Introduction
MicroRNAs (miRNAs) are a cluster of short non-coding
RNAs (~20 nucleotides long) (1).
miRNAs can post-transcriptionally regulate gene expression by
interacting with the 3′ untranslated region (3′-UTR) of targeted
messenger RNAs (mRNAs). Functionally, miRNAs were found to play
critical roles in various human diseases including human cancers.
Numerous miRNAs have been found to be aberrantly expressed in human
cancers and have been proposed as promising biomarkers and
prognostic predictors for cancer patients (2). Among these cancer-associated miRNAs,
miR-3653 is a novel cancer-associated miRNA. miRNA expression
profiles showed that miR-3653 is aberrantly expressed in lung
adenocarcinoma (3) and colon cancer
(4). A decreased expression level
of miR-3653 was found to be correlated with a poor prognosis of
lung adenocarcinoma patients.
Hepatocellular carcinoma (HCC) is a fatal malignancy
affecting millions of individuals worldwide. HCC ranks as the
second leading cause of cancer-related death (5). The overall survival for HCC patients
is unsatisfactory, and the 5-year survival rate of HCC patients is
less than 40%; and even worse for those in advanced stages
(6). Uncontrolled growth and
occurrence of local and systemic metastasis are the main reasons
for the poor prognosis of HCC patients. During the last two
decades, increasing studies have shown that miRNAs play critical
roles in the progression of HCC. miRNAs were found to regulate the
growth, metastasis and drug resistance of HCC cells. However, the
expression and biological functions of miR-3653 in HCC remain
unknown.
The present study demonstrated that compared with
adjacent non-tumor tissues, miR-3653 expression was significantly
decreased in HCC tissues. Patients with a low miR-3653 level showed
decreased overall and disease-free survival. Decreased expression
of miR-3653 was found to be associated with unfavorable
clinicopathological features (large tumor size and occurrence of
metastasis) of HCC patients. Using gain- and loss-of-function
assays, miR-3653 was found to inhibit the growth and metastasis of
HCC cells. In vivo assays showed that miR-3653 slowed down
the subcutaneous growth and reduced the lung metastasis of HCC
cells in nude mice. Mechanistically, the present study revealed
that integrin-β1 (ITGB1) was the downstream target of miR-3653 in
HCC cells. Moreover, we demonstrated that targeting ITGB1 was
critical for the biological functions of miR-3653 in HCC.
Materials and methods
Clinical tissues
HCC tissues along with adjacent non-tumor tissues
were collected from 60 HCC patients (37 male and 23 female
patients, average age 43.9±9.7 years) who received surgical
treatment at The Infectious Disease Center, The First Affiliated
Hospital of Xinjiang Medical University (Urumqi, Xinjiang) from
January 2002 to December 2010. All clinical tissues were
pathologically confirmed as HCC and maintained at −80°C before
being subjected to further experiments. Written informed consent
was obtained from every patient enrolled in this study. Ethical
protocols for using HCC patient samples were approved by the
Institutional Research Ethics Committee of the First Affiliated
Hospital of Xinjiang Medical University (Urumqi, China).
Cell culture
HCC cell lines including Hep3B, Huh7, MHCC97H and
HCCLM3 and the immortalized hepatocyte L-02 cell line were obtained
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) along with 10% fetal
bovine serum (10%) (FBS; Gibco; Thermo Fisher Scientific, Inc.) was
used for cell culture. Cell cultures were maintained in a cell
incubator at 37°C with 5% CO2.
Transfection of HCC cells
Transfection of HCC cells was performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
based on the manufacturer's instructions. miR-3653 mimic (50 nM;
product no. HMI0001-HMI2785) and non-targeting control (50 nM;
product no. HMC0002) were obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany), and transfected into HCCLM3 cells. miR-3653
inhibitor (50 nM; product no. HSTUD1287) and the corresponding
negative control (50 nM; product no. NCSTUD001) were obtained from
Sigma-Aldrich (Merck KGaA) and transfected into Hep3B cells. The
vector used for overexpression of ITGB1 was pcDNA 3.1 which was
obtained from Addgene (Cambridge, MA, USA). ITGB1 vector (1.5
µg/ml; cat. no. 51920) and the empty vector (1.5 µg/ml; cat. no.
52535) were obtained from Addgene and co-transfected with miR-3653
mimic or non-targeting control into HCCLM3 cells: HCCLM3 cells
co-transfected with non-targeting control (product no. HMC0002) and
control vector (cat. no. 52535), HCCLM3 cells transfected with
miR-3653 mimic (product no. HMI0001-HMI2785) and control vector
(cat. no. 52535), and HCCLM3 cells transfected with miR-3653 mimic
(product no. HMI0001-HMI2785) and ITGB1 vector (cat. no. 51920).
Forty-eight hours after the cellular transfection, these cells were
collected for western blot analysis, qRT-PCR, MTT, BrdU and
Transwell assays, and in vivo experiments. The efficacy of
cell transfection were confirmed by qRT-PCR or western blot
analysis.
Quantitative real-time reverse
transcription-PCR (qRT-PCR)
RNA in clinical tissues and HCC cells were extracted
using TRIzol and RNeasy Mini kit (Qiagen, Shanghai, China). The
Transcriptional First Strand cDNA Synthesis kit and SYBR-Green PCR
Master Mix (Applied Biosystems, Foster City, CA, USA) were used for
reverse transcription reactions and quantitative real-time PCR.
Primers for E-cadherin, N-cadherin, ITGB1, GAPDH, miR-3653 and U6
were obtained from Guangzhou GeneCopoeia (Guangzhou, China). GAPDH
was used as the internal controls for E-cadherin, N-cadherin and
ITGB1. U6 was used as the internal controls for miR-3653. Primer
sequences were listed as below: miR-3653 forward,
5′-TCTCCCGAGAGACATATTT-3′ and reverse, 5′-GATGAGAAGGTATGAATCA-3′;
U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; E-cadherin forward,
5′-CAGCATCACTGGCCAAGGAGCTGA-3′ and reverse,
5′-GACCACACTGATGACTCCTGTGTTCC-3′; N-cadherin forward,
5′-GTCATCTTGATCTCATAACGCTGG-3′ and reverse,
5′-AGCCCATCTGTACCTGTGGTTCA-3′; ITGB1 forward,
5′-TCAGAATTGGATTTGGCTCATTT-3′ and reverse,
5′-CCTGAGCTTAGCTGGTGTTGTG-3′; GAPDH forward,
5′-GGTCACCAGGGCTGCTTTTA-3′ and reverse,
5′-GGATCTCGCTCCTGGAAGATG-3′. The relative expression levels of
miRNAs or mRNAs were determined using ΔΔCq-based fold-change
calculations as previously described (7,8).
Western blot analysis
Proteins in clinical tissues and HCC cells were
extracted using RIPA buffer and subjected to concentration
measurements using BCA kit. After being separated in SDS-PAGE gels,
the protein (20 µg) on SDS-PAGE gels (4–20%) were transferred to
polyvinylidene fluoride membrane. These membranes were incubated
with 5% non-fat dry milk (diluted in TBST) at room temperature for
1 h and primary antibodies of E-cadherin (dilution 1:1,000; cat.
no. 3195; Cell Signaling Technologies, Inc., Danvers, MA, USA),
N-cadherin (dilution 1:500; cat. no. 4061; Cell Signaling
Technologies, Inc.), ITGB1 (dilution 1:1,000; cat. no. 4706; Cell
Signaling Technologies, Inc.) and GAPDH (dilution 1:2,000; cat. no.
sc-32233; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
overnight at 4°C. GAPDH was used as internal control. Protein
signals were detected using ECL reagents (Amersham Biosciences; GE
Healthcare, Chicago, IL, USA).
MTT and BrdU assays
For MTT assay, HCCLM3 cells overexpressing miR-3653
or those in the control group, and Hep3B cells with miR-3653
knockdown or those in the negative control group (5,000 cells/well)
were seeded into 96-well plates. Twenty-four, 28 and 72 h after
cell seeding, MTT (Sigma-Aldrich; Merck KGaA) was added into each
well and incubation was carried out for 4 h at 37°C. Absorbance at
490 nm was measured as the indicator of cell viability. For BrdU
assay, HCC cells described above were stained with
bromodeoxyuridine for 1 h at room temperature and then incubated
with anti-BrdUrd antibody (Sigma-Aldrich; Merck KGaA).
BrdU-positive cells were counted under fluorescence microscope
(Axioskop 2 Plus; Carl Zeiss Co., Ltd., Jena, Germany).
Transwell assay
The migratory and invasive abilities of HCC cells
were assessed by Transwell assay. The day before Transwell assay,
HCCLM3 cells overexpressing miR-3653 or those in the control group,
and Hep3B cells with miR-3653 knockdown or those in the negative
control group were starved in serum-free DMEM media overnight.
Then, the cells (3×104) resuspended in 200 µl basal DMEM
were added to the upper chamber of a Transwell insert. A total of
600 µl serum-containing DMEM (20% FBS) used as chemoattractant was
added to the lower chamber. For the invasion experiments, 100 µl
Matrigel (diluted in DMEM at the ratio of 1:8) was added to the
bottom of the upper chamber. Twenty-four hours later, cells that
had migrated through the membrane were stained with crystal violet.
The number of migrated or invaded cells was counted under a light
microscope at ×40 magnification.
Luciferase assay
3′-UTR of ITGB1 containing the binding sequences for
miR-3653 or the mutated 3′-UTR of ITGB1 was used to construct the
wild-type ITGB1-3′-UTR or mutant ITGB1-3′-UTR. HCC cells in 12-well
plates were transfected with wild-type or mutant 3′-UTR of ITGB1
along with miR-3653 mimic or inhibitor or the corresponding control
vector. After co-transfection, the luciferase activity was measured
by luciferase reporter kit (Promega Corporation, Madison, WI,
USA).
In vivo tumor growth and metastasis
assay
For the in vivo tumor growth studies, nude
mice (6-weeks of age, 4 female mice each group) were injected with
HCCLM3 cells overexpressing miR-3653 or cells in the control group
(1×106). The length and width of tumor nodules were
measured every 7 days. After 28 days, the nude mice were sacrificed
using cervical dislocation and the subcutaneous tumors were removed
and subjected to measurement of the volume (volume =
width2 × length/2). For tail vein injection model,
HCCLM3 cells overexpressing miR-3653 or cells in the control group
(5×104) were injected into nude mice (4 mice each group)
through the tail vein. All mice (Saierbio, Tianjing, China), with 4
female mice in each group, were maintained in specific
pathogen-free (SPF) rooms under a 12-h light/dark cycle, with two
mice in each static filter-topped cage. The initial weight of these
mice were approximately 20 g. Eight weeks after tail vein
injection, these nude mice were sacrificed using cervical
dislocation and the lungs were removed and subjected to H&E
staining. The protocols regarding the in vivo manipulations
were approved by the Animal Care Committee of the First Affiliated
Hospital of Xinjiang Medical University.
Statistical analysis
All quantitative data are presented as the mean ±
standard error of the mean (SEM) from at least three independent
replicates. SPSS software 13.0 (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis, a two-tailed Student t-test and
Kaplan-Meier analysis were used to analyze the differences between
two groups. One-way analysis of variance (ANOVA) was used to
examine the statistical difference between multiple groups with
least significant difference (LSD) test as post hoc test.
Differences between groups were considered statistically
significant at P<0.05.
Results
miR-3653 expression is decreased in
HCC tissues and cells
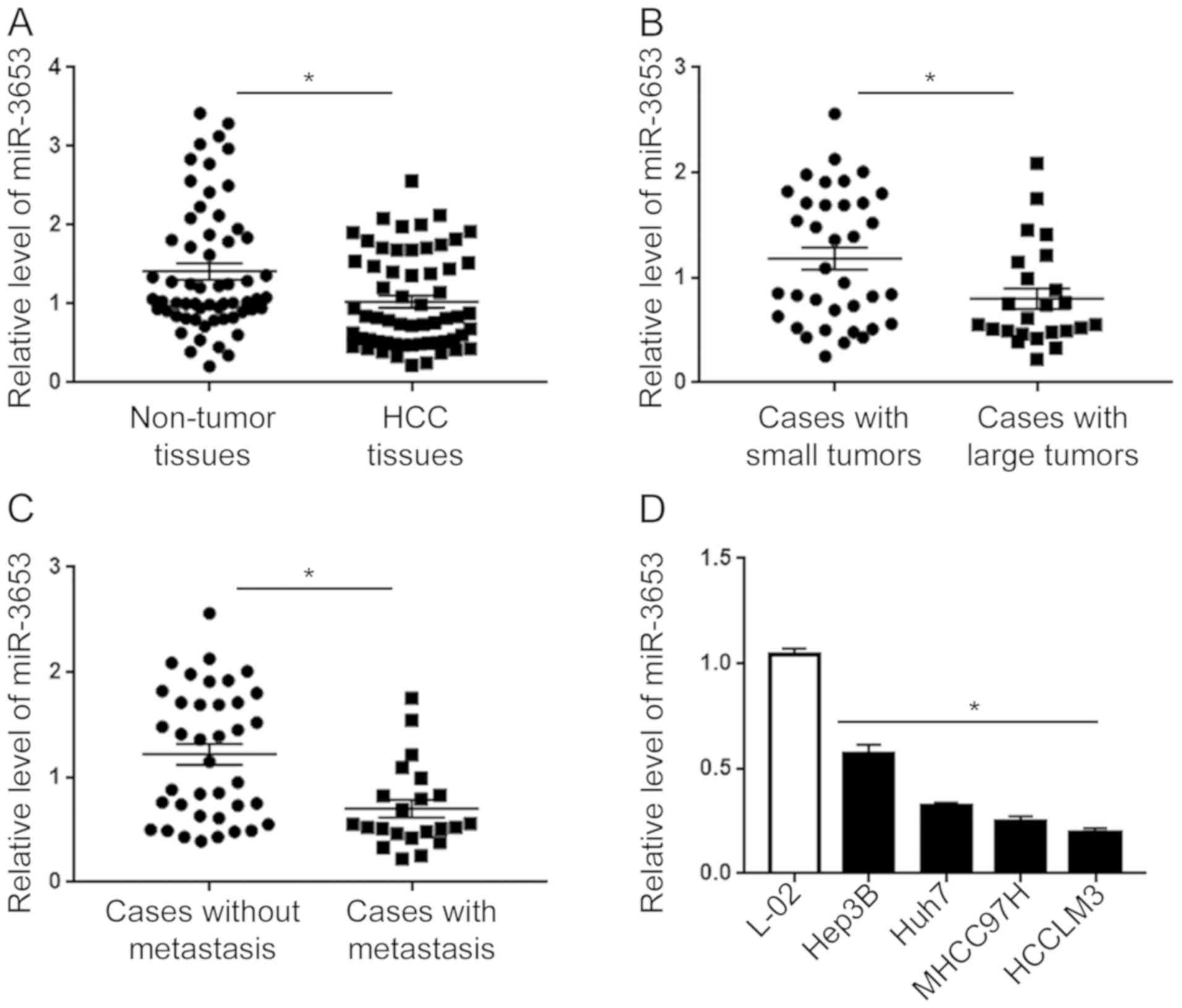
qRT-PCR of the clinical specimens showed that
compared with non-tumor tissues, HCC tissues showed a significantly
decreased level of miR-3653 (P<0.05; Fig. 1A). Subgroup analysis showed that
compared with tumors >5 cm, small tumors tumors ≤5 cm showed
significantly increased level of miR-3653 (P<0.05; Fig. 1B). Moreover, patients with local or
systemic metastasis showed a significantly decreased level of
miR-3653 than those without metastasis (P<0.05; Fig. 1C). Finally, we compared the
expression level of miR-345 in HCC cell lines and L-02 cells.
Compared with that in L-02 cells, the level of miR-3653 in the four
HCC cell lines was significantly decreased (P<0.01; Fig. 1D). Among the four HCC cell lines,
the expression level of miR-3653 was highest in the Hep3B cells and
lowest in the HCCLM3 cells. Furthermore, we investigated the
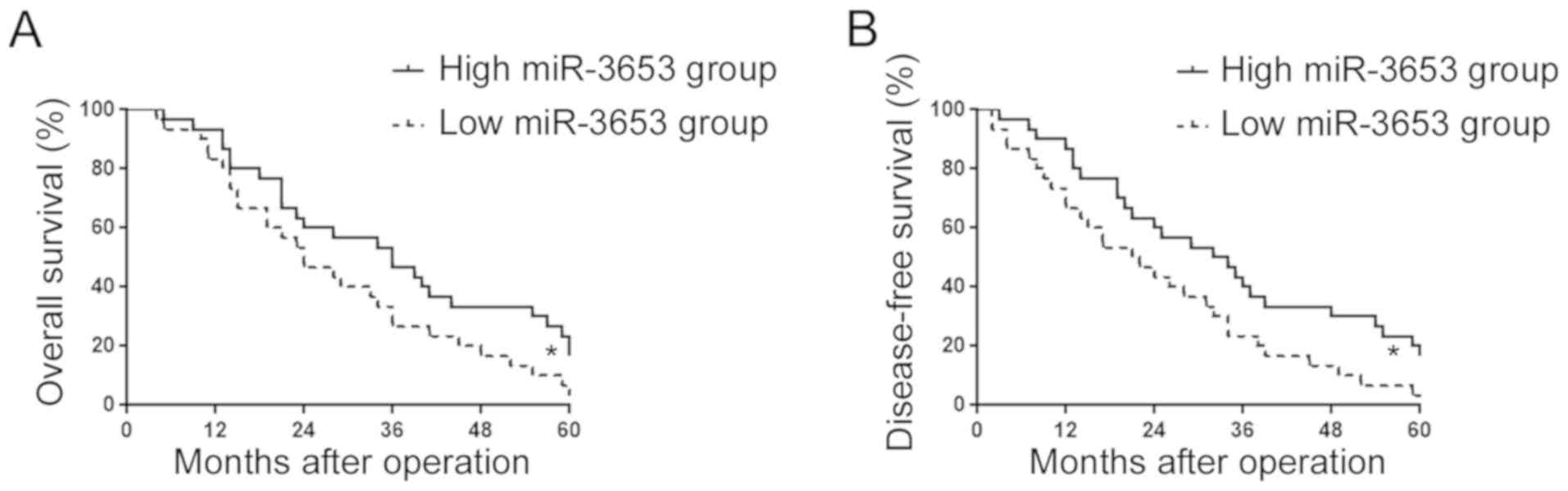
association between the miR-3653 level and patient prognosis.
Kaplan-Meier survival analysis showed that patients with a low
miR-3653 level had significantly decreased overall survival
(P<0.05; Fig. 2A) and
disease-free survival (P<0.05; Fig.
2B).
miR-3653 inhibits the growth and
metastatic ability of HCC cells in vitro
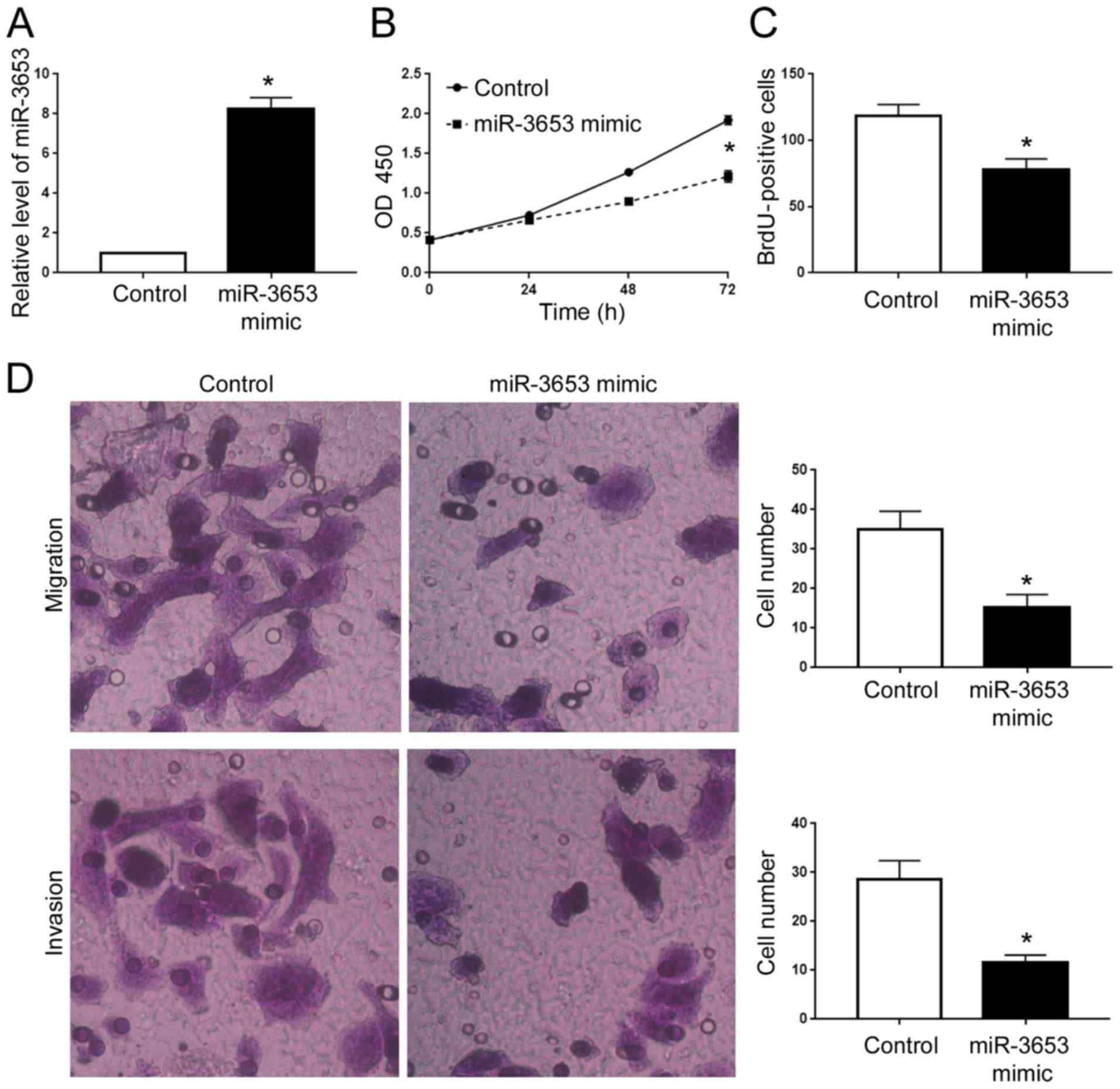
To determine the biological functions of miR-3653 in
HCC cells, we performed gain- and loss-of function experiments in
HCCLM3 and Hep3B cells, respectively. Transfection of miR-3653
mimics into HCCLM3 cells effectively increased the miR-3653 level
(P<0.05; Fig. 3A). MTT and BrdU
assays showed that overexpression of miR-3653 led to decreased cell
viability and proliferation of HCCLM3 cells (P<0.05; Fig. 3B and C). Transwell assay showed that
forced expression of miR-3653 led to significantly decreased
ability of cell migration and invasion (P<0.05; Fig. 3D). In contrast, we performed
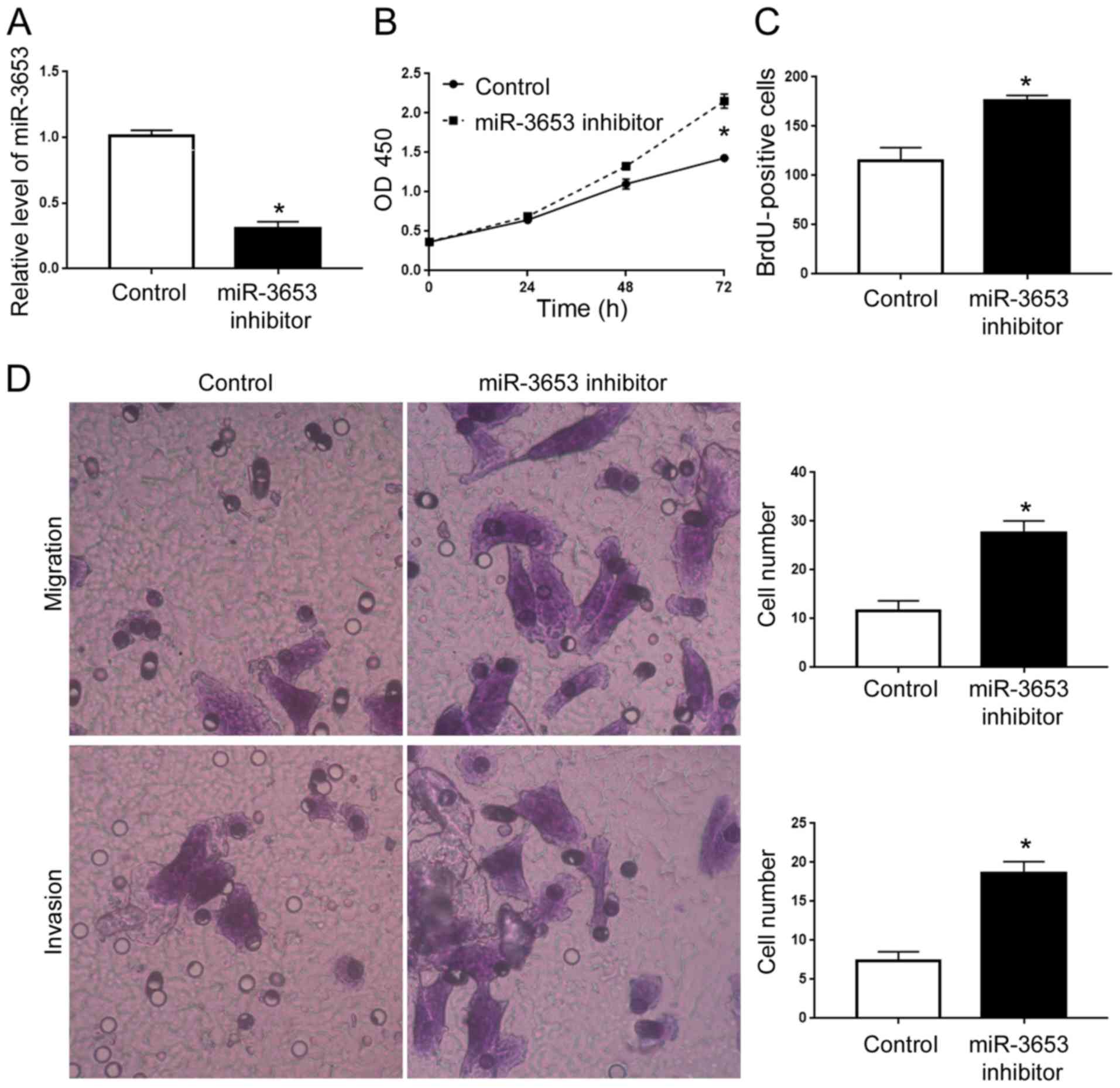
miR-3653 knockdown in Hep3B cells. miR-3653 inhibitor significantly
decreased the miR-3653 level in Hep3B cells (P<0.05; Fig. 4A), and led to increased cell
viability, proliferation, migration and invasion (P<0.05;
Fig. 4B-D).
miR-345 inhibits the EMT of HCC
cells
EMT has been widely accepted as a critical mechanism
for cancer metastasis (9).
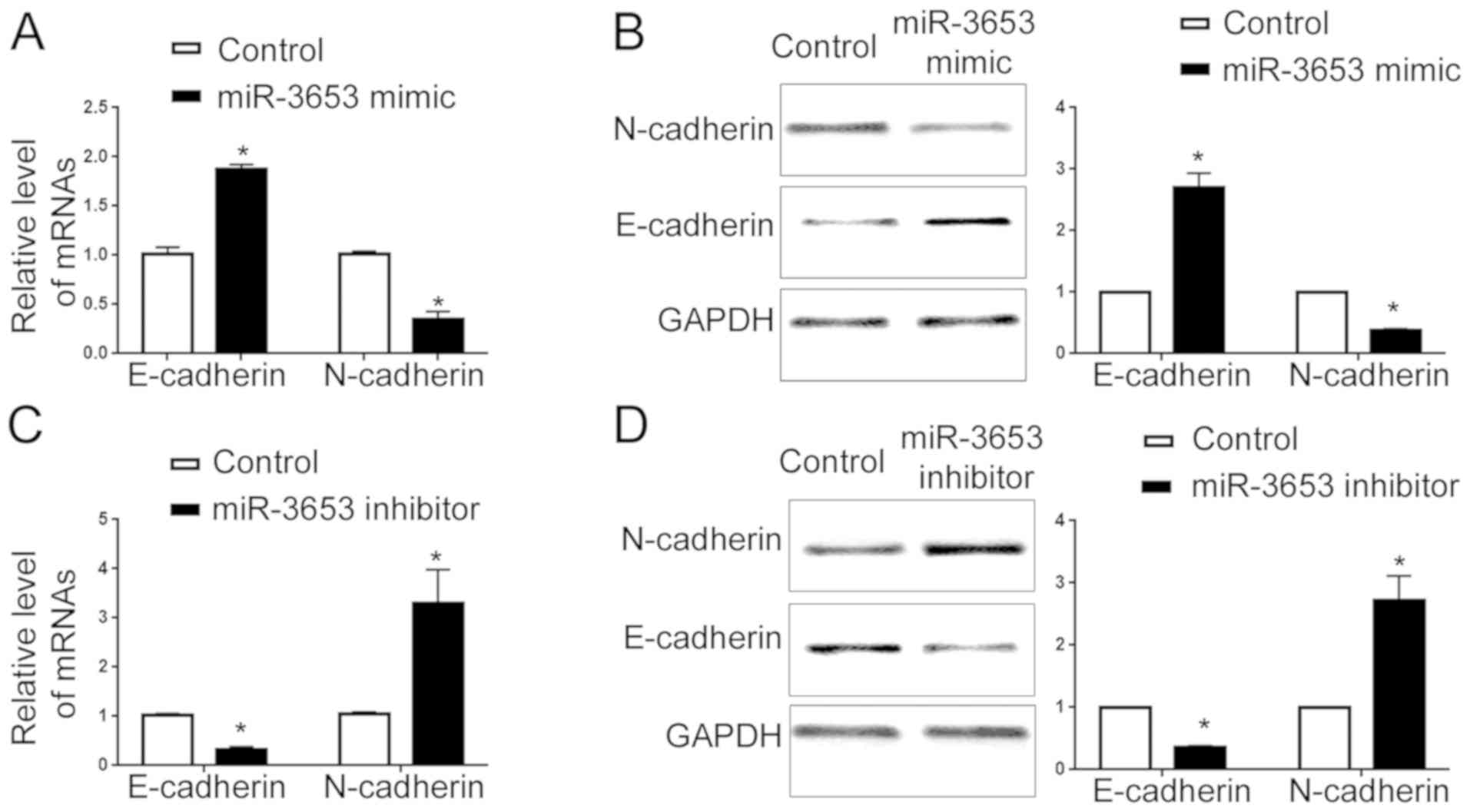
Therefore, we evaluated the EMT status after altering the miR-3653
level in HCCLM3 and Hep3B cells. qRT-PCR showed that overexpression
of miR-3653 increased E-cadherin mRNA and decreased N-cadherin mRNA
(P<0.05; Fig. 5A). Western blot
analysis showed that forced expression of miR-3653 led to elevated
E-cadherin protein and decreased N-cadherin protein (P<0.05;
Fig. 5B). In contrary, miR-3653
knockdown decreased the mRNA and protein level of E-cadherin
(P<0.05; Fig. 5C and D) and
increased N-cadherin expression (P<0.05; Fig. 5C and D).
miR-3653 inhibits the growth and
metastasis of HCCLM3 cells in nude mice
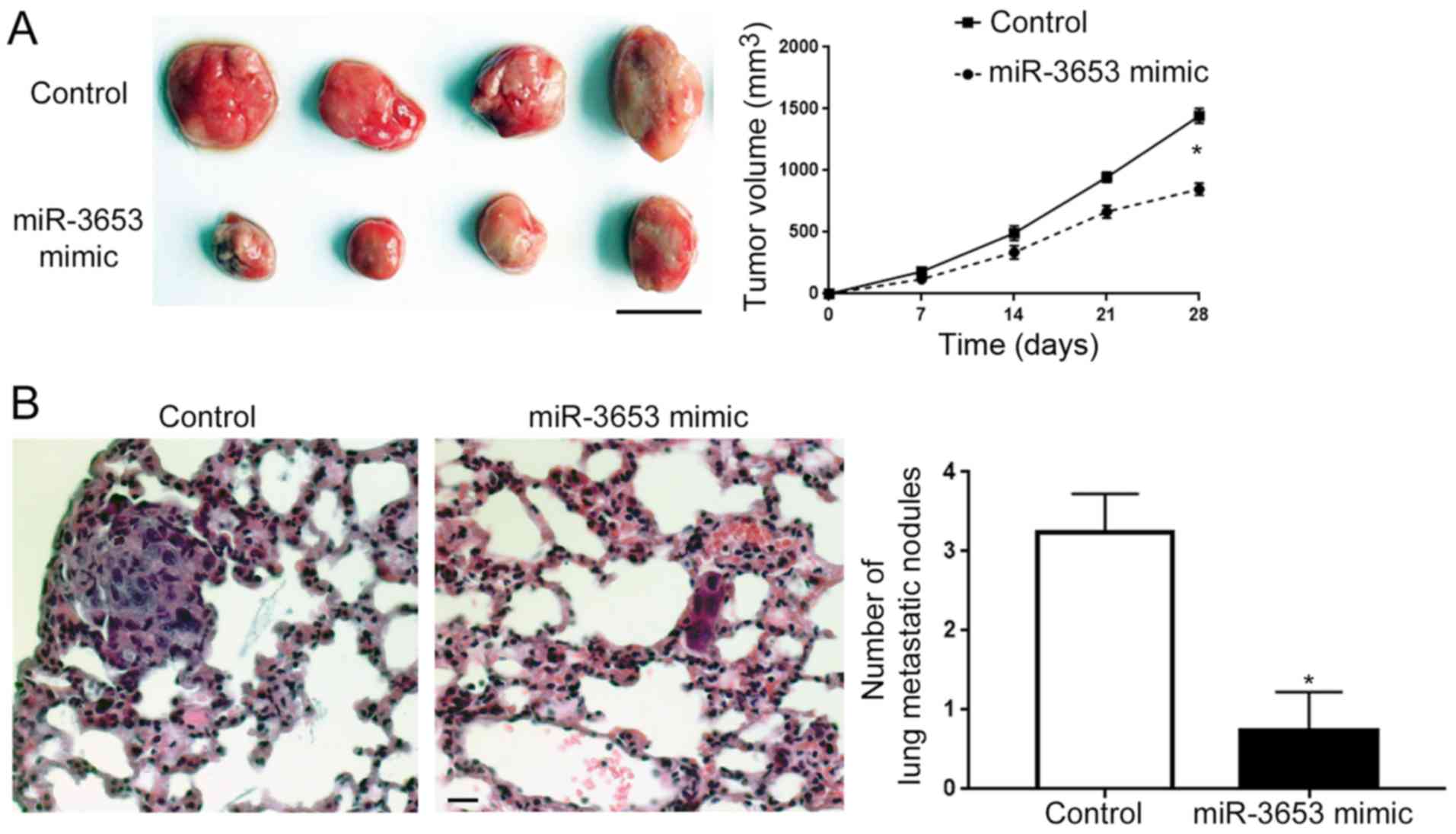
After confirming the in vitro influence of
miR-3653 on HCC cells, we further assessed the in vivo
function of miR-3653 in HCC. Subcutaneous injection of HCCLM3 cells
with or without miR-3653 overexpression showed that miR-3653
significantly slowed down the growth of HCCLM3 cell-derived tumors
in nude mice (P<0.05; Fig. 6A).
Tail vein injection model showed that overexpression of miR-3653
effectively reduced the number of lung metastatic nodules in nude
mice (P<0.05; Fig. 6B).
ITGB1 is the downstream target of
miR-3653 in HCC
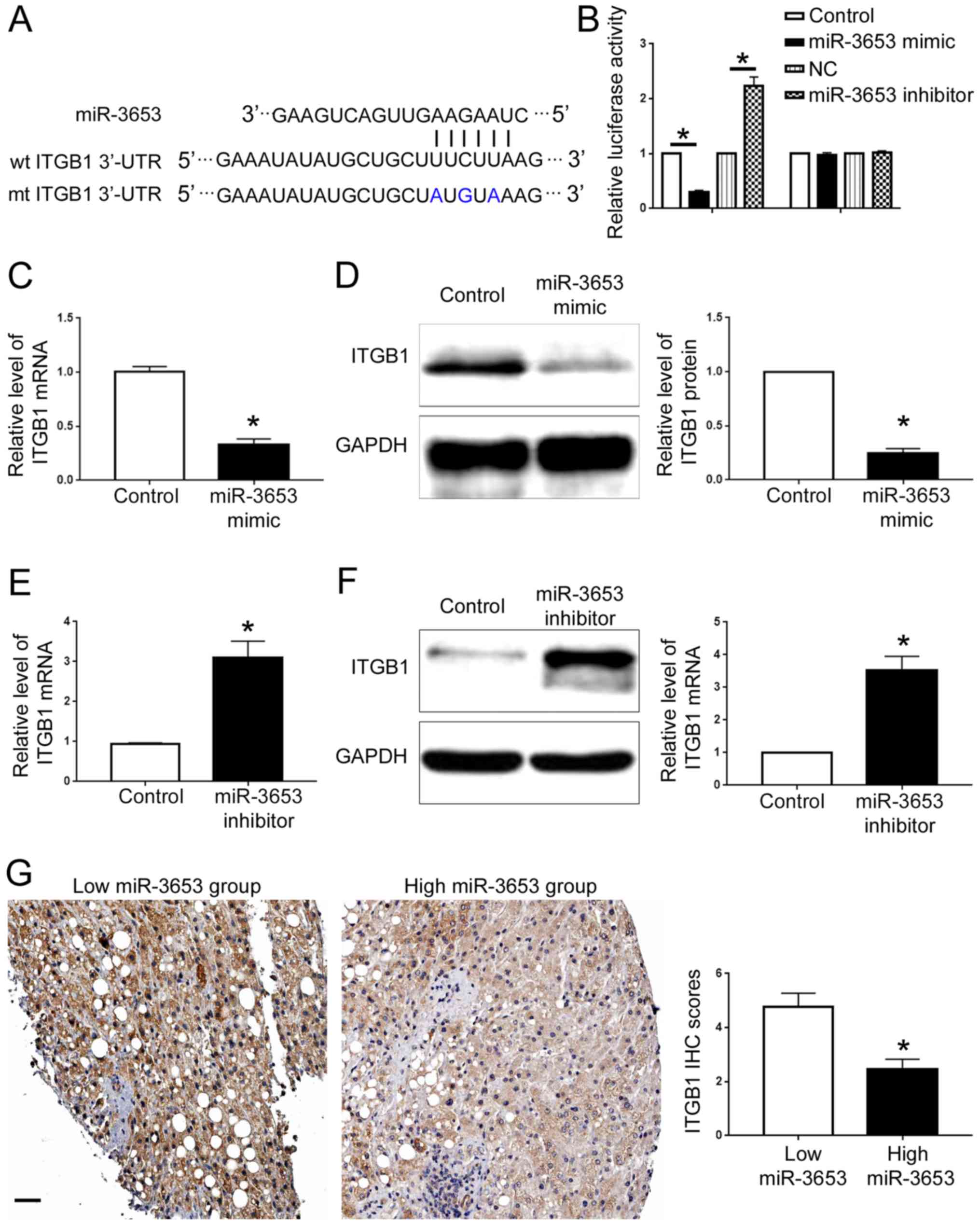
We further searched the public database TargetScan
(http://www.targetscan.org) to identify
potential downstream targets of miR-3653. ITGB1, a well-known
oncogenic protein which plays a critical role in HCC progression
(10), was one of the predicted
downstream targets of miR-3653. As shown in Fig. 7A, ITGB1 3′-UTR contained the binding
sequences for miR-3653. Luciferase assay showed that overexpression
of miR-3653 decreased the luciferase activity of wild-type ITGB1
3′-UTR while miR-3653 knockdown increased that of ITGB1 3′-UTR
(P<0.05; Fig. 7B). Neither
miR-3653 overexpression nor miR-3653 knockdown affected the
luciferase of mutated ITGB1 3′-UTR (Fig. 7B). qRT-PCR and western blot analysis
showed that miR-3653 overexpression decreased the mRNA and protein
level of ITGB1 in HCCLM3 cells (P<0.05; Fig. 7C and D). Knockdown of miR-3653
increased the expression of ITGB1 in Hep3B cells (P<0.05;
Fig. 7E and F). To further confirm
the regulatory effect of miR-3653 on ITGB1, we detected the ITGB1
expression in HCC tissues. Compared with those with high miR-3653
level, tissues with low miR-3653 level showed increased expression
of ITGB1. The IHC score of ITGB1 in HCC tissues with low miR-3653
level was significantly higher than that in tissues with high
miR-3653 level (P<0.05; Fig.
7G).
ITGB1 mediates the biological
functions of miR-3653 in HCC
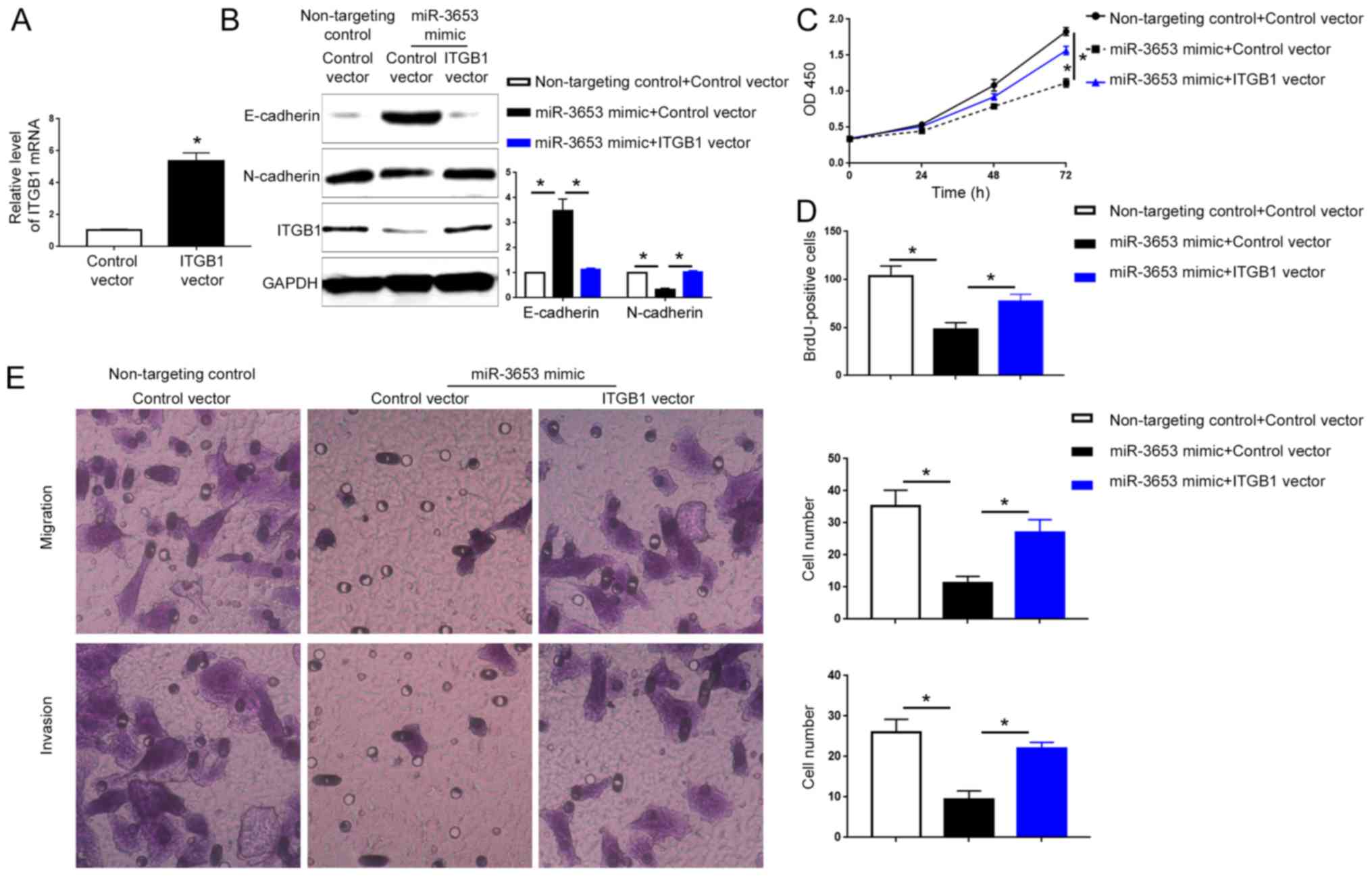
Since ITGB1 was confirmed as the downstream target
of miR-3653 in HCC, we further explored whether ITGB1 mediates the
biological functions of miR-3653 in HCC. qRT-PCR showed that
transfection of the ITGB1 vector significantly increased the ITGB1
mRNA in Hep3B cells (P<0.05; Fig.
8A). Western blot analysis showed that transfection of ITGB1
vector in HCCLM3 cells overexpressing miR-3653 significantly
increased ITGB1 expression and reversed the increase of E-cadherin
and the decrease of N-cadherin caused by miR-3653 overexpression
(P<0.05; Fig. 8B). Functionally,
forced expression of ITGB1 abrogated the decrease of cell viability
and proliferation caused by miR-3653 overexpression (P<0.05;
Fig. 8C and D). Transwell assay
showed that ITGB1 overexpression reversed the decrease of cell
migration and invasion induced by miR-3653 overexpression
(P<0.05; Fig. 8E).
Discussion
Hepatocellular carcinoma (HCC) is a fetal disease
affecting millions of individuals worldwide. For patients in
advanced stages, few effective options are available. The critical
molecular mechanisms responsible for HCC progression remain
unknown. Previous studies have confirmed that microRNAs (miRNAs)
play critical roles in HCC progression (11,12).
Numerous cancer-associated miRNAs have been found to affect the
growth, metastasis, stem cell formation and drug resistance of HCC
cells (13–16). miR-3653 is a novel cancer-associated
miRNA and has been found to be aberrantly expressed in different
types of human cancers (3,4). However, the expression of miR-3653 in
HCC remains unknown. The present study revealed for the first time
that miR-3653 expression is significantly decreased in HCC tissues
and cells. Patients with large tumor size or occurrence of
metastasis showed a significantly decreased miR-3653 level.
Survival analysis demonstrated that a decreased miR-3653 level is
correlated with a poor prognosis of HCC patients. These data
indicate that miR-3653 acts as a tumor suppressor in HCC.
Uncontrolled growth and local or systemic metastasis
are the main reasons for the poor survival of HCC patients. In this
study, using gain- and loss-of function experiments, we confirmed
that miR-3653 overexpression inhibited the cell viability,
proliferation, migration and invasion of HCCLM3 cells while
miR-3653 knockdown led to opposite functional consequences.
Previous studies demonstrated that EMT is an important mechanism of
cancer metastasis. This study found that overexpression of miR-3653
resulted in increased E-cadherin level and decreased N-cadherin
expression in HCCLM3 cells while knockdown of miR-3653 led to
opposite effects in Hep3B cells. Thus, miR-3653 inhibits the
process of EMT of HCC cells. Moreover, this study established a
subcutaneous injection model and tail vein injection model to
evaluate the influence of miR-3653 on the in vivo growth and
metastasis of HCC cells. In vivo assays showed that forced
expression of miR-3653 inhibited the growth and metastasis of
HCCLM3 cells in nude mice. Taken together, this study demonstrated
that miR-3653 inhibited the growth, metastasis and EMT of HCC
cells.
ITGB1 functions as an oncogene in different types of
human cancers (17–19). It was found to activate the FAK/AKT
pathway in cancer cells (20). As
for HCC, ITGB1 was found to promote the growth and metastasis of
HCC cells (10). In this study, the
data of luciferase assay, qRT-PCR and western blot analysis
consistently showed that miR-3653 could inhibit the expression of
ITGB1 in HCC cells by binding with the 3′-UTR of ITGB1. Rescue
experiments confirmed that ITGB1 overexpression could abrogate the
inhibitory effect of miR-3653 on EMT, cell viability, proliferation
and metastasis. Thus, ITGB1 is not only a downstream target of
miR-3653 but also a functional mediator of miR-3653 in HCC. It is
worth noting that ITGB1 was found to be under the control of many
other miRNAs including miR-29a (21) and miR-124 (19). These miRNAs were found to be
aberrantly expressed in HCC (22,23).
Therefore, the expression of ITGB1 in HCC is probably under the
control of many different miRNAs.
Collectively, the present study demonstrated that
miR-3653 expression is significantly decreased in HCC tissues and
cells. Decreased expression of miR-3653 is associated with poor
prognosis of HCC patients. miR-3653 was found to inhibit the
growth, metastasis and EMT of HCC cells. Moreover, we confirmed for
the first time that ITGB1 is a downstream target of miR-3653 and
mediates the biological functions of miR-3653 in HCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LZ, TZ and ZD acquired the data and created a draft
of the manuscript; LZ and ZD prepared the experimental materials
and performed the in vitro assays; LZ and LS interpreted the
data, performed the statistical analysis and analyzed the results;
LZ and LS revised and approved the final version of the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The protocol of the present study was approved by
the Institutional Research Ethics Committee of the First Affiliated
Hospital of Xinjiang Medical University and informed consent was
obtained from every patient enrolled in this study. The protocols
regarding the in vivo manipulations were approved by the
Animal Care Committee of the First Affiliated Hospital of Xinjiang
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nelson KM and Weiss GJ: MicroRNAs and
cancer: Past, present, and potential future. Mol Cancer Ther.
7:3655–3660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin K, Xu T, He BS, Pan YQ, Sun HL, Peng
HX, Hu XX and Wang SK: MicroRNA expression profiles predict
progression and clinical outcome in lung adenocarcinoma. Onco
Targets Ther. 9:5679–5692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu X, Li S, Xu X, Wu S, Chen R, Jiang Q,
Li Y and Xu Y: The potential value of miR-1 and miR-374b as
biomarkers for colorectal cancer. Int J Clin Exp Pathol.
8:2840–2851. 2015.PubMed/NCBI
|
|
5
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daniel R, Wu Q, Williams V, Clark G,
Guruli G and Zehner Z: A panel of MicroRNAs as diagnostic
biomarkers for the identification of prostate cancer. Int J Mol
Sci. 18(pii): E12812017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang YY, Kong LQ, Zhu XD, Cai H, Wang CH,
Shi WK, Cao MQ, Li XL, Li KS, Zhang SZ, et al: CD31 regulates
metastasis by inducing epithelial-mesenchymal transition in
hepatocellular carcinoma via the ITGB1-FAK-Akt signaling pathway.
Cancer Lett. 429:29–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gramantieri L, Fornari F, Callegari E,
Sabbioni S, Lanza G, Croce CM, Bolondi L and Negrini M: MicroRNA
involvement in hepatocellular carcinoma. J Cell Mol Med.
12:2189–2204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Braconi C, Henry JC, Kogure T, Schmittgen
T and Patel T: The role of microRNAs in human liver cancers. Semin
Oncol. 38:752–763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greenhill C: Hepatocellular carcinoma: New
insight into angiogenesis in hepatocellular carcinoma: Involvement
of microRNA-26a. Nat Rev Gastroenterol Hepatol. 11:32014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dou C, Wang Y, Li C, Liu Z, Jia Y, Li Q,
Yang W, Yao Y, Liu Q and Tu K: MicroRNA-212 suppresses tumor growth
of human hepatocellular carcinoma by targeting FOXA1. Oncotarget.
6:13216–13228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH,
Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX, et al: The putative
tumour suppressor microRNA-124 modulates hepatocellular carcinoma
cell aggressiveness by repressing ROCK2 and EZH2. Gut. 61:278–289.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kurozumi A, Goto Y, Matsushita R, Fukumoto
I, Kato M, Nishikawa R, Sakamoto S, Enokida H, Nakagawa M, Ichikawa
T and Seki N: Tumor-suppressive microRNA-223 inhibits cancer
cell migration and invasion by targeting ITGA3/ITGB1
signaling in prostate cancer. Cancer Sci. 107:84–94. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu C, Ni Z, Li BS, Yong X, Yang X, Zhang
JW, Zhang D, Qin Y, Jie MM, Dong H, et al: hTERT promotes the
invasion of gastric cancer cells by enhancing FOXO3a ubiquitination
and subsequent ITGB1 upregulation. Gut. 66:31–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hunt S, Jones AV, Hinsley EE, Whawell SA
and Lambert DW: MicroRNA-124 suppresses oral squamous cell
carcinoma motility by targeting ITGB1. FEBS Lett. 585:187–192.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang J, Hou Y, Zhou M, Wen S, Zhou J, Xu
L, Tang X, Du YE, Hu P and Liu M: Twist induces
epithelial-mesenchymal transition and cell motility in breast
cancer via ITGB1-FAK/ILK signaling axis and its associated
downstream network. Int J Biochem Cell Biol. 71:62–71. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He B, Xiao YF, Tang B, Wu YY, Hu CJ, Xie
R, Yang X, Yu ST, Dong H, Zhao XY, et al: hTERT mediates gastric
cancer metastasis partially through the indirect targeting of ITGB1
by microRNA-29a. Sci Rep. 6:219552016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furuta M, Kozaki KI, Tanaka S, Arii S,
Imoto I and Inazawa J: miR-124, miR-203 are epigenetically
silenced tumor-suppressive microRNAs in hepatocellular carcinoma.
Carcinogenesis. 31:766–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu XC, Dong QZ, Zhang XF, Deng B, Jia HL,
Ye QH, Qin LX and Wu XZ: microRNA-29a suppresses cell proliferation
by targeting SPARC in hepatocellular carcinoma. Int J Mol Med.
30:1321–1326. 2012. View Article : Google Scholar : PubMed/NCBI
|