Introduction
Lung cancer (LC) is one of the world's most
widespread cancers. More than 1.5 million people are diagnosed with
LC every year (1,2). Lung adenocarcinoma (LUAD) is the most
common histological subtype of LC (3,4). The
5-year survival rate for LUAD patients is less than 15%, as most
are diagnosed at advanced stages (5). Therefore, it is urgent to determine
the molecular mechanism of LUAD and identify an effective method
for early diagnosis and effective treatment.
MicroRNAs (miRNAs) are endogenous, non-coding, small
RNAs. They control gene expression by combining with the messenger
RNAs (mRNAs) of target genes, causing mRNA degradation or
translation suppression (6,7). Numerous studies have demonstrated that
the aberrant and disordered expression of miRNAs is involved in
many malignant tumors, including LCs (8–12).
Previous research has confirmed that miRNAs can be
divided into three different forms, as follows: primary
(pri-)miRNA, precursor (pre-)miRNA and mature miRNA. The mature
miRNAs designated as miRNA-3p and miRNA-5p are derived from the 3′
or 5′ arms, respectively, of their pre-miRNAs (13). Theoretically, therefore, all
pre-miRNAs can produce two types of mature miRNAs. Previous
research assumed that only one mature miRNA took part in regulating
target mRNAs; the other was thought to be a byproduct, and it was
regarded as functionally irrelevant (14). Increasingly, however, recent
investigations have confirmed that both miRNA-3p and miRNA-5p
originate from one pre-miRNA and can degrade various target mRNAs
(15). Studies also have found a
correlation and synergistic effects between miRNA-3p and −5p
produced by the same pre-miRNA (16). These findings suggest that miRNA-3p
and −5p both function during biological processes, implying that
they may co-regulate target genes in various diseases.
miRNA-126-3p and miRNA-126-5p (miRNA-126-3p/5p) are
both derived from pre-miRNA-126, which is located on human
chromosome 9. miRNA-126-3p and −5p have been identified as
essential biological factors in the development of certain
malignancies (17). Regarding LC,
studies with small sample sizes have confirmed that miRNA-126-3p is
downregulated in non-small cell lung cancers (NSCLCs), including
LUAD (18). Nevertheless, previous
studies concerning LC have mainly focused on miRNA-126-3p; no
research has demonstrated a relationship between miRNA-126-5p and
LC. It is also worth noting that LUAD research has not investigated
or clarified whether a correlation or synergistic effects between
miRNA-126-3p and −5p exist. Based on previous research, the authors
predicted that both miRNA-126-3p and −5p may be less expressed in
LUAD and may co-regulate key target genes of LUAD.
The present study aimed to explore the clinical
utility and investigate the correlation and synergistic effects of
miRNA-126-3p and miRNA-126-3p in LUAD. A total of 202 tissues (101
LUAD tissues and 101 adjacent normal lung tissues) were collected.
Real-time quantitative polymerase chain reaction (RT-qPCR) was
performed to explore the respective expression values of
miRNA-126-3p and −5p in the 202 tissues. A large sample size from
the Gene Expression Omnibus (GEO) database was obtained so that a
meta-analysis could be performed with the RT-qPCR data from the
present study to further identify miRNA-126-3p and −5p expression
in LUAD. In addition, 12 prediction software programs were employed
to obtain the target genes of miRNA-126-3p and −5p. Then,
bioinformatic analysis was conducted to identify the potential
molecular mechanisms of miRNA-126-3p and −5p in LUAD.
This is the first study to investigate the
co-regulation of miRNA-125-3p and −5p in LUAD. It is hoped that the
present study may demonstrate the molecular function and
synergistic effects of miRNA-126-3p and miRNA-126-3p. The obtained
research findings may reveal the importance of these miRNAs in LUAD
diagnosis and treatment.
Materials and methods
Clinical LUAD sample collection
Based on a previous study (19), 202 formalin-fixed, paraffin-embedded
(FFPE) tissues were collected at the First Affiliated Hospital of
Guangxi Medical University (Nanning, China). Of these 202 FFPE
tissues, 101 were LUAD tissues, while the remaining 101 tissues
were paired adjacent normal lung tissues. The research protocol for
this study has been ratified by the Ethics Committee of the First
Affiliated Hospital of Guangxi Medical University. Consent from all
patients was obtained at the time of the sample collection.
RT-qPCR
RT-qPCR is a sensitive technique for quantifying
specific RNA targets. RNA was removed from the FFPE sample tissues
using a miRNeasy FFPE kit (Qiagen, Venlo, The Netherlands) as
previously described (20,21). A NanoDrop 2000 spectrophotometer
(NanoDrop Technologies; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) was used to ensure the purity and concentration of the
extracted RNA from the FFPE tissues. The specific primers of
miRNA-126-3p and miRNA-126-5p were provided by TaqMan MicroRNA
Assays (4427975-000468; Applied Biosystems, Life Technologies
Europe B.V., Gent, Belgium). The reverse primers were included in
TaqMan MicroRNA Reverse Transcription kit (4366596; Applied
Biosystems, Life Technologies Europe B.V) (22). RNU6B was used as the internal
control. The forward primer sequences of RNU6B were
5′-CTCGCTTCGGCAGCACA-3′ and the reverse primer sequences were
5′-AACGCTTCACGAATTTGCGT-3′. The sequences of miRNA-126-3p were
5′-UCGUACCGUGAGUAAUAAUGCG-3′ and the sequences of miRNA-126-5p were
5′-CAUUAUUACUUUUGGUACGCG-3′. The thermocycling conditions were as
follows: denaturation at 95°C for 10 min, followed by 40 cycles of
95°C for 15 sec and 60°C for 60 sec. The RT-qPCR was analyzed with
Applied Biosystems PCR 7900 (Applied Biosystems; Thermo Fisher
Scientific, Inc.) to detect miRNA expression values. Relative
expression values were calculated using the 2−∆Cq method
(23).
GEO data extraction
GEO (https://www.ncbi.nlm.nih.gov/gds/) is the largest
fully disclosed high-throughput molecular abundance database; it is
mainly used to store gene expression data. All microarray datasets
related to LUAD were filtered out and downloaded from the GEO
database. The microarray dataset selection criteria were as
follows: i) the samples had to be human tissue; ii) the dataset had
to contain LUAD and non-cancerous lung tissue groups; iii) both the
LUAD and non-cancerous lung tissue groups had to include at least
two samples; and iv) expression values of miRNA-126-3p or −5p had
to be available.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
version 22.0 (IBM Corp., Armonk, NY, USA) software was applied to
analyze the RT-qPCR data. A quantitative variable was computed and
presented as the means ± standard deviation (SD). Student's t-test
was applied to evaluate the difference between two continuous
variables. A P-value of <0.05 was considered to indicate a
statistically significant result. A receiver operating
characteristic (ROC) curve based on the RT-qPCR data was applied to
estimate the respective distinguishing impact of miRNA-126-3p and
−5p on LUAD from non-cancerous tissues. Binary logistic regression
and ROC curve analyses were performed for evaluating the combined
distinguishing value of miRNA-126-3p and −5p (24,25).
Stata statistical software (version 12.0; Stata Corp., College
Station, TX, USA) was used to perform a meta-analysis of the GEO
data; the results were assessed using the standard mean difference
(SMD). The heterogeneity of the GEO data was assessed by
I2 statistics and a Q test. An I2 >50% or
a P-value <0.05 was deemed to indicate huge heterogeneity. A
summary ROC (SROC) analysis based on the selected microarray
datasets as well as the results from the present study was
performed to determine the potential distinguishing effect of
miRNA-126-3p and −5p on LUAD.
Predicting miRNA-126-3p and- 5p
targets
Twelve target gene prediction software programs
(TargetScan, miRWalk, Microt4, miRDB, miRanda, miRBridge, miRMap,
miRNAMap, PITA, PicTar2, RNA22 and RNAhybrid) (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/miRretsys-self.html)
were run to obtain the miRNA-126-3p and −5p target genes. Only
genes that appeared in more than two of the prediction programs
were selected as candidate targets. At the same time, an R function
package was used to screen all the overexpressed genes in LUAD from
The Cancer Genome Atlas (TCGA) database (https://cancergenome.nih.gov/). Fold change (FC) was
used to assess the level of gene expression in the TCGA data, and
genes were deemed to be overexpressed if the FC >2. Then, the
predicted target genes were overlapped with the overexpressed genes
to obtain the miRNA-126-3p and −5p target genes in LUAD.
Bioinformatic analysis
The Database for Annotation, Visualization, and
Integrated Discovery (DAVID) version 6.8 (https://david.ncifcrf.gov/) was employed for Gene
Ontology (GO) analysis, and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway analysis was used to analyze the function of the
above-identified genes in LUAD. A protein-protein interaction (PPI)
network based on the target genes of miRNA-126-3p and −5p was
established using the Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING) database (http://www.string-db.org/) and Cytoscape version 3.4.0
(26) was used to determine the
correlation between each target.
Results
Expression of miRNA-126-3p and
miRNA-126-5p in LUAD tissues according to RT-qPCR
Co-downregulation of the expression of
both miRNA-126-3p and miRNA-126-5p in LUAD
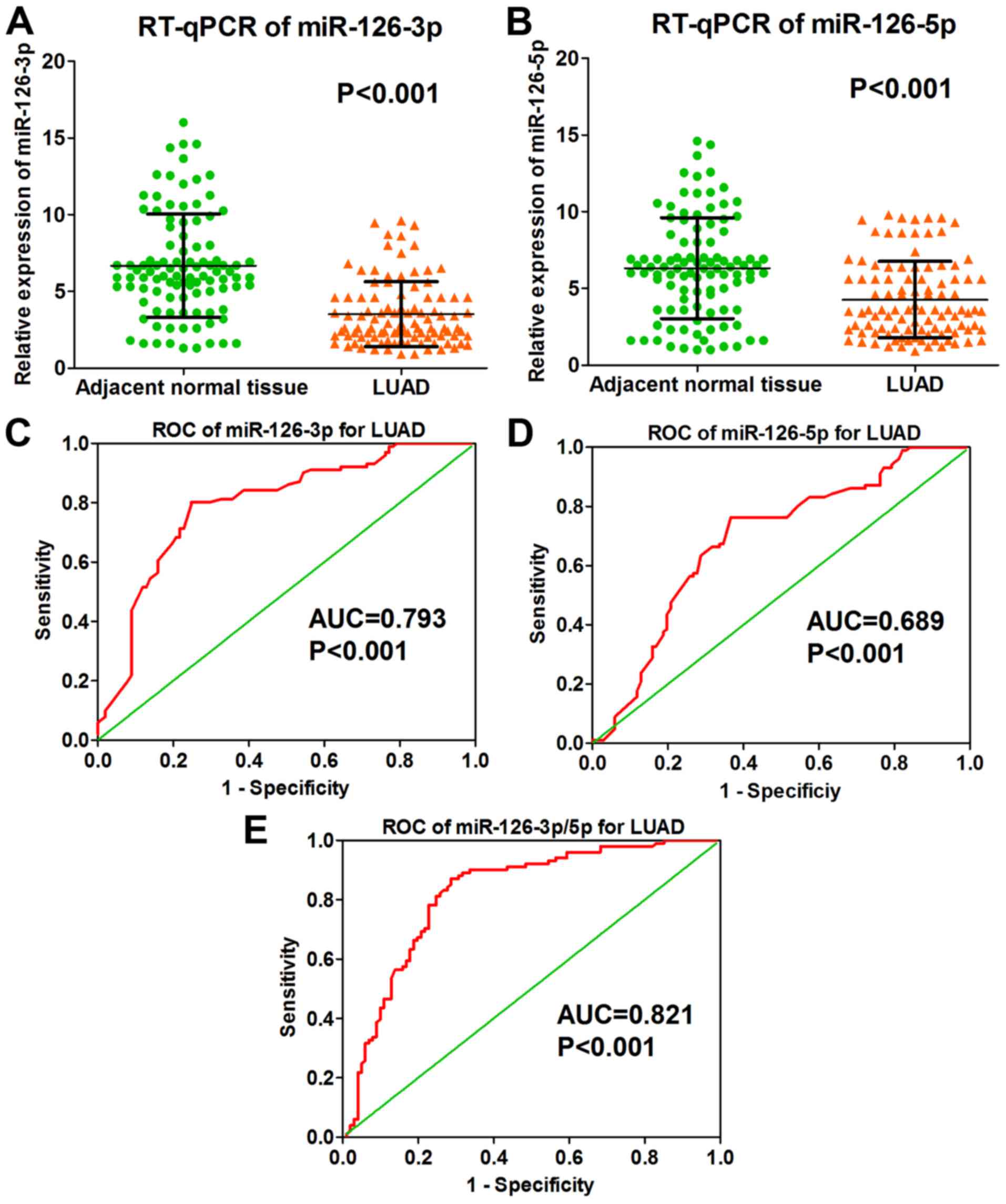
The results of the RT-qPCR performed on the 202
collected clinical samples showed that the level of miRNA-126-3p
was markedly lower in LUAD tissue than it was in adjacent normal
lung tissue (3.511±2.118 vs. 6.674±3.362, P<0.001; Fig. 1A and Table I); miRNA-126-5p expression was also
significantly lower in the LUAD tissues (4.271±2.501 vs.
6.314±3.289, P<0.001; Fig. 1B
and Table I). According to the
RT-qPCR data, the ROC curve of miRNA-126-3p in LUAD tissues showed
that the area under the curve (AUC) was 0.793 (P<0.001; Fig. 1C); the ROC curve of miRNA-126-5p in
LUAD tissues showed an AUC of 0.689 (P<0.001; Fig. 1D). The combined ROC curve of
miRNA-126-3p and −5p in LUAD tissues revealed an AUC of 0.821
(P<0.001; Fig. 1E).
 | Table I.Expression of miRNA-126-3p and
miRNA-126-5p in LUAD according to RT-qPCR data. |
Table I.
Expression of miRNA-126-3p and
miRNA-126-5p in LUAD according to RT-qPCR data.
|
|
| miRNA-126-3p | miRNA-126-5p |
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | N | Mean ± SD | t | P-value | Mean ± SD | t | P-value |
|---|
| Tissue |
|
LUAD | 101 | 3.511±2.118 | −8.003 | <0.001 | 4.271±2.501 | −4.970 | <0.001 |
|
Adjacent normal lung
tissue | 101 | 6.674±3.362 |
|
| 6.314±3.289 |
|
|
| Sex |
|
Male | 56 | 3.619±2.157 | 0.572 | 0.569 | 4.487±2.359 | 0.975 | 0.332 |
|
Female | 45 | 3.376±2.083 |
|
| 4.000±2.670 |
|
|
| Age (years) |
|
<60 | 41 | 3.549±1.897 | 0.149 | 0.881 | 4.194±2.081 |
|
|
|
≥60 | 60 | 3.484±2.271 |
|
| 4.323±2.535 |
|
|
| Smoking
history |
| + | 18 | 4.306±1.949 | 1.930 | 0.060 | 4.689±2.439 | −0.602 | 0.500 |
| − | 26 | 3.192±1.833 |
|
| 5.141±2.451 |
|
|
| Tumor size
(cm) |
| ≤3 | 53 | 3.373±2.250 | −0.685 | 0.495 | 4.450±2.665 | 0.755 | 0.452 |
|
>3 | 48 | 3.663±1.974 |
|
| 4.073±2.319 |
|
|
| Vascular
invasion |
| + | 31 | 3.074±1.697 | −1.384 | 0.169 | 3.045±1.544 | −4.195 | <0.001 |
| − | 70 | 3.704±2.263 |
|
| 4.814±2.657 |
|
|
| LNM |
| + | 56 | 3.161±1.679 | −1.798 | 0.076 | 3.671±2.090 | −3.197 | 0.002 |
| − | 45 | 3.946±2.514 |
|
| 5.142±2.713 |
|
|
| TNM stage |
| I +
II | 44 | 4.140±2.583 | 2.542 | 0.013 | 4.923±2.700 | 2.296 | 0.024 |
| III +
IV | 57 | 3.025±1.527 |
|
| 3.769±2.233 |
|
|
| Pathological
grade |
| I | 17 | 3.482±2.323 | F=1.2 | 0.305 | 4.147±2.543 | F=1.3 | 0.271 |
| II | 61 | 3.735±2.087 |
|
| 4.565±2.629 |
|
|
|
III | 23 | 2.935±2.024 |
|
| 3.583±2.034 |
|
|
Association between miRNA-126-3p and
miRNA-126-5p and the clinicopathological parameters of the LUAD
samples
The RT-qPCR analysis determined that miRNA-126-3p
levels differed significantly according to TNM stage, while
miRNA-126-5p levels differed significantly in regards to vascular
invasion, lymph node metastasis (LNM) and TNM stage (Table I). Compared with early TNM stages
(I–II) of LUAD, miRNA-126-3p was expressed lower in later TNM
stages (III–IV) of LUAD (4.140±2.583 vs. 3.025±1.527, P<0.001;
Fig. 2A). The ROC of miRNA-126-3p
for the TNM stages showed that the AUC was 0.608 (P=0.039; Fig. 2B). As for miRNA-126-5p, its level
was significantly lower in the samples with vascular invasion
(3.045±1.544 vs. 4.814±2.657, P<0.001; Fig. 2C). The ROC curve of miRNA-126-5p for
vascular invasion showed that the AUC was 0.693 (P=0.002; Fig. 2D). In addition, miRNA-126-5p
expression was lower in samples diagnosed with lymph node
metastasis (LNM) (3.671±2.090 vs. 5.142±2.713, P=0.002; Fig. 2E) and the ROC curve relevant to LNM
presented an AUC of 0.672 (P=0.003; Fig. 2F). miRNA-126-5p expression was also
downregulated in later TNM stages (III–IV) of LUAD (4.923±2.700 vs.
3.769±2.233, P=0.024; Fig. 2G),
while the relative ROC curve demonstrated that the AUC was 0.625
(P=0.032; Fig. 2H). No significant
difference was observed between miRNA-126-3p and −5p and other
clinicopathological features (all P>0.05; Table I).
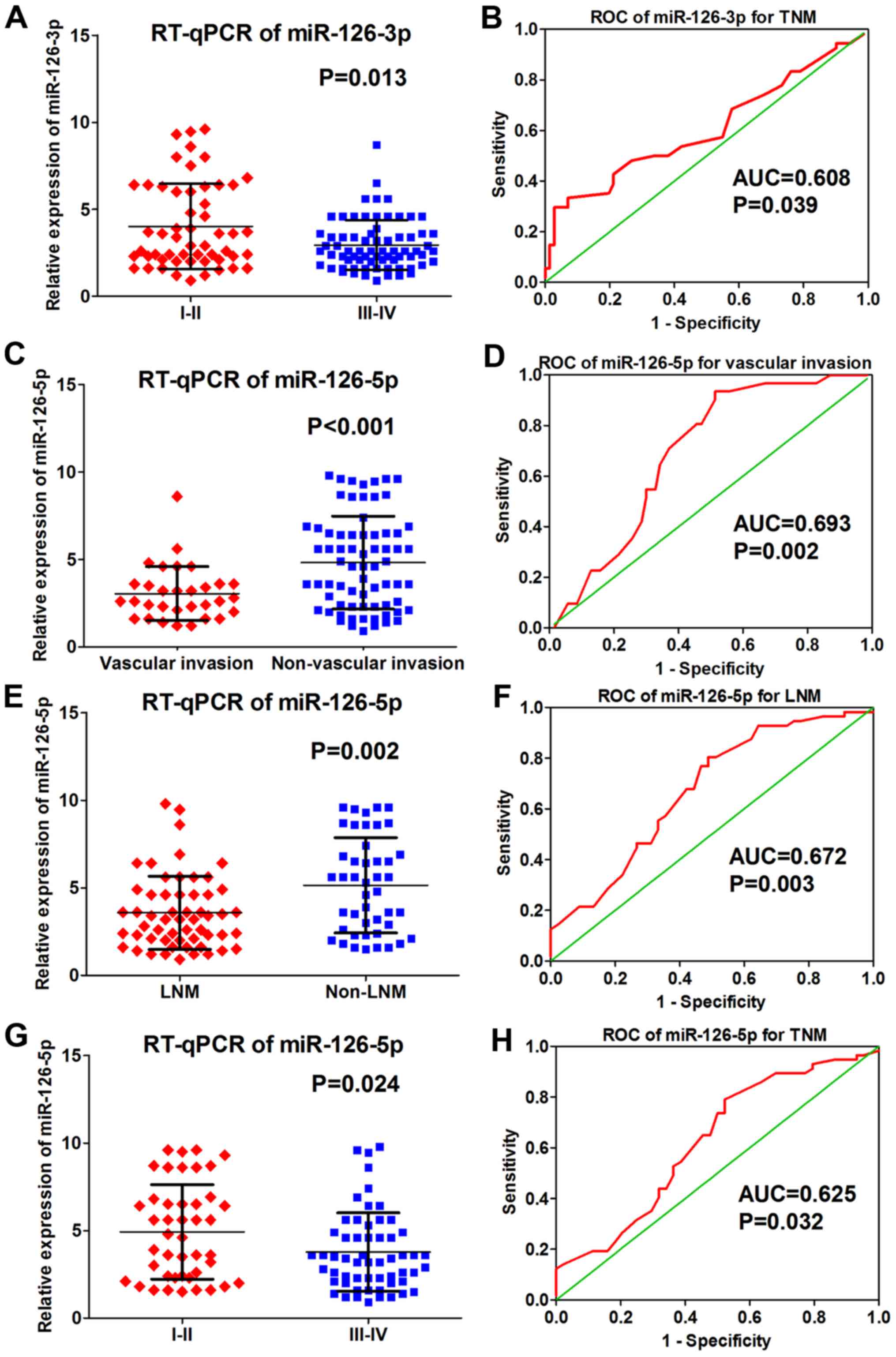 | Figure 2.Correlation between miRNA-126-3p and
miRNA-126-5p and clinical pathological parameters based on RT-qPCR
data. (A) Expression of miRNA-126-3p in early (I–II) and late
(III–IV) TNM stages of LUAD (4.140±2.583 vs. 3.025±1.527,
P<0.001). (B) ROC curve of miRNA-126-3p for TNM stages of LUAD
(AUC=0.608, P=0.039). (C) Expression of miRNA-126-5p in samples
with vascular invasion and samples without vascular invasion
(3.045±1.544 vs. 4.814±2.657, P<0.001). (D) ROC cure of
miRNA-126-5p for vascular invasion of LUAD (AUC=0.693, P=0.002).
(E) Expression of miRNA-126-5p in samples with LNM and samples
without LNM (3.671±2.090 vs. 5.142±2.713, P=0.002). (F) ROC of
miRNA-126-5p for LNM of LUAD (AUC=0.672, P=0.003). (G) Expression
of miRNA-126-5p in early TNM stage (I–II) and later TNM stage
(III–IV) of LUAD (4.923±2.700 vs. 3.769±2.233, P=0.024). (H) ROC of
miRNA-126-5p for TNM stage of LUAD (AUC=0.625, P=0.032). LUAD, lung
adenocarcinoma; AUC, area under the receiver operating
characteristic (ROC) curve. |
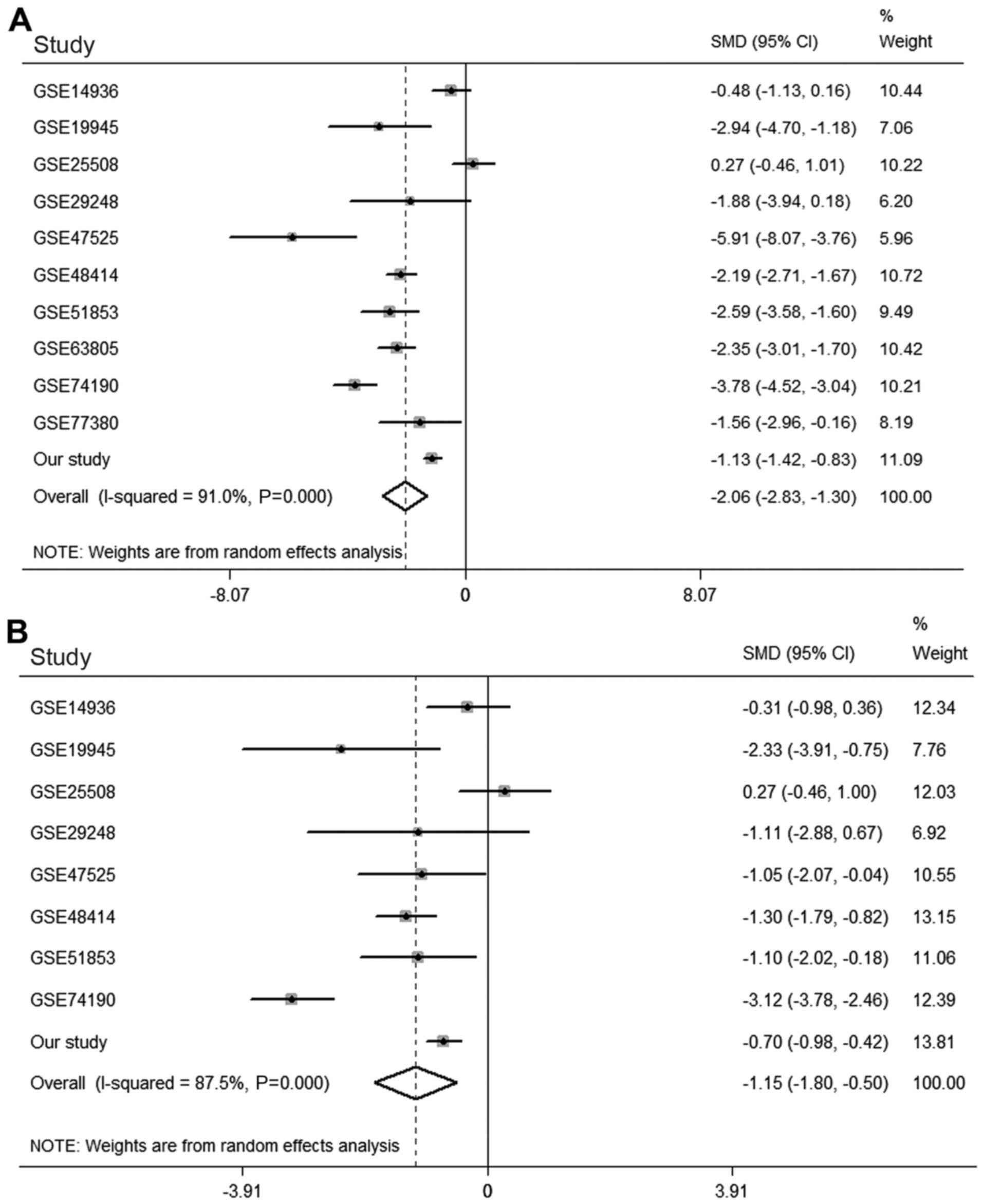
Meta-analysis of miRNA-126-3p and −5p
in LUAD based on the GEO databas
The ten relevant microarray datasets were the
following: GSE14936 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14936),
GSE19945 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE19945),
GSE25508 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE25508),
GSE29248 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE29248),
GSE47525 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE47525),
GSE48414 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE48414),
GSE51853 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE51853),
GSE63805 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE63805),
GSE74190 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE74190),
GSE77380 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE77380),
which contained the expression values of miRNA-126-3p in 388 LUAD
and 237 normal lung tissues, were identified and downloaded for
meta-analysis with the results from the present study (Table II) (27–35). A
random-effects model was used because major heterogeneity existed
(I2=91.0%, P<0.001). The meta-analysis demonstrated
that miRNA-126-3p expression was significantly lower in LUAD
tissues than it was in normal lung tissues [SMD=−2.063, 95%
confidence interval [CI]: −2.829 to −1.298, P<0.001; Fig. 3A].
 | Table II.The 10 selected microarray
datasets. |
Table II.
The 10 selected microarray
datasets.
| Authors | Microarray
datasets | Year | Country | LUAD tissues | Normal tissues | (Refs.) |
|---|
| Seike et
al | GSE14936 | 2009 | USA | 19 | 19 | (27) |
| Ohba et
al | GSE19945 | 2010 | Japan | 4 | 8 | Not published |
| Nymark et
al | GSE25508 | 2011 | Finland | 10 | 26 | (28) |
| Ma et
al | GSE29248 | 2011 | China | 3 | 3 | (29) |
| van Jaarsveld et
al | GSE47525 | 2014 | The
Netherlands | 6 | 14 | (30) |
| Bjaanaes et
al | GSE48414 | 2014 | Norway | 154 | 20 | (31) |
| Arima et
al | GSE51853 | 2014 | Japan | 76 | 5 | (32) |
| Robles et
al | GSE63805 | 2015 | USA | 32 | 30 | (33) |
| Jin et
al | GSE74190 | 2015 | China | 36 | 44 | (34) |
| Yoshimoto et
al | GSE77380 | 2018 | Japan | 3 | 12 | (35) |
Eight microarray datasets (GSE14936, GSE19945,
GSE25508, GSE29248, GSE47525, GSE48414, GSE51853 and GSE74190)
contained data concerning expression of miRNA-126-5p in 305 LUAD
and 139 normal lung tissues. As major heterogeneity also existed in
the meta-analysis of miRNA-126-5p (I2=87.5%,
P<0.001), a random-effects model was again performed. The
results showed that miRNA-126-5p also exhibited significantly low
expression in LUAD tissues (SMD=−1.152, 95% CI, −1.804 to −0.499,
P=0.001; Fig. 3B).
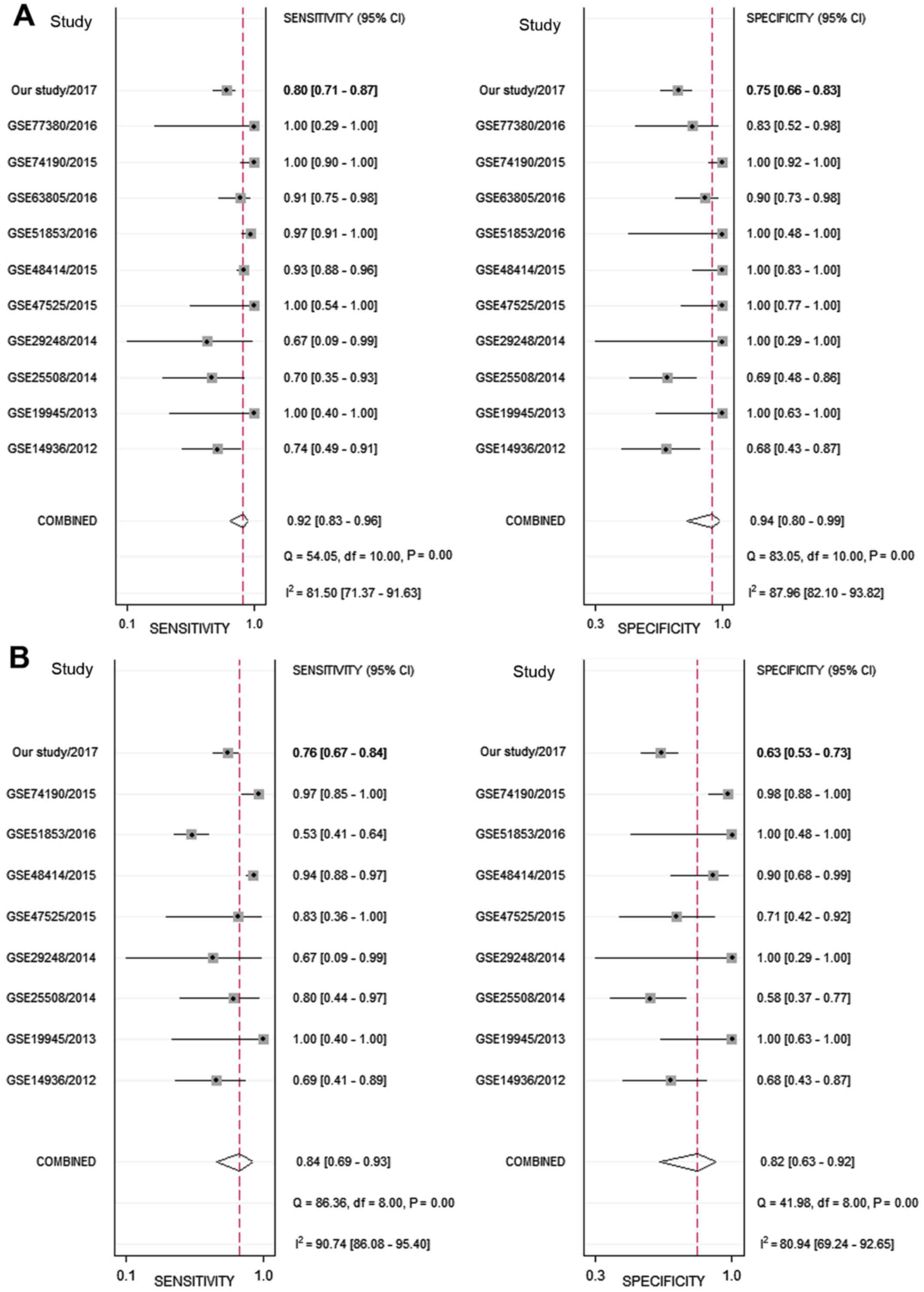
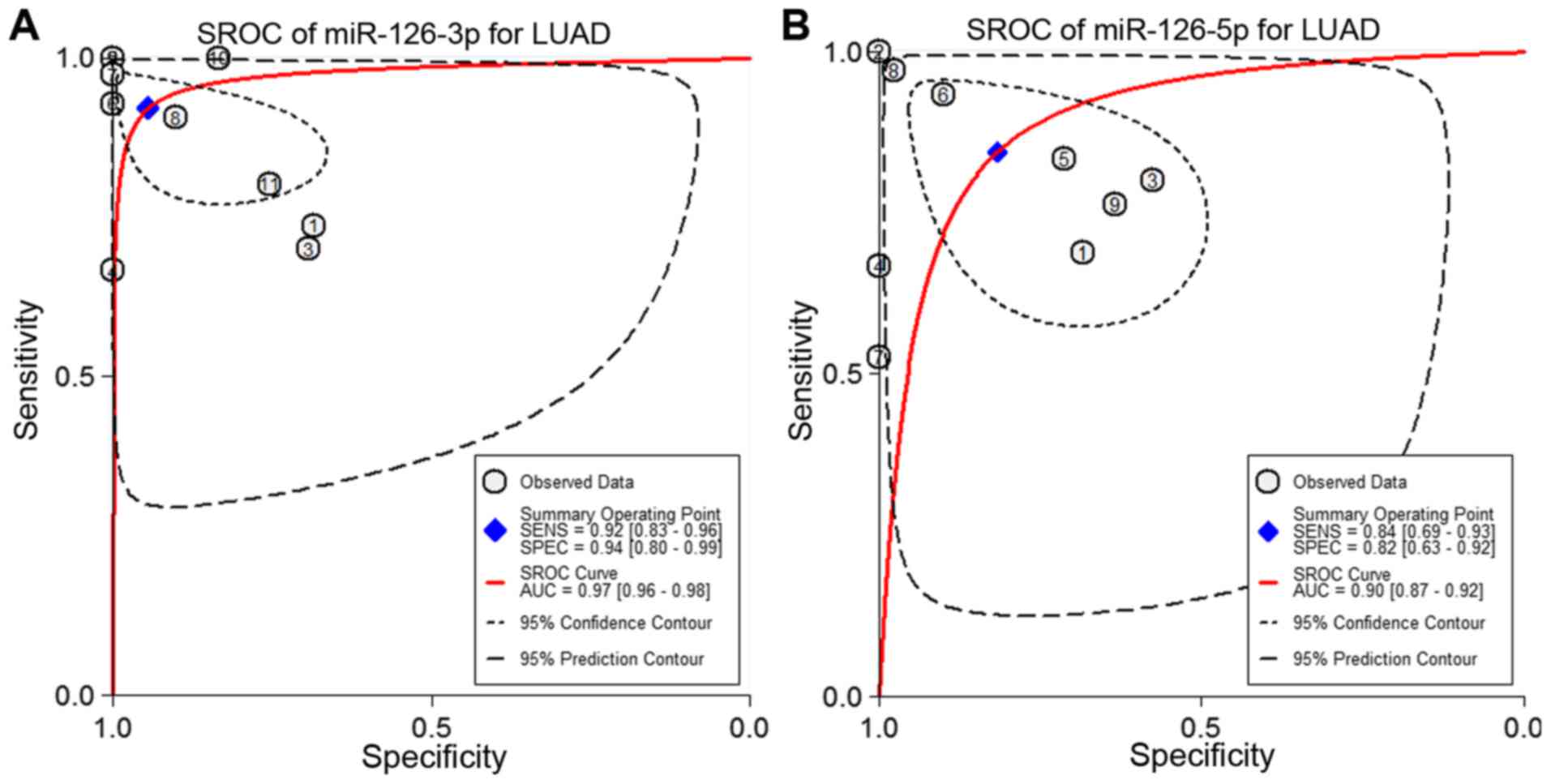
Next, the sensitivity and specificity of each
microarray dataset and the present study were extracted (Fig. 4). The SROC curves of miRNA-126-3p
and miRNA-126-5p, respectively, for LUAD tissues were performed
using the GEO data and the present study's RT-qPCR data. The SROC
of miRNA-126-3p showed an AUC of 0.97 (Fig. 5A), while the SROC of miRNA-126-5p
showed an AUC of 0.90 (Fig.
5B).
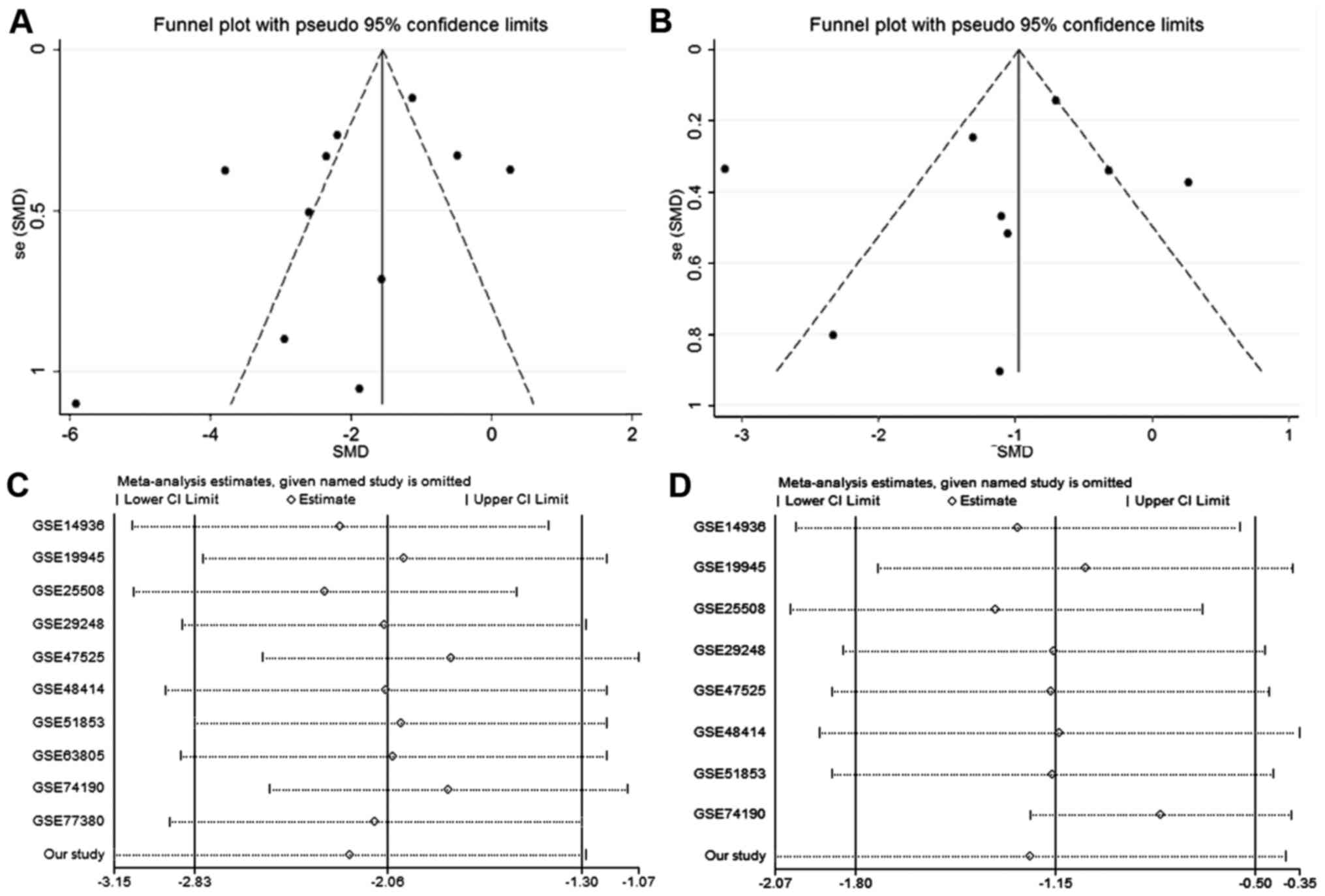
Funnel plots were employed to assess the publication
bias of the two meta-analyses (Fig. 6A
and B). The results of Begg's test (P=0.533 and P=0.533) and
Egger's test (P=0.592, P=0.307) showed no statistically significant
differences for either meta-analysis. Sensitivity analysis for
these two meta-analyses revealed that significantly lower
expression of miRNA-126-3p and miRNA-126-5p existed regardless of
which microarray datasets were removed (Fig. 6C and D).
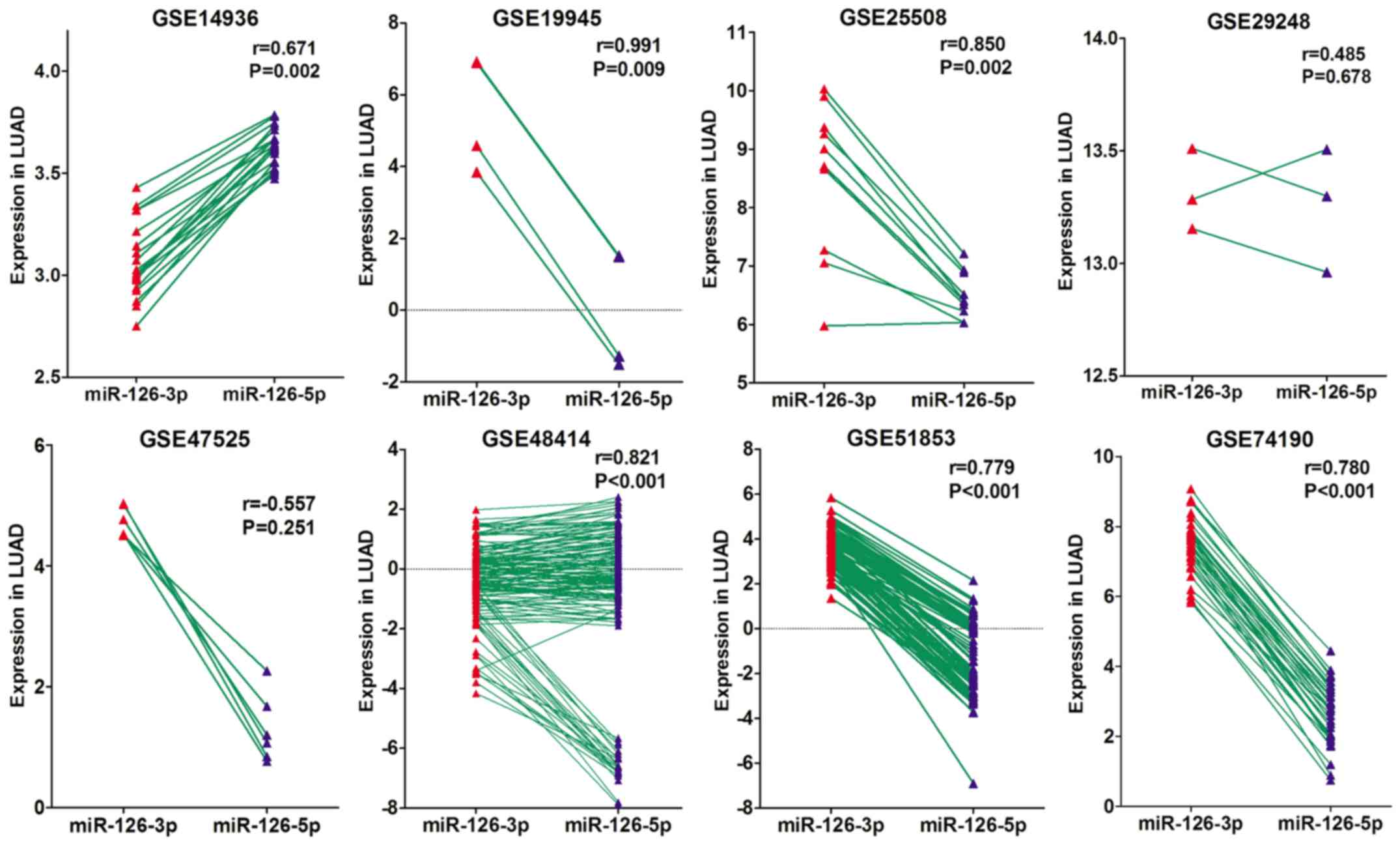
Correlation analysis between
miRNA-126-3p and miRNA- 126-5p
A Pearson correlation analysis was performed based
on the GEO data to assess the correlation between miRNA-126-3p and
miRNA-126-5p expression. Of the eight microarray datasets, six
showed significant positive correlations (r=0.671–0.991, all
P<0.05; Fig. 7), one suggested a
tendency toward a positive correlation (r=0.486; P=0.678) and one
suggested a trend toward a negative correlation (r=−0.557,
P=0.251).
Bioinformatic analysis based on the
target genes of miRNA-126-3p and miRNA-126-5p
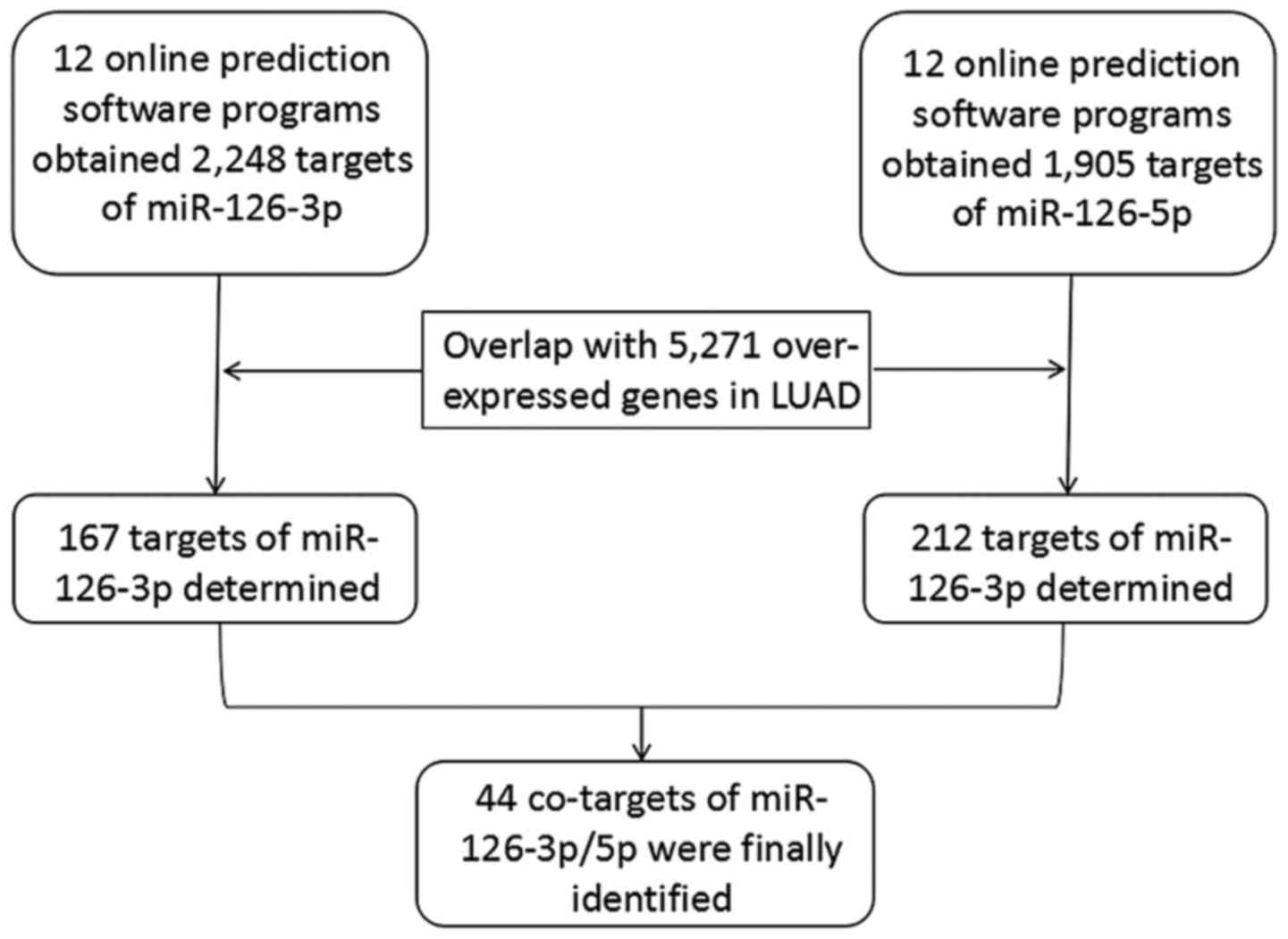
Prediction of the target genes
Twelve online prediction tools were employed for
acquiring the potential targets of miRNA-126-3p and −5p,
respectively. Initially, 2,248 genes and 1,905 genes were
separately collected as the target genes of miRNA-126-3p and −5p.
In addition, 5,271 upregulated genes in LUAD tissues were also
obtained from the TCGA database. After the upregulated genes were
overlapped with the 2,248 targets of miRNA-126-3p and the 1,905
targets of miRNA-126-5p, 167 genes and 212 genes, respectively,
were identified as the targets of miRNA-126-3p and −5p in LUAD.
Combining the 167 targets of miRNA-126-3p and 212 targets of
miRNA-126-5p produced 335 total target genes; 44 genes were
co-targets of both miRNA-126-3p and miRNA-126-5p (Fig. 8).
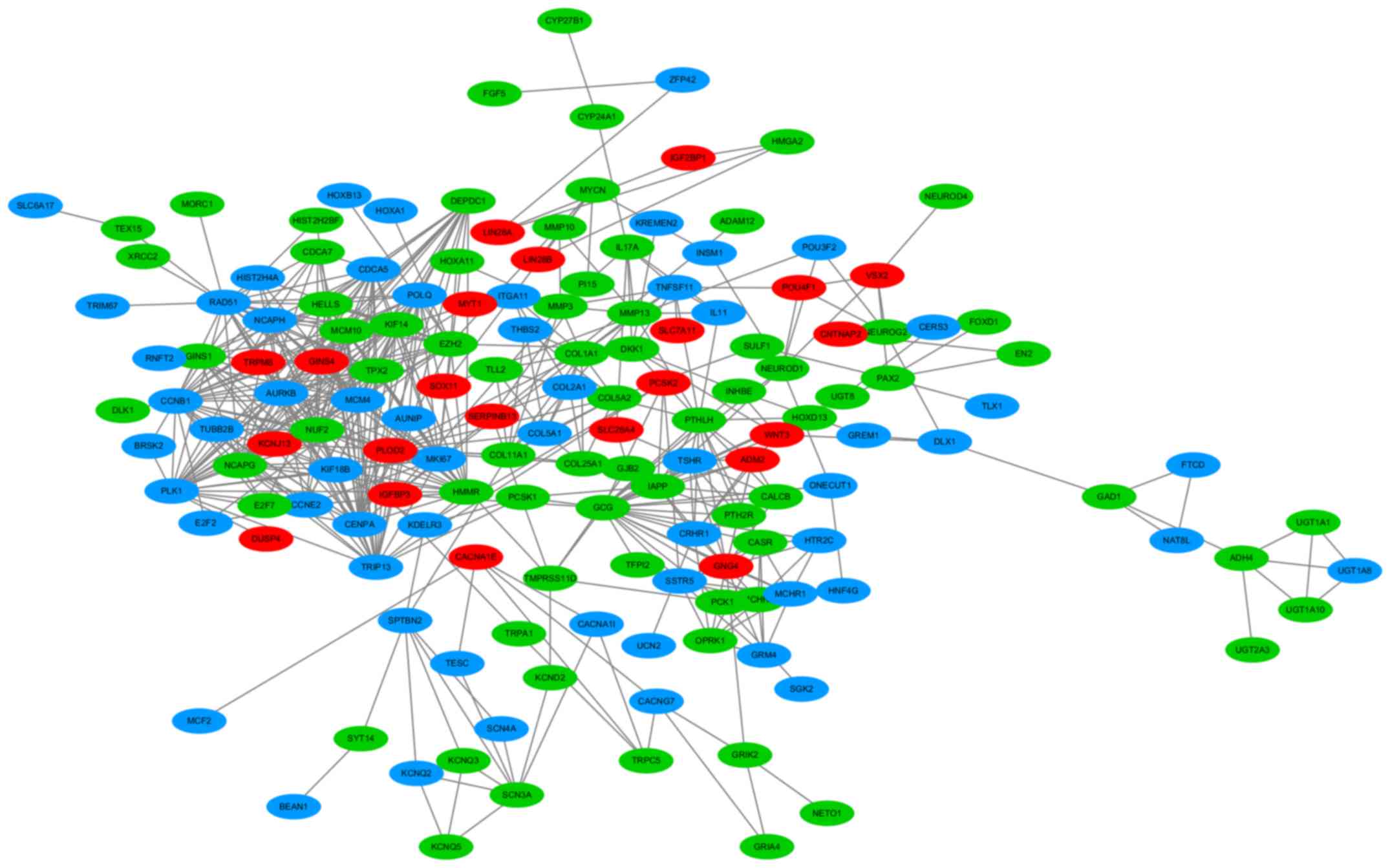
GO analysis, KEGG pathways and
PPI
Based on these 335 target genes, GO and KEGG pathway
analyses were conducted to determine their molecular biological
functions in LUAD. The results of the GO analysis indicated that
the targets were mainly enriched in cell-cell signaling of
biological processes (BPs), which are integral to the plasma
membrane of cell components (CCs) and channel activity of molecular
functions (MFs) (Table III). The
KEGG pathway analysis showed that target genes were significantly
enriched in neuroactive ligand-receptor interaction (Table IV). In addition, a PPI network of
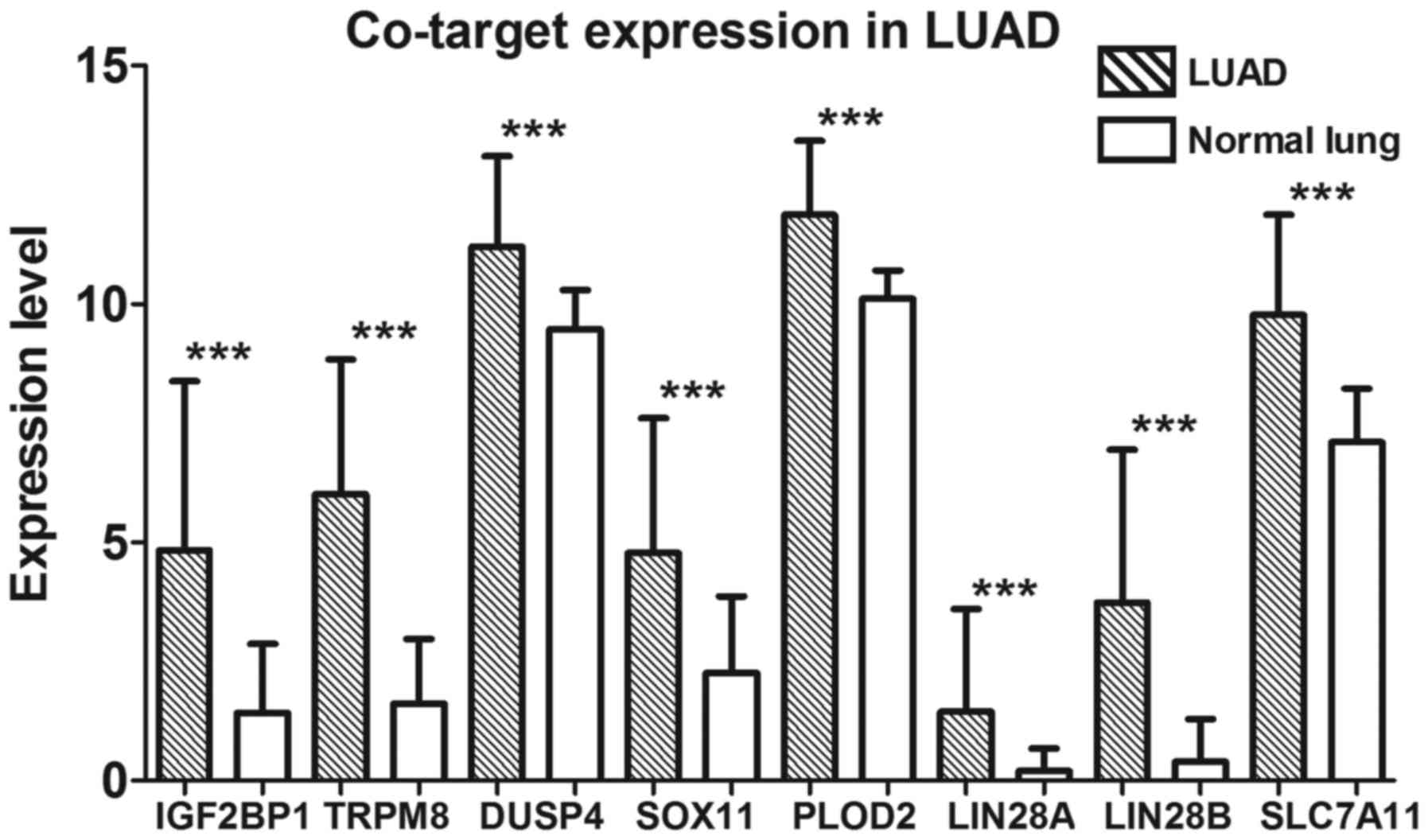
these 335 target genes was performed (Fig. 9). Eight crucial co-target genes
(IGF2BP1, TRPM8, DUSP4, SOX11, PLOD2, LIN28A, LIN28B and
SLC7A11) were selected for further analysis. All were
significantly highly expressed in LUAD (all P<0.001; Fig. 10).
 | Table III.GO functional annotation of the 335
target genes. |
Table III.
GO functional annotation of the 335
target genes.
| GO ID | Term | Count | P-value | Gene symbol |
|---|
| Biological
process |
| GO:0007267 | Cell-cell
signaling | 31 | 4.43E-07 | FGF5, GRIK2,
HOXA11, OPRK1, EFNA2, GLRA3, GREM1, IL11, PCSK1, KCNQ5, NPTX1,
BARX1, IL17A, and others |
| GO:0045165 | Cell fate
commitment | 13 | 8.31E-06 | ONECUT1, HOXA11,
ONECUT2, NEUROG2, PAX2, VSX2, NR2E1, DLX1, WNT3, NEUROD1, NEUROD4,
GAP43, TLX1 |
| GO:0060348 | Bone
development | 11 | 7.90E-05 | PTHLH, CYP24A1,
CASR, CYP27B1, TNFSF11, HOXA11, COL2A1, COL1A1, COL5A2, IGFBP3,
MMP13 |
| Molecular
function |
| GO:0005887 | Integral to plasma
membrane | 43 | 2.07E-05 | MCHR1, CASR,
SCN3A, GRIK2, ENPP3, OPRK1, GLRA3, ITGA11, PCDHA1, KCNJ13, KCNQ5,
UGT1A8, and others |
| GO:0034702 | Ion channel
complex | 15 | 2.35E-05 | KCND2, SCN3A,
GLRA3, CACNG7, CACNA1I, GABRA5, KCTD4, KCNJ13, KCNQ5, KCNQ3,
CHRNA5, and others |
| GO:0031226 | Intrinsic to plasma
membrane | 43 | 3.52E-05 | MCHR1, CASR,
SCN3A, GRIK2, ENPP3, OPRK1, GLRA3, ITGA11, PCDHA1, KCNJ13, KCNQ5,
UGT1A8, KCNQ3, and others |
| Cell component |
| GO:0015267 | Channel
activity | 23 | 7.61E-06 | KCND2, TRPM8,
SCN3A, TRPC5, GRIK2, GLRA3, CACNG7, GABRA5, TRPA1, CACNA1I, GRIA4,
CNGB3, KCTD4, and others |
| GO:0022803 | Passive
transmembrane transporter activity | 23 | 7.90E-06 | KCND2, TRPM8,
SCN3A, TRPC5, GRIK2, GLRA3, CACNG7, GABRA5, TRPA1, CACNA1I, GRIA4,
CNGB3, KCTD4, and others |
| GO:0005216 | Ion channel
activity | 22 | 9.34E-06 | KCND2, TRPM8,
SCN3A, TRPC5, GRIK2, GLRA3, CACNG7, GABRA5, TRPA1, CACNA1I, GRIA4,
CNGB3, KCTD4, and others |
 | Table IV.KEGG pathway analysis based on the
335 target genes. |
Table IV.
KEGG pathway analysis based on the
335 target genes.
| KEGG ID | Term | Count | P-value | Gene symbol |
|---|
| hsa04080 | Neuroactive
ligand-receptor interaction | 14 | 2.49E-04 | MCHR1, MCHR2,
PTH2R, GRIK2, GLRA3, OPRK1, GABRA5, GRIA4, CRHR1, SSTR5, GRM4,
HTR2C, TSHR, GABRP |
| hsa04512 | ECM-receptor
interaction | 8 | 4.23E-04 | ITGA11, COL2A1,
COL1A1, COL5A2, THBS2, COL11A1, COL5A1, HMMR |
| hsa04950 | Maturity onset
diabetes of the young | 4 | 7.69E-03 | ONECUT1, IAPP,
NEUROD1, HNF4G |
| hsa00140 | Steroid hormone
biosynthesis | 4 | 3.97E-02 | AKR1C2, UGT1A10,
UGT1A8, CYP21A2, UGT2A3, UGT1A1 |
| hsa00980 | Metabolism of
xenobiotics by cytochrome P450 | 4 | 7.60E-02 | AKR1C2, UGT1A10,
UGT1A8, ADH4, UGT2A3, UGT1A1 |
Discussion
In recent years, the effect of microRNAs (miRNAs) on
the pathogenesis of cancers has been well investigated and
clarified. However, even as the investigation of miRNAs has matured
and their functions have become clearer, the co-function of
miRNA-3p and −5p is rarely mentioned. The present study verified
the co-downregulation of the expression of miRNA-126-3p and
miRNA-126-5p in lung adenocarcinoma (LUAD) tissues using real-time
quantitative polymerase chain reaction (RT-qPCR) and the GEO
database. Bioinformatic analysis based on the obtained target genes
of miRNA-126-3p and −5p was performed to clarify their
co-regulation in LUAD.
The function of miRNA-126-3p has been articulated in
previous research. miRNA-126-3p levels have been shown to be
downregulated in various malignancies including colorectal,
esophageal, oral, liver, breast, thyroid and renal cancers
(36–42). In contrast, investigations of
miRNA-126-5p in malignant tumors have been rare and limited.
Several studies have reported that miRNA-126-5p is expressed at a
lower level in colorectal and breast cancers (43,44).
As for lung cancer (LC), several studies have reported that
miRNA-126-3p expression is downregulated in non-small cell lung
cancer (NSCLC) and small cell lung cancer (SCLC), suggesting that
it is an LC suppressor (45,46).
The targets of miRNA-126-3p have also been described in previous
studies. Liu and colleagues pointed out that miRNA-126-3p could
inhibit the growth of LC cell lines by targeting vascular
endothelial growth factor (VEGF) (47). Moreover, Song et al
demonstrated that miRNA-126-3p targeted PIK3R2 and affected the
PTEN/PI3K/AKT signaling pathway to suppress the growth of an NSCLC
cell line (48). As for the role
and effect of miRNA-126-5p in LC, available literature is
scarce.
All previous studies concerning miRNA-126 in LC
investigated miRNA-126-3p and ignored the function of miRNA-126-5p.
To the best of our knowledge, no previous study has explored the
co-function of miRNA-126-3p and −5p in LC. The present study first
concentrated on the co-function and co-targets of miRNA-126-3p and
−5p in LUAD. A total of 202 tissues and 11 microarray datasets were
collected to identify the co-expression of miRNA-126-3p and −5p in
LUAD. Bioinformatic analysis based on the potential target genes
was performed in the hopes of identifying the co-regulation and
synergistic effects of miRNA-126-3p and −5p in LUAD.
Both miRNA-126-3p and miRNA-126-5p are derived from
precursor miRNA-126 and are associated with angiogenesis and cell
proliferation in malignancies. The present study confirmed that
both miRNA-126-3p and −5p have lower expression in LUAD tissues,
indicating that both may be LUAD tumor suppressors. In addition,
expression levels of miRNA-126-3p and −5p were significantly
downregulated in late stage LUAD. The miRNA-126-5p expression
levels were significantly lower in samples with vascular invasion
and LNM, suggesting that miRNA-126-3p and −5p were related to the
development, invasion, and metastasis of LUAD. Lower expression of
miRNA-126-3p and −5p suggested a worse prognosis of LUAD.
In addition, correlation analysis showed a positive
correlation between miRNA-126-3p and −5p expression, meaning that
in LUAD, miRNA-126-5p levels decreased as miRNA-126-3p decreased.
Thus, miRNA-126-3p and −5p are not expressed independently in LUAD;
they are expressed consistently. Analysis of miRNA-126-3p and −5p
levels also showed that although these two miRNAs are expressed
consistently, the level of miRNA-126-5p was usually lower than that
of miRNA-126-3p in the same LUAD tissue sample. This observation
conforms to the rule of mature miRNA expression: When miRNA-3p and
−5p are developed from the same precursor, one of them is often
more highly expressed than the other (14). Previous research improperly assumed
that the lower-expressed miRNA was functionally irrelevant, while
the higher-expressed one played a leading role in biological
processes (13,14). In fact, additional research has
demonstrated the important function of both miRNA-3p and −5p
(49). The present study confirmed
the significantly lower expression of both miRNA-126-3p and −5p in
LUAD and demonstrated their association with TNM stage, invasion
and metastasis; it also confirmed that both of them perform
important functions. Their consistent expression may indicate a
synergistic effect in LUAD.
The target predictions further demonstrated the
synergistic effects of miRNA-126-3p and −5p. Combining the 167
target genes of miRNA-126-3p with the 212 targets of miRNA-126-5p
revealed that these two miRNAs share 44 co-targets. In other words,
26% (44/167) of miRNA-126-3p targets and 20% (44/212) of
miRNA-126-5p targets were co-targets of both. This high target
overlap provides credible evidence for their co-regulation in LUAD.
The combined 335 targets were mainly enriched in neuroactive
ligand-receptor interaction and ECM-receptor interaction of the
KEGG pathway. However, when the 167 miRNA-126-3p targets and 212
miRNA-126-5p targets were separately considered to explore the KEGG
pathway, the results also showed that the targets were mainly
enriched in neuroactive ligand-receptor interaction and
ECM-receptor interaction. Thus, miRNA-126-3p and −5p may influence
LUAD development together via a similar signaling pathway.
More importantly, many of these co-targets have been
identified as key genes in LC development. IGF2BP1 has been
proven to be a target gene of miRNA-491-5p. miRNA-491-5p can
downregulate IGF2BP1 to suppress the growth of NSCLC
(50). TRPM8 was found to
contribute to an invasive phenotype in LC (51). Furthermore, DUSP4 was shown
to function as a suppressor and can inhibit the growth of
EGFR-mutant LUAD (52).
SOX11 was found to exhibit significantly higher expression
in LC and is associated with a prognosis of neuroendocrine tumors
of the lung (53). PLOD2 was
proven to be regulated by the PI3K/AKT-FOXA1 axis to promote the
metastasis of LC, and its high expression is indicative of a worse
prognosis of LUAD (54). miRNA-98
may suppress the expression of LIN28A to decrease the
invasion and metastasis of LC cells (55), while LIN28B expression is
high in tumor-initiating cells from NSCLC and functions in the
tumorigenesis of lung cancer cells (56). The overexpression of SLC7A11
was shown to be associated with poor prognosis of LC (57). According to TCGA data, all these
co-targets play critical roles and are overexpressed in LUAD.
miRNA-126-3p and −5p may directly target these key genes at the
same time to co-regulate the development of LUAD. Downregulation of
the expression of miRNA-126-3p and −5p reduces their combination
with these targets, which decreases the degradation of these target
genes and causes them to be highly expressed in LUAD. It will be
meaningful to deeply research these potential targets and further
validate that they are the downstream targets of miRNA-126-3p and
−5p in LUAD. However, the core point of this study was to
demonstrate the co-regulation as well as the synergistic effects of
miRNA-125-3p and −5p in LUAD, while elucidating the downstream
target genes is a secondary viewpoint of our study. Thus, we
completed a preliminary identification of these potential targets.
Further and intensive investigation is needed to validate them in
the future.
It is worth mentioning that the co-function of
miRNA-3p and −5p has only recently begun to be recognized and
investigated. In fact, many miRNA-3p and −5p pairs have been found
to co-regulate the same malignant tumors (49). The present study demonstrated the
co-regulation of miRNA-126-3p and −5p in LUAD. The present results
and the PPI network provide clear evidence of the existence of the
synergistic effects between miRNA-126-3p and −5p in LUAD. The
co-regulation of miRNA-126-3p and −5p may be a fail-proof mode of
the regulation of miRNAs in LUAD or other malignancies, which can
ensure that if either miRNA-126-3p or −5p is disabled by
transcriptional inhibition or mutation, the associated biological
function may still proceed stably and normally (58). In the regulation of a malignant
tumor, this fail-proof mode may also exist between pairings other
than miRNA-3p and −5p or even between miRNAs from two different
families.
In conclusion, the present study verified the
co-downregulation of the expression of miRNA-126-3p and −5p in LUAD
tissues and discovered the existence of co-target genes between
miRNA-126-3p and −5p, many of which have been confirmed to be
closely related to the proliferation, invasion, metastasis and poor
prognosis of LC. These results indicate that the co-regulation of
miRNA-126-3p and −5p plays a significant role in the development of
LUAD, which also implies a fail-proof mode between miRNA-3p and
−5p. While miRNA-126-5p is by no means equivalent to miRNA-126-3p,
the two miRNAs possess vital associations and valuable clinical
significance in LUAD and various other malignancies.
Acknowledgements
Not applicable.
Funding
The present study was supported by funds from the
National Natural Science Foundation of China (NSFC 81560469), the
Natural Science Foundation of Guangxi, China (nos.
2017GXNSFAA198016 and 2015GXNSFCA139009), the Guangxi Medical
University Training Program for Distinguished Young Scholars,
Medical Excellence Award Funded by the Creative Research
Development Grant from the First Affiliated Hospital of Guangxi
Medical University, and the Guangxi Zhuang Autonomous Region
University Student Innovative Plan (no. 201710598064).
Availability of data and materials
The datasets used and analyzed in the current study
are available from the corresponding authors on reasonable
request.
Authors' contributions
PC designed the study, analyzed the main data and
wrote the main part of the manuscript. YYG and FCM carried out the
detection of miRNA-126-3p and −5p expression in LUAD, collected the
data from GEO database and contributed to the writing of the
manuscript. RQH analyzed the data and revised the manuscript. ZYL
completed the detection of miRNA-126-3p and −5p expression and
analysed the data. GQZ and XL performed the bioinformatics
analyses. XHH designed the study and revised the manuscript. LJP
and GC designed the study, supervised all experiments and revised
the manuscript. All authors read and approved the final manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The research protocol for this study has been
ratified by the Ethics Committee of the First Affiliated Hospital
of Guangxi Medical University. All patients content was obtained at
the time of the sample collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests, and all authors confirm its accuracy.
References
|
1
|
Blanco-Prieto S, Barcia-Castro L, Páez de
la Cadena M, Rodríguez-Berrocal FJ, Vázquez-Iglesias L, Botana-Rial
MI, Fernández-Villar A and De Chiara L: Relevance of matrix
metalloproteases in non-small cell lung cancer diagnosis. BMC
Cancer. 17:8232017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan Y, Wu J, Li B, Niu J, Tan H and Qiu
S: Regulation of signaling pathways involved in the
anti-proliferative and apoptosis-inducing effects of M22 against
non-small cell lung adenocarcinoma A549 cells. Sci Rep. 8:9922018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen J, Wang B, Zhang T, Zhu N, Wang Z,
Jin J, He Y and Hu M: Suppression of non-small cell lung cancer
growth and metastasis by a novel small molecular activator of RECK.
Cell Physiol Biochem. 45:1807–1817. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Han J, Zhu H, Peng L and Chen Z:
MiR181b5p mediates TGFbeta1-induced epithelial-to-mesenchymal
transition in non-small cell lung cancer stem-like cells derived
from lung adenocarcinoma A549 cells. Int J Oncol. 51:158–168. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Denisenko TV, Budkevich IN and Zhivotovsky
B: Cell death-based treatment of lung adenocarcinoma. Cell Death
Dis. 9:1172018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y and Zhou S: MicroRNA-29a inhibits
mesenchymal stem cell viability and proliferation by targeting
Roundabout 1. Mol Med Rep. 12:6178–6184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou S, Ye W, Zhang Y, Yu D, Shao Q, Liang
J and Zhang M: MiR-144 reverses chemoresistance of hepatocellular
carcinoma cell lines by targeting Nrf2-dependent antioxidant
pathway. Am J Transl Res. 8:2992–3002. 2016.PubMed/NCBI
|
|
8
|
Gamazon ER, Trendowski MR, Wen Y, Wing C,
Delaney SM, Huh W, Wong S, Cox NJ and Dolan ME: Gene and microRNA
perturbations of cellular response to pemetrexed implicate
biological networks and enable imputation of response in lung
adenocarcinoma. Sci Rep. 8:7332018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao F, Ge YZ, Zhou LH, Xu LW, Xu Z, Ping
WW, Wang M, Zhou CC, Wu R and Jia RP: Identification of hub miRNA
biomarkers for bladder cancer by weighted gene coexpression network
analysis. Onco Targets Ther. 10:5551–5559. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cochetti G, Poli G, Guelfi G, Boni A,
Egidi MG and Mearini E: Different levels of serum microRNAs in
prostate cancer and benign prostatic hyperplasia: Evaluation of
potential diagnostic and prognostic role. Onco Targets Ther.
9:7545–7553. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang L, Wei DM, Li JJ, Luo DZ, Chen G,
Dang YW and Cai XY: Prognostic microRNAs and their potential
molecular mechanism in pancreatic cancer: A study based on The
Cancer Genome Atlas and bioinformatics investigation. Mol Med Rep.
17:939–951. 2018.PubMed/NCBI
|
|
12
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer-a brief overview. Adv Biol Regul. 57:1–9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mitra R, Lin CC, Eischen CM, Bandyopadhyay
S and Zhao Z: Concordant dysregulation of miR-5p and miR-3p arms of
the same precursor microRNA may be a mechanism in inducing cell
proliferation and tumorigenesis: A lung cancer study. RNA.
21:1055–1065. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okamura K, Phillips MD, Tyler DM, Duan H,
Chou YT and Lai EC: The regulatory activity of microRNA* species
has substantial influence on microRNA and 3′UTR evolution. Nat
Struct Mol Biol. 15:354–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo L, Yu J, Yu H, Zhao Y, Chen S, Xu C
and Chen F: Evolutionary and expression analysis of miR-#-5p and
miR-#-3p at the miRNAs/isomiRs levels. BioMed Res Int.
2015:1683582015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu T, Li J, Yan M, Liu L, Lin H, Zhao F,
Sun L, Zhang Y, Cui Y, Zhang F, et al: MicroRNA-193a-3p and 5p
suppress the metastasis of human non-small-cell lung cancer by
downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway.
Oncogene. 34:413–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y,
Li C, Chong M, Ibrahim T, Mercatali L, et al: MiR-126 and miR-126*
repress recruitment of mesenchymal stem cells and inflammatory
monocytes to inhibit breast cancer metastasis. Nat Cell Biol.
15:284–294. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Q, Hu H, Jiao D, Yan J, Xu W, Tang X,
Chen J and Wang J: miR-126-3p and miR-451a correlate with
clinicopathological features of lung adenocarcinoma: The underlying
molecular mechanisms. Oncol Rep. 36:909–917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang R, Zhong T, Dang Y, Zhang X, Li P and
Chen G: Association between downexpression of miR-203 and poor
prognosis in non-small cell lung cancer patients. Clin Transl
Oncol. 18:360–368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verderio P, Bottelli S, Ciniselli CM,
Pierotti MA, Gariboldi M and Pizzamiglio S: NqA: An R-based
algorithm for the normalization and analysis of microRNA
quantitative real-time polymerase chain reaction data. Anal
Biochem. 461:7–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Benes V and Castoldi M: Expression
profiling of microRNA using real-time quantitative PCR, how to use
it and what is available. Methods. 50:244–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen G, Umelo IA, Lv S, Teugels E, Fostier
K, Kronenberger P, Dewaele A, Sadones J, Geers C and De Grève J:
MiR-146a inhibits cell growth, cell migration and induces apoptosis
in non-small cell lung cancer cells. PLoS One. 8:e603172013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ivo D, Urso P, Fernando D'Urso O, Damiano
Gianfreda C, Mezzolla V, Storelli C and Marsigliante S: miR-15b and
miR-21 as circulating biomarkers for diagnosis of glioma. Curr
Genomics. 16:304–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang XN, Guo RJ, Li S, Zheng ZM and Liang
HD: Binary logistic regression analysis of solid thyroid nodules
imaged by high-frequency ultrasonography, acoustic radiation force
impulse, and contrast-enhanced ultrasonography. Eur Rev Med
Pharmacol Sci. 18:3601–3610. 2014.PubMed/NCBI
|
|
26
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seike M, Goto A, Okano T, Bowman ED,
Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, et
al: MiR-21 is an EGFR-regulated anti-apoptotic factor in lung
cancer in never-smokers. Proc Nal Acad Sci USA. 106:12085–12090.
2009. View Article : Google Scholar
|
|
28
|
Nymark P, Guled M, Borze I, Faisal A,
Lahti L, Salmenkivi K, Kettunen E, Anttila S and Knuutila S:
Integrative analysis of microRNA, mRNA and aCGH data reveals
asbestos- and histology-related changes in lung cancer. Genes
Chromosome Cancer. 50:585–597. 2011. View Article : Google Scholar
|
|
29
|
Ma L, Huang Y, Zhu W, Zhou S, Zhou J, Zeng
F, Liu X, Zhang Y and Yu J: An integrated analysis of miRNA and
mRNA expressions in non-small cell lung cancers. PLoS One.
6:e265022011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Jaarsveld MT, Wouters MD, Boersma AW,
Smid M, van Ijcken WF, Mathijssen RH, Hoeijmakers JH, Martens JW,
van Laere S, Wiemer EA and Pothof J: DNA damage responsive
microRNAs misexpressed in human cancer modulate therapy
sensitivity. Mol Oncol. 8:458–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bjaanaes MM, Halvorsen AR, Solberg S,
Jørgensen L, Dragani TA, Galvan A, Colombo F, Anderlini M,
Pastorino U, Kure E, et al: Unique microRNA-profiles in
EGFR-mutated lung adenocarcinomas. Int J Cancer. 135:1812–1821.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arima C, Kajino T, Tamada Y, Imoto S,
Shimada Y, Nakatochi M, Suzuki M, Isomura H, Yatabe Y, Yamaguchi T,
et al: Lung adenocarcinoma subtypes definable by lung
development-related miRNA expression profiles in association with
clinicopathologic features. Carcinogenesis. 35:2224–2231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Robles AI, Arai E, Mathé EA, Okayama H,
Schetter AJ, Brown D, Petersen D, Bowman ED, Noro R, Welsh JA, et
al: An integrated prognostic classifier for stage I lung
adenocarcinoma based on mRNA, microRNA, and DNA methylation
biomarkers. J Thorac Oncol. 10:1037–1048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin Y, Liu Y, Zhang J, Huang W, Jiang H,
Hou Y, Xu C, Zhai C, Gao X, Wang S, et al: The expression of
miR-375 is associated with carcinogenesis in three subtypes of lung
cancer. PLoS One. 10:e01441872015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshimoto T, Motoi N, Yamamoto N, Nagano
H, Ushijima M, Matsuura M, Okumura S, Yamaguchi T, Fukayama M and
Ishikawa Y: Pulmonary carcinoids and low-grade gastrointestinal
neuroendocrine tumors show common MicroRNA expression profiles,
different from adenocarcinomas and small cell carcinomas.
Neuroendocrinology. 106:47–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiong Y, Kotian S, Zeiger MA, Zhang L and
Kebebew E: MiR-126-3p inhibits thyroid cancer cell growth and
metastasis, and is associated with aggressive thyroid cancer. PLoS
One. 10:e01304962015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Du C, Lv Z, Cao L, Ding C, Gyabaah OA, Xie
H, Zhou L, Wu J and Zheng S: MiR-126-3p suppresses tumor metastasis
and angiogenesis of hepatocellular carcinoma by targeting LRP6 and
PIK3R2. J Transl Med. 12:2592014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu W, Chen H, Wong N, Haynes W, Baker CM
and Wang X: Pseudohypoxia induced by miR-126 deactivation promotes
migration and therapeutic resistance in renal cell carcinoma.
Cancer Lett. 394:65–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Ping JL, Ma B, Chen YR and Li LQ:
Deregulation of miR-126-3p in basal-like breast cancers stroma and
its clinical significance. Pathol Res Pract. 213:922–928. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fiala O, Pitule P, Hosek P, Liska V,
Sorejs O, Bruha J, Vycital O, Buchler T, Poprach A, Topolcan O and
Finek J: The association of miR-126-3p, miR-126-5p and miR-664-3p
expression profiles with outcomes of patients with metastatic
colorectal cancer treated with bevacizumab. Tumour Biol.
39:10104283177092832017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kong R, Ma Y, Feng J, Li S, Zhang W, Jiang
J, Zhang J, Qiao Z, Yang X and Zhou B: The crucial role of miR-126
on suppressing progression of esophageal cancer by targeting
VEGF-A. Cell Mol Biol Lett. 21:32016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sasahira T, Kurihara M, Bhawal UK, Ueda N,
Shimomoto T, Yamamoto K, Kirita T and Kuniyasu H: Downregulation of
miR-126 induces angiogenesis and lymphangiogenesis by activation of
VEGF-A in oral cancer. Br J Cancer. 107:700–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Paszek S, Gabło N, Barnaś E, Szybka M,
Morawiec J, Kołacińska A and Zawlik I: Dysregulation of microRNAs
in triple-negative breast cancer. Ginekol Pol. 88:530–536. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jinushi T, Shibayama Y, Kinoshita I,
Oizumi S, Jinushi M, Aota T, Takahashi T, Horita S, Dosaka-Akita H
and Iseki K: Low expression levels of microRNA-124-5p correlated
with poor prognosis in colorectal cancer via targeting of SMC4.
Cancer Med. 3:1544–1552. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Peng Z, Pan L, Niu Z, Li W, Dang X, Wan L,
Zhang R and Yang S: Identification of microRNAs as potential
biomarkers for lung adenocarcinoma using integrating genomics
analysis. Oncotarget. 8:64143–64156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jusufović E, Rijavec M, Keser D, Korošec
P, Sodja E, Iljazović E, Radojević Z and Košnik M: let-7b and
miR-126 are down-regulated in tumor tissue and correlate with
microvessel density and survival outcomes in non-small-cell lung
cancer. PLoS One. 7:e455772012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu B, Peng XC, Zheng XL, Wang J and Qin
YW: MiR-126 restoration down-regulate VEGF and inhibit the growth
of lung cancer cell lines in vitro and in vivo. Lung Cancer.
66:169–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Song L, Li D, Gu Y, Wen ZM, Jie J, Zhao D
and Peng LP: MicroRNA-126 targeting PIK3R2 inhibits NSCLC A549 cell
proliferation, migration, and invasion by regulation of
PTEN/PI3K/AKT pathway. Clin Lung Cancer. 17:e65–e75. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang CJ, Nguyen PN, Choo KB, Sugii S, Wee
K, Cheong SK and Kamarul T: Frequent co-expression of miRNA-5p and
−3p species and cross-targeting in induced pluripotent stem cells.
Int J Med Sci. 11:824–833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gong F, Ren P, Zhang Y, Jiang J and Zhang
H: MicroRNAs-491-5p suppresses cell proliferation and invasion by
inhibiting IGF2BP1 in non-small cell lung cancer. Am J Transl Res.
8:485–495. 2016.PubMed/NCBI
|
|
51
|
Du GJ, Li JH, Liu WJ, Liu YH, Zhao B, Li
HR, Hou XD, Li H, Qi XX and Duan YJ: The combination of TRPM8 and
TRPA1 expression causes an invasive phenotype in lung cancer.
Tumour Biol. 35:1251–1261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin H, Qiu S, Xie L, Liu C and Sun S:
Nimbolide suppresses non-small cell lung cancer cell invasion and
migration via manipulation of DUSP4 expression and ERK1/2
signaling. Biomed Pharmacother. 92:340–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Walter RF, Mairinger FD, Werner R, Ting S,
Vollbrecht C, Theegarten D, Christoph DC, Zarogoulidis K, Schmid
KW, Zarogoulidis P and Wohlschlaeger J: SOX4, SOX11 and PAX6 mRNA
expression was identified as a (prognostic) marker for the
aggressiveness of neuroendocrine tumors of the lung by using
next-generation expression analysis (NanoString). Future Oncol.
11:1027–1036. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Du H, Chen Y, Hou X, Huang Y, Wei X, Yu X,
Feng S, Wu Y, Zhan M, Shi X, et al: PLOD2 regulated by
transcription factor FOXA1 promotes metastasis in NSCLC. Cell Death
Dis. 8:e31432017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu WL, Chang JM, Chong IW, Hung YL, Chen
YH, Huang WT, Kuo HF, Hsieh CC and Liu PL: Curcumin inhibits
LIN-28A through the activation of miRNA-98 in the lung cancer cell
line A549. Molecules. 22:E9292017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang WC, Shyh-Chang N, Yang H, Rai A,
Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, et al: Glycine
decarboxylase activity drives non-small cell lung cancer
tumor-initiating cells and tumorigenesis. Cell. 148:259–272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cohen AS, Khalil FK, Welsh EA, Schabath
MB, Enkemann SA, Davis A, Zhou JM, Boulware DC, Kim J, Haura EB and
Morse DL: Cell-surface marker discovery for lung cancer.
Oncotarget. 8:113373–113402. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Choo KB, Soon YL, Nguyen PN, Hiew MS and
Huang CJ: MicroRNA-5p and −3p co-expression and cross-targeting in
colon cancer cells. J Biomed Sci. 21:952014. View Article : Google Scholar : PubMed/NCBI
|