley C and Philpott A: Co-ordination of
cell cycle and differentiation in the developing nervous system.
Biochem J. 444:375–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robertson JD and Orrenius S: Role of
mitochondria in toxic cell death. Toxicology. 181–182. 491–496.
2002.PubMed/NCBI
|
|
24
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Basu-Roy U, Basilico C and Mansukhani A:
Perspectives on cancer stem cells in osteosarcoma. Cancer Lett.
338:158–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shangguan WJ, Li H and Zhang YH: Induction
of G2/M phase cell cycle arrest and apoptosis by ginsenoside Rf in
human osteosarcoma MG-63 cells through the mitochondrial pathway.
Oncol Rep. 31:305–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Xu Y, Reiter RJ, Pan Y, Chen D, Liu
Y, Pu X, Jiang L and Li Z: Inhibition of ERK1/2 signaling pathway
is involved in melatonin's antiproliferative effect on human MG-63
osteosarcoma cells. Cell Physiol Biochem. 39:2297–2307. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang HW, Tang JY, Ou-Yang F, Wang HR,
Guan PY, Huang CY, Chen CY, Hou MF, Sheu JH and Chang HW: Sinularin
selectively kills breast cancer cells showing G2/M arrest,
apoptosis, and oxidative DNA damage. Molecules. 23:E8492018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Xu W, Yan Z, Zhao W, Mi J, Li J
and Yan H: Metformin induces autophagy and G0/G1 phase cell cycle
arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways.
J Exp Clin Cancer Res. 37:632018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saunders EJ, Dadaev T, Leongamornlert DA,
Al Olama AA, Benlloch S, Giles GG, Wiklund F, Gronberg H, Haiman
CA, Schleutker J, et al: Gene and pathway level analyses of
germline DNA-repair gene variants and prostate cancer
susceptibility using the iCOGS-genotyping array. Br J Cancer.
114:945–952. 2016. View Article : Google Scholar : PubMed/NCBI
|