Introduction
For adenovirus replication, several early gene
products, such as E1A, E1B and E4, are necessary to change the
environment within host cells (1).
The E4 region of adenoviruses encodes multiple proteins and these
proteins are required for DNA replication, late gene expression and
host cell shutoff (2). The largest
protein encoded in the E4 region is E4orf6, which is required for
productive adenovirus replication (1). E4orf6 forms a complex with another
early gene product, E1B55k (3), and
this complex associates with cellular proteins such as cullin 5,
elongins B and C and Rbx, to form E3 ubiquitin ligase (4,5). This
ligase targets p53 (4–7), the MRN complex (8), DNA ligase IV (9) and integrin α3 (10) for proteasomal degradation. This
ubiquitin ligase activity is known to be required for the nuclear
export of viral late mRNAs (11,12).
Several viral proteins encoded in the E4 region,
such as E4orf1, E4orf3 and E4or6 are known to have oncogenic
activities (13–17). E4orf1 of adenovirus type 9, which
belongs to adenovirus subgroup D, has been shown to be involved in
mammary tumorigenesis (14,18). E4orf3 and E4orf6 proteins of
subgroup C adenovirus type 5 have the potential to transform cells
in cooperation with E1A and E1B proteins and enhance the growth of
tumors transplanted in nude mice (15–17).
In a previous report, we demonstrated that in cells transformed
with adenovirus type 5 E4orf6, cellular pp32 protein associates
with E4orf6 and AU-rich element (ARE)-containing mRNAs are exported
to the cytoplasm in a chromosome region maintenance 1
(CRM1)-independent manner (19).
Furthermore, the exported ARE-mRNAs are stabilized and those mRNAs
acquire the potential to transform cells (20).
AREs usually exist in the 3′-untranslated region
(UTR) of certain mRNAs encoding early response genes or
growth-related genes, such as proto-oncogenes and growth factors
(21,22). AREs are targets for rapid
degradation of mRNA (21,23) and the fate of ARE-mRNA is controlled
by several RNA-binding proteins, such as AUF1, tristetraprolin
(TTP) and HuR (24). HuR, which is
a member of the embryonic lethal abnormal vision (ELAV) family of
RNA-binding proteins, binds to AREs in order to protect ARE-mRNA
from rapid degradation (23,24).
HuR is able to shuttle between the nucleus and cytoplasm, whereas
it is mainly located in the nucleus. HuR-mediated stabilization of
ARE-mRNA depends on HuR localization in the cytoplasm (24,25).
In normal cells, HuR transiently relocalizes to the cytoplasm under
conditions of stress. On the other hands, HuR constitutively
accumulates in the cytoplasm of cancer cells and the cytoplasmic
expression of HuR is thought to be involved in malignant
transformation of cancer cells (25,26).
These facts suggest that the E4orf6-deleted mutant
adenovirus is able to proliferate selectively in cancer cells in
which ARE-mRNA is stabilized. In the present study, we examined the
oncolytic activity of the adenovirus E4orf6-deleted mutant, dl355.
The ability of this virus to replicate was markedly increased in
cancer cells compared with that in normal cells. dl355 showed
cytolytic activity for cancer cells in vitro and in
vivo. The propagation and cytolytic activity of this virus were
higher than those of an E1B55k-deleted adenovirus. These findings
indicate that dl355 is a potential oncolytic virus.
Materials and methods
Cell lines, viruses and
antibodies
The human lung cancer cell lines, A549 and H1299;
cervical carcinoma cell lines HeLa, HeLa S3 and C33A; African green
monkey kidney (Vero) cells carrying an integrated copy of the Ad5
E4 region, W162; human embryonal kidney cell line 293 (transformed
by the adenovirus E1 gene); human foreskin fibroblast cell line,
BJ; and normal human lung primary cell line, WI38 were used in the
present study. All cells were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA) and cultured in
Dulbecco's modified Eagle's medium (DMEM; Sigma-Ardrich; Merck
KGaA, Darmstadt, Germany) containing 10% fetal bovine serum (FBS;
Biowest, Nuaille, France) with antibiotics at 37°C in a 5%
CO2 atmosphere under humidified conditions.
Wild-type adenovirus type 5 (WT300), E4orf6-deleted
mutant adenovirus (dl355) (generous gift from Dr T. Shenk;
Princeton University) and E1B55k-deleted mutant adenovirus (dl1520)
(generous gift from Dr A.J. Berk; University of California) were
used in the present study.
A western blot analysis was performed as previously
described (27) using antibodies
specific to E1A (M73; generous gift from Dr T. Shenk, Princeton
University), E4orf6 (RSA#3; generous gift from Dr T. Shenk;
Princeton University), actin (dilution 1:1,000; cat. no. sc-1616;
Santa Cruz Biotechnology, Dallas, TX, USA) and HuR (dilution
1:2,500; cat. no. sc-5261; Santa Cruz Biotechnology) and β-tubulin
(dilution 1:1,000; cat. no. 05-661; EMD Millipore Corp., Darmstadt,
Germany) as primary antibodies. The secondary antibody was
horseradish peroxidase-conjugated anti-goat IgG (dilution 1:5,000;
cat. no. 805-035-180; Jackson ImmunoResearch Laboratories, West
Grove, PA, USA) and horseradish peroxidase-conjugated anti-mouse
IgG (dilution 1:5,000; cat. no. 115-035-062; Jackson ImmunoResearch
Laboratories). Antibody binding was visualized using SuperSignal
West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Preparation of dl355 and dl1520
lysates
To prepare virus lysates, dl355-infected W162 cells
and dl1520 or WT300-infected 293 cells were subjected to three
cycles of freezing and thawing. Virus concentrations [virus
particles (vp)/ml] were then determined by a QuickTiter Adenovirus
Quantitation kit (Cell Biolabs, San Diego, CA, USA). Viral titers
[infectious units (ifu)/ml] were determined using the Adeno-X™
Rapid Titer kit (Clontech Laboratories, Inc., Mountain View, CA,
USA) according to the manufacturer's instructions. To use a virus
in in vivo experiments, its extract was purified using a
Fast-Trap Adenovirus Purification and Concentration kit (Millipore,
Billerica, MA, USA) according to the manufacturer's protocols.
Cytopathic effect assay and cell
viability assay
Human cancer and normal cells were plated on 24-well
plates (5×104 cells/well). Twenty-four hours later, the
cells were infected with dl355 at a multiplicity of infection (MOI)
of 0.1, 0.5, 1, 10 or 100 vp/cell and maintained for an additional
7 days. Cells were then fixed and stained with Coomassie brilliant
blue.
A 2-3-bis
[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide
inner salt assay was used to examine cell metabolic activity.
Cancer and normal cells were seeded on 96-well plates at a density
of 3.0×103 cells/well. Twenty-four hours later, the
cells were infected with dl355, dl1520 or WT300 at an MOI of 100
vp/cell. Cell metabolic activity was determined using an XTT assay
on days 1, 3, 5 and 7 with the Cell Proliferation kit II (Roche
Diagnostics, Basel, Switzerland) according to the manufacturer's
protocol.
In vitro virus proliferation
assay
Cancer and normal cells were seeded at
5.0×104 cells/well 24 h before the infection. Cells were
infected with dl355, dl1520, or WT300 at an MOI of 1 vp/cell. These
cells were incubated at 37°C for 48 h, after which cells were
collected and a virus lysate was prepared as described above. Viral
titers (ifu/ml) were determined using the Adeno-X Rapid Titer kit
(Clontech Laboratories).
HuR depletion
For RNA interference analysis, Lipofectamine RNAiMAX
(Invitrogen; Thermo Fisher Scientific) was used to transfect HeLa
cells with 20 nM of each small interfering RNA (siRNA) targeting
HuR (5′-TTCGTAAGTTATTTCCTTTAATT-3′) or with a negative control
siRNA (5′-TCTTAATCGCGTATAAGGCTT-3′; Qiagen, Hilden, Germany). After
48 h of transfection, HeLa cells were infected with dl355. After 24
h of infection, all cells were collected and the virus lysate was
prepared using three freeze-thaw cycles. Viral titers were
determined using the Adeno-X Rapid Titer kit (Clontech
Laboratories) and 293 cells.
For heat shock treatment, HeLa cells were incubated
at 43°C for 2 h immediately after dl355 infection, and the infected
cells were heat shocked (2 h) again at 24 h after infection. Cells
were harvested at 28 h after infection and the viral titers were
determined as described above.
In vivo human tumor model
Female BALB/c nu/nu mice (purchased from Hokudo,
Sapporo, Japan) were housed under specific pathogen-free
conditions. The temperature was 26–28°C, and the light/dark cycle
was 10-h/14-h cycle, food and water were taken ad libitum.
HeLa S3 cells (1.0×106 cells/mouse) were injected
subcutaneously into the flanks of mice (5 week old and 20–24 g) and
permitted to grow to ~5–6 mm in diameter. The mice were randomly
divided into two groups (5 per group) and 109 vp (100
µl) of dl355 or the same volume of PBS was injected twice (days 0
and 3) directly into the tumors. The perpendicular diameters of the
tumors were measured every 3 or 4 days and tumor volumes were
calculated using the following equation: Volume (mm3) =
A × B2 × 0.5 (A is the longest diameter, B is the
shortest diameter). The mice were sacrificed by cervical
dislocation after 30 days of injection of virus. All procedures
performed in this study involving animals were in accordance with
the ethical standards of the Animal Care and Use Committee of the
Hokkaido University (Sapporo, Japan).
Results
Selective dl355 replication in cancer
cells
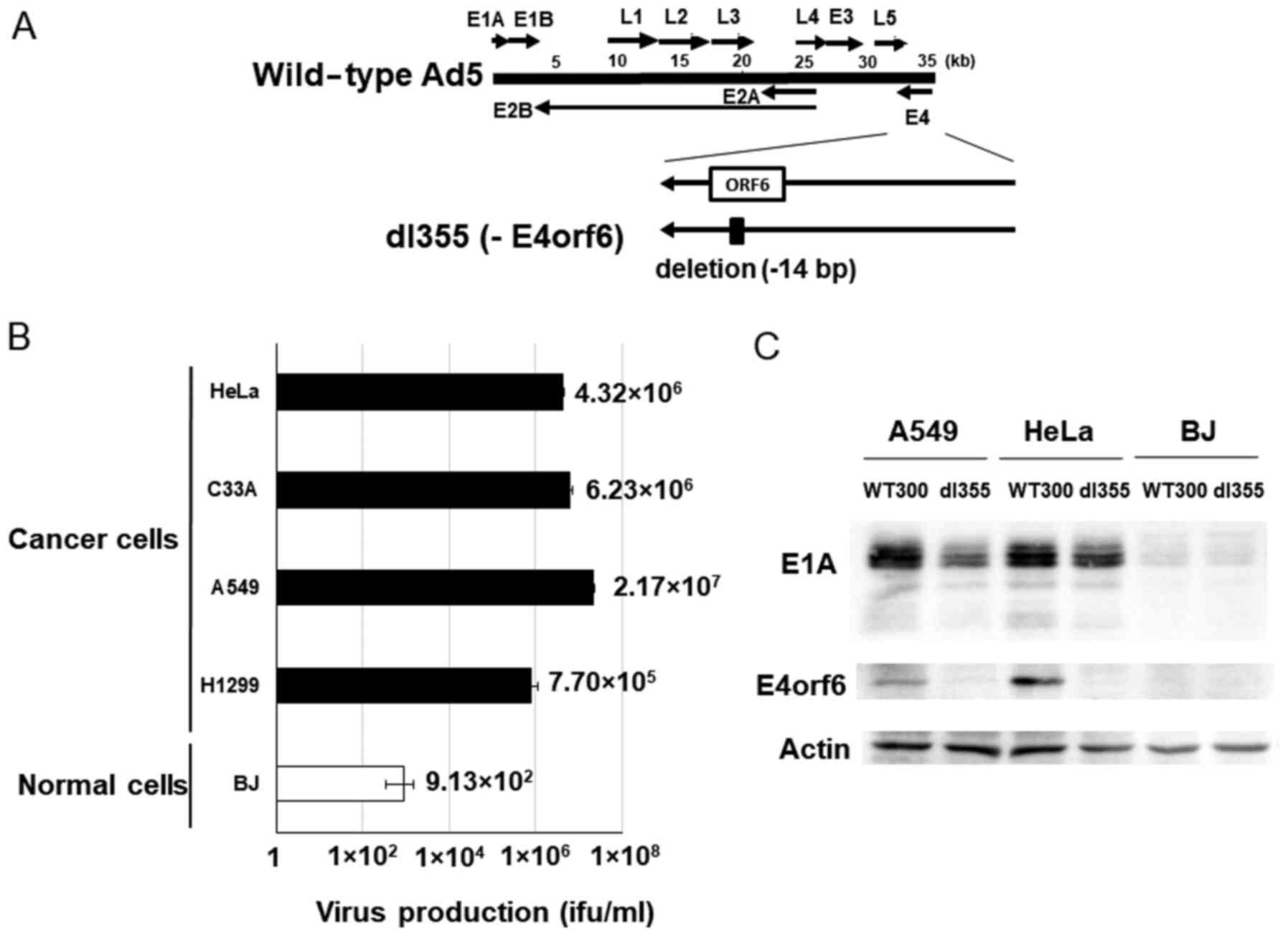
dl355 (Fig. 1A) has
a 14-bp deletion in the E4orf6 gene and was constructed in
1985 to investigate the functions of genes located in the E4 region
in adenovirus-infected host cells. This virus showed deficient
virus DNA replication, accumulation of late viral mRNAs, and
shutoff of host cell mRNAs compared to wild-type adenovirus type 5
(Ad5) (2). To examine the
productive efficiency of dl355, cancer cells (HeLa, C33A, A549 and
H1299) and normal cells (BJ) were infected with dl355 at an MOI of
1 virus particle (vp)/cell and virus titers generated after 48 h
were detected by staining the hexon protein of virus particles in
293 cells. In these cancer cells, the propagation of dl355 was very
high, with titers from 7.70×105 to 2.17×107
ifu/ml. On the other hand, the titer of dl355 in normal cells (BJ;
foreskin fibroblasts) was 3 to 5 logs lower (9.13×102
ifu/ml) than in cancer cells (Fig.
1B). We examined the expression of E1A protein, which is
expressed first after infection. The amount of E1A protein was at a
high level in dl355-infected A549 and HeLa cells, although the
level was low in normal BJ cells (Fig.
1C). These results suggest that dl355 is selectively produced
in cancer cells.
Since dl355 was thought to propagate in cancer cells
in which ARE-mRNA is stabilized, we examined whether the ARE-mRNA
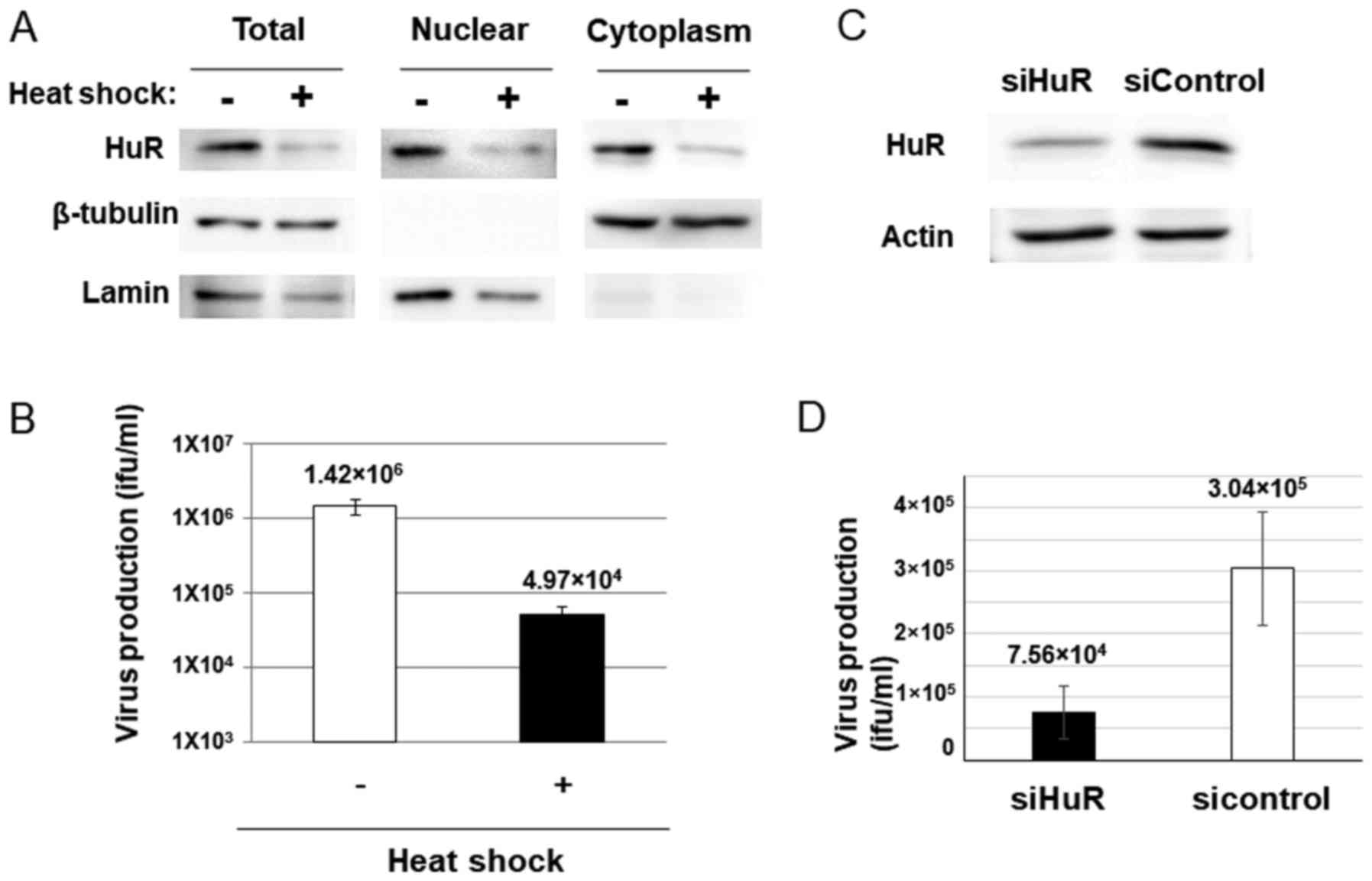
stabilization system was required for dl355 replication. To
evaluate this, we confirmed adenovirus production in HuR-depleted
cells, because decreased HuR inhibits ARE-mRNA stabilization
(28). Heat shock (HS) treatment is
known to downregulate HuR by ubiquitin-mediated proteolysis and
HuR-targeted mRNA is also decreased in HS-treated cells (29). If dl355 replicates using the
ARE-mRNA stabilization system, the virus titer is expected to
reduce with HS treatment. HeLa cells were subjected to HS, as
described in Materials and methods, and were then examined for HuR
protein and virus production. As expected, a 2-h HS treatment of
HeLa cells resulted in reduced expression of HuR protein in the
cytoplasm of HeLa cells (Fig. 2A).
Furthermore, HS-treated cells showed a significant reduction in
virus production compared to the non-treated cells (~1/28.6;
Fig. 2B).
Since HS treatment affects have many influences
other than HuR proteolysis in cells, we confirmed the HuR-depletion
effect by HuR knockdown (KD). The virus production in siRNA for HuR
introduced cells (Fig. 2C) was much
less than that of the cells with control siRNA transfected cells
(~1/4; Fig. 2D). These results
suggest that HuR plays an important role in dl355 replication.
In vitro and in vivo cytolytic
potential of dl355
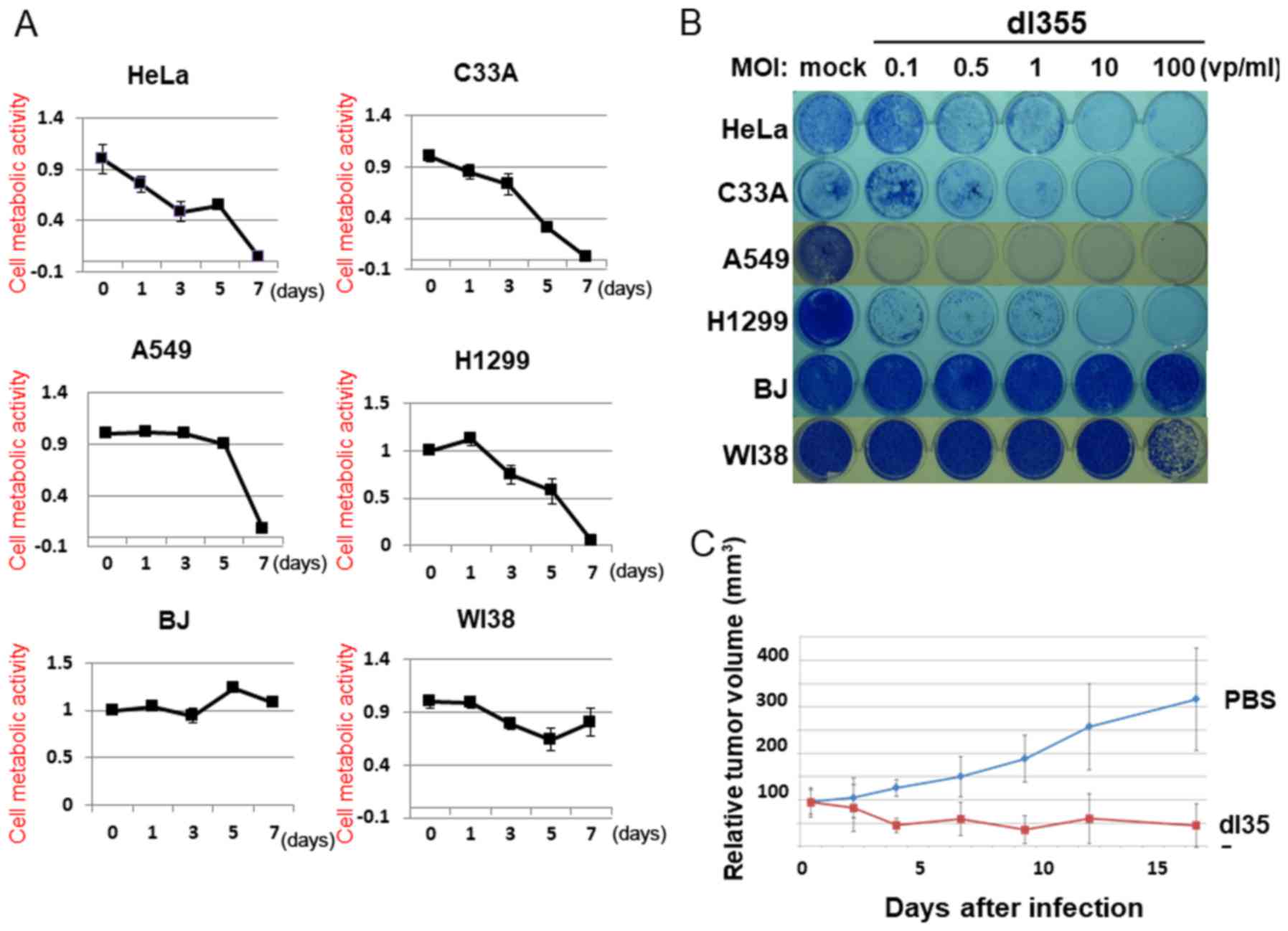
To estimate the cell lysis activity of dl355, we
examined cell viability using the XTT assay. HeLa, A549, C33A,
H1299, BJ and WI38 cells were infected with dl355 at an MOI of 100
(vp/cells) and the XTT assay was performed 1, 3, 5 and 7 days after
infection (Fig. 3A). In the case of
HeLa cells, dl355 infection resulted in reduced cell viability 3
days after infection and most cells died after 7 days. HeLa cells
showed the earliest effects, but in other cancer cells, nearly all
cells died on day 7. In contrast, most normal cells infected with
dl355 did not die even after 7 days.
To assess the cell lysis activity of dl355 further,
we examined the infected cells using a cytopathic effects (CPE)
assay. Four different cancer cell types (HeLa, C33A, A549 and
H1299) and two normal cell types (BJ and WI38) were infected with
dl355 at MOIs of 0.1, 0.5, 1, 10 or 100. Cytotoxicity was estimated
by staining the remaining cells with Coomassie brilliant blue 7
days after infection (Fig. 3B).
Although several cell lines survived with low MOIs, all cancer
cells were killed by dl355 in a dose-dependent manner. On the other
hand, most normal cells survived in all infections. Taken together,
these results demonstrate the in vitro-selective cytolytic
activity of dl355.
We estimated the therapeutic effect of dl355 for
human cancers using a tumor xenograft model. In order to examine
this, HeLa S3 cells were implanted into the hind flanks of
5-week-old female BALB/c nu/nu mice. When the tumors grew to ~5–6
mm in diameter, 109 vp dl355 (100 µl) or the same volume
of phosphate-buffered saline (PBS) (as a control) were injected
twice (days 0 and 3) directly into the tumor. Tumor growth was
significantly suppressed by injection with dl355 whereas the
PBS-injected tumors grew almost three times as large in 18 days
(Fig. 3C). Thus, dl355 exerted
significant effects on human tumor xenografts in nude mice.
Comparison of the oncolytic effects of
dl355 and dl1520
Since an E1B-55k gene deleted-adenovirus has been
developed as an oncolytic adenovirus (30) and is already applied clinically, we
next compared the oncolytic activity of dl355 with that of the
E1B-55k-deleted adenovirus, dl1520. Using cancer cells, HeLa, C33A,
A549 and H1299, and normal BJ cells, dl355, dl1520 and WT300 as a
control of infectivity were infected at an MOI of 1 and virus
titers were estimated 48 h after infection. Since different cell
lines (293 and W162 cells) were used to determine the virus
production rate (ifu/ml) of each virus, we compared the productive
efficiencies of both viruses with viral particles (vp/ml). As shown
in Fig. 4A, dl355 virus production
was significantly higher (1–3 logs higher) than dl1520 virus
production in all cancer cells.
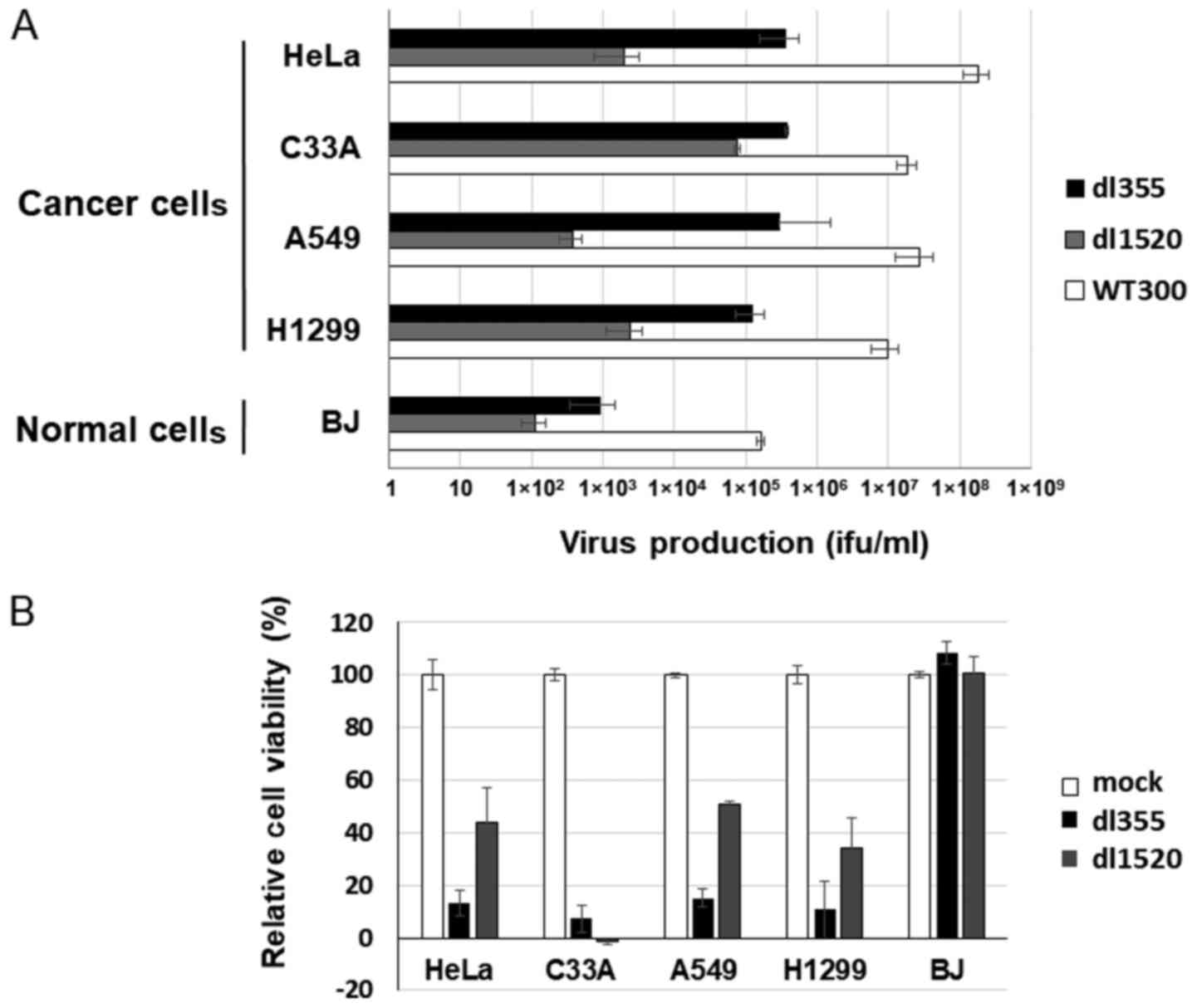 | Figure 4.Comparison of virus production and
cell lysis activity between dl355 and dl1520. (A) Cancer (HeLa,
C33A, A549 and H1299) and normal (BJ) cells were infected with
dl355, dl1520, or WT300 at an MOI of 1 vp/cell and virus production
was determined as described in Materials and methods. Each titer
(vp/ml) is indicated on the graph. Data are shown as the mean ±
standard deviation of three independent experiments. (B) The same
cancer and normal cells were infected with dl355 or dl1520 at an
MOI of 100 vp/cell and cell viabilities were estimated 7 days after
infection. Data are shown as the mean ± standard deviation of three
independent experiments. WT300, wild-type adenovirus type 5; dl355,
E4orf6-deleted mutant adenovirus; dl1520, E1B55k-deleted mutant
adenovirus; MOI, multiplicity of infection; vp, virus particles;
ifu, infectious units. |
To compare cell lysis activity, both viruses (MOI
100) were infected into the same cancer and normal cells and the
XTT assay was performed 7 days after infection. Except for C33A
case, dl355 showed stronger cell death activity than dl1520 in
cancer cells (Fig. 4B). However, in
normal cells, both viruses showed almost no cell death effect.
Taken together, these data indicate that dl355 has a stronger
oncolytic effect than dl1520 in at least several cancer cell
lines.
Discussion
In the present study, we described the oncolytic
potential of the E4orf6-deleted adenovirus, dl355. The productive
efficiencies of this virus with cancer cells were approximately
102 to 103 times higher from that of normal
cells (Fig. 1B). It showed a high
level of cytolytic activity for cervical and lung cancer cells
in vitro and the same effect was evident in a tumor
xenograft model. Furthermore, dl355 has oncolytic activity that may
be superior to dl1520, which is currently used clinically. These
findings indicate that dl355 is a potential oncolytic virus.
Many types of conditionally replicative adenoviruses
(CRAds) targeted to cancer cells are being developed and several
viruses are currently in clinical trials (31). Oncolytic adenoviruses can be divided
into at least two types (32), one
of which has mutations in genes required for viral replication. For
example, the E1A or E1B-55k gene deleted-virus has
been developed as a CRAd and it has oncolytic effects for pRB- or
p53-deficient tumor cells (30,33).
The other group consists of viruses that possess a cancer-specific
transcription system in the virus genes required for replication
such as E1A. For example, promoters of the telomerase gene
(34) or prostate-specific antigen
gene (35) are inserted into the
5′-untranslated region (UTR) of the E1A gene to produce CRAds,
which are specifically activated in cancer cells. There are few
reports describing oncolytic viruses with tumor selectivity based
on the level of mRNA stability. Thus, dl355 is a rare type of
oncolytic virus that its replication is controlled by RNA
modulation.
As we have shown in a previous study (28), HuR KD attenuates the export and
stabilization of ARE-mRNA. This is due to the fact that HuR is the
only protein that binds directly to ARE, so the ARE-mRNA failed to
be exported to the cytoplasm if HuR disappears. In this study,
virus proliferation was downregulated under HuR HS or HuR KD
conditions. These results suggest that the export and stabilization
of ARE-mRNA is essential for the growth of dl355. In a previous
study (36), it was clarified that
various stimuli promote the relocalization of HuR to the cytoplasm
and the stabilization of ARE-mRNA. Therefore, if stimulation is
added to enhance export and stabilization of ARE-mRNA, the
production efficiency of dl355 increases, indicating the
possibility of obtaining a stronger tumor lysis activity.
We used various types of cancer cells in this study
and it is expected that the infection efficiency of the virus was
different in each cell line. We examined the expression of E1A
protein, which is expressed first after infection, to evaluate the
infectivity of dl355. As the amount of E1A protein was almost the
same between dl355-infected A549 and HeLa cells (Fig. 1C), the infectivity can be considered
to be equivalent. Furthermore, as shown in Fig. 4A, the virus production of the WT300
infected in different types of cancer cells was not significantly
different, thus the infection efficiency was not so different.
These data showed the validity of comparing the production
efficiency of dl355 and dl1520.
In the vast majority of cancer cells, ARE-mRNAs
relocate to the cytoplasm with HuR and are constitutively
stabilized (26). We showed that
dl355 replication depends on the ARE-mRNA stabilization system,
since the replication of dl355 is downregulated in HuR-depleted
cancer cells (Fig. 2). These facts
suggest that dl355 has the potential to be effective for many types
of cancer cells.
Acknowledgements
The authors thank Dr T. Shenk (Princeton University)
for providing dl355 and wild-type (WT300) adenoviruses and
antibodies and Dr A.J. Berk (University of California) for
providing the mutant adenovirus, dl1520, and the members of our
laboratories for their helpful discussions and support.
Funding
The present study was supported by a Grant-in-Aid
for Scientific Research from the Ministry of Education, Science and
Culture of Japan (nos. 26293423 and 23659928).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
FH, AYM and YM conceived and designed the research
and contributed to the writing of the paper. AYM and YM mainly
conducted the research. UH, TK and MTA conducted the in vivo
analysis. MY and UH contributed to the production of the virus. FH,
MY, YK, KM and MS assisted with statistical analysis and also
analyzed the data. TK, YK and MS proofread the paper. KM and MTA
revised the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the study are appropriately investigated and resolved.
Ethics approval and consent to
participate
All the animal experiments performed in this study
were in accordance with the ethical standards of the Animal Care
and Use Committee of Hokkaido University (Sapporo, Hokkaido,
Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shenk T: Adenoviridae: The viruses and
their replication. Fundamental Virology. Knipe DM and Howley PM:
4th. Lippincott Williams & Wilkins Ltd.; Philadelphia: pp.
1053–1088. 2001
|
|
2
|
Halbert DN, Cutt JR and Shenk T:
Adenovirus early region 4 encodes functions required for efficient
DNA replication, late gene expression, and host cell shutoff. J
Virol. 56:250–257. 1985.PubMed/NCBI
|
|
3
|
Sarnow P, Hearing P, Anderson CW, Halbert
DN, Shenk T and Levine AJ: Adenovirus early region 1B 58,000-dalton
tumor antigen is physically associated with an early region 4
25,000-dalton protein in productively infected cells. J Virol.
49:692–700. 1984.PubMed/NCBI
|
|
4
|
Querido E, Blanchette P, Yan Q, Kamura T,
Morrison M, Boivin D, Kaelin WG, Conaway RC, Conaway JW and Branton
PE: Degradation of p53 by adenovirus E4orf6 and E1B55K proteins
occurs via a novel mechanism involving a Cullin-containing complex.
Genes Dev. 15:3104–3117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harada JN, Shevchenko A, Shevchenko A,
Pallas DC and Berk AJ: Analysis of the adenovirus E1B-55K-anchored
proteome reveals its link to ubiquitination machinery. J Virol.
76:9194–9206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo K, Ehrlich E, Xiao Z, Zhang W, Ketner
G and Yu XF: Adenovirus E4orf6 assembles with
Cullin5-ElonginB-ElonginC E3 ubiquitin ligase through an HIV/SIV
Vif-like BC-box to regulate p53. FASEB J. 21:1742–1750. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng CY, Blanchette P and Branton PE: The
adenovirus E4orf6 E3 ubiquitin ligase complex assembles in a novel
fashion. Virology. 364:36–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stracker TH, Carson CT and Weitzman MD:
Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair
complex. Nature. 418:348–352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baker A, Rohleder KJ, Hanakahi LA and
Ketner G: Adenovirus E4 34k and E1b 55k oncoproteins target host
DNA ligase IV for proteasomal degradation. J Virol. 81:7034–7040.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dallaire F, Blanchette P, Groitl P, Dobner
T and Branton PE: Identification of integrin alpha3 as a new
substrate of the adenovirus E4orf6/E1B 55-kilodalton E3 ubiquitin
ligase complex. J Virol. 83:5329–5338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woo JL and Berk AJ: Adenovirus
ubiquitin-protein ligase stimulates viral late mRNA nuclear export.
J Virol. 81:575–587. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blanchette P, Kindsmüller K, Groitl P,
Dallaire F, Speiseder T, Branton PE and Dobner T: Control of mRNA
export by adenovirus E4orf6 and E1B55K proteins during productive
infection requires E4orf6 ubiquitin ligase activity. J Virol.
82:2642–2651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Täuber B and Dobner T: Adenovirus early E4
genes in viral oncogenesis. Oncogene. 20:7847–7854. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Javier RT: Adenovirus type 9 E4 open
reading frame 1 encodes a transforming protein required for the
production of mammary tumors in rats. J Virol. 68:3917–3924.
1994.PubMed/NCBI
|
|
15
|
Nevels M, Täuber B, Kremmer E, Spruss T,
Wolf H and Dobner T: Transforming potential of the adenovirus type
5 E4orf3 protein. J Virol. 73:1591–1600. 1999.PubMed/NCBI
|
|
16
|
Moore M, Horikoshi N and Shenk T:
Oncogenic potential of the adenovirus E4orf6 protein. Proc Natl
Acad Sci USA. 93:11295–11301. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nevels M, Rubenwolf S, Spruss T, Wolf H
and Dobner T: The adenovirus E4orf6 protein can promote
E1A/E1B-induced focus formation by interfering with p53 tumor
suppressor function. Proc Natl Acad Sci USA. 94:1206–1211. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Javier R, Raska K Jr and Shenk T:
Requirement for the adenovirus type 9 E4 region in production of
mammary tumors. Science. 257:1267–1271. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higashino F, Aoyagi M, Takahashi A, Ishino
M, Taoka M, Isobe T, Kobayashi M, Totsuka Y, Kohgo T and Shindoh M:
Adenovirus E4orf6 targets pp32/LANP to control the fate of
ARE-containing mRNAs by perturbing the CRM1-dependent mechanism. J
Cell Biol. 170:15–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuroshima T, Aoyagi M, Yasuda M, Kitamura
T, Jehung JP, Ishikawa M, Kitagawa Y, Totsuka Y, Shindoh M and
Higashino F: Viral-mediated stabilization of AU-rich element
containing mRNA contributes to cell transformation. Oncogene.
30:2912–2920. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen CY and Shyu AB: AU-rich elements:
Characterization and importance in mRNA degradation. Trends Biochem
Sci. 20:465–470. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jacobson A and Peltz SW:
Interrelationships of the pathways of mRNA decay and translation in
eukaryotic cells. Annu Rev Biochem. 65:693–739. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brennan CM and Steitz JA: HuR and mRNA
stability. Cell Mol Life Sci. 58:266–277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hinman MN and Lou H: Diverse molecular
functions of Hu proteins. Cell Mol Life Sci. 65:3168–3181. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
López de Silanes I, Lal A and Gorospe M:
HuR: Post-transcriptional paths to malignancy. RNA Biol. 2:11–13.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
López de Silanes I, Fan J, Yang X,
Zonderman AB, Potapova O, Pizer ES and Gorospe M: Role of the
RNA-binding protein HuR in colon carcinogenesis. Oncogene.
22:7146–7154. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aoyagi M, Higashino F, Yasuda M, Takahashi
A, Sawada Y, Totsuka Y, Kohgo T, Sano H, Kobayashi M and Shindoh M:
Nuclear export of adenovirus E4orf6 protein is necessary for its
ability to antagonize apoptotic activity of BH3-only proteins.
Oncogene. 22:6919–6927. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kakuguchi W, Kitamura T, Kuroshima T,
Ishikawa M, Kitagawa Y, Totsuka Y, Shindoh M and Higashino F: HuR
knockdown changes the oncogenic potential of oral cancer cells. Mol
Cancer Res. 8:520–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abdelmohsen K, Srikantan S, Yang X, Lal A,
Kim HH, Kuwano Y, Galban S, Becker KG, Kamara D, de Cabo R, et al:
Ubiquitin-mediated proteolysis of HuR by heat shock. EMBO J.
28:1271–1282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bischoff JR, Kirn DH, Williams A, Heise C,
Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, et al:
An adenovirus mutant that replicates selectively in p53-deficient
human tumor cells. Science. 274:373–376. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Larson C, Oronsky B, Scicinski J, Fanger
GR, Stirn M, Oronsky A and Reid TR: Going viral: A review of
replication-selective oncolytic adenoviruses. Oncotarget.
6:19976–19989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bressy C and Benihoud K: Association of
oncolytic adenoviruses with chemotherapies: An overview and future
directions. Biochem Pharmacol. 90:97–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heise C, Hermiston T, Johnson L, Brooks G,
Sampson-Johannes A, Williams A, Hawkins L and Kirn D: An adenovirus
E1A mutant that demonstrates potent and selective systemic
anti-tumoral efficacy. Nat Med. 6:1134–1139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kawashima T, Kagawa S, Kobayashi N,
Shirakiya Y, Umeoka T, Teraishi F, Taki M, Kyo S, Tanaka N and
Fujiwara T: Telomerase-specific replication-selective virotherapy
for human cancer. Clin Cancer Res. 10:285–292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rodriguez R, Schuur ER, Lim HY, Henderson
GA, Simons JW and Henderson DR: Prostate attenuated replication
competent adenovirus (ARCA) CN706: A selective cytotoxic for
prostate-specific antigen-positive prostate cancer cells. Cancer
Res. 57:2559–2563. 1997.PubMed/NCBI
|
|
36
|
Wang J, Guo Y, Chu H, Guan Y, Bi J and
Wang B: Multiple functions of the RNA-binding protein HuR in cancer
progression, treatment responses and prognosis. Int J Mol Sci.
14:10015–10041. 2013. View Article : Google Scholar : PubMed/NCBI
|