Introduction
Therapeutic resistance of common types of solid
cancer to diverse cytotoxic agents is a challenge in medicine, and
is primarily acquired through mechanisms including the upregulation
of xenobiotic efflux pumps and enhancement of DNA repair (1,2).
Glutathione S-transferase (GST) in the cytosol has various isozymes
that may be further assigned into sub-isoforms of splice variants
(3). The upregulation of these GST
isozymes has been proposed to cause drug resistance through the
enhanced catalytic detoxification of antineoplastic agents and the
modulations of apoptotic signaling pathways (4–6). The
suppression of GST activity is thus expected to sensitize
drug-resistant solid cancer cells to cytotoxic agents (2,6–12).
However, GSTs play complicated physiological roles in cells, and
the expression profiles of GST isozymes in cancer cells have been
associated with sex, tissues and organs (4,13,14).
The incidence of solid cancers in different origins is associated
with various GST isozymes (4).
Therefore, the cost and time of screening for potent inhibitors
against such GST isozymes to sensitize common types of
drug-resistant solid cancer is a challenge; no notable progress has
been made yet regarding the use of selective GST isozyme inhibitors
in the treatment of common types of drug-resistant solid
cancer.
The recognition of GST isozymes predominantly
involved in drug resistance of common types of solid cancer
facilitates the development of potent selective inhibitors to be
used as general sensitizers of such types of drug-resistant solid
cancer. To recognize a GST isozyme responsible for drug resistance,
the effects of selective inhibition of its actions on drug toxicity
should be assessed. To this end, the most straightforward method is
the detection of drug action after the selective inhibition of each
GST isozyme; however, the selective inhibitors for each GST isozyme
are yet to be developed. Current inhibitors for GST isozymes are
not satisfactory with regard to their isozyme-selectivity,
inhibition potency and membrane permeability (7–12).
Therefore, alternative methods have to be sought to determine GST
isozymes associated with drug resistance of common types of solid
cancer.
Short interfering RNAs (siRNAs) can selectively
block the action of a specified protein by inhibiting the
expression and/or translation of the target gene. As a result,
siRNAs are promising tools for downregulating the expression of GST
isozymes in order to detect their roles in drug resistance
(15,16) and to identify GST isozymes that may
be suitable targets to sensitize common types of drug-resistant
solid cancer to cytotoxic agents. Studies on different cancer cells
and tissues have identified the contributions of various GST
isozymes to the incidence of drug resistance in common types of
solid cancer (4,13,14).
However, for drug-resistant cancer cells or tissues, the available
studies have been investigating the roles of GST isozyme(s) in one
type of drug-resistant solid cancer, and few have been examining
the roles of different GST isozymes in different types of
drug-resistant solid cancer. Lung, ovarian and stomach cancer types
exhibit high rates of drug resistance, and therefore high mortality
rates (17). Cisplatin (DDP) alone
or in combination with other cytotoxic agents is the first-line
chemotherapy for such types of solid cancer; however, the
therapeutic efficiency is usually hindered due to the development
of acquired drug resistance (2,18) and
the upregulated expression of glutathione S-transferase pi 1
(GSTP1), glutathione S-transferase mu 2 (GSTM2) and glutathione
S-transferase alpha 1 (GSTA1) (4,19–23).
To ascertain the hypothesis that there may be a specific GST
isozyme that is predominantly involved in the drug resistance of
common types of solid cancer to DDP, siRNAs of GSTP1, GSTM2 and
GSTA1 were transfected into DDP-resistant solid cancer cells,
A549/DDP, SKOV3/DDP and SGC7901/DDP, which were originated from the
lungs, ovaries and stomach, respectively; cells after further
treatment with DDP were then examined for proliferation and
apoptosis.
Materials and methods
Materials
Lipofectamine 2000 was purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Human non-small
cell lung cancer cell line A549 (TCHu150) and human gastric cancer
cell lines SGC7901 (TCHu 46) were purchased from the Cell Bank of
the Chinese Academy of Sciences (Shanghai, China);
Cisplatin-resistant A549/DDP (BNCC341254) and cisplatin-resistant
SGC7901/DDP (BNCC342230) were purchased from the BeNa Culture
Collection (Beijing Bei Na Chuanglian Biotechnology Research
Institute, Beijing, China). Cisplatin-sensitive human ovarian cell
line SKOV3 and their cisplatin-resistant clones SKOV3/DDP were
obtained from the Chinese Academy of Medical Sciences and Peking
Union Medical College (Beijing, China). Rabbit polyclonal primary
antibody against GSTM2 (cat. no. YN2960) and rabbit polyclonal
primary antibody against β-actin (cat. no. A283), were purchased
from ImmunoWay Biotechnology Co., Ltd. (Plano, TX, USA). Rabbit
polyclonal primary antibody against GSTP1 (cat. no. D222453) was
purchased from Sangon Biotechnology Co., Ltd. (Shanghai, China),
rabbit monoclonal primary antibody against GSTA1 (cat. no.
ab207413) was purchased from Abcam (Cambridge, MA, USA).
HRP-labeled goat anti-rabbit IgG (cat. no. A25222) was purchased
from Abbkine Scientific Co., Ltd. (Wuhan, China).
1-Chloro-2,4-dinitrobenzene (CDNB) was obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Reduced glutathione (GSH) was
obtained from Beijing Dingguo Changsheng Biotechnology Co., Ltd.
(Beijing, China). Other chemicals were analytical reagents, unless
otherwise stated.
Cell culture and siRNA
transfection
Three candidate sequences of siRNA for silencing
GSTP1, GSTM2 and GSTA1 and negative siRNA were designed and
synthesized by Suzhou GenePharma Co., Ltd. (Suzhou, China) and the
most potent siRNA was screened. Cells were cultured in Roswell Park
Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal
bovine serum (FBS; Capricorn Scientific GmbH Ebsdorfergrund,
Germany), 100 kU/l penicillin and 100 mg/l streptomycin in an
atmosphere containing 5% CO2 at 37°C. Cells were grown
in the complete medium for 24 h and subsequently transfected with
GST isozyme siRNAs. Following pre-incubation at room temperature
for 20 min, cells were seeded in 6-well plates to 60–70% confluence
and treated with a solution of 0.2 µg siRNA and 5 µl Lipofectamine
2000 in Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.). The final
concentration of siRNA used was 50 pmol/l. Cells were incubated
with medium containing siRNA and Lipofectamine complex for 6 h.
Following this, the transfection medium was replaced with complete
medium free of antibiotics. The transfected cells were incubated at
37°C in an atmosphere containing 5% CO2 for 48 h.
Candidate sequences for siRNA of
GSTP1, GSTM2 and GSTA1
GSTP1 sequence 1: Sense, 5′-CCUACACCGUGGUCUAUUUTT-3′
and antisense, 5′-AAAUAGACCACGGUGUAGGTT-3′; GSTP1 sequence 2:
Sense, 5′-CCUCAUCUACACCAACUAUTT-3′ and antisense,
5′-AUAGUUGGUGUAGAUGAGGTT-3′; GSTP1 sequence 3: Sense,
5′-GCUGAUCCAUGAGGUCCUATT-3′; and antisense,
5′-UAGGACCUCAUGGAUCAGCTT-3′; GSTM2 sequence 1: Sense,
5′-GGAUUUCAUCGCUUAUGAUGUTT-3′ and antisense,
5′-ACAUCAUAAGCGAUGAAAUCCTT-3′; GSTM2 sequence 2: Sense,
5′-GCACUCCCUGAAAUGCUGAAGTT-3′ and antisense,
5′-CUUCAGCAUUUCAGGGAGUGCTT-3′; GSTM2 sequence 3: Sense,
5′-GAUUUGAGGCUUGGAGAAGATT-3′ and antisense,
5′-UCUUCUCCAAGCCCUCAAAUCTT-3′; GSTA1 sequence 1: Sense,
5′-CCACAGUGAAGAAGUUUCUTT-3′ and antisense:
5′-AGAAACUUCUUCACUGUGGTT-3′; GSTA1 sequence 2: Sense,
5′-CCAAGCUUGCCUUGAUCAATT-3′ and antisense,
5′-UUGAUCAAGGCAAGCUUGGTT-3′; GSTA1 sequence 3: Sense,
5′-GGAGCUUGACUCCAGUCUUTT-3′ and antisense,
5′-AAGACUGGAGUCAAGCUCCTT-3′.
Western blot analysis
DDP-susceptible or DDP-resistant cells were detached
with trypsin, centrifuged at 4°C and washed three times with
pre-chilled phosphate-buffered saline (PBS). RIPA lysis buffer
(cat. no. P0013K; Beyotime Institute of Biotechnology, Haimen,
Jiangsu, China) plus PMSF (cat. no. ST506; Beyotime Institute of
Biotechnology), was subsequently added and the cells were incubated
on ice for 30 min. The supernatants containing proteins were
collected by centrifugation at 13,000 × g for 20 min. Protein
concentration in each cell lysate was determined using the Bradford
assay with BSA as the reference (24). A total of 50 µg protein was
separated from each group using SDS-PAGE (10% gels) and transferred
to a polyvinylidene fluoride (PVDF) membrane (0.45 µm; GE
Healthcare, Chicago, IL, USA). The membrane was soaked in 5% bovine
serum albumin (BSA) (in TBS at pH 7.4 containing 0.1% Tween-20) for
2 h and incubated with rabbit polyclonal/monoclonal antibodies
against GST isozymes and β-actin (all 1:1,000 dilution) at 4°C
overnight. Following this, the PVDF membrane was washed and
subsequently incubated with goat anti-rabbit IgG conjugated to
horseradish peroxidase (dilution 1:5,000; cat. no. A25222; from
Abbkine Scientific Co., Ltd., Wuhan, China) at 37°C for 1 h. The
ChemiDoc MP Imaging System (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and BeyoCEL Plus (Beyotime Institute of Biotechnology,
Haimen, China) were used to detect chemiluminescence under the
catalytic action of horseradish peroxidase conjugated to the
adsorbed goat anti-rabbit IgG. Densitometry analysis was performed
using ImageJ software (version 1.46; National Institutes of Health,
Bethesda, MD, USA).
Assay of GST activity in lysates
As described by Habig et al (25), in 1.0 ml of 20 mmol/l phosphate
buffer (pH 6.5) containing GSH and CDNB (final concentration, 1.0
mmol/l, 30 µl of cell lysate was added to initiate the reaction,
and the absorbance at 340 nm was recorded for 3 min at 10-sec
intervals, following a lagging time of 15 sec. One unit of GST
activity was defined as the amount of GST enzyme that resulted in 1
µmol product/min at 25°C (pH 6.5). Apparent specific activities in
cell lysates were calculated using the concentrations of total
proteins (24).
Cell proliferation assay
Cell proliferation was assessed using the Cell
Counting Kit-8 assay (CCK-8; Biotool, Houston, TX, USA). Following
transfection with GST isozyme positive siRNAs or negative siRNAs
(sicontrol), A549/DDP, SKOV3/DDP and SGC7901/DDP cells were seeded
in 96-well plates at a density of 5.0×107 cells/l.
Following 24 h of incubation, DDP was added at a final
concentration of 0–80 µmol/l for A549/DDP, 0–40 µmol/l for
SKOV3/DDP and 0–20 µmol/l for SGC7901/DDP. Cells were subsequently
incubated for 72 h (15,16,20–22).
CCK-8 was added according to the manufacturer's instructions in
order to detect the absorbance at 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc.).
Apoptosis assay
Cells transfected with a positive siRNA against an
indicated GST isozyme or negative siRNA (sicontrol) were further
treated for 72 h with DDP (10 µmol/l for A549/DDP but 5 µmol/l for
SKOV3/DDP and SCG7901/DDP). To examine apoptosis, DDP
concentrations were set to maintain >60% survival of the tested
solid cancer cells when treated with DDP alone (15,16,20–22).
Cells after the treatment were collected and washed with pre-cooled
PBS prior to staining using Annexin Cy5 apoptosis assay kit (cat.
no. ab14150) and propidium iodide flow cytometric kit (cat. no.
ab139418; Abcam, Cambridge, MA, USA) according to the
manufacturer's instructions. Following this, analysis was conducted
using flow cytometry (FACSVantage SE system; BD Biosciences,
Franklin Lakes, NJ, USA). Data acquisition and analysis were
performed using CellQuest Pro software (version 5.1; BD
Biosciences).
Hoechst 33342 staining
Cells after silencing of an indicated GST isozyme
and the control cells were seeded in 24-well plates at a density of
5,000 cells/well for 24 h-incubation and subsequently treated with
DDP further as described above for 72 h. Cells were fixed with 50%
methanol at 4°C for 30 min and stained with 10 µl Hoechst 33342
(Invitrogen; Thermo Fisher Scientific, Inc.) for 10 min at 4°C in
the dark, according to the manufacturer's instructions. The
supernatant was discarded and the cells were washed three times
with pre-cooled PBS. Cells were then observed under an inverted
microscope (Nikon Corp., Tokyo, Japan).
Statistical analysis
SPSS software (version 19.0; IBM Corp., Armonk, NY,
USA) was used for statistical analysis. Experimental data were
performed in triplicate and presented as the mean ± standard
deviation. The Student's t-test was used for analyzing the
differences between groups and one-way ANOVA followed by a
Newman-Keuls post hoc test was used for the analysis of the
differences among groups. P<0.05 was considered to indicate a
statistically significant result.
Results
Expression and activity of GSTs
As upregulated GST expression levels are commonly
associated with the incidence of DDP-resistance, the activities and
expression levels of GSTP1, GSTM2 and GSTA1 in the three tested
types of DDP-resistant cancer cells were compared with those in
their parental DDP-susceptible cells. All DDP-resistant cancer
cells exhibited some increased GST activities compared with the
parental DDP-susceptible cells (Table
I). Notably, a significant increase of GST activity was
observed in SKOV3/DDP cells compared with that in SKOV3 cells.
Compared with those in the parental DDP-susceptible cells, the
protein expression levels of GSTP1, GSTM2 and GSTA1 were
upregulated in SKOV3/DDP according to western blot analysis
(Fig. 1B); the protein expression
levels of GSTP1 and GSTA1 were increased, while that of GSTM2 was
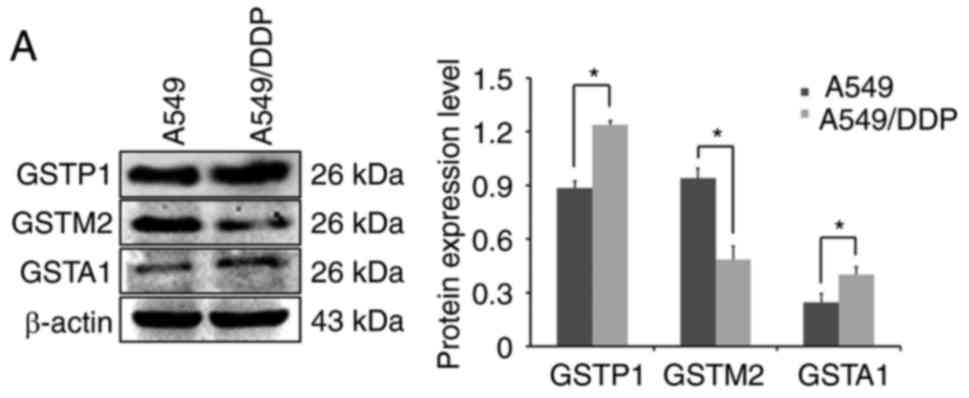
decreased in A549/DDP cells (Fig.
1A). These results were in line with data revealing GSTP
expression to be significantly upregulated in a variety of
drug-resistant solid cancer types (originating from the breast,
colon, pancreas, liver, lung, ovary and stomach), and GSTA and GSTM
expression to be induced by lower extents in some types of such
drug-resistant solid cancers (4,12–15,18).
However, the protein levels of GSTM2 and GSTA1 were decreased in
SGC7901/DDP cells compared with those in parental DDP-susceptible
cells. Furthermore, the protein expression of GSTP1 was decreased
to a lower degree in SGC7901/DDP cells compared with that in
parental DDP-susceptible cells (Fig.
1C), potentially due to lower quantification accuracy of
western blot analysis.
 | Table I.Comparison of the GST activity in
cell lysates. |
Table I.
Comparison of the GST activity in
cell lysates.
| Cell lines | GST activity in
cell lysates (U/mg) | Ratioa |
|---|
| A549 | 0.19±0.02 |
|
| A549/DDP | 0.24±0.02 | 1.3 |
| SGC7901 | 0.14±0.01 |
|
| SGC7901/DDP | 0.18±0.02 | 1.3 |
| SKOV3 | 0.43±0.04 |
|
| SKOV3/DDP |
1.25±0.10b | 2.9 |
Screening for siRNA against GST
isozymes
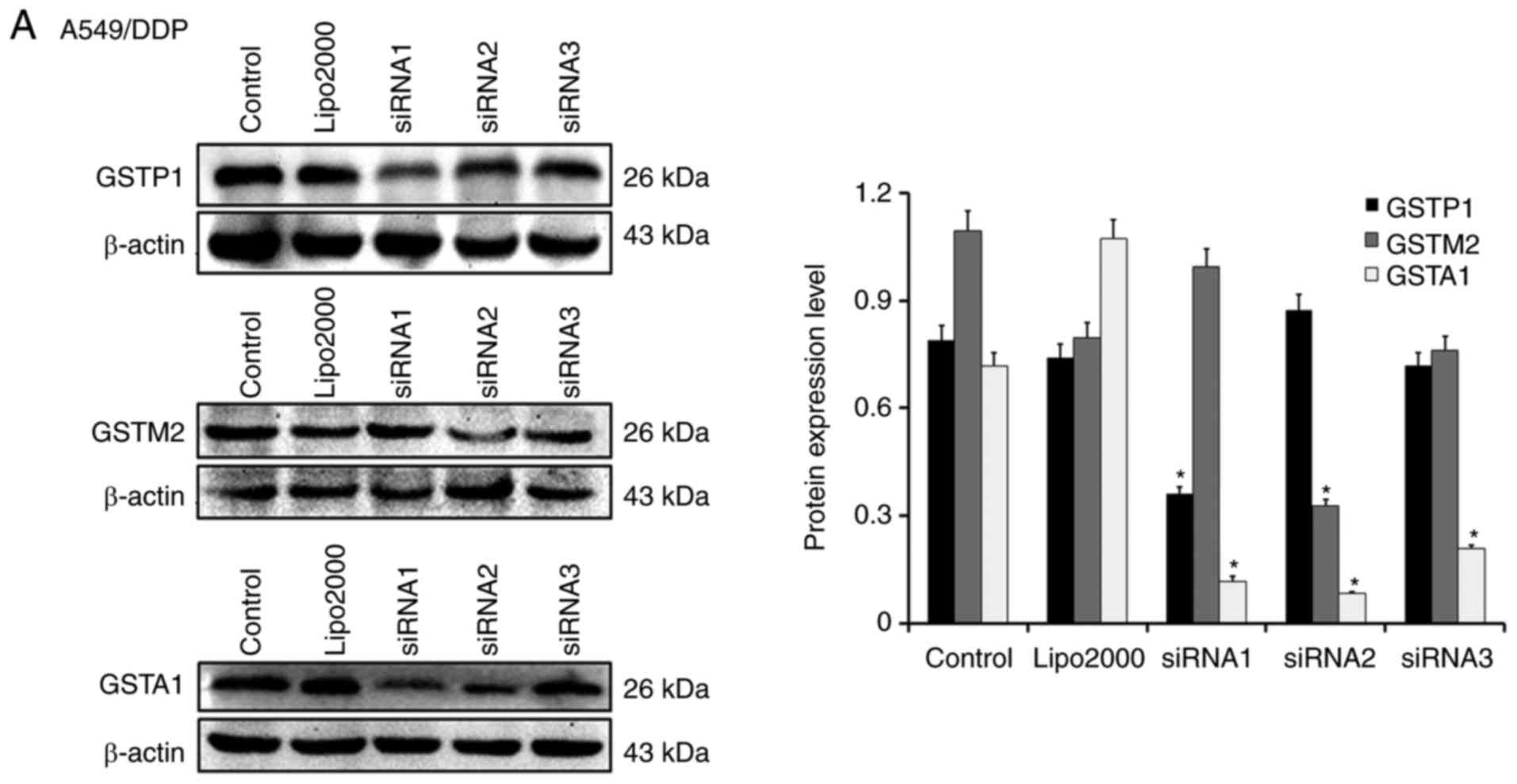
Three candidate sequences of siRNA against GSTM2,
GSTP1 and GSTA1 were designed by Suzhou GenePharma Co., Ltd.
A549/DDP, SKOV3/DDP and SGC7901/DDP cells were separately
transfected with each of those three candidates of siRNA for GSTP1,
GSTM2, or GSTA1 in liposome, and the expression profiles of GSTs
were compared by western blot analysis for screening the sequence
causing the largest reduction in the protein expression of each GST
isozyme. Compared with the untreated cells or those treated solely
with Lipofectamine 2000, different candidate sequences of siRNAs
resulted in varying reductions of each targeted GST isozyme
(Fig. 2). Accordingly, those
producing the most significant reductions of protein expression in
each of the three types of cancer cells, i.e., sequence 1
for si-GSTP1, sequence 2 for si-GSTM2, and sequence 2 for si-GSTA1
in A549/DDP (Fig. 2A), sequence 3
for si-GSTP1, sequence 2 for si-GSTM2, and sequence 1 for si-GSTA1
in SKOV3/DDP (Fig. 2B), and
sequence 3 for si-GSTP1, sequence 1 for si-GSTM2 and sequence 2 for
si-GSTA1 in SGC7901/DDP (Fig. 2C),
were selected for subsequent experiments.
Effects of silencing GST isozymes on
DDP toxicity to DDP-resistant cancer cells
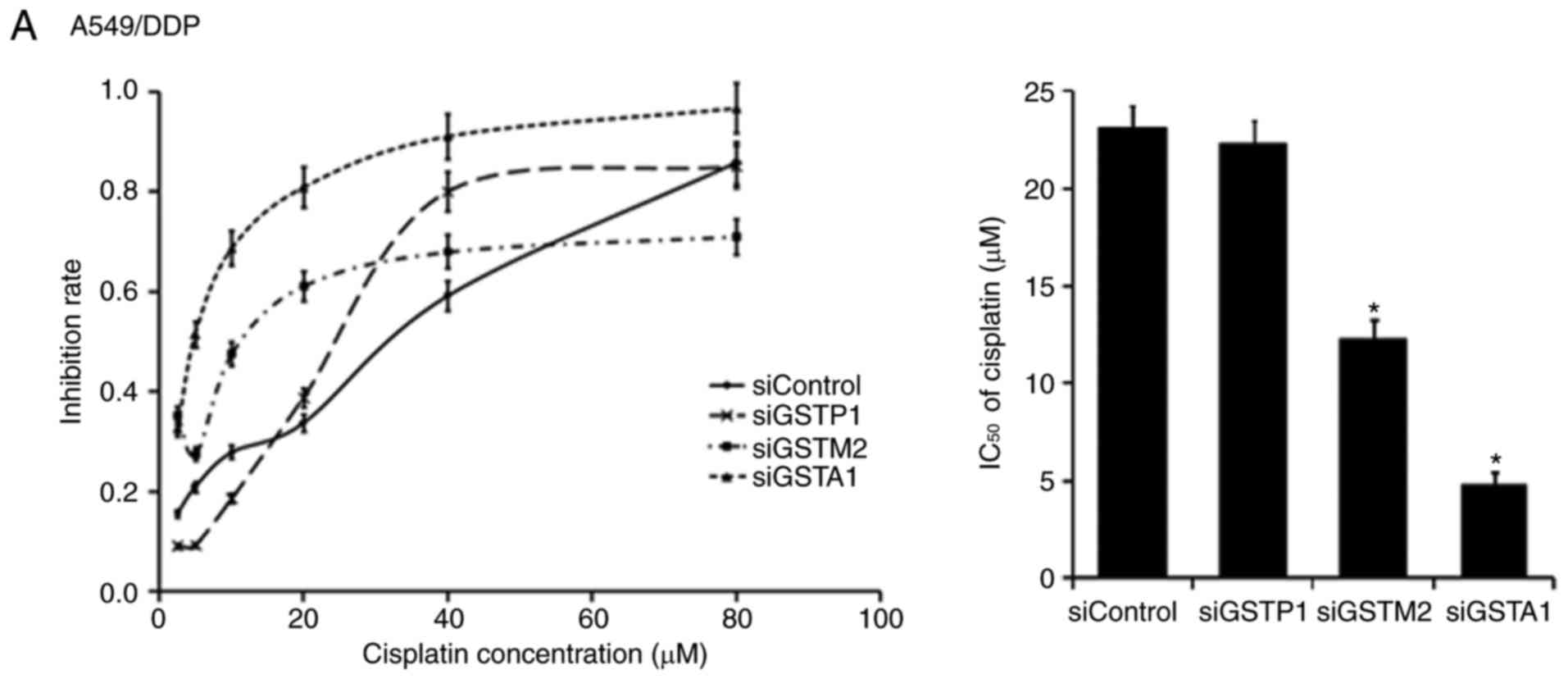
To investigate whether siRNAs of GSTP1, GSTM2 and
GSTA1 modulate differently the susceptibility of A549/DPP,
SKOV3/DDP and SGC7901/DDP cells to DDP, those cells after the
transfection with positive or negative siRNAs were further treated
with DDP. Of the three tested types of DDP-resistant cancer cells
transfected with positive and negative siRNAs, the half maximal
inhibition concentration (IC50) values of DDP were
compared. For each of the three types of DDP-resistant cancer
cells, the treatment with Lipofectamine 2000 alone or the
transfection with a negative siRNA resulted in a survival rate
consistent with that of the untreated cells (data not shown). In
comparison with cells after the combination treatment with DDP and
a negative siRNA against a certain GST isozyme, IC50 of
DDP was decreased differently in each DDP-resistant cell type after
the combination treatment with DDP and the positive siRNA against
the indicated GST isozyme (Fig. 3).
In detail, in A549/DDP cells, the silencing of GSTA1 resulted in a
5-fold decrease whereas the silencing of GSTM2 resulted in a 2-fold
decrease in the IC50 of DDP, but the silencing of GSTP1
had no significant effect on the IC50 of DDP (Fig. 3A). In SKOV3/DDP cells, the silencing
of GSTM2 and GSTA1 resulted in 2-fold decreases in the
IC50 of DDP, whereas the silencing of GSTP1 had
negligible effect on the IC50 of DDP (Fig. 3B). In SGC7901/DDP cells, the
silencing of GSTA1 and GSTP1 resulted in 6- and 4-fold decreases,
respectively, whereas the silencing of GSTM2 provided a marginal
reduction in the IC50 of DDP (Fig. 3C). Therefore, these GST isozymes may
have different roles in DDP resistance of the tested three types of
DDP-resistant solid cancer. Notably, the silencing of GSTA1
produced the highest sensitizing effects on DDP cytotoxicity in the
examined three types of DDP-resistant solid cancer cells,
indicating that GSTA1 may be a suitable target for the development
of potent selective inhibitors to sensitize various types of
drug-resistant solid cancer.
Effects of silencing of GSTs on cell
apoptosis
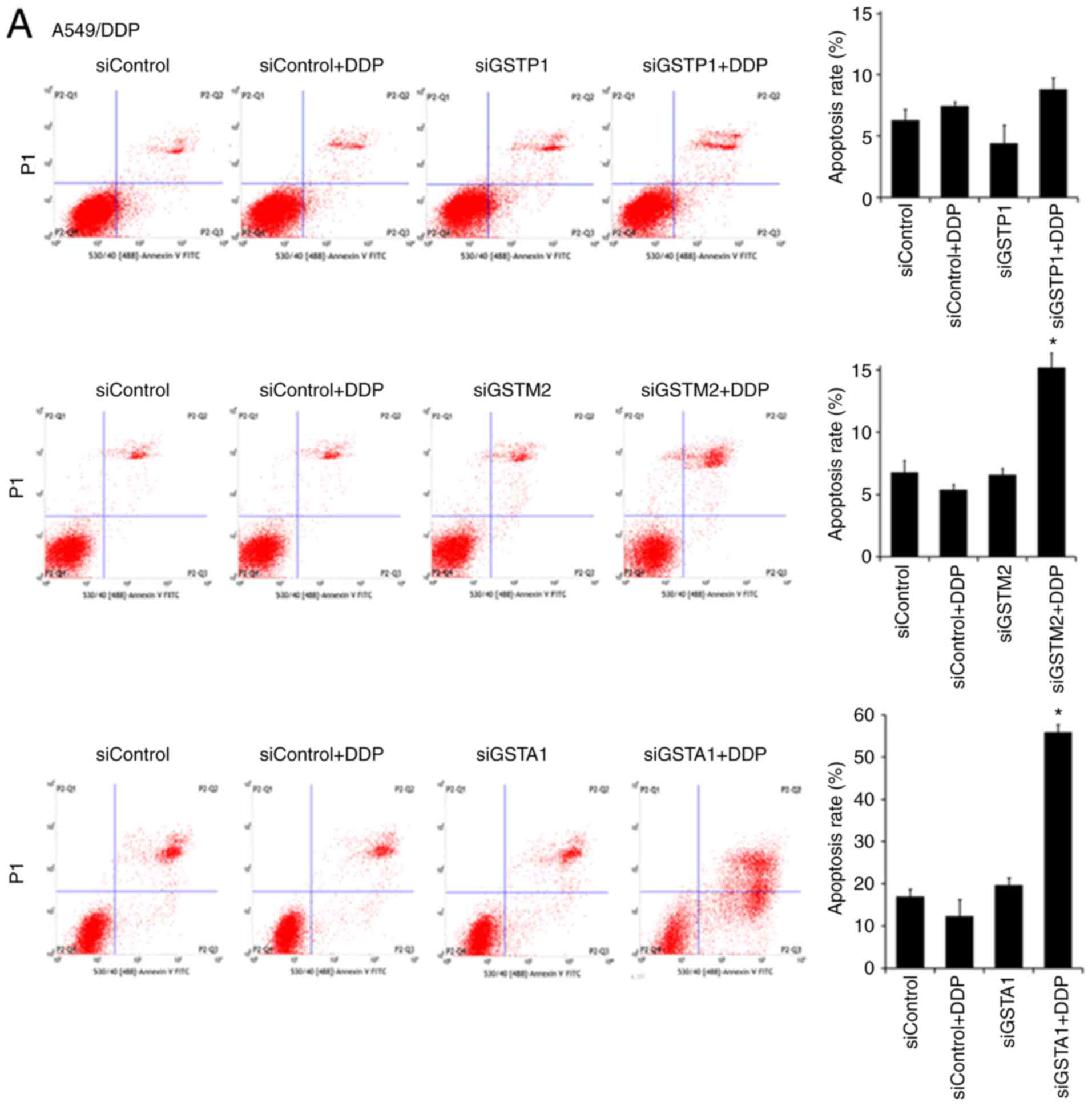
To assess the cellular mechanism associated with the
sensitization effects of GST silencing on DDP toxicity, the
apoptotic rates of A549/DDP, SKOV3/DDP and SGC7901/DDP cells were
determined using flow cytometry and the morphological changes were
observed using Hoechst 33342 staining. On the apoptosis rate of
each tested type of DDP-resistant cells, the effect of the combined
treatment with a positive siRNA against a certain GST isozyme and
DDP at a final concentration smaller than the IC50 was
compared with that of the combined treatment with DDP at the same
level and a negative siRNA. Of either of those three tested types
of DDP-resistant cancer cells, the treatment with only a negative
siRNA against a certain GST isozyme or Lipofectamine 2000 alone
produced an apoptosis rate consistent with that of the untreated
cells (data not shown). However, the effective silencing of GSTP1,
GSTM2 and GSTA1 with positive siRNAs had different synergistic
effects with DDP treatment on the apoptosis rates of the tested
types of DDP-resistant cancer cells (Fig. 4). In detail, in A549/DDP cells, the
treatment with DDP after the effective silencing of GSTP1, GSTM2
and GSTA1 resulted in apoptosis rates that were 1- (insignificant),
3- and 4-fold of those in the same cells after the combination
treatment with DDP and the negative siRNAs, correspondingly
(Fig. 4A). In SKOV3/DDP cells, the
combination treatment with DPP and the positive siRNAs of GSTP1,
GSTM2 and GSTA1 resulted in apoptosis rates that were ~1.2-
(insignificant), 6- and 13-fold of those in the same cells after
the combination treatment with DDP and the negative siRNAs,
respectively (Fig. 4B). In
SGC7901/DDP cells, the combination treatment with DDP and the
positive siRNAs of GSTP1, GSTM2 and GSTA1 resulted in apoptosis
rates that were 2-, 1.4- and 3-fold of those in the same cells
treated with DDP and the negative siRNAs, correspondingly (Fig. 4C). Clearly, of the tested
DDP-resistant cancer cells after the combination treatment with DDP
and the positive siRNAs, the apoptotic rates were inversely
associated with their IC50 values of DDP (Fig. 3), among which the silencing of GSTA1
consistently exhibited the highest percentage of cell apoptosis.
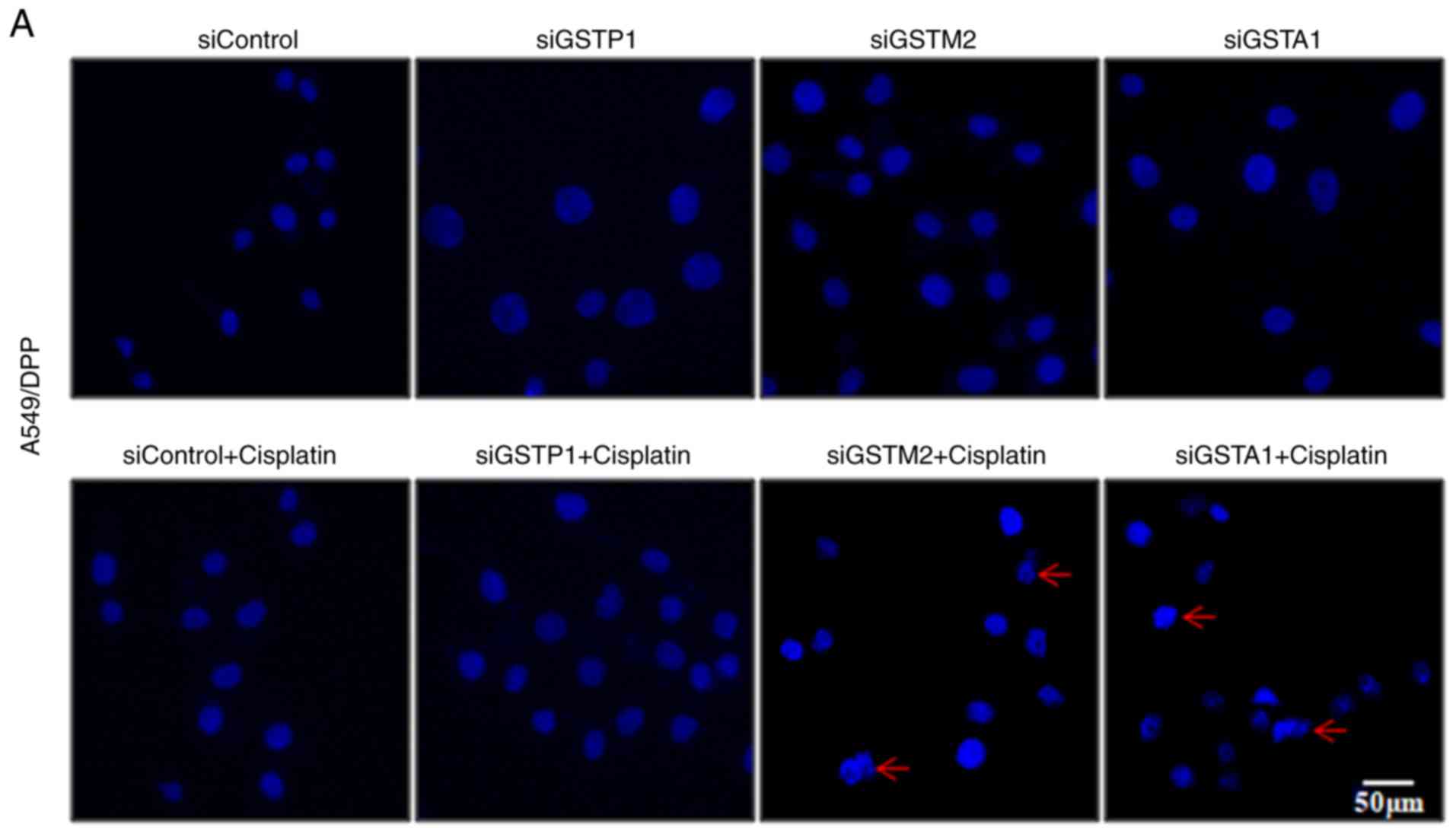
Furthermore, staining with Hoechst 33342 revealed some
characteristic features of apoptosis, such as pyknotic and
condensed nuclei, in each type of DDP-resistant cells subjected to
the combination treatment of the positive siRNAs and DDP (Fig. 5). Therefore, of the three tested
types of DDP-resistant solid cancer cells, GSTA1 was the
predominant GST isozyme associated with their DDP-resistance and
the downregulation of GSTA1 expression levels may enhance DDP
cytotoxicity by the promotion of cell apoptosis.
Discussion
DDP is a first-line anticancer agent that is widely
used for the treatment of various types of solid cancer, but
frequently confronts with the challenge of DDP-resistance.
Inhibition of the expression of a specified GST isozyme by
chemicals or siRNA has been found to reverse DDP-resistance in
A549/DDP, SCG7901/DDP or SKOV3-/DDP cells, but there are few
studies on any of the same GST isozymes potentially involved in
DDP-resistance of different types of solid cancer (15,16,20–22).
The present study indicated that there were some increases in the
activities of GSTs and the differently induced expression of tested
GST isozymes in SGC7901/DDP, A549/DDP and SKOV3/DDP cells. Notably,
the transfection with positive siRNA against GSTA1 resulted in the
largest enhancements of DDP toxicity and apoptotic rates of all the
tested three types of DDP-resistant solid cancer cells, in
comparison to the transfections with siRNAs against GSTP1 and
GSTM2. Therefore, GSTA1 may be a rational target for the
development of GSTA1-selective inhibitors to sensitize all the
three types of, and even other types of, DDP-resistant cancer
cells.
However, the exact action mechanisms of GST
isozymes, especially GSTA1, in DDP resistance of SGC7901/DDP,
A549/DDP and SKOV3/DDP cells remain unclear. The mechanism of DDP
action which can be intervened by GST isozymes putatively involves
two ways; one is the initiation of some toxic consequences that can
be alleviated by the catalytic actions of GST isozymes, the other
is the induction of apoptosis primarily through the activation of
the mitogen-activated protein kinase signaling pathway involving
the interactions with c-Jun N-terminal kinase (JNK) whose actions
can be modulated by GST isozymes (2,18,26).
In general, the effects of siRNAs cannot discriminate the roles of
these two ways, since siRNA reduced the protein expression levels
of GST isozymes, and thus decreased both their catalytic actions
and their interactions with signaling proteins involved in cell
apoptosis. In fact, GSTs are a family of detoxification enzymes
that can be upregulated in response to xenobiotics stress (4,5), and
putatively catalyze the conjugation of cytotoxic drugs with GSH to
drive the efflux of their conjugates through transporters (2,6,7). With
regard to biotransformations, GSTP1 and GSTA1 preferrentially act
on DDP and doxorubicin, while GSTM1 has stronger actions on
nitrosourea and nitrogen mustards (4–7,27). In
cancer cells, DDP produces a toxic product of lipid peroxidation,
4-hydroxyheptanene (4HNE), which at high levels activates JNK and
caspase-3 to induce apoptosis (28). When GSH is heavily consumed, 4HNE
acts as a small electrophile to form conjugates with proteins and
DNA that also induce apoptosis (29). Notably, various studies on GSTA
subtypes have suggested that they regulate the physiological
effects of oxidative stress by limiting the actions of 4HNE
(28,30). For instance, GSTA1-1 and GSTA2-2
were found to limit the formation of 4HNE by catalyzing the
metabolism of lipid hydroperoxides that were the precursors of
4HNE, and GSTA4-4 was identified to reduce 4HNE levels via the
direct catalysis on the conjugation of 4HNE and GSH (4,28,30).
As a result, the reductions of 4HNE-mediated cell apoptosis may
account principally for the catalysis-dependent mechanism of GSTA1
in DDP resistance, indicating that GST isozymes are indirect
determinants of DDP resistance of solid cancer cells. On the other
hand, there has also been increasing evidence to suggest that GST
isozymes are direct determinants of drug resistance of solid cancer
cells since such enzymes as proteins are components in
protein-protein interaction networks regulating cell apoptosis
(3,4,18). The
formation of GSTP1-JNK-c-Jun and GSTA-JNK complexes may inhibit
c-Jun phosphorylation and thus inhibit the JNK signaling pathway
that promotes cell apoptosis; the binding of GSTP1-1 to TRAF2, and
GSTM1-1 to ASK1, as both TRAF2 and ASK1 are the upstream activators
of JNK, blocked the MAPK/JNK signaling cascade that promotes
apoptosis of cancer cells (3,4,27,31).
The interactions of GSTP or GSTA with signaling proteins can be
disrupted upon ligand binding in the active sites of GSTs through
the induced changes of GST conformations. For example,
membrane-permeable GST inhibitor
6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)-hexanol (NBDHEX) (32) and GSTP1 inhibitors TLK199 and TLK286
have been found to be bound in the active sites of GSTs and induce
cell death through both the inhibition of their catalytic
activities and the dissociation of the JNK:GSTP1 complex (6,7).
Notably, significant allosteric conformation changes in GSTs were
observed upon the binding of NBDHEX into their active sites
(32,33), indicating that the sensitization
actions of isozyme-selective inhibitors of GST on DDP-resistant
solid cancer cells may be initiated by allosteric effects upon
their bindings to activate signaling pathways promoting cell
apoptosis. The action mechanisms of GST isozymes in DDP resistance
should thus be associated with both their catalytic detoxification
actions on cytotoxic agents and/or small apoptosis signaling
mediators, and their direct interactions with signaling proteins in
pathways promoting cancer cell apoptosis; the contributions of such
two ways may vary with regard to types of solid cancers and
cytotoxic agents.
The development of selective GST isozyme inhibitors
as general sensitizers on a wide spectrum of drug-resistant solid
cancer cells is surely absorbing in the field. However, no such
isozymes have been demonstrated as rational targets. Data in the
present study indicated that GSTA1 was involved in DDP resistance
of cancer cells originated from the lung, ovary and stomach;
GSTA-specific inhibitors may be useful in sensitizing the three
tested types of and even other types of DDP-resistant solid cancer
cells to DDP. Certainly, other pathways associating with cellular
responses to the stress of cytotoxic agents, such as the
downregulation of MRP/P-gp and the inhibition of other GST
isozymes, may also play important roles in the resistance of cancer
cells to cisplatin (3,18); other types of solid cancers
resistant to cisplatin and/or other chemotherapy agents still
warrant studies of the roles of GST isozymes in drug resistance.
The development of GSTA-selective inhibitors bearing demonstrated
actions to sensitize diverse DDP-resistant solid cancers are highly
desired to support the conclusion in this study and the handling of
DDP-resistance of some types of solid cancer in practice.
Collectively, with the use of siRNAs to compare the roles of GSTP1,
GSTM2 and GSTA1 in three representative cisplatin-resistant solid
cancer cells, it is preliminarily concluded that GSTA predominates
over the other two isozymes in the DDP resistance of the tested
types of solid cancer cells; GSTA may be a rational target for the
development of potent isozyme-selective inhibitors to overcome drug
resistance of common types of solid cancer.
Acknowledgements
The authors would like to thank Professor JingYuan
Wan at the College of Pharmacy of Chongqing Medical University
(Chongqing, China) for his technical assistance.
Funding
The present study was supported by the Natural
Sciences Foundation of China (grant nos. 31570862 and
81773625).
Availability of data and materials
The datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
MZ, XH, BX, XW, FL and XY made substantial
contributions to the conception, design and intellectual content of
this study. MZ and TT performed the experiments, and MZ wrote the
manuscript. YJ, LX, WZ, JL, XW and FL made key contributions to the
acquisition, analysis, and interpretation of the data. TT, YJ, LX,
WZ, JL, XY and FL revised critically the manuscript for important
intellectual content. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chang A: Chemotherapy, chemoresistance and
the changing treatment landscape for NSCLC. Lung Cancer. 71:3–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Board PG and Menon D: Glutathione
transferases, regulators of cellular metabolism and physiology.
Biochim Biophys Acta. 1830:3267–3288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh S: Cytoprotective and regulatory
functions of glutathione S-transferases in cancer cell
proliferation and cell death. Cancer Chemother Pharmacol. 75:1–15.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clapper ML and Szarka CE: Glutathione
S-transferases-biomarkers of cancer risk and chemopreventive
response. Chem Biol Interact. 111:377–388. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahajan S and Atkins WM: The chemistry and
biology of inhibitors and pro-drugs targeted to glutathione
S-transferases. Cell Mol Life Sci. 62:1221–1233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sau A, Pellizzari Tregno F, Valentino F,
Federici G and Caccuri AM: Glutathione transferases and development
of new principles to overcome drug resistance. Arch Biochem
Biophys. 500:116–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruzza P and Calderan A: Glutathione
transferase (GST)-activated prodrugs. Pharmaceutics. 5:220–231.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johansson K, Ito M, Schophuizen CM, Mathew
Thengumtharayil S, Heuser VD, Zhang J, Shimoji M, Vahter M, Ang WH,
et al: Characterization of new potential anticancer drugs designed
to overcome glutathione transferase mediated resistance. Mol Pharm.
8:1698–1708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ertan-Bolelli T, Musdal Y, Bolelli K,
Yilmaz S, Aksoy Y, Yildiz L, Aki-Yalcin E and Yalcin I: Synthesis
and biological evaluation of
2-substituted-5-(4-nitrophenylsulfonamido) benzoxazoles as human
GST P1-1 inhibitors, and description of the binding site features.
Chem Med Chem. 9:984–992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koehler RT, Villar HO, Bauer KE and
Higgins DL: Ligand-based protein alignment and isozyme specificity
of glutathione S-transferase inhibitors. Proteins. 28:202–216.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morgan AS, Ciaccio PJ, Tew KD and Kauvar
LM: Isozyme-specific glutathione S-transferase inhibitors
potentiate drug sensitivity in cultured human tumor cell lines.
Cancer Chemother Pharmacol. 37:363–370. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mohana K and Achary A: Human cytosolic
Glutathione-S-transferases: Quantitative analysis of expression,
comparative analysis of structures and inhibition strategies of
isozymes involved in drug resistance. Drug Metab Rev. 49:318–337.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tew KD, Monks A, Barone L, Rosser D,
Akerman G, Montali JA, Wheatley JB and Schmidt DE Jr:
Glutathione-associated enzymes in the human cell lines of the
National Cancer Institute Drug Screening Program. Mol Pharmacol.
50:149–159. 1996.PubMed/NCBI
|
|
15
|
Lin C, Xie L, Lu Y, Hu Z and Chang J:
miR-133b reverses cisplatin resistance by targeting GSTP1 in
cisplatin-resistant lung cancer cells. Int J Mol Med. 41:2050–2058.
2018.PubMed/NCBI
|
|
16
|
Liu H, Yang Z, Zang L, Wang G, Zhou S, Jin
G, Yang Z and Pan X: Downregulation of Glutathione S-transferase A1
suppressed tumor growth and induced cell apoptosis in A549 cell
line. Oncol Lett. 16:467–474. 2018.PubMed/NCBI
|
|
17
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moscow JA and Dixon KH:
Glutathione-related enzymes, glutathione and multidrug resistance.
Cytotechnology. 12:155–170. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y and Zhu Z, Cai H, Liu Q, Zhou H and
Zhu Z: SKI-II reverses the chemoresistance of SGC7901/DDP gastric
cancer cells. Oncol Lett. 8:367–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Xie Z, Sun G, Yang P, Li J, Yang
H, Xiao S, Liu Y, Qiu H, Qin L, et al: Reversing drug resistance of
cisplatin by hsp90 inhibitors in human ovarian cancer cells. Int J
Clin Exp Med. 8:6687–6701. 2015.PubMed/NCBI
|
|
22
|
Ye LY, Hu S, Xu HE, Xu RR, Kong H, Zeng
XN, Xie WP and Wang H: The effect of tetrandrine combined with
cisplatin on proliferation and apoptosis of A549/DDP cells and A549
cells. Cancer Cell Int. 17:402017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang W, Liu F, Wang C, Wang C, Tang Y and
Jiang Z: Glutathione S-transferase A1 mediates nicotine-induced
lung cancer cell metastasis by promoting epithelial-mesenchymal
transition. Exp Ther Med. 14:1783–1788. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bradford MA: Rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Habig WH, Pabst MJ and Jakoby WB:
Glutathione S-transferases: The first enzymatic step in mercapturic
acid formation. J Biol Chem. 249:7130–7139. 1974.PubMed/NCBI
|
|
26
|
Mansoori B, Mohammadi A, Davudian S,
Shirjang S and Baradaran B: The different mechanisms of cancer drug
resistance: A brief review. Adv Pharm Bull. 7:339–348. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Allocati N, Masulli M, Di Ilio C and
Federici L: Glutathione transferases: Substrates, inihibitors and
pro-drugs in cancer and neurodegenerative diseases. Oncogenesis.
7:82018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singhal SS, Singh SP, Singhal P, Horne D,
Singhal J and Awasthi S: Antioxidant role of glutathione
S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated
signaling. Toxicol Appl Pharmacol. 289:361–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rudd LP, Kabler SL, Morrow CS and Townsend
AJ: Enhanced glutathione depletion, protein adduct formation, and
cytotoxicity following exposure to 4-hydroxy-2-nonenal (HNE) in
cells expressing human multidrug resistance protein-1 (MRP1)
together with human glutathione S-transferase-M1 (GSTM1). Chem Biol
Interact. 194:113–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Li Y, Chen W, Wang Y, Hui L, Liu
J, Li N, Zhang L, Zou Y and Wang F: Nrf-2/Gst-α mediated imatinib
resistance through rapid 4-HNE clearance. Exp Cell Res. 353:72–78.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pajaud J, Kumar S, Rauch C, Morel F and
Aninat C: Regulation of signal transduction by glutathione
transferases. Int J Hepatol. 2012:1376762012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ricci G, De Maria F, Antonini G, Turella
P, Bullo A, Stella L, Filomeni G, Federici G and Caccuri AM:
7-Nitro-2,1,3-benzoxadiazole derivatives, a new class of suicide
inhibitors for glutathione S-transferases. Mechanism of action of
potential anticancer drugs. J Biol Chem. 280:26397–26405. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Turella P, Cerella C, Filomeni G, Bullo A,
De Maria F, Ghibelli L, Ciriolo MR, Cianfriglia M, Mattei M,
Federici G, et al: Proapoptotic activity of new glutathione
S-transferase inhibitors. Cancer Res. 65:3751–3761. 2005.
View Article : Google Scholar : PubMed/NCBI
|