Introduction
Lung cancer is a malignant tumor with a particularly
high incidence rate, and its poor prognosis makes it one of the
primary causes for cancer-associated mortality in the world
(1). Among them, 85% of patients
with lung cancer suffer from non-small cell lung carcinoma (NSCLC),
which is commonly diagnosed at an advanced stage of disease
(2). The 5-year overall survival
rates of patients with lung cancer is <15% in spite of the
recent advances in targeted therapies (1). Although several studies have reported
various protein-coding genes, which are differentially expressed in
NSCLC tissues, their relatively low specificity and insufficient
sensitivity have presented difficulties with attempting to obtain
an early diagnosis, prognosis evaluation, and recurrence
prediction. Consequently, there is a requirement to develop novel
molecular markers for NSCLC diagnosis and treatment (3,4).
A class of non-coding RNA, termed long non-coding
RNAs (lncRNAs) due to their length of >200 nucleotides, have
been the focus of research (5).
LncRNAs transcriptionally activate genes that do not code for
proteins, and these account for >80% of all genes (6). It is well-established that lncRNAs
serve essential roles in regulating diverse biological process,
including cell proliferation, apoptosis and differentiation, and
tumorigenesis and metastasis in tumor tissues, which may predict
the overall prognosis in certain types of cancer (7). It has been hypothesized that lncRNAs
are a promising source of diagnostic biomarkers and therapeutic
targets for human cancer (8).
A member of the long intergenic non-coding RNA
(lincRNA) family, lincRNA-p21, was reported to be able to restrain
the invasion and metastasis of various types of cancer, including
colorectal cancer and hepatocellular carcinoma, and has been
demonstrated to be associated with the enhancement of
epithelial-mesenchymal transition (EMT) (9,10).
Furthermore, it has been reported that lincRNA-p21 is able to
induce EMT by regulating its downstream miRNA, ultimately affecting
hepatocellular carcinoma tumor growth (10). For example, lincRNA-p32 is
downregulated in human hepatocellular carcinoma (HCC) tissues, and
its upregulation remarkably suppresses the migration and invasion
of HCC cells (10). Furthermore,
lncRNA-p21 was demonstrated to be able to negatively regulate the
expression level of microRNA (miR)-9, which directly targets
E-cadherin (11). Through this
interaction, the progression of HCC is inhibited by lincRNA-p21 via
the miR-9/E-cadherin cascade signaling pathway (11). Previous research also identified the
abnormal expression of lincRNA-p21 in NSCLC tissues (12). However, the underlying molecular
mechanism of lincRNA-p21 in the initiation, development and
metastasis of NSCLC remains unclear.
The present study aimed to highlight the important
role of lincRNA-p21 in NSCLC tumors. Firstly, the expression of
lincRNA-p21 in clinical lung cancer tissues was measured by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), and
was demonstrated to be negatively associated with the advanced
clinical pathology of NSCLC. Furthermore, the potential mechanism
of lincRNA-p21 in regulating the proliferation and apoptosis of the
lung cancer cells was explored by transfection with lincRNA-p21
small interfering (siRNA) or an overexpression plasmid.
Additionally, a panel of factors associated with proliferation,
apoptosis, and migration, including B-cell lymphoma-2 matrix
metallopeptidase 9, were quantified by western blot analysis and
RT-qPCR. Through bioinformatics analysis, it was hypothesized that
the downstream miRNA of lincRNA-p21 is miR-17-5p. The role of
lincRNA-p21 in regulating miR-17-5p was further demonstrated at a
cellular level, and the direct interaction of lincRNA-p21 and
miR-17-5p were further validated by a dual luciferase reporter
assay. The inhibitory effect of lincRNA-p21 on lung tumor growth
was also verified by in vivo studies. The results of the
present study suggest a novel regulatory function of lincRNA-p21 in
NSCLC and provides a potential therapeutic target for the treatment
of NSCLC.
Materials and methods
Patients and clinical tissue
samples
A total of 40 pairs of lung cancer tissue samples
and adjacent tissue samples were obtained from patients with NSCLC
in Guangdong General Hospital (Guangzhou, China). Among them, 29
patients were male and 11 patients were female (age range, 25–45
years old; mean age, 36 years old). All the collected cases were
diagnosed as NSCLC pathologically in Southern Medical University
(Guangzhou, China), and patients did not undergo preoperative
radiotherapy and/or chemotherapy prior to resection. All samples
were collected with informed consent obtained from each patient and
approval from the Southern Medical University Institutional Review
Board.
Cell culture and transfection
Human NSCLC cell lines A549 and PC9 (American Type
Culture Collection, Manassas, VA, USA) were cultivated in RPMI-1640
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with
10% of fetal bovine serum (FBS; Invitrogen; Thermo Fisher
Scientific, Inc.) in a humidified incubator with 5% CO2
at 37°C. A total of 1×104 A549 and PC9 cells were seeded
into 24-well plates, and once cells achieved 85% confluence, they
were transfected with 10 nM pcDNA3.1-lincRNA-p21 overexpression
plasmid or lincRNA-p21 siRNA (5′-UGAAAAGAGCCGUGAGCUA-3′) (both from
Shanghai GenePharma Co., Ltd., Shanghai, China) using Lipofectamine
3000 (Thermo fisher Scientific, Inc.), according to the
manufacturer's protocol. The empty plasmid pcDNA3.1 and lincRNA-p21
scrambled siRNA sequence (5′-AGCCUGCAGGUGAGACCAGAACUG-3′) (both
from Shanghai GenePharma Co., Ltd.) were used as negative control
(NC) groups for the overexpression and knockdown experiments,
respectively.
RT-qPCR
Total RNA was first extracted from A459 and PC9
cells or clinical tissue samples using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Total cDNA was reversed transcribed from isolated RNA
using the PrimeScript RT Master mix (Takara Biotechnology Co.,
Ltd., Dalian, China). The thermocycling conditions maintained were
as follows: 30°C for 10 min, then 42°C for 30 min, followed by 95°C
for 5 min. The expression levels of lincRNA-p21 were detected by
qPCR on the ABI Biosystems (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using SYBR Premix Ex Taq (Takara Biotechnology
Co., Ltd.). The RT-qPCR primers used were as follows: lincRNA-p21
forward, 5′-CCTGTCCCACTCGCTTTC-3′ and reverse,
5′-GGAACTGGACACGGAATGTC-3′; GAPDH forward,
5′-TGTTCGTCATGGGTGTGAAC-3′ and reverse, 5′-ATGGCATGGACTGTGGTCAT-3′.
The thermocycling conditions maintained were as follows: 95°C for
30 sec, then 40 cycles of 95°C for 5 sec followed by 60°C for 30
sec. The relative expression level of lincRNA-p21 was normalized to
internal control GAPDH, and quantified using the 2−ΔΔCq
cycle threshold method (13).
Cell proliferation analysis
At 72 h following transfection, the effects of
lincRNA-p21 on the proliferation of A549 and PC9 cells were
analyzed using a Cell Counting Kit-8 assay (CCK-8; Beyotime
Institute of Biotechnology, Shanghai, China) according to the
manufacturer's protocol. Briefly, A549 cells were washed with PBS
buffer (pH 7.4) and harvested by trypsinization. A total of
1×104 cells were reseeded into a 96-well plate. The
plate was then incubated in a 5% CO2 humidified
incubator at 37°C. Following the incubation, 10 µl of the CCK-8
solution was added to each well and the plate was incubated for 2
h. The measurements were performed by detecting the absorbance at
450 nm with a microplate reader.
Apoptosis analysis
An Annexin V-FITC and propidium iodide (PI) staining
kit (Dead Cell Apoptosis kit with Annexin V Alexa Fluor™
488 & PI; Thermo Fisher Scientific, Inc.) was used to detect
the cell apoptosis according to the manufacturer's protocol.
Briefly, A549 and PC9 cells were transfected with lincRNA-p21
overexpression plasmid or lincRNA-p21 siRNA for 72 h, the cells
were collected and washed with cold PBS twice. Cells were stained
with Annexin-FITC and PI for 5 min at room temperature in the dark,
and the cells were subjected to flow cytometric analysis.
Western blot analysis
Total proteins were isolated from A549 cells and PC9
cells using a Protease Inhibitor cocktail (Thermo Fisher
Scientific, Inc.). Protein concentrations were quantified using a
Pierce BCA Protein assay kit (Thermo Fisher Scientific, Inc.). A
total of 100 µg of protein/lane was resolved by 10% SDS-PAGE and
then transferred onto a polyvinylidene fluoride (PVDF) membrane,
followed by blocking in 5% non-fat dry milk in Tris-buffered saline
containing 0.05% Tween 20 with a pH value of 7.4 overnight at 4°C.
Subsequently, the PVDF membrane was blotted with the primary
antibodies at 4°C overnight against Bcl-2 (1:1,000 dilution; cat.
no. 4223; rabbit; Cell Signaling Technology, Inc., Danvers, MA,
USA), MMP9 (1:1,000 dilution; cat. no. 13667; rabbit; Cell
Signaling Technology, Inc.), SPARC-related modular calcium binding
1 (SMOC1; 1:1,000 dilution; cat. no. ab200219; rabbit; Abcam,
Cambridge, UK) and GAPDH (1:1,000 dilution; cat. no. 5174; rabbit;
Cell Signaling Technology, Inc.). Chemiluminescence signals were
detected incubated with mouse anti-rabbit (1:5,000 dilution; cat.
no. ab99696) and rat anti-mouse (1:5,000 dilution; cat. no.
ab99616) horseradish peroxidase-conjugated secondary rabbit
antibodies (both from Abcam) for 1 h at 4°C. The expression levels
of the target proteins were visualized by electrochemiluminescence
(cat. no. 32109; Thermo Fisher Scientific, Inc.). The relative
protein expression levels were evaluated through the gray value
ratio of each protein and GAPDH using ImageJ (version 2.0; National
Institutes of Health, Bethesda, MD, USA).
Transwell assay
Transwell chambers were warmed and placed into a
24-well plate at 37°C. The upper and lower chambers were hydrated
with 0.5 ml pre-heated RPMI-1640 culture medium with 1% FBS in an
incubator for 2 h at 37 °C followed by the removal of the solution
in the upper and lower chambers. The cells were digested using
trypsin and a cell suspension was prepared to make up a total of
5×104 cells/ml. The hydrated chambers were transferred
into a 24-well plate containing 0.5 ml RPMI-1640 complete medium.
The chambers were incubated with 0.5 ml diluted cell suspension for
24 h at 37°C and the liquid in the upper and lower chambers were
removed. The upper surface cells on the membrane were gently wiped
off and washed three times in PBS. The cells that had migrated to
the lower surface were fixed with precooled 100% methanol for 30
min, stained with 1% crystal violet for 1 h at room temperature,
and washed with running water. Following the removal the crystal
violet by PBS, the dried cells were counted in five randomly
selected fields using a high-powered confocal microscope
(magnification ×200; Quantity One system; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The mean number of cells passing through
the basement membrane was recorded and imaged. The experiment was
performed in triplicate.
Dual-luciferase reporter analysis
Bioinformatics analysis (miRDB: http://www.mirdb.org/; starBase v2.0; http://starbase.sysu.edu.cn/) was used to predict
targets of miR-17-5p. To verify whether miR-17-5p was a direct gene
target of lincRNA-p21, pmirGLO was used to construct a lincRNA-p21
luciferase reporter with binding site for miR-17-5p
(pmirGLO-lincRNA-p21). lincRNA-p21 and its interacting miRNAs were
inserted into the luciferase reporters (Promega Corporation,
Madison, WI, USA) using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Cells were transfected with luciferase
reporters, internal control and indicating RNAs. At 72 h following
transfection, luciferase activities were monitored using the
Dual-Luciferase® Reporter assay system (Promega
Corporation) according to the manufacturer's protocol. Final
results were normalized to Renilla luciferase and analyzed
statistically. The firefly luciferase acted as the internal
reference of 3′UTR wt and the expression of the carrier 3′UTR
wt+miR-NC acted as the control.
Tumor xenograft in vivo model
In order to establish an in vivo tumor model,
a total of 18 BALB/c female nude mice (4–5 weeks old; mean weight,
20 g) were purchased from the Animal Center at Southern Medical
University and maintained under pathogen-free conditions according
to the protocols. The animals were maintained on a 12/12 h
light/dark cycle at a constant temperature of 22°C and humidity,
50–60%. Free access to chow and water were provided. The use of
animals in the present study was approved by the Institution of
Animal Ethical and Welfare Committee. The size of tumors was
measured using calipers and calculated as follows: (Large diameter)
× [2 × (short diameter)]/2 and expressed in mm3. Twelve
mice were randomly divided into three groups (4 mice/group):
pcDNA3.1-vector, lincRNA-p21 and lincRNA-p21 plus miR-17 mimics.
A549 cells were treated with lentivirus vector or lentivirus
lincRNA-p21, using Lipofectamine 3000 as aforementioned. Treated
cells were injected into the posterior flank of each mouse
subcutaneously. Mice in the vector group were administered with
A549 treated with pcDNA3.1-vector, mice in the lincRNA-p21 group
were administered with A549 treated with pcDNA3.1-lincRNA-p21,
while lincRNA-p21 and miR-17 mimics group was injected with A549
cells treated with pcDNA3.1-lincRNA-p21, followed by injection of
miR-17 mimics on the site of tumor after 7 days. To examine the
impact of lincRNA-p21 and miR-17 in tumorigenesis, the tumor growth
within the mice were monitored every other day. Tumor volumes were
measured at using a caliper at the time of sacrifice, 28 days after
the cell injection.
Statistical analysis
Experiments were all repeated at least three times.
A two-tailed Student's t-test was applied to analysis of two groups
and difference between multiple groups was analyzed by one-way
analysis of variance followed by Dunnett's test using IBM SPSS
Statistics software (version 17.0; SPSS, Inc., Chicago, IL, USA).
All data are expressed as mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
lincRNA-p21 expression in NSCLC tumor
tissues is significantly decreased
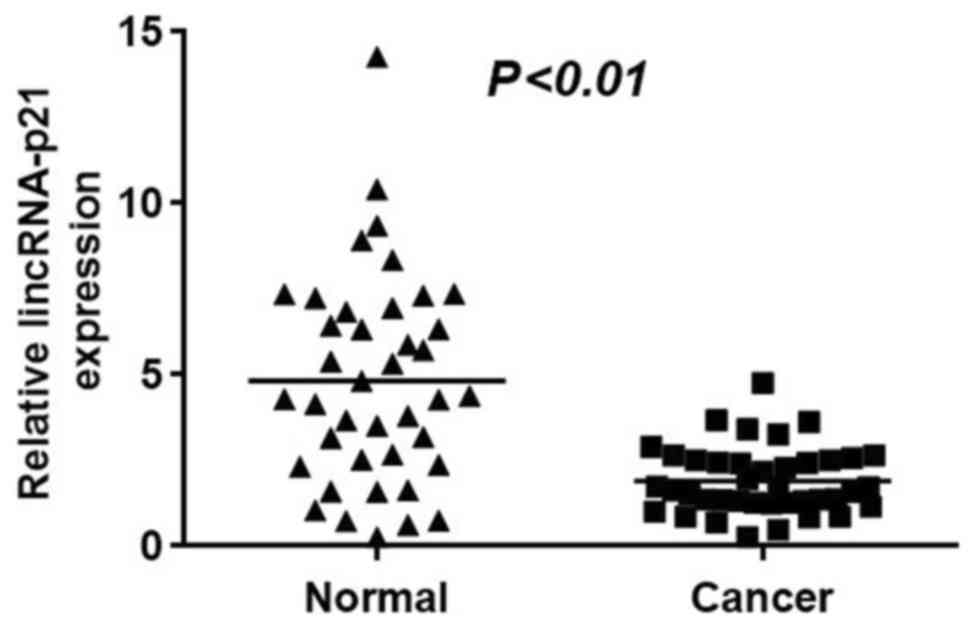
The RT-qPCR results in Fig. 1 illustrate the relative expression
levels of lincRNA-p21 in NSCLC tumor tissues and surrounding normal
tissues. The data indicated that lincRNA-p21 exhibited a
significantly higher expression level in normal tissues compared
with tumor tissues (Fig. 1;
P<0.01). Thus, lincRNA-p21 appears to be downregulated in NSCLC
tumor tissues. Furthermore, lincRNA-p21 expression was analyzed
according to the clinicopathological parameters of 40 patients with
NSCLC (Table I). The mean
expression value for lincRNA-p21 was used to distinguish low or
high expression. Notably, low lincRNA-p21 expression was associated
with distance metastasis and advanced TNM stages, and was
independent of smoking status, sex, age and lymph node
metastasis.
 | Table I.Association between lincRNA-p21
expresion and clinicopathological parameters of patients with
NSCLC. |
Table I.
Association between lincRNA-p21
expresion and clinicopathological parameters of patients with
NSCLC.
| Parameters | No. of
patients | lincRNA-p21 | P-value |
|---|
| Smoking status |
|
| 0.158 |
|
Yes | 28 | 1.454±0.908 |
|
| No | 12 | 1.912±0.954 |
|
| Sex |
|
| 0.178 |
|
Male | 29 | 1.464±0.839 |
|
|
Female | 11 | 1.930±0.999 |
|
| Age, years |
|
| 0.231 |
|
<50 | 10 | 1.324±0.655 |
|
|
>50 | 30 | 1.680±1.004 |
|
| Lymph node
metastasis |
|
| 0.136 |
| N0 | 8 | 1.378±0.647 |
|
|
N1-N3 | 32 | 1.965±0.751 |
|
| Distant
metastasis |
|
| 0.046a |
| M0 | 29 | 1.782±0.678 |
|
| M1 | 11 | 1.023±0.887 |
|
| TNM stage |
|
| 0.034a |
|
0–II | 25 | 1.913±0.789 |
|
|
III–IV | 15 | 0.987±0.861 |
|
lincRNA-p21 levels in different lung
cancer cell lines and expression levels following transfection
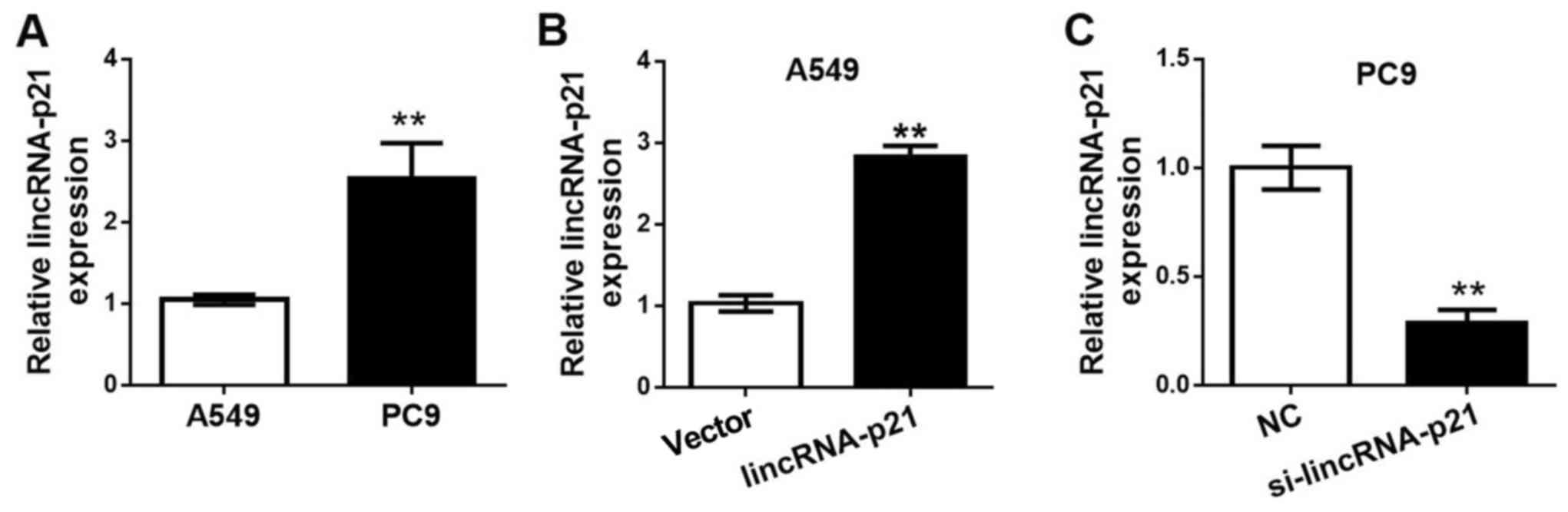
As illustrated in Fig.
2A, the expression level of lincRNA-p21 in the human lung
cancer cell line A549 was significantly lower compared with that of
the human lung cancer cell line PC9. Thus, the A549 lung cancer
cells were used as low lincRNA-p21 expression level cells for the
transfection of lincRNA-p21, while the PC9 lung cancer cells were
used as high lincRNA-p21 expression level cells for the
transfection of lincRNA-p21 siRNA to downregulate lincRNA-p21
expression. The RT-qPCR results in Fig.
2B illustrate the lincRNA-p21 expression levels in each cell
group following transfection. In Fig.
2C, the A549 cells transfected with the lincRNA-p21 plasmid had
a significantly higher lincRNA-p21 expression level, compared with
the control group, which was transfected with the vector alone.
Furthermore, the PC9 cells transfected with lincRNA-p21 siRNA
exhibited a significant reduction in lincRNA-p21 expression when
compared with the NC group in PC9 cells, indicating an effective
knockdown of lincRNA-p21.
Overexpression of lincRNA-p21 inhibits
and knockdown of lincRNA-p21 induces lung cancer cell
proliferation
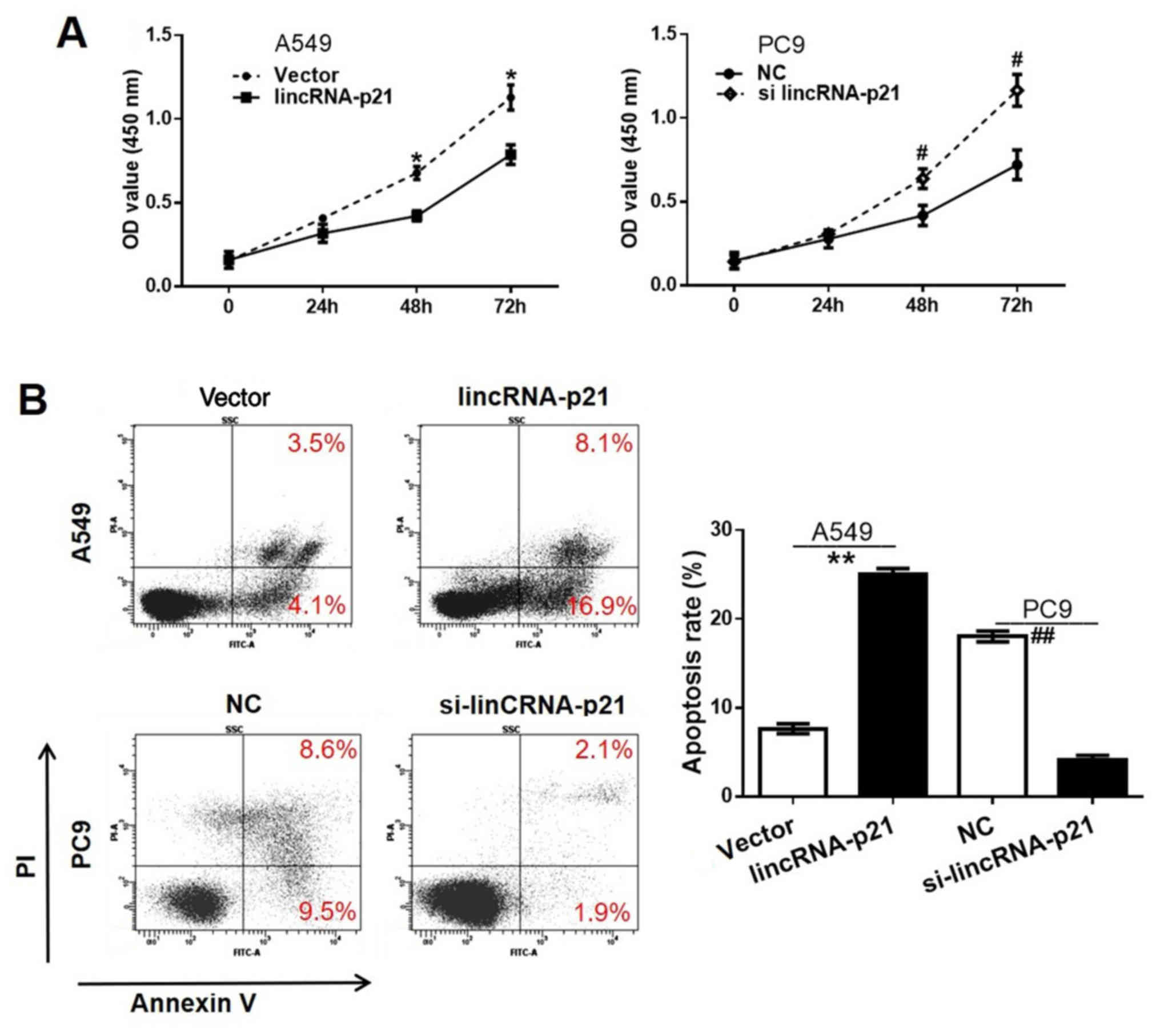
The CCK-8 assay results in Fig. 3A demonstrate the optical density at
450 nm which reflects the cell proliferation rates for different
cell groups during the 0, 24, 48 and 72 h intervals. The
proliferation rate of A549 cells transfected with lincRNA-p21 was
significantly lower compared with the vector-transfected group,
demonstrating the inhibitory effect of lincRNA-p21 on human lung
cancer cell proliferation. Conversely, the PC9 cell line with
si-lincRNA-p21 transfection exhibited a significantly higher
proliferation rate, compared with the NC group, providing evidence
for the suppressive effect of lincRNA-p21 on human lung cancer cell
proliferation.
Cell apoptosis rate is increased in
the lincRNA-p21 overexpression group and decreased in the
lincRNA-p21 knockdown group
The results demonstrated that the cell apoptosis
rate in the A549 lincRNA-p21 group was 16.9%, which was
significantly higher compared with that of the A549 vector group
(4.1%) (P<0.05), while the cell apoptosis rate in the PC9
si-lincRNA-p21 group was 1.9%, which was significantly reduced
compared with that of the PC9 NC group (9.5%) (Fig. 3B). This indicated that the
overexpression of lincRNA-p21 may facilitate cell apoptosis in lung
cancer cells.
Cell migration is decreased in the
lincRNA-p21 overexpression group and increased in the lincRNA-p21
knockdown group
The Transwell analysis results as presented in
Fig. 3C demonstrated that the A549
lincRNA-p21 group had a significantly reduced number of invading
cells (~150 cells) compared with that of the A549 vector group
(~300 cells), while the PC9 si-lincRNA-p21 group had a significant
increase in the number of invading cells (~400 cells) compared with
the PC9 NC group (~170 cells) (P<0.005). Taken together these
results demonstrated that the overexpression of lincRNA-p21
sufficiently inhibited the migration of lung cancer cells.
lincRNA-p21 regulates the expression
of miR-17-5p in lung cancer cells
Investigations on the association between the
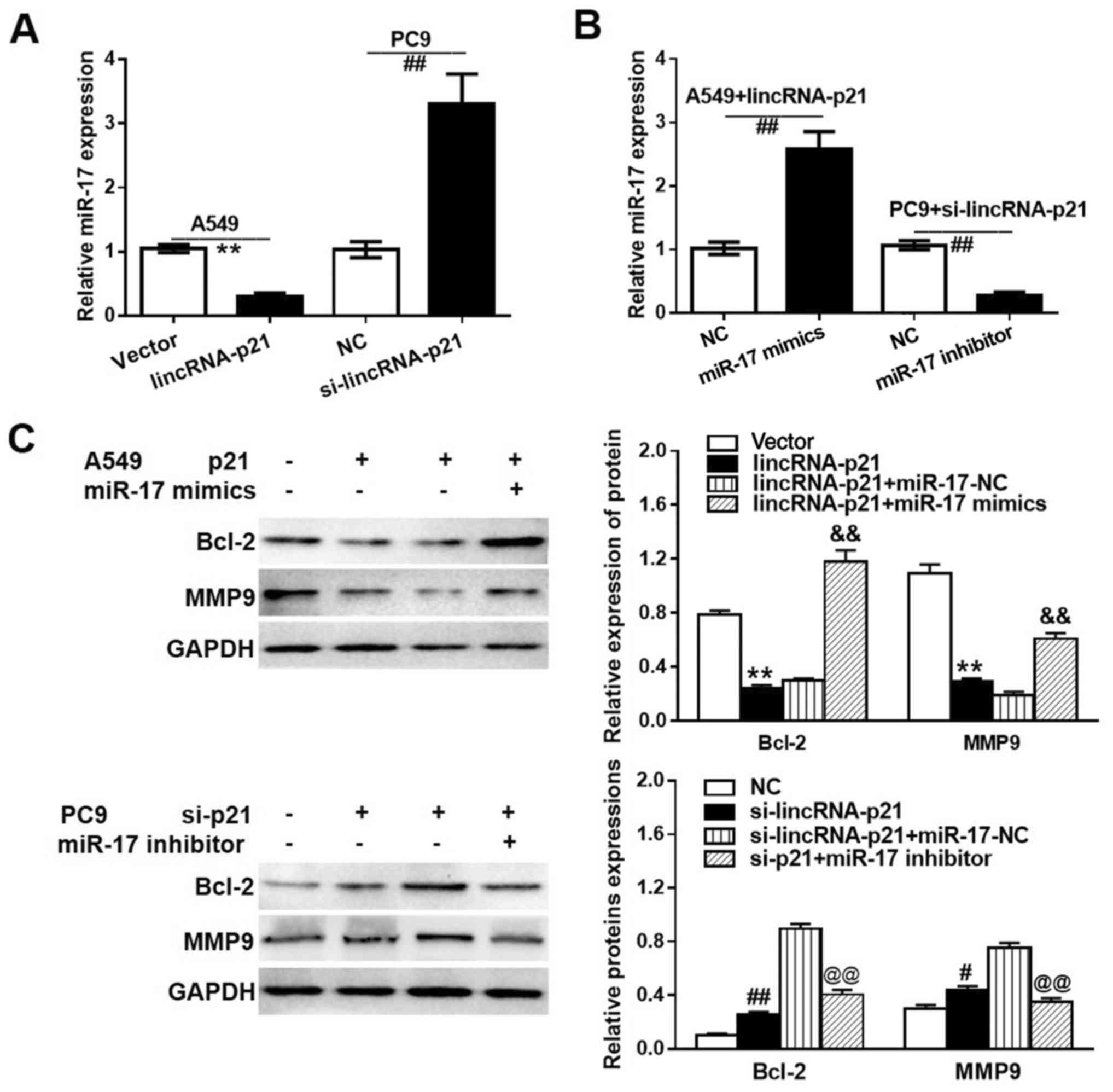
expression levels of lincRNA-p21 and miR-17-5p revealed that
lincRNA-p21 was able to regulate the expression of miR-17-5p in
lung tumor cells. Fig. 4A and B
illustrate the effect of lincRNA-p21 on miR-17-5p expression. It
was identified that cells with overexpressed lincRNA-p21 (A549
lincRNA-p21 group) had a significantly lower miR-17-5p expression
level compared with the low lincRNA-p21 expression cells
(A549-vector group), and cells with downregulated lincRNA-p21
expression (PC9 si-lincRNA-p21 group) had a significantly higher
miR-17-5p expression level compared with the control group cells
(PC9-NC group). The aforementioned observations were further
confirmed by the relative miR-17-5p expression results in the A549
lincRNA-p21 and PC9 si-lincRNA-p21 groups following the addition of
a miR-17-5p mimic and inhibitor, respectively. The western blot
analysis results in Fig. 4C
demonstrate that the overexpressed lincRNA-p21 significantly
suppressed the expression levels of Bcl-2 and MMP9 compared with
the control group, and following the addition of miR-17-5p mimics,
the expression levels of Bcl-2 and MMP9 were partially recovered,
whereby the expression levels of Bcl-2 and MMP9 were upregulated.
Furthermore, the knockdown of si-lincRNA-p21 resulted in a
significant increase in Bcl-2 and MMP9 levels compared with the NC
group, whereas the addition of miR-17-5p inhibitors caused a
reduction in the expression levels of Bcl-2 and MMP9 compared with
that in the si-lincRNA-p21+miR-17-5p NC group. These results
indicated that lincRNA-p21 affected lung cancer cells through
altering the expression of Bcl-2 and MMP9.
lincRNA-p21 affects the proliferation
and migration of lung cancer cells via regulating miR-17-5p
The CCK-8 assay results for the A549 lincRNA-p21
group, PC9 si-lincRNA-p21 group and following the addition of a
miR-17-5p mimic and inhibitor, respectively are presented in
Fig. 5A. Treatment of the A549
lincRNA-p21-transfected cells with miR-17 mimic significantly
reduced the cell proliferation rate compared with the NC group.
Additionally, treatment of the PC9 si-lincRNA-p21 group with
miR-17-5p inhibitors significantly reduced the proliferation of
cells compared with the corresponding NC group. The results from
cell apoptosis analysis demonstrated that the miR-17-5p mimics
significantly suppressed cell apoptosis in
lincRNA-p21-overexpressed A549 cells, whereas the miR-17-5p
inhibitor partially recovered the apoptosis rate in the PC9 cell
line with si-lincRNA-p21 transfection. The Transwell assay results
presented in Fig. 5C confirmed that
miR-17-5p significantly accelerated cell migration, while
inhibiting miR-17-5p suppressed cell migration, which were opposing
to the actions of lincRNA-p21.
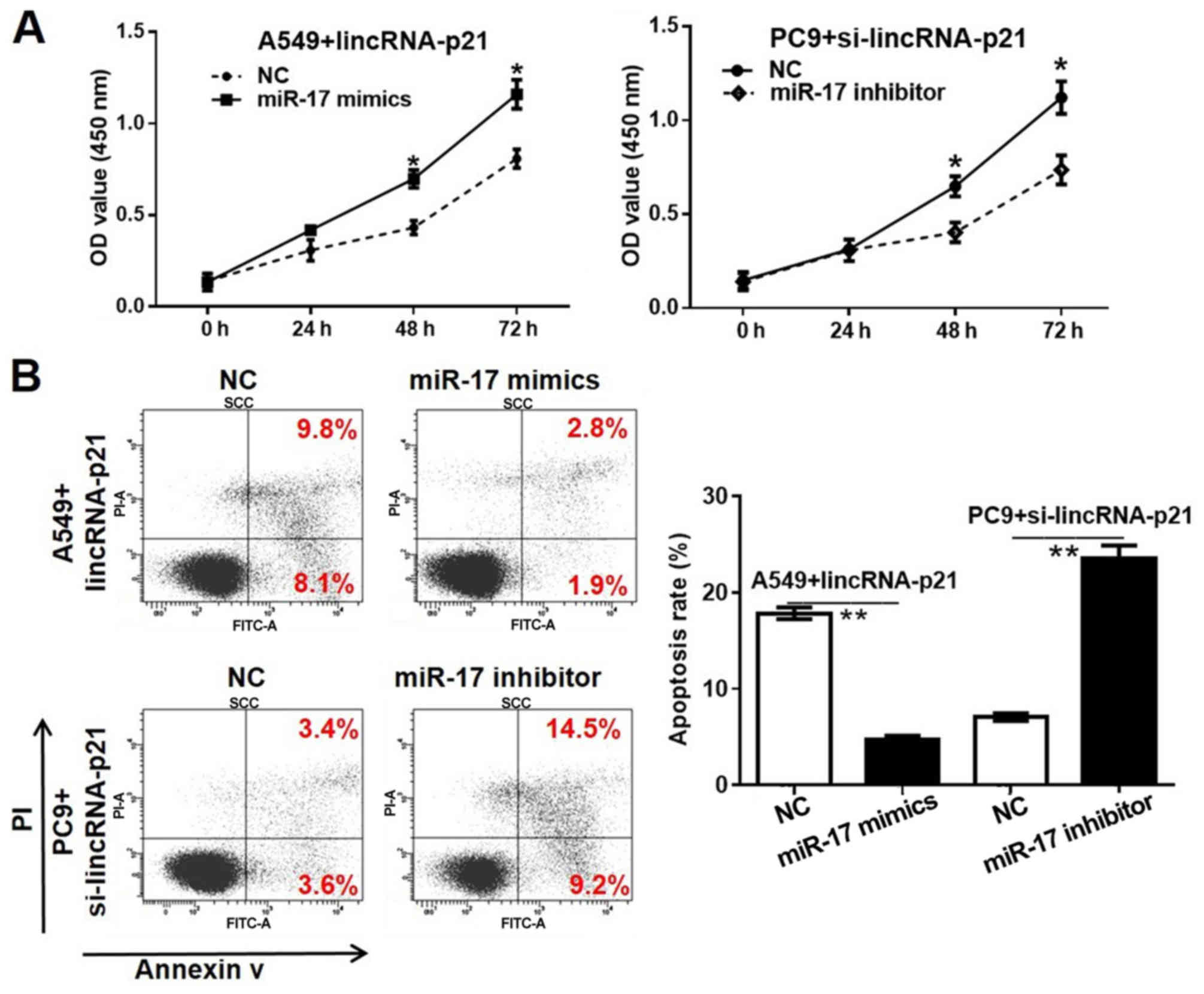 | Figure 5.Effects of miR-17-5p on lung cancer
cell proliferation, apoptosis and migration. (A) lincRNA-p21
overexpression plasmid- or lincRNA-p21 siRNA-transfected lung
cancer cells were treated miR-17-5p mimics or inhibitor for 72 h,
and cell proliferation was detected using a Cell Counting Kit-8
assay. (B) lincRNA-p21 overexpression plasmid- or lincRNA-p21
siRNA-transfected lung cancer cells were treated miR-17-5p mimics
or inhibitor for 72 h, and the cells apoptosis were detected by
flow cytometric analysis. (C) lincRNA-p21 overexpression plasmid-
or lincRNA-p21 siRNA-transfected lung cancer cells were treated
miR-17-5p mimics or inhibitor for 24 h, and the cells were
subjected to Transwell analysis (magnification, ×200). *P<0.05
and **P<0.01 vs. NC group. lincRNA, long intergenic non-coding
RNA; siRNA/si, small interfering RNA; NC, negative control; miR,
microRNA; PI, propidium iodide; OD, optical density. |
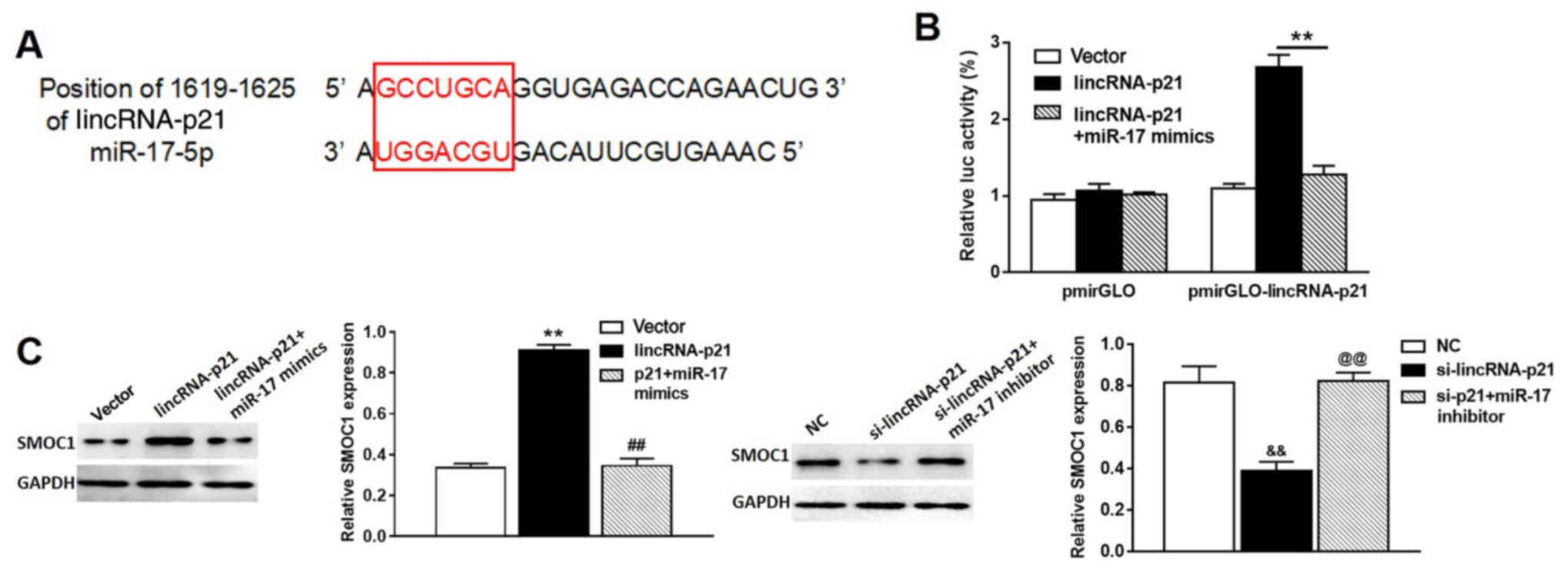
miR-17-5p is a direct gene target of
lincRNA-p21
Bioinformatics analysis (miRDB: http://www.mirdb.org/; starBase v2.0; http://starbase.sysu.edu.cn/) was first used to
generate a lincRNA-p21 recognition sequence and a putative target
site for miR-17-5p was revealed (Fig.
6A). To verify whether miR-17-5p was a direct gene target of
lincRNA-p21, pmirGLO was used to construct a lincRNA-p21 luciferase
reporter with binding site for miR-17-5p (pmirGLO-lincRNA-p21). As
shown in Fig. 6B, lincRNA-p21
transfection caused a significant increase in luciferase activity
in the pmirGLO-lincRNA-p21 group compared with the pmirGLO control
group, and the addition of miR-17-5p mimics reverse this effect.
Furthermore, western blot analysis showed that SMOC1 protein, the
target gene of miR-17, was significantly increased in A549 cells
treated with pcDNA3.1-lincRNA-p21, compared with that in A549 cells
treated with pcDNA3.1 vector only, while miR-17 mimics were able to
rescue the enhanced expression of SMOC1 triggered by lincRNA-p21
overexpression (Fig. 6C, left
panel). Consistently, SMOC1 protein was significantly inhibited in
A549 cells treated with si-lincRNA-p21, compared with that in A549
cells treated with siRNA control, whereas miR-17 inhibitor was able
to rescue the reduced expression of SMOC1 induced by knockdown of
lincRNA-p21 (Fig. 6C, right
panel).
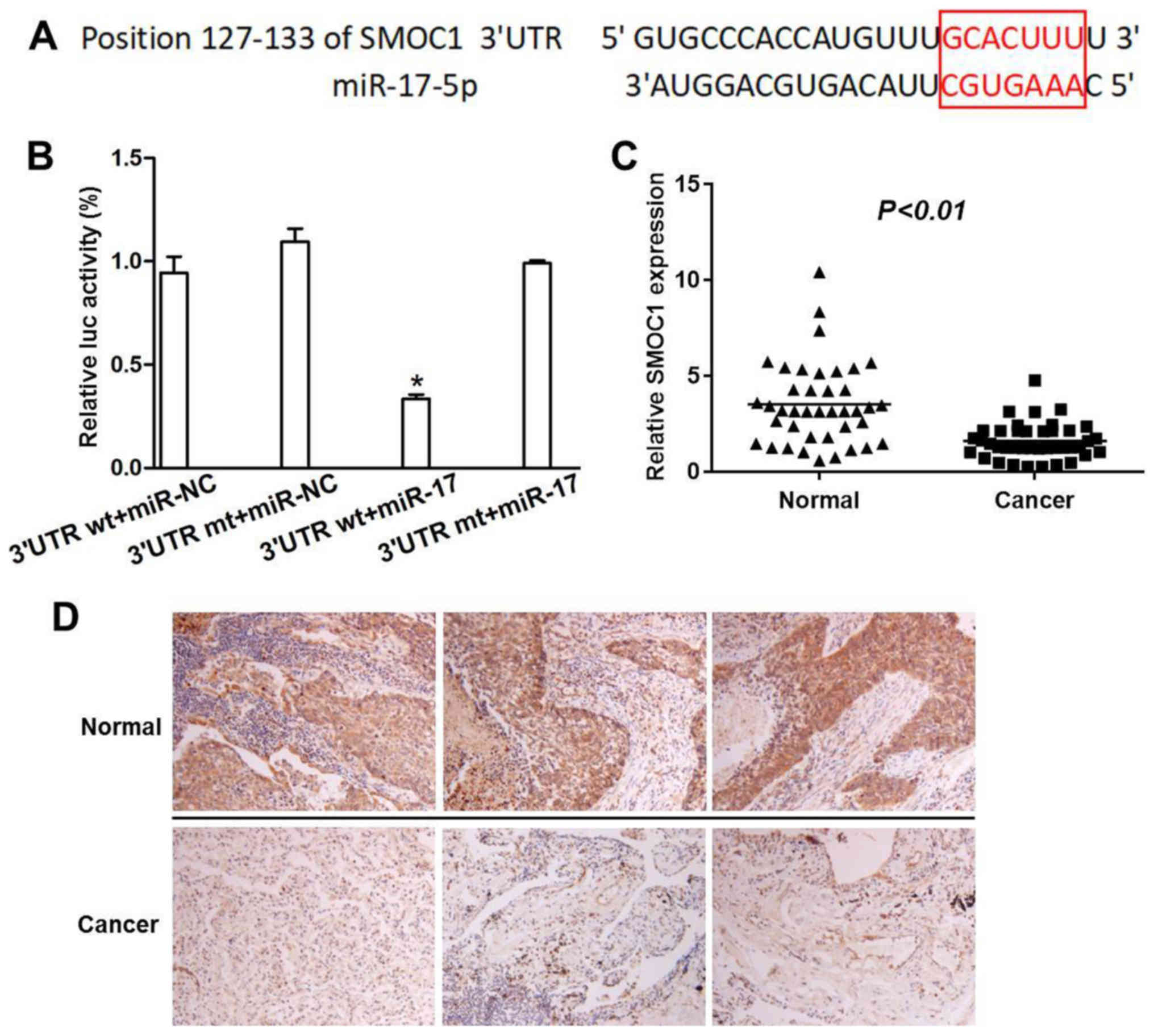
SMOC1 is a direct gene target of
miR-17-5p
As presented in Fig.
7A, the bioinformatics analysis results identified the
recognition sequence position 127–133 of SMOC1 for miR-17-5p. The
dual-luciferase reporter analysis results provided evidence that
SMOC1 was a direct gene target for miR-17-5p. As illustrated in
Fig. 7B, the relative luciferase
activity in the wild type SMOC1 3′untranslated region (UTR) group
was significantly suppressed by miR-17-5p, while no significant
difference was observed in the relative luciferase activity in the
mutant type SMOC1 3′UTR group following miR-17-5p treatment
compared with the wild and mutant SMOC1 3′UTR NC groups.
Furthermore, SMOC1 expression was significantly reduced in NSCLC
tumor tissues compared with adjacent normal tissues as demonstrated
by RT-qPCR (P<0.01; Fig. 7C).
This result was further validated using immunohistochemical
staining of NSCLC tumor tissues and adjacent normal tissues, which
demonstrated a substantial abundance of SMOC1 expression in
adjacent normal tissues, compared with NSCLC tumor tissues
(Fig. 7D). All these results
suggest that miR-17-5p can directly target the binding sites on
SMOC1 in NSCLC tumor.
lincRNA-p21 inhibits lung tumor growth
in vivo
Further investigation of the contribution of
lincRNA-p21 to lung tumor growth was performed in vivo. Nude
mice were administered with A549 stably transfected with a vector
or lincRNA-p21, or lincRNA-p21 together with miR-17-5p mimics.
Subsequently, tumor growth was monitored over time for a total of 4
weeks. The tumor volume (Fig. 8A and
B) and tumor weight (Fig. 8C)
in the lincRNA-p21 treatment group were significantly decreased
compared with the vector group, and the tumor treated with
lincRNA-p21 miR-17-5p mimics were smaller compared with the vector
group; however, notably larger compared with the lincRNA-p21
treatment group. Furthermore, the expression of lincRNA-p21 was
significantly increased in the lincRNA-p21 group, compared with the
vector control group (P<0.01; Fig.
8D). Consistently, the expression level of miR-17 was
significantly inhibited in the lincRNA-p21 group, compared with the
vector control group (Fig. 8E). In
addition, the expression of SMOC1 protein was remarkably higher in
tumor tissues isolated from the lincRNA-p21 group, compared with
the vector control group. Nevertheless, miR-17 mimics were able to
reverse the elevation in SMOC1 expression induced by lincRNA-p21
overexpression in the tumor tissue (Fig. 8F). These results demonstrated that
lincRNA-p21 inhibited lung tumor growth via regulating SMOC1 in
vivo.
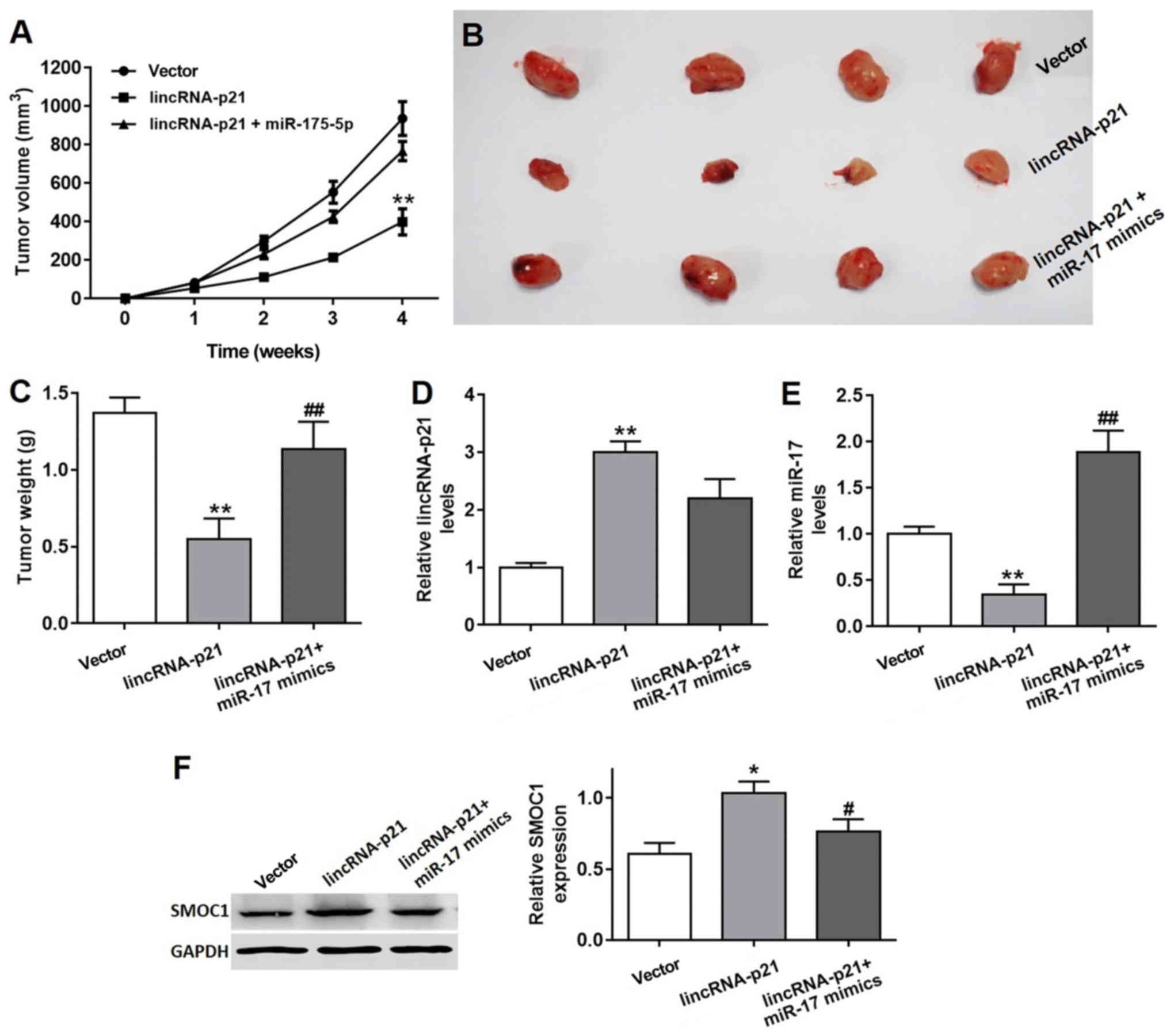 | Figure 8.Inhibitory effect of lincRNA-p21 on
lung tumor growth in vivo. (A) Tumor volumes of different
groups were monitored over 4 weeks. **P<0.01 vs. vector group.
(B) Tumor tissues from vector control, lincRNA-p21 treated,
lincRNA-p21 and miR-17-5p treated groups were isolated and (C)
weighed. The relative levels of (D) lincRNA-p21 and (E) miR-17 in
tumors isolated from the vector, lincRNA-p21 and lincRNA-p21+miR-17
mimics groups, as demonstrated by RT-qPCR. **P<0.01 vs. vector
control group, ##P<0.01 vs. lincRNA-p21 group. (F)
The SMOC1 protein expression in tumors isolated from the vector,
lincRNA-p21 and lincRNA-p21+miR-17 mimics groups, as demonstrated
by RT-qPCR. *P<0.05 vs. vector control group,
#P<0.05 vs. lincRNA-p21 group. RT-qPCR, reverse
transcription quantitative polymerase chain reaction; lincRNA, long
intergenic non-coding RNA; siRNA/si, small interfering RNA; NC,
negative control; miR, microRNA; SMOC1, SPARC-related modular
calcium binding 1. |
Discussion
At present, NSCLC remains difficult to treat with a
5-year survival rate <15%, which is notably lower compared with
that of other common cancer types, for example breast and prostate
cancer (12). The principal cause
of mortality is tumor invasion and metastasis, which are signs of
disease progression and the principal cause of treatment failure.
Although a number of studies on tumor invasion and metastasis have
been performed, the exact mechanism remains to be elucidated
(14,15). Previous studies have reported that
the development of lung cancer and malignant metastasis is
influenced by genetic factors and small non-coding RNA, in which
lncRNAs serve an important role (16–18).
lincRNA-p21 has been recently reported to be
associated with pulmonary fibrosis in acute respiratory distress
syndrome (19), and is
significantly increased in hepatocytes during liver fibrosis
(20). However, an evident decrease
in lincRNA-p21 in human fibrotic liver and cirrhotic liver was also
reported by a recent study (21),
indicating the elusive deregulation of lincRNA-p21 in disease. In
the present study, an evident reduction in the expression of
lincRNA-p21 was identified in NSCLC tumor tissues compared with
that in healthy tissues, and the survival percentage of patients in
various clinical stages was significantly higher in lincRNA-p21
overexpression cases compared with patients with low lincRNA-p21
expression. In addition, the inhibitory effect of lincRNA-p21 on
lung tumor was verified by investigations on different human lung
cancer cells. The proliferation and migration of
lincRNA-p21-overexpressed cells were significantly inhibited, and
conversely, were promoted in lincRNA-p21 knockdown cells compared
with the vector group. Furthermore, the overexpression of
lincRNA-p21 was observed to significantly increase the rate of lung
cancer cell apoptosis, while the knockdown of lincRNA-p21 inhibited
the apoptotic rate compared with the vector group. From the results
of the present study, it is evident that lincRNA-p21 effectively
inhibited the progression of lung cancer.
A previous study reported that under different
conditions, the regulation of lincRNA-p21 is associated with
various signaling pathways (22).
Studies have demonstrated that lincRNA-p21 regulates the
proliferation and apoptosis of various cell types, for example
vascular smooth muscle cells and keratinocytes, by mediating p53
activity (23–25). It has also been reported that
lincRNA-p21 may inhibit the activity of β-catenin signaling to
attenuate the tumorigenicity of colorectal cancer stem cells
(26). Furthermore, a recent study
reported that lincRNA-p21 inhibited the Wnt/β-catenin signaling
pathway by regulating the miR-17-5p level in activated hepatic
stellate cells (27). miR-17-5p is
reported to be one of most essential miRNAs in lung cancer cells,
and the regulation of miR-17-5p contributes to cell proliferation
and invasion in NSCLC (28–30). The present study investigated the
association between lincRNA-p21 and miR-17-5p expression in NSCLC
tumor cells to explore the possible regulating mechanism. An
evident negative association between lincRNA-p21 and miR-17-5p
expression levels was observed. The miR-17-5p expression level was
significantly reduced in lincRNA-p21-overexpressed cells, and
significantly increased in lincRNA-p21 knockdown cells; however,
these effects were reversed following the addition of a miR-17-5p
mimic or inhibitor.
The present study also demonstrated that the
inhibitory effect of overexpressed lincRNA-p21 on lung cancer cell
proliferation and migration was partially counteracted by treatment
with miR-17-5p mimics, and following the addition of miR-17-5p
inhibitors the inhibitory effect of lincRNA-p21 was partially
reversed in the lincRNA-p21 knockdown group. The upregulated cell
apoptotic rate caused by lincRNA-p21 overexpression was suppressed
by miR-17-5p mimics and the reduced cell apoptotic rate in
lincRNA-p21 knockout cells was increased by miR-17-5p inhibitors.
Based on the observed negative association between lincRNA-p21 and
miR-17-5p, further studies to verify the direct binding sites for
miR-17-5p on lincRNA-p21 are required. The bioinformatics results
predicted that miR-17-5p may be a direct target for lincRNA-p21 and
the luciferase reporter analysis confirmed that lincRNA-p21 was
able to bind with miR-17-5p directly leading to a mutual inhibiting
effect. In addition, in vivo studies verified that
lincRNA-p21 significantly inhibited lung tumor growth. The
mechanism of lincRNA-p21 deregulation in various diseases is
primarily imprecise; however, the present study provides, to the
best of our knowledge, the first evidence for an inhibitory effect
of lincRNA-p21 in the progression of NSCLC via direct targeting of
miR-17-5p. The underlying mechanism for the regulation of
lincRNA-p21 towards lung cancer cells is suggested to be associated
with miR-17-5p associated signaling pathways. However, there is a
possibility that other mechanisms may coexist, thus further
researches are required for clarification.
In conclusion, the dysregulated expression of
lincRNA-p21 in NSCLC tumor tissues was observed, and the functional
role in NSCLC tumor cell progression and the underlying signaling
pathway have been investigated in the present study. It was
demonstrated that lincRNA-p21 is downregulated in NSCLC tumor
tissues, and its overexpression has an inhibitory effect on lung
cancer cell proliferation and migration while its knockdown
accelerates NSCLC cell migration. Furthermore, the inhibitory
effect of overexpressed lincRNA-p21 on NSCLC progression was
partially counteracted by miR-17-5p mimics. A negative association
between the expression levels of lincRNA-p21 and miR-17-5p was
observed, and the results demonstrated that miR-17-5p is a direct
target of lincRNA-p21. In conclusion, the present study revealed
that lincRNA-p21 inhibits the progression of NSCLC via targeting a
miR-17-5p associated signaling pathway; this provides a cornerstone
for future researches regarding the pathology and therapeutic
methods of NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the General guide
project of Guangzhou health and family planning science and
technology project (grant no. 20181A011099).
Availability of data and materials
All data sets used in this study are available from
the corresponding author on reasonable request.
Authors' contributions
MJ and GC designed the study. MJ, XA, JZ, HL and HX
performed the experiments and analyzed the data. HX was the major
contributor in developing the first draft of this manuscript. GC
reviewed and approved the final draft of the manuscript prior to
submission. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval for the study was provided by the
Clinical Research and Ethics Committee at Guangdong General
Hospital.
Patient consent for publication
All patients provided written informed consent for
the publication of all associated data in this study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung carcinoma
|
|
lincRNA
|
long intergenic non-coding RNA
|
|
RT-qPCR
|
reverse transcription quantitative
polymerase chain reaction
|
|
siRNA/si
|
small interfering RNA
|
|
NC
|
negative control
|
|
PI
|
propidium iodide
|
|
miR
|
microRNA
|
|
Bcl-2
|
B-cell lymphoma-2
|
|
MMP9
|
matrix metalloproteinase
|
|
SMOC1
|
SPARC-related modular calcium binding
1
|
|
UTR
|
untranslated region
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gridelli C, Rossi A, Carbone DP, Guarize
J, Karachaliou N, Mok T, Petrella F, Spaggiari L and Rosell R:
Non-small-cell lung cancer. Nat Rev Dis Primers. 1:150092015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
D'Addario G, Früh M, Reck M, Baumann P,
Klepetko W and Felip E: ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 21 Suppl
5:v116–v119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giovannetti E, Toffalorio F, De PT and
Peters GJ: Pharmacogenetics of conventional chemotherapy in
non-small-cell lung cancer: A changing landscape? Pharmacogenomics.
13:1073–1086. 2015. View Article : Google Scholar
|
|
5
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Engreitz JM, Ollikainen N and Guttman M:
Long non-coding RNAs: Spatial amplifiers that control nuclear
structure and gene expression. Nat Rev Mol Cell Biol. 17:756–770.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi P and Du X: The long non-coding RNAs, a
new cancer diagnostic and therapeutic gold mine. Mod Pathol.
26:155–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang G, Li Z, Zhao Q, Zhu Y, Zhao C, Li X,
Ma Z, Li X and Zhang Y: LincRNA-p21 enhances the sensitivity of
radiotherapy for human colorectal cancer by targeting the
Wnt/β-catenin signaling pathway. Oncol Rep. 31:1839–1845. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia M, Jiang L, Wang YD, Huang JZ, Yu M
and Xue HZ: LincRNA-p21 inhibits invasion and metastasis of
hepatocellular carcinoma through Notch signaling-induced
epithelial-mesenchymal transition. Hepatol Res. 46:1137–1144. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding G, Peng Z, Shang J, Kang Y, Ning H
and Mao C: LincRNA-p21 inhibits invasion and metastasis of
hepatocellular carcinoma through miR-9/E-cadherin cascade signaling
pathway molecular mechanism. OncoTargets Ther. 10:3241–3247. 2017.
View Article : Google Scholar
|
|
12
|
Castellano JJ, Navarro A, Viñolas N,
Marrades RM, Moises J, Cordeiro A, Saco A, Muñoz C, Fuster D,
Molins L, et al: LincRNA-p21 impacts prognosis in resected
non-small cell lung cancer patients through angiogenesis
regulation. J Thorac Oncol. 11:2173–2182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Li Y, Li YW, Zhang HB, Gong H,
Yuan Y, Li WT, Liu HY and Chen J: HOTAIR lncRNA SNPs rs920778 and
rs1899663 are associated with smoking, male gender, and squamous
cell carcinoma in a Chinese lung cancer population. Acta Pharmacol
Sin. Aug 28–2018.(Epub ahead of print): View Article : Google Scholar
|
|
14
|
Fang L, Wu S, Zhu X, Cai J, Wu J, He Z,
Liu L, Zeng M, Song E, Li J, et al: MYEOV functions as an amplified
competing endogenous RNA in promoting metastasis by activating
TGF-β pathway in NSCLC. Oncogene. Sep 4–2018.(Epub ahead of
print) View Article : Google Scholar
|
|
15
|
Matsukuma S, Kono T, Takeo H, Hamakawa Y
and Sato K: Tumor-to-tumor metastasis from lung cancer: A
clinicopathological postmortem study. Virchows Arch. 463:525–534.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei L, Wu T, He P, Zhang JL and Wu W:
LncRNA ATB promotes the proliferation and metastasis of lung cancer
via activation of the p38 signaling pathway. Oncol Lett.
16:3907–3912. 2018.PubMed/NCBI
|
|
17
|
Qi L, Liu F, Zhang F, Zhang S, Lv L, Bi Y
and Yu Y: lncRNA NEAT1 competes against let-7a to contribute to
non-small cell lung cancer proliferation and metastasis. Biomed
Pharmacother. 103:1507–1515. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao Y, Cheng S, Xiang J and Luo C: lncRNA
CCHE1 increased proliferation, metastasis and invasion of non-small
lung cancer cells and predicted poor survival in non-small lung
cancer patients. Eur Rev Med Pharmacol Sci. 22:1686–1692.
2018.PubMed/NCBI
|
|
19
|
Zhou WQ, Wang P, Shao QP and Wang J:
Lipopolysaccharide promotes pulmonary fibrosis in acute respiratory
distress syndrome (ARDS) via lincRNA-p21 induced inhibition of
Thy-1 expression. Mol Cell Biochem. 419:19–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tu X, Zhang Y, Zheng X, Deng J, Li H, Kang
Z, Cao Z, Huang Z, Ding Z, Dong L, et al: TGF-β-induced hepatocyte
lincRNA-p21 contributes to liver fibrosis in mice. Sci Rep.
7:29572017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng J, Dong P, Mao Y, Chen S, Wu X, Li
G, Lu Z and Yu F: lincRNA-p21 inhibits hepatic stellate cell
activation and liver fibrogenesis via p21. FEBS J. 282:4810–4821.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang F, Zhang H, Mei Y and Wu M:
Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the
Warburg effect. Mol Cell. 53:88–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen
C, Cai Y, Huang H, Yang Y, Liu Y, et al: lincRNA-p21 regulates
neointima formation, vascular smooth muscle cell proliferation,
apoptosis and atherosclerosis by enhancing p53 activity.
Circulation. 130:1452–1465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hall J, Messenger ZJ, Tam HW, Phillips SL,
Recio L and Smart RC: Long non-coding RNA lincRNA-p21 is the major
mediator of UVB-induced and p53-dependent apoptosis in
keratinocytes. Cell Death Dis. 6:e17002016. View Article : Google Scholar
|
|
25
|
Bao X, Wu H, Zhu X, Guo X, Hutchins AP,
Luo Z, Song H, Chen Y, Lai K, Yin M, et al: The p53-induced
lincRNA-p21 derails somatic cell reprogramming by sustaining
H3K9me3 and CpG methylation at pluripotency gene promoters. Cell
Res. 25:80–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Lei ZJ, Guo Y, Wang T, Qin ZY,
Xiao HL, Fan LL, Chen DF, Bian XW, Liu J and Wang B:
miRNA-regulated delivery of lincRNA-p21 suppresses β-catenin
signaling and tumorigenicity of colorectal cancer stem cells.
Oncotarget. 6:37852–37870. 2015.PubMed/NCBI
|
|
27
|
Yu F, Guo Y, Chen B, Shi L, Dong P, Zhou M
and Zheng J: LincRNA-p21 inhibits the Wnt/β-catenin pathway in
activated hepatic stellate cells via sponging MicroRNA-17-5p. Cell
Physiol Biochem. 41:1970–1980. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang G, An X and Zhao H, Zhang Q and Zhao
H: Long non-coding RNA HNF1A-AS1 promotes cell proliferation and
invasion via regulating miR-17-5p in non-small cell lung cancer.
Biomed Pharmacother. 98:594–599. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chatterjee A, Chattopadhyay D and
Chakrabarti G: miR-17-5p downregulation contributes to paclitaxel
resistance of lung cancer cells through altering beclin1
expression. PLoS One. 9:e957162014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Lin J, Wang P and Sun J:
miR-17-5p down-regulation contributes to erlotinib resistance in
non-small cell lung cancer cells. J Drug Target. 25:125–131. 2017.
View Article : Google Scholar : PubMed/NCBI
|