Introduction
Urothelial carcinoma of the bladder (UCB) is the
fourth most common cancer among men and the second most frequent
malignancy of the urogenital tract. It is estimated that globally
76,960 patients were newly diagnosed with UCB and 16,390 patients
succumbed to the disease in 2016 (11,820 men and 4,570 women)
(1). In Japan, the estimated number
of new cases and deaths from UCB in 2017 were 21,000 and 8,800
(6,100 men and 2,700 women), respectively (2). Approximately 75% of UCB cases are
diagnosed as non-muscle invasive bladder cancer (NMIBC) consisting
of tumors staged as Ta, T1 and carcinoma in situ (3). Transurethral resection of the bladder
tumor, followed by the administration of adjuvant intravesical
treatment with bacillus Calmette-Guerin (BCG) or chemotherapeutic
agents such as mitomycin C (MMC) and adriamycin (ADM), is the
gold-standard treatment for NMIBC (4). Despite improved management for
decreasing the recurrence rate and prolonging the progression-free
interval, NMIBC exhibits significant potential of recurrence
intravesically and progression to muscle-invasive bladder cancer
(5,6). Although intravesical treatment with
BCG represents the most effective and common form of adjuvant
therapy for high risk NMIBC, BCG-failure NMIBC is the one of main
problems in the management of UCB (7–9). Thus,
clinical management of high-risk NMIBC remains challenging and
further advancements in treatment initiation and maintenance are
urgent.
Intravesical sequential treatment with BCG and
chemotherapeutic agents could be a strategy to improve outcomes for
patients with BCG-failure NMIBC (10–18).
Previous studies have revealed that intravesical sequential
treatment with BCG and chemotherapeutic agents (MMC and ADM)
decreased the recurrence rates of NMIBC, compared with BCG
treatment alone (15–17). The roles of chemotherapeutic agents
in sequential treatment have direct antitumor effects, which leads
to a reduction in tumor cells and tissue-scarifying of the bladder
surface. BCG binds to the urothelial lining and tumor cells via
fibronectin attachment protein on bacilli and then elicits a
non-specific immune response within the bladder wall, involving the
activation of multiple types of immune cells and cytokines.
Intravesical chemotherapies damage the bladder wall, enhancing the
expression of fibronectin, and rendering BCG easier to attach,
resulting in the enhanced antitumor activity of BCG (12–14,16,19–21).
Our previous study on 154 NMIBCs revealed that high
counts of tumor-associated macrophages (TAMs) and regulatory T
cells (Tregs) were associated with shorter recurrence-free
survival, while high Treg counts were an independent predictor for
recurrence. The presence of TAM and Treg in the tumor
microenvironment led to a poor response to the treatment with BCG
(22). In an animal model,
intravesical treatment with MMC and ADM also caused a reduction of
TAMs and Tregs in the tumor microenvironment and induced systemic
changes to cytokines, including interleukin (IL)-17 and
granulocyte-colony stimulating factor (G-CSF) (23). There are limited studies describing
details regarding immunoreactions induced by treatments with BCG
and chemotherapeutic agents, and their efficacy from an
immunological standpoint. The aim of the present study was to
immunologically evaluate the efficacy of intravesical
chemotherapeutic agents, MMC or ADM, combined with BCG using an
N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN)-induced orthotopic
bladder cancer model.
Materials and methods
Animals
Animal care was in compliance with the
recommendations of The Guide for Care and Use of Laboratory Animals
(National Research Council) and approval for the animal studies was
obtained from the Ethics Committee on Animal Research of Nara
Medical University (reference no. 11649). Thirty-six 6-week-old
female C57BL/6J mice weighing 25 g were obtained from Oriental Bio
Service (Kyoto, Japan). They were kept in a temperature- and
humidity-controlled room, with a 12-h light/dark cycle and food
were given ad libitum.
Reagents
BBN (B0938; Tokyo Chemical Industry, Tokyo, Japan)
was used to prepare an orthotopic murine bladder cancer model. To
treat mice bearing BBN-induced bladder cancer with intravesical
instillation, the following agents, which are commonly used in a
clinical setting for NMIBC, were prepared: BCG (Nippon Kayaku,
Tokyo, Japan), MMC (Kyowa Hakko Kirin, Tokyo, Japan) and ADM (Wako,
Osaka, Japan). Each agent was diluted in sterile saline solution
according to the manufacturer's instructions, and the dosage of
each agent was determined by our previous study (23). Sterile phosphate-buffered saline
(PBS) was used as a control.
Murine orthotopic bladder cancer model
and intravesical treatment
Fig. 1A displays a
schematic representation of the study. After allowing mice to
acclimate to our facility for a week, they received 0.05% BBN in
drinking water, continuously for 16 weeks, to induce the
development of NMIBC as previously described (23). Mice were then allowed to resume
drinking BBN-free water after 17 weeks and were randomly divided
into six groups (N=6 mice/group) as follows: Control (PBS), BCG (10
µg/kg), MMC (5 µg/kg), ADM (5 µg/kg), MMC-BCG (5 and 10 µg/kg,
respectively), and ADM-BCG (5 and 10 µg/kg, respectively).
Intravesical treatment was initiated from week 17 and administered
once a week for six weeks. The treatment schedule was determined
based on our previous study that suggested that intravesical
treatment with MMC and ADM suppressed the expression of TAMs and
Tregs (23). Our hypothesis was
that the administration of MMC and ADM prior to BCG treatment could
modulate the tumor microenvironment to produce conditions allowing
BCG to produce a greater effect. Intravesical instillation regimens
of each group are presented in Fig.
1A. In regimens containing BCG, mice were treated with BCG at
weeks 2, 3, 5 and 6. In regimens containing MMC or ADM, mice were
treated with MMC or ADM at weeks 1 and 4. Fig. 1B illustrates the procedure of
catheterization and occlusion with a purse-string suture. All
bladder instillations were performed under anesthesia with
isoflurane, whereby a 24-gauge Teflon angiocatheter was placed into
the bladder via the urethra. Urine was completely drained from the
bladder, and then PBS, BCG, MMC and/or ADM were delivered by
transurethral instillation through the catheter and allowed to
dwell in the bladder by occlusion of the urethra with a
purse-string suture. After 2 h, the suture was removed and mice
were stimulated to expel bladder contents. One week after the last
intravesical treatment, all mice were euthanized by exsanguination
under anesthesia with isoflurane and tissues (bladders and whole
blood by cardiac puncture) were harvested for the following
experiments. Resected bladder weights were assessed by an
electronic precision weighing scale and then examined by
hematoxylin and eosin (H&E) staining and immunohistochemical
(IHC) staining analysis. Treatment-related changes in IL-17 and
G-CSF in sera were evaluated by enzyme-linked immunosorbent assay
(ELISA)-based analysis. The reason why we focused on IL-17 and
G-CSF is that our previous study revealed systemic changes in these
two cytokines (23).
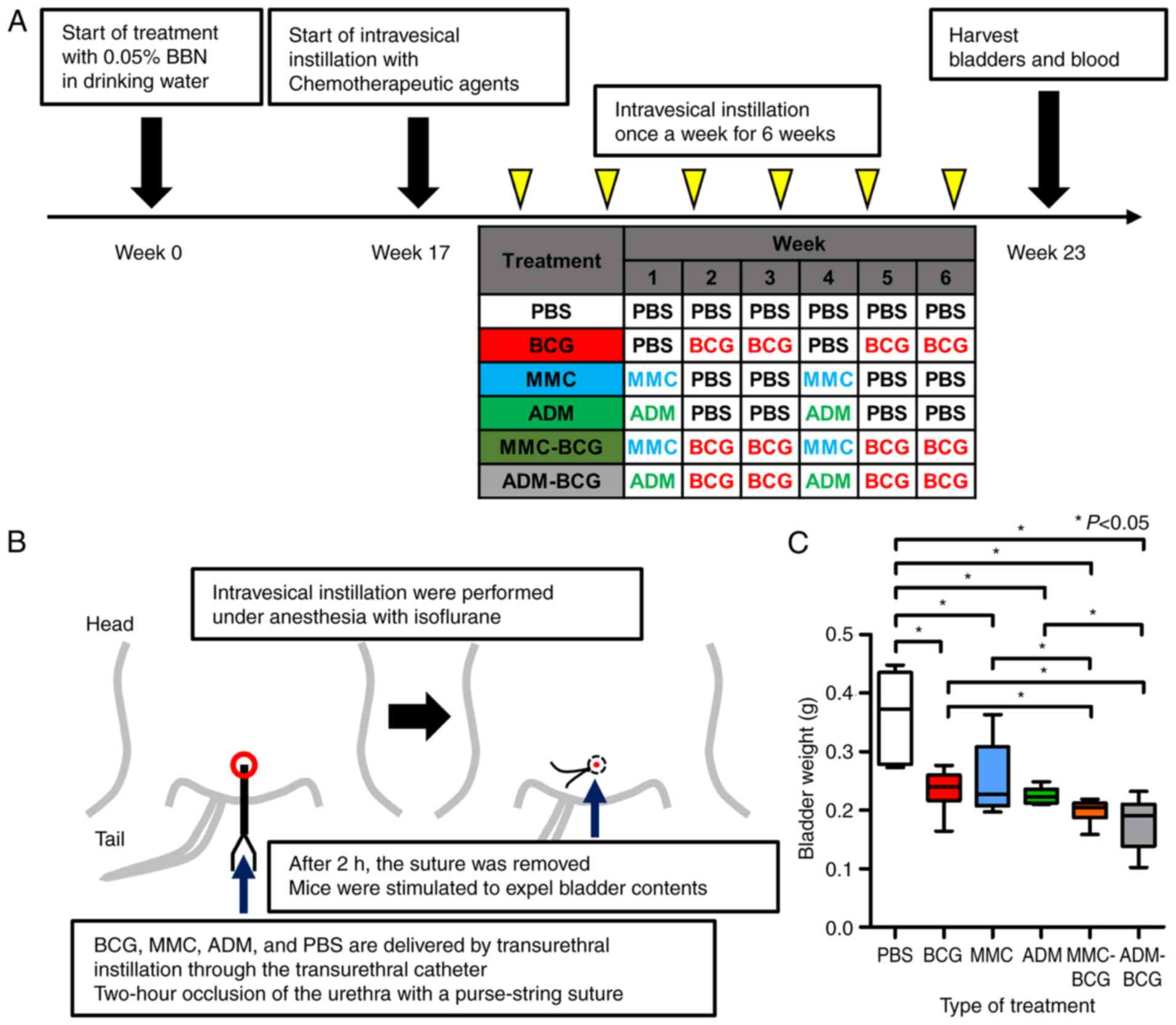 | Figure 1.Study treatment schema and resected
bladder tissues. (A) Schematic diagram illustrating the study
workflow. Mice were administered 0.05% BBN in drinking water
continuously for 16 weeks and were then randomly divided into six
groups (N=6/group). Starting at week 17, mice were treated once a
week for 6 weeks with PBS, BCG, MMC, or ADM. One week after the
last treatment, mice were euthanized for harvest of the bladder and
whole blood by cardiac puncture. Anti-CD56, CD204, and Foxp3
antibodies were used to evaluate natural killer cells,
tumor-associated macrophages, and regulatory T cells, respectively,
in resected bladder samples. Serum was used to perform
enzyme-linked immunosorbent assays. (B) Illustration revealing the
procedure of intravesical instillation and occlusion of the urethra
with a purse-string suture. All intravesical treatments were
performed under anesthesia with isoflurane, and all drugs were
delivered by transurethral instillation through a catheter and
allowed to dwell in the bladder. After 2 h, sutures were removed
and mice were allowed to urinate. (C) Comparison between resected
bladder weights of each treatment group. All intravesical
treatments had antitumor activity similar to BCG. The intravesical
combination treatment with BCG and MMC or ADM caused significant
bladder weight loss compared with that with BCG alone or the
chemotherapeutic agent alone. *P<0.05. (D) Representative images
of hematoxylin and eosin-stained bladder samples from each group.
BBN-induced bladder cancer was observed in all resected bladders.
BBN, N-butyl-N-(4-hydroxybutyl) nitrosamine; PBS,
phosphate-buffered saline; BCG, bacillus Calmette-Guerin; MMC,
mitomycin C; ADM, adriamycin. |
IHC staining analysis
Tumors were examined by IHC staining analysis as
previously described (24,25). All resected bladders were filled
with 150 µl of 10% neutral buffered formalin, and entire specimens
were placed in 10% neutral buffered formalin. Bladders in formalin
were embedded in paraffin and then subjected to IHC staining for
cell surface and immunological markers, CD56 [natural killer (NK)
cell], CD204 (M2 macrophage, known as TAM), and Foxp3 (Treg).
Paraffin blocks were cut and placed on SuperFrost Plus microslides
(Thermo Fisher Scientific, Inc., Yokohama, Japan). Sections were
deparaffinized and antigen retrieval was carried out in citric acid
buffer (pH 6.0) using an autoclave. IHC staining was performed
using a Histofine ABC kit (Nichirei Biosciences, Tokyo, Japan)
according to the manufacturer's instructions. Briefly, slides were
incubated overnight at 4°C with mouse monoclonal antibodies against
CD56 (1:500 dilution; cat. no. MA1-70100; Thermo Fisher Scientific,
Inc.), CD204 (1:2,000 dilution; cat. no. KT022; TransGenic, Inc.,
Kobe, Japan), and Foxp3 (1:500 dilution; ab20034; Abcam, Cambridge,
UK). The slides were counterstained with Mayer's hematoxylin,
dehydrated, and sealed with a cover slide. In each case, positive
cells were evaluated by two investigators (MM and YT), who were
blinded to the information pertaining to the treatment. Positive
cells from each specimen were counted from a minimum of four
randomly selected fields per high power field (HPF; magnification,
×400; 0.0625 µm2) and compared with the control by
calculating the average number of cells.
Assessment of serum IL-17 and G-CSF by
ELISA
Serum was collected in tubes, centrifuged at 10,000
× g for 15 min, and the supernatant was stored at −80°C. All 36
serum samples were thawed just before use and analyzed for their
concentrations of IL-17 (BMS6001; Affymetrix eBioscience; Thermo
Fisher Scientific, Inc.) and G-CSF (ELM-GCSF; RayBiotech, Norcross,
GA, USA). Samples were developed with horseradish
peroxidase-conjugated secondary antibodies. After adding the
substrate and stop solution, a Tecan microplate reader (Tecan, San
Jose, CA, USA) was used to assess the absorbance at 450 nm.
Statistical analysis
Statistical analyses and figure plotting were
performed using GraphPad Prism 5.0 (GraphPad Software, Inc., San
Diego, CA, USA). Data are expressed as bar charts or box plots and
the Student's t-test or the Mann-Whitney U test was applied for
statistical analysis, as appropriate. A P-value <0.05 was
considered to indicate a statistically significant result in all
analyses.
Results
Antitumor effects of intravesical
treatment with BCG, MMC, ADM and the sequential treatment
All mice were intravesically treated for six weeks
with PBS, BCG, MMC, ADM, MMC-BCG, or ADM-BCG according to the
regimens displayed in Fig. 1A. All
treatments were well tolerated, with no body weight loss except
minor hematuria. A week after the termination of treatment,
significant bladder weight loss was observed in the BCG, MMC, ADM,
MMC-BCG, and ADM-BCG treatment groups compared with the control
(P=0.0043, P=0.041, P=0.0023, P=0.0021, P=0.0020, respectively;
Fig. 1C). Moreover, significant
differences were observed between the bladder weights of mice
receiving BCG monotherapy or MMC/ADM monotherapy and sequential
treatments with BCG and MMC/ADM (BCG vs. MMC-BCG, P=0.042; BCG vs.
ADM-BCG, P=0.026; MMC vs. MMC-BCG, P=0.041; ADM vs. ADM-BCG,
P=0.0040). These results indicated that the antitumor effects were
increased by combining BCG with chemotherapeutic agents. Fig. 1D displays representative H&E
staining images for each treatment group and the development of
NMIBC was confirmed in all samples.
Features of immune-related cells
induced by intravesical sequential treatment with BCG and
chemotherapeutic agents
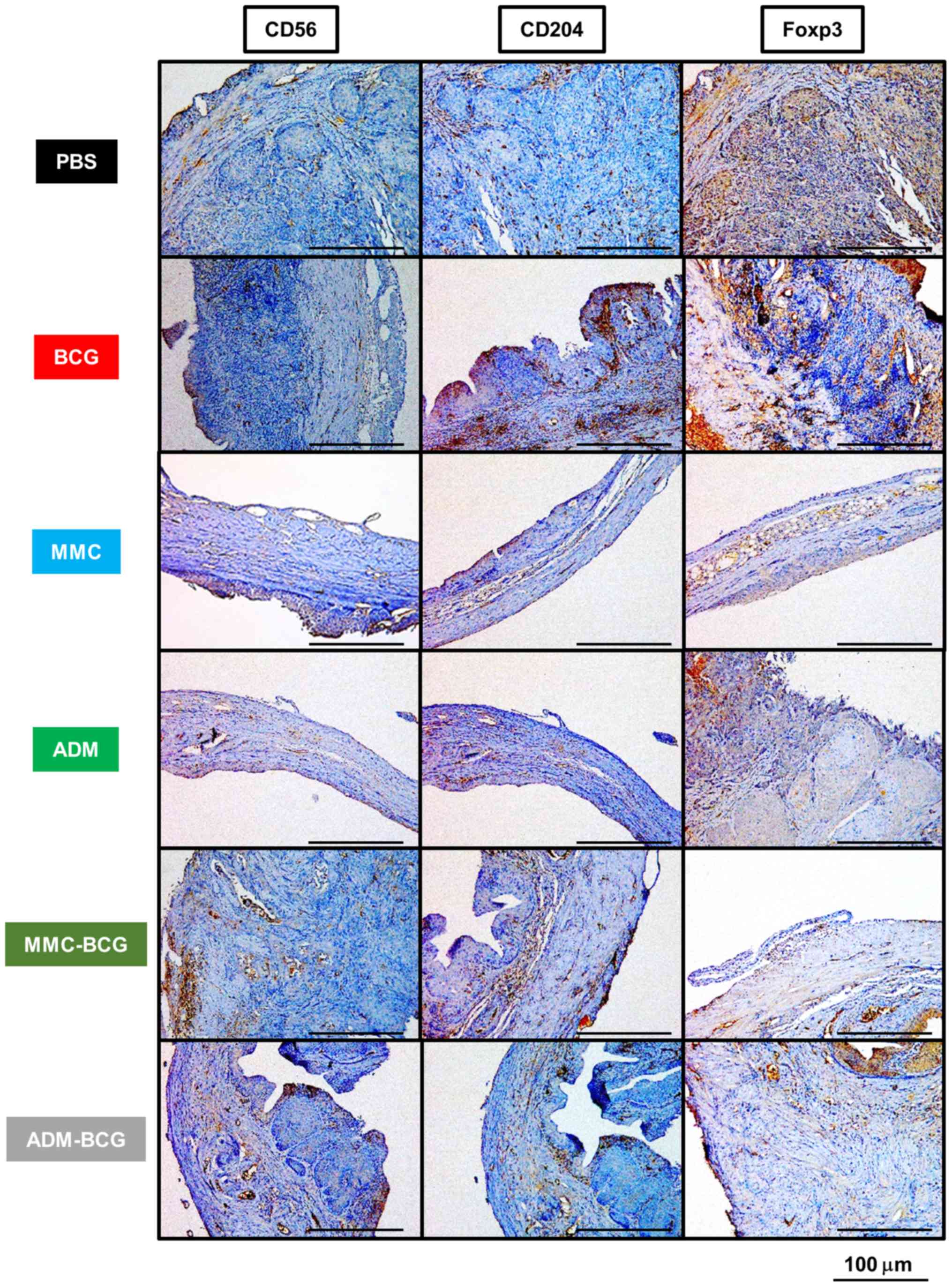
To investigate the effects of intravesical
sequential treatments with BCG and MMC/ADM on immune-related cells,
IHC staining for three markers (CD56, CD204 and Foxp3) was
performed. Representative images of antibody-stained resected
bladders are presented in Fig. 2
and the results are summarized in Table
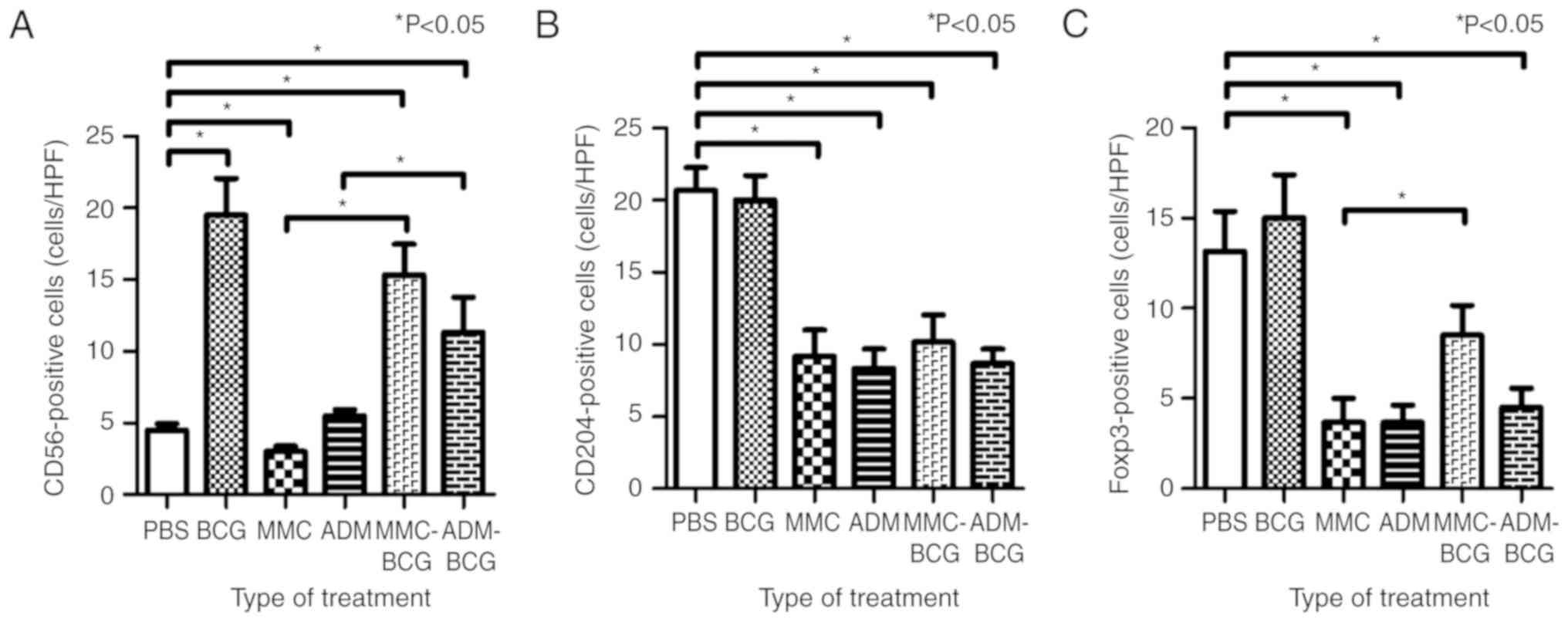
I. NK cells were significantly induced by treatment regimens
containing BCG and were reduced by treatment with MMC monotherapy,
compared with the control (BCG, P=0.0049; MMC-BCG, P=0.0048;
ADM-BCG, P=0.0062; MMC, P=0.039; and ADM, P=0.16; Fig. 3A). NK cell levels were highest after
treatment with BCG monotherapy, compared to all other treatment
groups. Although NK cells were not recruited after treatment with
MMC/ADM monotherapy, NK cells were significantly induced in the
tumor microenvironment by sequential treatment with BCG and MMC/ADM
(MMC-BCG, P=0.0048; and ADM-BCG, P=0.015; Fig. 3A). TAMs were significantly reduced
by the treatment regimens containing chemotherapeutic agents,
compared with the control (MMC, P=0.0043; ADM, P=0.0022; MMC-BCG,
P=0.010; and ADM-BCG, P=0.0021; Fig.
3B). Although TAMs were not reduced by the treatment with BCG
monotherapy, the sequential treatment with BCG and MMC/ADM reduced
TAMs, indicating that intravesical treatment with chemotherapeutic
agents prevented the recruitment of TAMs and/or killed TAMs in the
tumor microenvironment. With regard to Tregs, treatment regimens
containing ADM, as well as treatment with MMC monotherapy,
significantly reduced Tregs in the tumor microenvironment, compared
with the control (ADM, P=0.0064; ADM-BCG, P=0.010; and MMC,
P=0.016; Fig. 3C). Similar to TAMs,
Tregs tended to be reduced after treatment with chemotherapeutic
agents.
 | Table I.Summary of immunohistochemical
staining and ELISA assay of serum. |
Table I.
Summary of immunohistochemical
staining and ELISA assay of serum.
|
| Immunohistochemical
staining analysis of the treated bladder (vs. the control) ELISA of
serum (vs. the control) |
|---|
|
|
|
|---|
| Treatment | CD56 | CD204 | Foxp3 | IL-17 | G-CSF |
|---|
| BCG | Up | NS | NS | Up | Up |
| MMC | Down | Down | Down | NS | NS |
| ADM | NS | Down | Down | NS | Up |
| MMC-BCG | Up | Down | NS | Up | NS |
| ADM-BCG | Up | Down | Down | Up | Up |
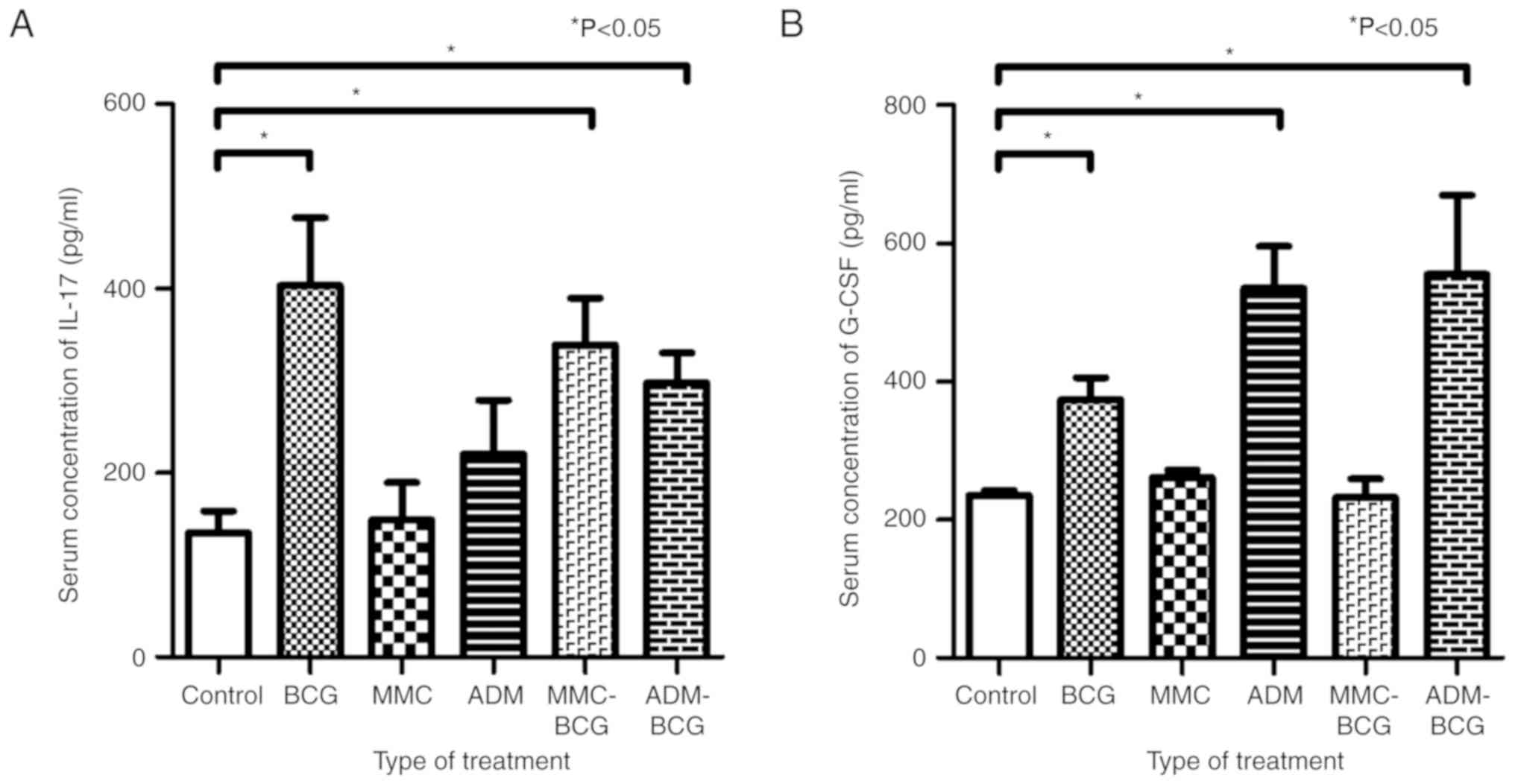
Systemic changes of IL-17 and G-CSF
levels in serum caused by intravesical sequential treatment with
BCG and chemotherapeutic agents
To investigate the association between intravesical
treatment and systemic changes to IL-17 and G-CSF levels, ELISA was
performed using the serum obtained from the euthanized mice. The
serum concentration of IL-17 was increased by treatment with BCG,
MMC-BCG, and ADM-BCG, compared with the control (P=0.0043, P=0.0042
and P=0.0087, respectively; Fig.
4A). The treatment regimens containing BCG had an effect on the
systemic induction of IL-17, indicating that instillation of BCG is
an important factor for induction of IL-17, systemically. In
contrast, the serum concentration of G-CSF was increased by
treatment with BCG, ADM, and ADM-BCG, compared with the control
(P=0.0087, P=0.0022, and P=0.0021, respectively; Fig. 4B). The treatment regimens containing
ADM exhibited greater increase of G-CSF, indicating that
instillation of ADM was a key factor for induction of G-CSF,
systemically. The results are summarized in Table I.
Discussion
The present study revealed that intravesical
sequential treatment with BCG and chemotherapeutic agents (MMC and
ADM) elicited characteristic changes to immune cells in the tumor
microenvironment of UCB. Although NK cells are induced by treatment
with BCG monotherapy, treatment with chemotherapeutic agents
followed by BCG also induced the recruitment of NK cells in the
tumor microenvironment. The recruitment of TAMs and Tregs was
inhibited by treatment with chemotherapeutic agents, except for
treatment with MMC-BCG. Our previous study indicated that
intravesical chemotherapy induced systemic changes in IL-17 and
G-CSF levels, and that these cytokines had a role in promoting
neutrophils, NK cells, and cytotoxic T lymphocytes (CTLs) in the
tumor microenvironment, resulting in antitumor effects (23). Sequential treatment with BCG and
chemotherapeutic agents, particularly ADM, induced IL-17 and G-CSF
systemically. The summary of this study is displayed in Fig. 5. Intravesical sequential treatment
with BCG and chemotherapeutic agents have direct antitumor effects
upon T lymphocyte-dependent cytokines [particularly T helper 1
(Th-1) dependent cytokines such as IL-2, IL-12, and interferon
(INF)-γ], immune-related cells (NK cells, CTL and macrophages), and
apoptotic pathways (tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL) and fatty-acid synthase (Fas)
ligand) (26). BCG treatment may
also have indirect antitumor effects induced by chemotherapeutic
agents, including the modulation of the tumor environment via the
reduction of TAMs and Tregs.
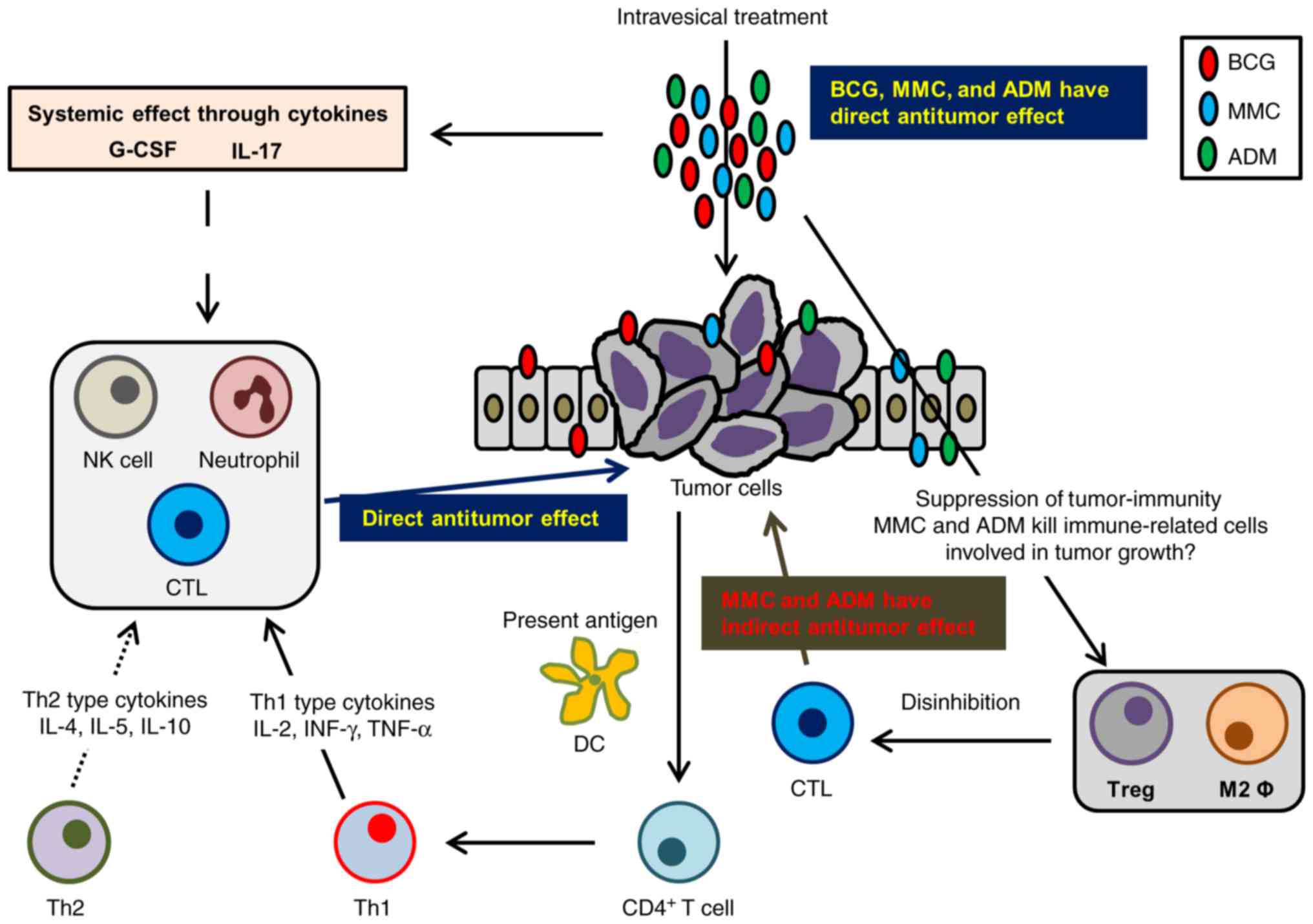 | Figure 5.Schematic summary of the study.
Intravesical treatment with chemotherapeutic agents demonstrates
antitumor activity through direct cytotoxicity and indirectly
through modulation of antitumor immunity. CTLs recognize
cancer-specific antigens from apoptotic cancer cells and eliminate
them. In addition, DC capture these antigens and present them to
CD4+ T cells. Activated CD4+ T cells produce
cytokines resulting in the activation of Th-1 cells and the
recruitment of CTLs, NK cells, and macrophages. The treatment with
BCG enhances this pathway through activation of CTLs and NK cells.
In contrast, intravesical treatment with chemotherapeutic agents
modifies the tumor microenvironment to consist of less
immunosuppressive cells, including a reduction in TAMs and Tregs.
As a result, intravesical treatment with BCG works more
effectively. IL-17 and G-CSF are induced by intravesical treatment,
recruiting neutrophils to the tumor microenvironment. Neutrophils
eliminate tumor cells by activating apoptotic pathways.
Intravesical treatment with BCG and chemotherapeutic agents can be
a novel treatment strategy for patients with BCG-failure non-muscle
invasive bladder cancer by immunomodulation of the tumor
microenvironment. CTLs, cytotoxic T cells; DC, dendritic cells;
Th-1, helper T1; NK, natural killer; TAMs, tumor-associated
macrophages; Tregs, regulatory T cells; BCG, bacillus
Calmette-Guerin; IL, interleukin; G-CSF, granulocyte-colony
stimulating factor. |
Table II shows a
summary of previous prospective studies involving intravesical
sequential treatment with BCG and chemotherapeutic agents for
patients with NMIBC (10–17,19–21,27–32).
Intravesical sequential treatment has been studied since the 1990s,
with the studies mainly focusing on the effects of chemotherapeutic
agents, such as direct antitumor effects, tissue-scarifying of the
bladder surface for attachment of BCG to the bladder wall, and the
reduction of adverse events induced by BCG. Solsona et al
reported that the 5-year disease-free interval (DFI) of sequential
treatment with BCG and MMC, and treatment with BCG monotherapy, was
20.6 and 3.9%, respectively. This was the largest study of its kind
and revealed that treatment with BCG monotherapy had a long
disease-free interval and a low rate of adverse events, compared
with sequential treatment with BCG and MMC (11). In contrast, Di Stasi et al,
which had the longest follow-up period, reported that DFI was
significantly increased in patients treated sequentially with BCG
and MMC, compared to treatment with BCG monotherapy (15). The extent of the antitumor activity
and decrease in adverse events following intravesical sequential
treatment remains unclear due to the controversial results of each
study. The antitumor effects of chemotherapeutic agents are mainly
demonstrated by blocking DNA synthesis, RNA synthesis, or cell
division. Our hypothesis was that intravesical treatment with
chemotherapeutic agents modified the tumor microenvironment to
conditions in which tumor cells found it difficult to proliferate
and/or host immune-related cell attacks on tumor cells. Previous
studies focusing on aspects involving immunomodulation of the tumor
microenvironment are limited. The reduction of TAMs and Tregs,
along with the potential effects of chemotherapeutic agents such as
direct cytotoxicity and tissue-scarifying, demonstrated antitumor
effects. Ghiringhelli et al reported that pretreatment with
cyclophosphamide, which suppresses Tregs, enhanced the effects of
subsequent immunotherapy in colorectal cancer (33). The treatment schedules of previous
reports varied widely. It is our opinion, that the administration
of MMC or ADM followed by BCG would be a better schedule, and that
treatment should be repeated for several cycles. In addition, most
of the previous prospective randomized trials were focused on
sequential treatment with BCG and MMC, thus sequential treatment
with BCG and ADM has not yet been verified by randomized trials.
Our results indicated that sequential treatment with BCG and ADM
inhibited the recruitment of Tregs to the tumor microenvironment,
compared with BCG and MMC treatment. Verification of combination
treatment with BCG and ADM is therefore necessary in the near
future.
 | Table II.Review of literature involved in
intravesical sequential treatment with bacillus Calmette-Guerin and
chemotherapeutic agents. |
Table II.
Review of literature involved in
intravesical sequential treatment with bacillus Calmette-Guerin and
chemotherapeutic agents.
|
|
|
|
|
|
|
| Outcome (%) | AE (n) |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Year | Author | Objectives | Treatment
regimen | Size | Induction dose and
schedule | F/u (year) | RR | PR | DSM | TCR | F | C | H | (Refs.) |
|---|
| 1994 | Uekado et
al | pTa/pT1 | BCG+Epi | 29 | BCG+Epi: 80 mg
Tokyo strain + 40 mg (Epi immediately after TURBT, and then weekly
BCG for 6 weeks) | 1.6 | 3.4 | ND | ND | 96.6 | 4 | 25 | 1 | (27) |
| 1994 | Erol et
al | pTa/pT1 | BCG+Epi | 14 | BCG+Epi: 80 mg
Connaught strain + 50 mg (weekly Epi immediately followed by weekly
BCG for 6 weeks) | 1.2 | 11 | ND | ND | 64.3 | 14 | 14 | 12 | (28) |
| 1995 | Rintala et
al | pTis | MMC | 40 | MMC: 20–40 mg (MMC
1/week for 4 weeks, and | 2.8 | a, b | 10 | ND | 95 | ND | ND | ND | (19) |
|
|
|
| vs. | vs. | 1/month for 1 year,
and then 1/3 months for 1 year) |
| 1.8 | vs. |
| vs. |
|
|
|
|
|
|
|
| BCG+MMC | 28 | BCG+MMC: 75 mg
Pasteur strain + 20–40 mg |
| vs. | 7.1 |
| 100 |
|
|
|
|
|
|
|
|
|
| (MMC 1/week for 4
weeks as induction, followed by MMC and BCG alternately by 1 month
for 12 months, then by 3 months for 12 months) |
| 0.9 |
|
|
|
|
|
|
|
| 1996 | Rintala et
al | pTa/pT1 | MMC | 93 | MMC: 20–40 mg (MMC
1/week for 4 weeks, and | 2.8 | 64 | 3.2 | ND | 79.6 | ND | ND | ND | (14) |
|
|
|
| vs. | vs. | 1/month for 1 year,
and then 1/3 months for 1 year) |
| vs. | vs. |
| vs. |
|
|
|
|
|
|
|
| BCG+MMC | 95 | BCG+MMC: 75 mg
Pasteur strain + 20–40 mg (MMC 1/week for 4 weeks as induction,
followed by MMC and BCG alternately by 1 month for 12 months, then
by 3 for 12 months) |
| 62 | 2.1 |
| 81.1 |
|
|
|
|
| 1996 | Van der Epi for 4
weeks followed by weekly BCG for 6 weeks) | pTa/pT1 | BCG+MMC | 32 | BCG+Epi:
5×108 CFU RIVM strain + 40 mg (weekly Meijden et
al | ND | 9.4 | 3.1 | 0 | 96.9 | 31 | 31 | 31 | (21) |
| 1998 | Witjes et
al | pTa/pT1 | MMC | 92 | MMC: 40 mg (weekly
MMC for 10 weeks) | 2.7 | 46 | 4 | 8.7 | 90.2 | 3 | 60 | ND | (12) |
|
|
|
| vs. | vs. | BCG+MMC:
5×108 CFU Tice strain + 40 mg |
| vs. | vs. | vs. | vs. | vs. | vs. |
|
|
|
|
|
| BCG+MMC | 90 | (weekly MMC for 4
weeks, followed by weekly BCG for 6 weeks) |
| 39 | 6 | 5.6 | 94.4 | 11 | 44 |
|
|
| 1999 | Ali-El-Dein | pT1 | BCG vs. | 58 | BCG: 150 mg Pasteur
strain (weekly BCG for 6 weeks, | 2.5 | 20.7 | 8.6 | ND | 83 | 3 | 36 | 4 | (29) |
|
| et al |
| BCG+Epi | vs. | followed by monthly
for 10 months) |
| vs. | vs. |
| vs. | vs. | vs. | vs. |
|
|
|
|
|
| 66 | BCG+Epi: 150 mg
Pasteur strain + 50 mg (alternating BCG and Epi 1/week for 6 weeks,
followed by monthly for 10 months) |
| 10.6 | 4.6 |
| 95.7 | 0 | 18 | 0 |
|
| 2000 | Kaasinen | pTa/pT1 | BCG+MMC | 118 | BCG: 81 mg
Connaught strain (weekly BCG for 6 weeks) | 2.6 | b28.4 | 2.9 | ND | 99 | ND | ND | ND | (13) |
|
| et al |
| vs. MMC+ | vs. | BCG+MMC: 81 mg
Connaught strain + 10 or 30 mg |
| vs. | vs. |
| vs. |
|
|
|
|
|
|
|
| IFN-a2b±BCG | 118 | (MMC instillated 1
day before BCG, MMC: 1/2 weeks for 6 weeks, BCG: 1/week for 6
weeks) |
| 68 | 3.9 |
| 97.1 |
|
|
|
|
| 2000 | Bilen et
al | pT1 | BCGvs. | 21 | BCG: 81 mg
Connaught strain (weekly BCG for | 1.5 | 19 | 10 | ND | 100 | 3 | 21 | 8 | (17) |
|
|
|
| BCG+Epi | vs. | 6 weeks) BCG+Epi:
81 mg Connaught strain + 50 mg |
| vs. | vs. |
| vs. | vs. | vs. | vs. |
|
|
|
|
|
| 20 | (weekly for 12
weeks as follows:
Epi/Epi/Epi/Epi/BCG/BCG/BCG/Epi/BCG/BCG/BCG/Epi) |
| 15 | 4.7 |
| 90 | 2 | 20 | 4 |
|
| 2000 | Bono et
al | pT1 | BCG+Epi | 81 | BCG+Epi: 81 mg
Connaught strain + 50 mg (weekly Epi immediately followed by
BCG) | 4 | 22.4 | 7.4 | 4.9 | 98.8 | 8 | 13 | 4 | (30) |
|
|
|
|
|
| (BCG: 1/week for 6
weeks, Epi: 1/week for 8 weeks) |
|
|
|
|
|
|
|
|
|
| 2002 | Kaasinen | Ta/T1 | BCG+MMC | 102 | BCG: 120 mg
Connaught strain (weekly BCG | 3.9 | b34.3 | ND | ND | ND | ND | ND | ND | (20) |
|
| et al | without | vs. MMC+ | vs. | for 6 weeks,
followed by monthly for 1 year) |
| vs. |
|
|
|
|
|
|
|
|
|
| CIS | IFN-a2b±BCG | 103 | BCG+MMC: 120 mg
Connaught strain + 40 mg (MMC 1/week for 6 weeks, followed by
alternating BCG and MMC monthly for 1 year) |
| 73.8 |
|
|
|
|
|
|
|
| 2003 | Kaasinen | pTis | BCGvs. | 157 | BCG: 120 mg
Connaught strain(weekly BCG for | 4.7 | b44.8 | 13.8 | 6.9 | 74.5 | ND | ND | ND | (16) |
|
| et al |
| BCG+MMC | vs. | 6 weeks, followed
by monthly for 1 year) |
| vs. | vs. | vs. | vs. |
|
|
|
|
|
|
|
|
| 165 | BCG+MMC: 120 mg
Connaught strain + 40 mg (MMC 1/week for 6 weeks, followed by
alternating BCG and MMC monthly for 1 year) |
| 54.7 | 21.4 | 8.2 | 93.7 |
|
|
|
|
| 2006 | Di Stasi et
al | pT1 | BCG | 105 | BCG: 81 mg
Connaught strain (1/week for 6 weeks, | 7.3 | b58.1 | 21.9 | 16.2 | 97.1 | 24 | 60 | 61 | (15) |
|
|
|
| vs. | vs. | followed by monthly
for 10 months) BCG+MMC: |
| vs. | vs. | vs. | vs. | vs. | vs. | vs. |
|
|
|
|
| BCG+MMC | 107 | 81 mg Connaught
strain + 40 mg (1/week for 9 weeks as follows:
BCG/BCG/MMC/BCG/BCG/MMC/BCG/BCG/MMC, followed by 1/month for 9
months: 3 cycles of MMC, MMC and BCG) |
| 42.1 | 9.3 | 5.6 | 97.2 | 21 | 65 | 64 |
|
| 2008 | Cai et
al | pTa/pT1 | BCG | 81 | BCG:
5×108 CFU Tice strain (1/week for 6 weeks, | ND | 49.4 | 4.9 | ND | ND | ND | ND | ND | (31) |
|
|
|
| vs. | vs. | and then
maintenance for 3 years) BCG+Epi: 5×108 |
| vs. | vs. |
|
|
|
|
|
|
|
|
|
| BCG+Epi | 80 | colony-forming
units Tice strain + 80 mg (Epi immediately after TURBT, followed by
BCG 1/week for 6 weeks, and then maintenance for 3 years |
| 42.5 | 2.5 |
|
|
|
|
|
|
| 2011 | Oosterlinck | pTis | BCG | 48 | BCG:
5×108 CFU TICE strain (weekly BCG for | 4.7 | 54.2 | 10.4 | 12.5 | 97.8 | ND | ND | ND | (10) |
|
| et al
(10) |
| vs. | vs. | 6 weeks, followed
by 3 weeks of rest, and then by |
| vs. | vs. | vs. | vs. |
|
|
|
|
|
|
|
| BCG+MMC | 48 | 3 weeks of BCG)
BCG+MMC: 5×108 CFU TICE strain + 40 mg weekly MMC for 6
weeks, followed by 6 weekly BCG |
| 47.9 | 4.2 | 0 | 95.7 |
|
|
|
|
| 2012 | Järvinen | pTis | MMC | 40 | MMC: 40 mg (weekly
MMC for 4 weeks, followed by | 7.2 | 88 | 35 | 30 | ND | ND | ND | ND | (32) |
|
| et al |
| vs. | vs. | MMC monthly for 2
years) BCG+MMC: 75 mg Pasteur |
| vs. | vs. | vs. |
|
|
|
|
|
|
|
|
| BCG+MMC | 28 | strain + 40 mg
(weekly MMC for 4 weeks, followed by alternating BCG and MMC
monthly for 1 year and alternating BCG and MMC 1/3 months for
additional 1 year) |
| 68 | 28 | 29 |
|
|
|
|
|
| 2015 | Solsona | pTa/pT1/ | BCG | 196 | BCG: 81 mg
Connaught strain (weekly BCG for 6 weeks) | 7.1 | b34.6 | 12.2 | 7.6 | 95.8 | ND | ND | ND | (11) |
|
| et al | pTis | vs. | vs. | BCG+MMC: 81 mg
Connaught strain + 10 or 30 mg |
| vs. | vs. | vs. | vs. |
|
|
|
|
|
|
|
| BCG+MMC | 211 | (MMC instillated 1
day before BCG, MMC: ½ weeks for 6 weeks, BCG: 1/week for 6
weeks) |
| 20.8 | 12.3 | 4.7 | 80 |
|
|
|
|
In the tumor microenvironment, activated Th-1
produces immune-activating cytokines such as IL-2 and INF-γ, while
stimulated CTLs play an important role in tumor immunity. Contrary
to this, tumor cells produce immunosuppressive cytokines such as
IL-10 and TNF-β and induce immunosuppressive cells such as TAMs,
Tregs, and myeloid-derived suppressor cells (MDSCs) (26,34–36).
Various previous studies have suggested that intravesical
chemotherapy modifies the tumor microenvironment into a less
immunosuppressive condition, involving the induction of TAMs
towards antitumoral M1 macrophages, proliferation of CTLs, an
increase in the permeability of tumor cells to CTL-produced
granzyme B, selective reduction of MDSCs, and recruitment of NK
cells and CTLs expressing perforin, granzyme B, and IFN-γ (37–40).
Treatment for patients with BCG failure is still challenging. High
numbers of TAMs and Tregs in bladder tissue have been suggested to
have a substantial correlation with BCG-failure in patients with
NMIBC (22,23). Intravesical treatment with MMC and
ADM prior to BCG can be a novel strategy for patients with high
numbers of Tregs and TAMs in their transurethrally resected
tissue.
With regard to the cytokines evaluated in the
present study, the role of IL-17 in cancer remains unclear. The
balance of Th-1, Th-2, and Th-17, which produce IL-17, is important
for antitumor activity. Treatment with BCG was revealed to induce
IL-17 and Th-17, resulting in antitumor activity (41). G-CSF could also be a marker for
intravesical recurrence after intravesical treatment with BCG
(42). Intravesical sequential
treatment with BCG and chemotherapeutic agents induced IL-17 and
G-CSF in this study. Thus, the increase in these cytokines leads to
the subsequent induction of neutrophils and facilitates antitumor
activity through Fas and/or TRAIL apoptotic pathways. In addition,
these cytokines could be involved in immunomodulation via a
reduction in immunosuppressive cells.
The present study has some limitations. The
administration of BBN was not by oral gavage but was received in
drinking water. Thus, the BBN intake varied among the mice and this
could lead to variability in the tumor size and/or tumor behavior
of each mouse. A confirmatory study with human bladder cancer
specimens should be performed. A treatment schedule, controlling
the timing of administration of BCG and chemotherapeutic agents,
the number of treatment cycles, and the necessity of maintenance
treatment, should be considered to achieve maximum efficacy of
intravesical combination treatment. Further experiments are
required to establish a novel treatment strategy for patients with
NMIBC, particularly for patients with BCG-failure, based on
immunological and molecular aspects.
In conclusion, intravesical sequential treatment
with BCG and chemotherapeutic agents has more effective antitumor
activity than that of BCG or chemotherapeutic agent monotherapy in
a BBN-induced bladder cancer mouse model. Intravesical chemotherapy
modified the tumor microenvironment to suppress pro-tumoral
immunity and enhance antitumor immunity, increasing the efficiency
of BCG treatment. These findings indicated that intravesical
combination treatment could suppress resistance to BCG through
enhancement of antitumor immunity (induction of NK cells) and
inhibition of pro-tumoral immunity (reduction of TAMs and Tregs)
and may be a novel strategy for BCG-failure NMIBC. Further studies,
including clinical trials, may be required to establish appropriate
strategies for intravesical sequential treatment, based on the
immunomodulation of the tumor microenvironment.
Acknowledgements
We thank Mrs. Aya Asano (Department of Pathology,
Nara Medical University, Nara, Japan) for giving us substantial
help with the intravesical treatment.
Funding
The present study was supported by the JSPS KAKENHI
grant nos. 15K10605 (KF) and 16K20159 (MM), and the Fiscal Years
2015–2016 Nara Medical University Grant-in-Aid for Collaborative
Research Projects (KF and MM).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are not publicly available due to our hospital
policy, but are available from the corresponding author upon
reasonable request.
Authors' contributions
SH, MM and KF contributed to the design of the study
and writing of the manuscript. SH, YT, YM, SO, KO, KI, DG, and YI
carried out the animal experiments and molecular biology studies.
MM, YN, and NT performed the statistical tests. MM and KF assisted
with the writing of the manuscript. MM and YT reviewed the
pathological diagnosis of bladder tissues. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The Ethics Committee on Animal Research of Nara
Medical University (Kashihara, Japan) approved this animal study.
The reference number is 11649.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
UCB
|
urothelial carcinoma of the
bladder
|
|
NMIBC
|
non-muscle invasive bladder cancer
|
|
BCG
|
bacillus Calmette-Guerin
|
|
MMC
|
mitomycin C
|
|
ADM
|
adriamycin
|
|
TAM
|
tumor-associated macrophage
|
|
Treg
|
regulatory T cell
|
|
IL
|
interleukin
|
|
G-CSF
|
granulocyte-colony stimulating
factor
|
|
BBN
|
N-butyl-N-(4-hydroxybutyl)
nitrosamine
|
|
PBS
|
phosphate-buffered saline
|
|
H&E
|
hematoxylin and eosin
|
|
IHC
|
immunohistochemistry
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
NK
|
natural killer
|
|
HPF
|
high power field
|
|
CTL
|
cytotoxic T lymphocyte
|
|
Th
|
T-helper
|
|
IFN
|
interferon
|
|
TNF
|
tumor necrosis factor
|
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
|
Fas
|
fatty-acid synthetase
|
|
DFI
|
disease-free interval
|
|
MDSC
|
myeloid-derived suppressor cell
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Editorial board of the cancer statistics
in japan. Cancer statistic in Japan 2017. Tokyo: Foundation for
promotion of cancer research; 2017. https://ganjoho.jp/data/reg_stat/statistics/bro-chure/2017/cancer_statistics_2017.pdf16–July.
2018
|
|
3
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A,
Palou Redorta J, et al: EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder: Update 2013. Eur Urol.
64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miyake M, Fujimoto K and Hirao Y: Active
surveillance for nonmuscle invasive bladder cancer. Investig Clin
Urol. 57 Suppl 1:S4–S13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herr HW: Intravesical bacillus
Calmette-Guerin outcomes in patients with bladder cancer and
asymptomatic bacteriuria. J Urol. 187:435–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shahin O, Thalmann GN, Rentsch C,
Mazzucchelli L and Studer UE: A retrospective analysis of 153
patients treated with or without intravesical bacillus
Calmette-Guerin for primary stage T1 grade 3 bladder cancer:
Recurrence, progression and survival. J Urol. 169:96–100. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herr HW and Morales A: History of Bacillus
Calmette-Guerin and bladder cancer: An immunotherapy success story.
J Urol. 179:53–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamm DL, Blumenstein BA, Crissman JD,
Montie JE, Gottesman JE, Lowe BA, Sarosdy MF, Bohl RD, Grossman HB,
Beck TM, et al: Maintenance bacillus Calmette-Guérin immunotherapy
for recurrent TA, T1 and carcinoma in situ transitional cell
carcinoma of the bladder: A randomized Southwest Oncology Group
Study. J Urol. 163:1124–1129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakai Y, Anai S, Tanaka N, Chihara Y,
Haramoto M, Otani T, Nakagawa Y, Hirao Y, Konishi N and Fujimoto K:
Insignificant role of bacillus Calmette-Guérin maintenance therapy
after complete transurethral resection of bladder tumor for
intermediate- and high-risk non-muscle-invasive bladder cancer:
Results from a randomized trial. Int J Urol. 23:854–860. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oosterlinck W, Kirkali Z, Sylvester R, da
Silva FC, Busch C, Algaba F, Collette S and Bono A: Sequential
intravesical chemoimmunotherapy with mitomycin C and bacillus
Calmette-Guérin and with bacillus Calmette-Guérin alone in patients
with carcinoma in situ of the urinary bladder: Results of an EORTC
genito-urinary group randomized phase 2 trial (30993). Eur Urol.
59:438–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Solsona E, Madero R, Chantada V, Fernandez
JM, Zabala JA, Portillo JA, Alonso JM, Astobieta A, Unda M,
Martinez-Piñeiro L, et al: Sequential combination of mitomycin C
plus bacillus Calmette-Guérin (BCG) is more effective but more
toxic than BCG alone in patients with non-muscle-invasive bladder
cancer in intermediate- and high-risk patients: Final outcome of
CUETO 93009, a randomized prospective trial. Eur Urol. 67:508–516.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Witjes JA, Caris CT, Mungan NA, Debruyne
FM and Witjes WP: Results of a randomized phase III trial of
sequential intravesical therapy with mitomycin C and bacillus
Calmette-Guerin versus mitomycin C alone in patients with
superficial bladder cancer. J Urol. 160:1668–1671. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaasinen E, Rintala E, Pere AK, Kallio J,
Puolakka VM, Liukkonen T and Tuhkanen K: Weekly mitomycin C
followed by monthly bacillus Calmette-Guerin or alternating monthly
interferon-alpha2B and bacillus Calmette-Guerin for prophylaxis of
recurrent papillary superficial bladder carcinoma. J Urol.
164:47–52. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rintala E, Jauhiainen K, Kaasinen E, Nurmi
M and Alfthan O: Alternating mitomycin C and bacillus
Calmette-Guerin instillation prophylaxis for recurrent papillary
(stages Ta to T1) superficial bladder cancer. Finnbladder Group. J
Urol. 156:56–60. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Stasi SM, Giannantoni A, Giurioli A,
Valenti M, Zampa G, Storti L, Attisani F, De Carolis A, Capelli G,
Vespasiani G, et al: Sequential BCG and electromotive mitomycin
versus BCG alone for high-risk superficial bladder cancer: A
randomised controlled trial. Lancet Oncol. 7:43–51. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaasinen E, Wijkström H, Malmström PU,
Hellsten S, Duchek M, Mestad O and Rintala E; Nordic Urothelial
Cancer Group, : Alternating mitomycin C and BCG instillations
versus BCG alone in treatment of carcinoma in situ of the urinary
bladder: A nordic study. Eur Urol. 43:637–645. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bilen CY, Ozen H, Aki FT, Aygün C, Ekici S
and Kendi S: Clinical experience with BCG alone versus BCG plus
epirubicin. Int J Urol. 7:206–209. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hilton WM, Ercole B, Parekh DJ, Sonpavde
G, Ghosh R and Svatek RS: Efficacy of combined intravesical
immunotherapy and chemotherapy for non-muscle invasive bladder
cancer. Expert Rev Anticancer Ther. 11:949–957. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rintala E, Jauhiainen K, Rajala P, Ruutu
M, Kaasinen E and Alfthan O: Alternating mitomycin C and bacillus
Calmette-Guerin instillation therapy for carcinoma in situ of the
bladder. The Finnbladder Group. J Urol. 154:2050–2053. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaasinen E, Rintala E, Hellström P,
Viitanen J, Juusela H, Rajala P, Korhonen H and Liukkonen T;
FinnBladder Group, : Factors explaining recurrence in patients
undergoing chemoimmunotherapy regimens for frequently recurring
superficial bladder carcinoma l. Eur Urol. 42:167–174. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van der Meijden AP, Hall RR, Macaluso MP,
Pawinsky A, Sylvester R and Van Glabbeke M: Marker tumour responses
to the sequential combination of intravesical therapy with
mitomycin-C and BCG-RIVM in multiple superficial bladder tumours.
Report from the European Organisation for Research and Treatment on
Cancer-Genitourinary Group (EORTC 30897). Eur Urol. 29:199–203.
1996.PubMed/NCBI
|
|
22
|
Miyake M, Tatsumi Y, Gotoh D, Ohnishi S,
Owari T, Iida K, Ohnishi K, Hori S, Morizawa Y, Itami Y, et al:
Regulatory T cells and tumor-associated macrophages in the tumor
microenvironment in non-muscle invasive bladder cancer treated with
intravesical bacille Calmette-Guérin: A long-term follow-up study
of a Japanese cohort. Int J Mol Sci. 18:E21862017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hori S, Miyake M, Tatsumi Y, Onishi S,
Morizawa Y, Nakai Y, Tanaka N and Fujimoto K: Topical and systemic
immunoreaction triggered by intravesical chemotherapy in an
N-butyl-N-(4-hydroxybutyl) nitorosamine induced bladder cancer
mouse model. PLoS One. 12:e01754942017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hori S, Miyake M, Tatsumi Y, Morizawa Y,
Nakai Y, Onishi S, Onishi K, Iida K, Gotoh D, Tanaka N and Fujimoto
K: Gamma-Klotho exhibits multiple roles in tumor growth of human
bladder cancer. Oncotarget. 9:19508–19524. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hori S, Miyake M, Onishi S, Tatsumi Y,
Morizawa Y, Nakai Y, Anai S, Tanaka N and Fujimoto K: Clinical
significance of α- and β-Klotho in urothelial carcinoma of the
bladder. Oncol Rep. 36:2117–2125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kitamura H and Tsukamoto T: Immunotherapy
for urothelial carcinoma: Current status and perspectives. Cancers.
3:3055–3072. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uekado Y, Hirano A, Shinka T and Ohkawa T:
The effects of intravesical chemoimmunotherapy with epirubicin and
bacillus Calmette-Guérin for prophylaxis of recurrence of
superficial bladder cancer: A preliminary report. Cancer Chemother
Pharmacol. 35 Suppl:S65–S68. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Erol A, Ozgür S, Basar M and Cetin S:
Trial with bacillus Calmette-Guérin and epirubicin combination in
the prophylaxis of superficial bladder cancer. Urol Int. 52:69–72.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ali-El-Dein B, Nabeeh A, Ismail EH and
Ghoneim MA: Sequential bacillus Calmette-Guerin and epirubicin
versus bacillus Calmette-Guerin alone for superficial bladder
tumors: A randomized prospective study. J Urol. 162:339–342. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bono AV, Lovisolo JA and Saredi G:
Transurethral resection and sequential chemo-immunoprophylaxis in
primary T1G3 bladder cancer. Eur Urol. 37:478–483. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai T, Nesi G, Tinacci G, Zini E, Mondaini
N, Boddi V, Mazzoli S and Bartoletti R: Can early single dose
instillation of epirubicin improve bacillus Calmette-Guerin
efficacy in patients with nonmuscle invasive high risk bladder
cancer? Results from a prospective, randomized, double-blind
controlled study. J Urol. 180:110–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Järvinen R, Kaasinen E, Rintala E and
Group TF: Long-term results of maintenance treatment of mitomycin C
or alternating mitomycin C and bacillus Calmette-Guérin
instillation therapy of patients with carcinoma in situ of the
bladder: A subgroup analysis of the prospective FinnBladder 2 study
with a 17-year follow-up. Scand J Urol Nephrol. 46:411–417. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghiringhelli F, Larmonier N, Schmitt E,
Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E,
Bonnotte B and Martin F: CD4+CD25+ regulatory
T cells suppress tumor immunity but are sensitive to
cyclophosphamide which allows immunotherapy of established tumors
to be curative. Eur J Immunol. 34:336–344. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakaguchi S, Sakaguchi N, Shimizu J,
Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M and
Takahashi T: Immunologic tolerance maintained by CD25+
CD4+ regulatory T cells: Their common role in
controlling autoimmunity, tumor immunity, and transplantation
tolerance. Immunol Rev. 182:18–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gabrilovich DI and Nagarai S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao Y, Wang D, Xu T, Liu P, Cao Y, Wang
Y, Yang X, Xu X, Wang X and Niu H: Bladder cancer cells re-educate
TAMs through lactate shuttling in the microfluidic cancer
microenvironment. Oncotarget. 6:39196–39210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Svatek RS, Zhao XR, Morales EE, Jha MK,
Tseng TY, Hugen CM, Hurez V, Hernandez J and Curiel TJ: Sequential
intravesical mitomycin plus Bacillus Calmette-Guérin for
non-muscle-invasive urothelial bladder carcinoma: Translational and
phase I clinical trial. Clin Cancer Res. 21:303–311. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Obeid M, Tesniere A, Ghiringhelli F, Fimia
GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T,
Casares N, et al: Calreticulin exposure dictates the immunogenicity
of cancer cell death. Nat Med. 13:54–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ramakrishnan R, Assudani D, Nagaraj S,
Hunter T, Cho HI, Antonia S, Altiok S, Celis E and Gabrilovich DI:
Chemotherapy enhances tumor cell susceptibility to CTL-medeated
killing during cancer immunotherapy in mice. J Clin Invest.
120:1111–1124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alizadeh D, Trad M, Hanke NT, Larmonier
CB, Janikashvili N, Bonnotte B, Katsanis E and Larmonier N:
Doxorubicin eliminates myeloid-derived suppressor cells and
enhances the efficacy of adoptive T-cell transfer in breast cancer.
Cancer Res. 74:104–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saban MR, Simpson C, Davis C, Wallis G,
Knowlton N, Frank MB, Centola M, Gallucci RM and Saban R:
Discriminators of mouse bladder response to intravesical Bacillus
Calmette-Guerin (BCG). BMC Immunol. 8:62007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shintani Y, Sawada Y, Inagaki T, Kohjimoto
Y, Ueoka Y and Shinka T: Intravesical instillation therapy with
bacillus Calmette-Guérin for superficial bladder cancer: Study of
the mechanism of bacillus Calmette-Guérin immunotherapy. Int J
Urol. 14:140–146. 2007. View Article : Google Scholar : PubMed/NCBI
|