Introduction
As the nutritional status, sanitation, refrigeration
and access to fresh fruits and vegetables have improved; the
incidence of gastric cancer (GC) appears to be decreasing in many
nations over the last few decades (1–3). GC
represents the third most common cause of cancer-associated
mortality worldwide (4). The
etiological agent of GC is complicated, and ascribed to assorted
diet and environmental and genetic factors (5). Multifarious treatments including
surgical resection, neoadjuvant chemical therapy, radiation
treatment and targeted molecular therapy have become pivotal
treatments to prevent disease evolvement. The 5-year survival rate
of patients with GC is <20% worldwide, particularly in eastern
Asia and China (6–8). Traditionally, cancer prognosis is
based on the depth of tumor infiltration, the occurrence of lymph
node and long-distance metastases, which can be evaluated by
microscopic examination of pathology (9). The unfavorable prognosis associated
with GC has compelled for the development of novel diagnostic
markers. However, the potential pathogenic mechanisms underlying GC
progression have not been completely elucidated. As a result, it is
essential to illuminate neoteric molecular approaches to ameliorate
existing prognostic stratification and provide relevant critical
clinical insights into assessing the outcome of patients with
GC.
Cell division cycle-associated 3 (CDCA3), also known
as Tome-1 (trigger of mitotic entry), was initially detected during
the degradation process of intra-cellular protein induced by
APCCDH1 (10,11). CDCA3 of human beings is located on
chromosome 12p12, is composed of 268 amino acids and has a
molecular weight of 29 kDa (12).
CDCA3 contains an F-box motif, which is available to combine with
Skp1 and cullin, and to regulate numerous physiological and
pathological processes in the human body by stimulating the
degradation of certain proteins such as cell cycle-regulating
proteins, transcription factors and signal transduction molecules
(13,14). Recent investigations have suggested
that CDCA3 serves a key role in the development of various types of
cancers (15–19). Recent studies demonstrated that
there is a high expression of CDCA3 in oral squamous cell
carcinoma, and it has the capacity to promote the growth and
proliferation of tumor cells by regulating mitosis (20). O'Byrne et al reported that
CDCA3 was markedly upregulated and associated with poor prognosis
in non-small cell lung cancer (NSCLC) and highlighted CDCA3 as a
novel factor in mediating NSCLC cell proliferation (21). CDCA3 was revealed to serve a key
role in tumorigenesis and the development of esophageal carcinoma,
and was involved in the cell cycle and endocytosis (22). High expression of CDCA3 has also
been observed in liver cells, and has prognostic significance in
patients with hepatocellular carcinoma (15,18).
In addition, prostate cancer progression could be promoted by
upregulating CDCA3 expression, and CDCA3 may serve as a potential
therapeutic target for human prostate cancer (17). However, the expression of CDCA3 in
GC cell lines and tissues and the association between CDCA3
expression and the survival outcome of patients with GC remain
poorly understood, and furthermore, the precise effect of CDCA3 on
GC progression remains obscure.
In the present study, a novel oncogene was
identified and its expression profile in GC tissues and cell lines
was evaluated by employing reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and immunohistochemical
analysis. An association between CDCA3 expression and
clinicopathological features of patients with GC was discovered,
and the merits of prognosis for the accurate predictability for the
survival of patients with GC was analyzed. The present study
demonstrated that the expression of CDCA3 influenced GC cell
proliferation in vitro and vivo, in particular with
respect to its signaling pathways by which CDCA3 may mediate GC
cell progression. These findings demonstrated that CDCA3 may
provide an increased understanding into predicting a poor outcome,
and be applied to the diagnosis and treatment of patients with
GC.
Materials and methods
Tissue specimens and cell lines
GC tissues and paired normal adjacent mucosa tissues
were derived from 150 patients with GC who had undergone surgical
resection at the Department of General Surgery, The Affiliated
Hospital of Nantong University (Nantong, China). Histological
classifications and tumor-node-metastasis (TNM) stages were
independently determined by two senior pathologists according to
the classification criteria of the American Joint Committee on
Cancer. Resected samples were snap-frozen in liquid nitrogen until
RNA and protein extraction could be performed. Tissue specimens
were flash frozen immediately following surgery and stored in
liquid nitrogen until RNA extraction. All patients provided written
informed consent prior to surgery and the Ethics Committee of
Affiliated Hospital of Nantong University, approved the present
study. Signed informed consents were obtained from all subjects and
no scientific research was conducted without the informed contents.
Nantong University approved the present study. The study was
approved by the Ethics Committee of the Affiliated Hospital of
Nantong University. All procedures performed in this study were in
accordance with the 1964 Helsinki Declaration and its later
amendments.
Five human GC cell lines (BGC823, AGS, MKN45, MKN28
and SGC7901) and a human normal gastric epithelial cell line
(GES-1) were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA), and the National Infrastructure of Cell
Line Resource (Beijing, China). The cell lines were maintained in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS),
ampicillin and streptomycin, in a humidified chamber at 37°C with
5% CO2.
RT-qPCR
Total RNA was isolated from paired GC and adjacent
non-tumor tissues, 5 human GC cell lines and GES-1 cells by
employing TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
PrimeScript RT reagent (Takara Bio, Inc., Otsu, Japan) was used to
transcribe isolated total RNA into cDNA and then SYBR Premix Ex Taq
(Takara Bio, Inc.) was used to evaluate mRNA expression levels by
quantitative real-time PCR assays. The primer sequences were as
follows: CDCA3 sense, 5′-TGGTATTGCACGGACACCTA-3′ and antisense,
5′-TGTTTCACCAGTGGGCTTG-3′; β-actin sense,
5′-AGAGCCTCGCCTTTGCCGATCC-3′ and antisense,
5′-CTGGGCCTCGTCGCCCACATA-3′. All experiments were run in
triplicate.
Immunohistochemical (IHC)
staining
Paraffin-embedded sections (4-µm thick) were
incubated with polyclonal rabbit anti-CDCA3 antibody (dilution
1:200; cat. no. ab167037; Abcam, Cambridge, UK) at 4°C overnight
using SP-9000 Histostain™-Plus kits (ZSGB-BIO; OriGene
Technologies, Inc., Beijing, China) according to the manufacturer's
protocol. The immunoreactive scores (IRS) for the proportion of
positive cells and he staining grade were calculated for each
specimen. The quantity score evaluating the proportion of positive
cells of CDCA3 was scored as 0 (negative), 1 (1–10% labeled cells),
2 (11–50% labeled cells), 3 (>50% labeled cells). The intensity
score evaluating the intensity of staining was scored as 0
(negative staining), 1 (weakly positive), 2 (moderately positive)
and 3 (strongly positive). Multiplication of the extent scores and
the staining intensity was performed to calculate the IRS and 3 was
the optimal cutoff value: Samples with an IRS ≥3 were defined as
high CDCA3 expression and samples with an IRS <3 were regarded
as low CDCA3 expression.
Construction of recombinant
plasmids
Based on the CDCA3 nucleotide sequence from the
human cDNA library (GenBank: NM_001297602.2), the full-length open
reading frame (ORF) of the human CDCA3 gene was amplified using
polymerase chain reaction according to the manufacturer's protocol
(PrimeStar PCR; Takara Bio, Co., Ltd., Dalian, China). For the
construction of CDCA3, the expression vector pcDNA3.1B containing
the full-length open reading frame (ORF) of the human CDCA3 gene
was used to generate pcDNA3.1B-CDCA3. The sequence of the forward
primer was as follows:
5-EcoRI-AGAGAATTCATGGGCTCAGCCAAGAGCGT-3, and sequence of the
reverse primer was 5-BamHI-AGAGGATCCCTAGCTCTCCACCAAGGGA-3.
The construct was verified by sequencing.
Short hairpin RNA preparation
Small interference RNAs were chemically synthesized
(Shanghai GenePharma Co., Ltd., Shanghai, China). The sequence of
siRNA-1069 was: 5′-GGGUACCCAGUUAUCUGUUGAGGAAdTdT-3′ (sense) and
5′-UUCCUCAACAGAUAACUGGGUACCCdTdT-3′ (antisense). Negative control
(NC) siRNA synthesized by Shanghai GenePharma Co. was used as a
control. The sequence of si-NC was as follows:
5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAATT-3′
(antisense). Synthesized DNA nucleotide fragment encoding shRNA
against endogenous CDCA3 was designed according to the Invitrogen
(Thermo Fisher Scientific, Inc.) and synthesized by GenePharma Co.,
Ltd. The sequences were incorporated into the Vector p-SUPER
(OligoEngine, Seattle, WA, USA) to generate p-SUPER-sh-1069. The
sequence of sh-1069 was as follows:
5′-GATCCCCGGGTACCCAGTTATCTGTTGAGGAATTCAAGAGATTCCTCAACAGATAACTGGGTACCCTTTTTGGAAA-3′
(sense) and
5′-AGCTTTTCCAAAAAGGGTACCCAGTTATCTGTTGAGGAATCTCTTGAATTCCTCAACAGATAACTGGGTACCCGGG-3′
(antisense). The sequence of sh-NC was as follows:
5′-GATCCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTGGAAA-3′
(sense) and
5′-AGCTTTTCCAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAGGG-3′
(antisense). The constructs were verified by sequencing.
Cell transfection
BGC823 and SGC7901 cells were plated in each well of
6-well plates at a density of 5×104 cells/well. BGC823
cells were transfected with p-SUPER-sh-1069 and p-SUPER-sh-NC,
SGC7901 cells were transfected with pcDNA3.1B-CDCA3 and pcDNA3.1B,
respectively. Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used according to the manufacturer's protocol
and transfection was conducted when cells reached ~80% confluency.
After transfection, we determined transfection efficiency and
conducted subsequent functional experiments.
Cell proliferation assay
The transfected cells were seeded at a density of
2,000 cells/well in 96-well plates at daily intervals (every 24 h
for 5 days) and Cell Counting Kit-8 (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used according to the
manufacturer's protocol. The absorbance was measured at 450 nm to
evaluate the growth curve of transfected cells. All experiments
were run in triplicate.
Colony formation assay
Each type of SGC7901 (SGC7901 transfected with
pcDNA3.1B-CDCA3 SGC7901 transfected with pcDNA3.1B) and BGC823
(BGC823 transfected with p-SUPER-sh-1069 BGC823 transfected with
p-SUPER-sh-NC) cells were seeded into 6-well plates at a density of
500 cells/well, and then maintained for 3 weeks in RPMI-1640 medium
supplemented with 10% FBS, ampicillin and streptomycin containing
G418 (1,000 µg/ml). Paraformaldehyde (4%) (Invitrogen; Thermo
Fisher Sceintific, Inc.) was employed to fix proliferating colonies
for 30 min at room temperature, which were then stained with 1%
crystal violet for 120 min at room temperature. The stained
colonies were photographed under a light microscope (Leica
Microsystems GmbH, Wetzlar, Germany; magnification, ×40), the
numbers of colonies (>50 cells/colony) were counted. All
experiments were run in triplicate.
Tumorigenicity assay in nude mice
Sixty BALB/c nude male mice, 4-weeks old, were
purchased from the Department of Laboratory Animal Center, Nantong
University (Nantong, China). Animal experiments were performed
according to the protocol approved by the Nantong University Ethics
Committee. A total of 100 µl phosphate-buffered saline (PBS) mixed
with 5×106 control and transfected cells (SGC7901
transfected with pcDNA3.1B-CDCA3; BGC823 transfected with
p-SUPER-sh-1069) were separately and subcutaneously inoculated into
each flank of nude mice. The tumor size of inoculated nude mice was
monitored and measured every third day following injection. The
mice were sacrificed and the subcutaneous tumors were dissected and
weighed following 3 weeks. During the experiment, the nude mice
were exposed to a natural light-dark cycle at room temperature and
a specific-pathogen-free (SPF) environment, and were allowed to
feed and drink water ad libitum. Carbon dioxide was used to
euthanize the mice and the subcutaneous tumors were dissected out
and weighed after 3 weeks. The tumor volume was calculated using
the following formula: Tumor volume = length × width2 ×
0.5. Care of experimental animals was in accordance with
institutional animal care and use committee guidelines.
Flow cytometric analysis
Transfected cells were seeded at a density of
1×105 cells/well into 6-well plates, incubated overnight
at 37°C, harvested by trypsinization without EDTA and the cells
were washed two times with ice-cold PBS. Following treatment with
75% ethanol at 4°C overnight, the cells were incubated on ice for 1
h. Subsequently, the fixed cells were stained at room temperature
for 30 min with 0.5 ml PBS solution containing 0.2 mM EDTA, 20
mg/ml of propidium iodide and 0.2% Triton X-100. The proportion of
cells in the G0/G1, S and G2/M
phases of the cell cycle was evaluated employing the Beckman
Gallios Flow Cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Western blot analysis
A total of 25 mmol/l Tris-Cl (pH 7.5), 5 mmol/l
EDTA, 1% SDS and 1% protein lysate with protease inhibitor were
used to extract protein, and we determined the protein
concentration using a BCA kit. Total cellular proteins (10 µl
protein/lane) were separated by SDS-polyacrylamide gel
electrophoresis (10% separation gel and 4% stacking gel), blotted
onto polyvinylidene difluoride (PVDF) membranes, the PVDF membranes
were blocked by 5% skimmed milk, at room temperature for 1 h and
then were incubated with specific primary antibodies at 4°C
overnight. Following incubation for 1 h at room temperature with
horseradish peroxidase (HRP)-conjugated secondary antibody
(dilution 1:2,000; cat. no. Ab205718), each membrane was detected
using an enhanced chemiluminescence (ECL) detection system (Pierce
Biotechnology, Inc., Rockford, IL, USA) according to the
manufacturer's protocol. The inclusion of the primary antibodies
used in the present study as follows: Anti-CDCA3 (dilution 1:1,000;
cat. no. ab167037), anti-p-RAS (dilution 1:1,000; cat. no.
ab214100), anti-t-RAS (dilution 1:5,000; cat. no. ab52939),
anti-p-MEK (dilution 1:1,000; cat. no. ab194754), anti-t-MEK
(dilution 1:5,000; cat. no. ab178876), anti-p-ERK (dilution
1:1,000; cat. no. ab201015), anti-t-ERK (dilution 1:1,000; cat. no.
ab17942), anti-p21Cip1 (dilution 1:1,000; cat. no. ab109520),
anti-cyclin D1 (dilution 1:200; cat. no. ab16663), anti-cyclin E
(dilution 1:1,000; cat. no. ab33911) and anti-β-actin (dilution
1:1,000; cat. no. ab8226) antibody (all purchased from Abcam).
Follow-up of patients
GC tissues and paired normal adjacent mucosa tissues
were derived from 150 patients with GC who had undergone surgeries
at the Affiliated Hospital of Nantong University between April 2010
and March 2011. The lump and the regional lymph nodes were
radically dissected and the edge of the resection was tumor-free,
which was deemed to be curatively resected. Patients with GC with
distant metastasis were not included in the present study.
Follow-up visits of 150 GC patients ranged from April 2011 to March
2016 and the specified clinicopathological features of the 150
patients with GC are listed Table
I.
 | Table I.Association of CDCA3 expression in
tumorous tissues with clinicopathological characteristics in GC
patients. |
Table I.
Association of CDCA3 expression in
tumorous tissues with clinicopathological characteristics in GC
patients.
|
|
| CDCA3 |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathologic
characteristics | n | Low or no
expression | High
expression | Pearson
χ2 | P-value |
|---|
| Total | 150 | 64 (42.67) | 86 (57.33) |
|
|
| Sex |
|
|
| 0.492 | 0.483 |
|
Male | 96 | 43 (44.79) | 53 (55.21) |
|
|
|
Female | 54 | 21 (38.89) | 33 (61.11) |
|
|
| Age at diagnosis
(years) |
|
|
| 0.060 | 0.807 |
|
≤60 | 65 | 27 (41.54) | 38 (58.46) |
|
|
|
>60 | 85 | 37 (43.53) | 48 (56.47) |
|
|
| Tumor size
(cm) |
|
|
| 6.968 | 0.008a |
|
<3 | 46 | 27 (58.70) | 19 (41.30) |
|
|
| ≥3 | 104 | 37 (35.58) | 67 (64.42) |
|
|
| Location |
|
|
| 2.194 | 0.334 |
|
Lower | 102 | 45 (44.12) | 57 (52.88) |
|
|
|
Middle | 31 | 10 (32.26) | 21 (67.74) |
|
|
|
Upper | 17 | 9 (52.94) | 8 (47.06) |
|
|
|
Differentiation |
|
|
| 8.012 | 0.005a |
| Low
grade | 90 | 30 (33.33) | 60 (66.67) |
|
|
| Middle
and high grade | 60 | 34 (56.67) | 26 (43.33) |
|
|
| Primary tumor |
|
|
| 7.492 | 0.024a |
| T1 | 33 | 19 (57.58) | 14 (42.42) |
|
|
| T2 | 30 | 16 (53.33) | 14 (46.67) |
|
|
|
T3+T4 | 87 | 29 (33.33) | 58 (66.67) |
|
|
| Lauren type |
|
|
| 0.109 | 0.741 |
|
Intestinal | 96 | 40 (41.67) | 56 (58.33) |
|
|
|
Diffuse/other | 54 | 24 (44.44) | 30 (55.56) |
|
|
| Lymph node
metastasis |
|
|
| 13.252 | 0.001a |
|
N0 | 71 | 40 (56.34) | 31 (43.66) |
|
|
|
N1 | 19 | 9 (47.37) | 10 (52.63) |
|
|
|
N2+N3 | 60 | 15 (25.00) | 45 (75.00) |
|
|
| Stage grouping with
TNM |
|
|
| 23.720 | 0.001a |
| I | 50 | 29 (58.00) | 21 (42.00) |
|
|
| II | 33 | 21 (63.64) | 12 (36.36) |
|
|
|
III+IV | 67 | 14 (20.90) | 53 (79.10) |
|
|
Statistical analysis
The study data were analyzed using SPSS 19.0 and
GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA). A two-tailed Student's t-test was employed to analyze
differences between 2 groups. Multiple comparisons between groups
was performed using analysis of variance (ANOVA) followed by
Student-Newman-Keuls test. The χ2 test was employed to
determine categorical data. The Kaplan-Meier method was employed to
estimate survival rates and the log-rank test was used to compare
the significance between survival times. A Univariate Cox
regression model was used to investigate factors of prognostic
significance and then the factors were further investigated using a
multivariate Cox regression model. Quantitative results were
expressed as the mean ± SD. P<0.05 was considered to indicate a
statistically significant difference.
Results
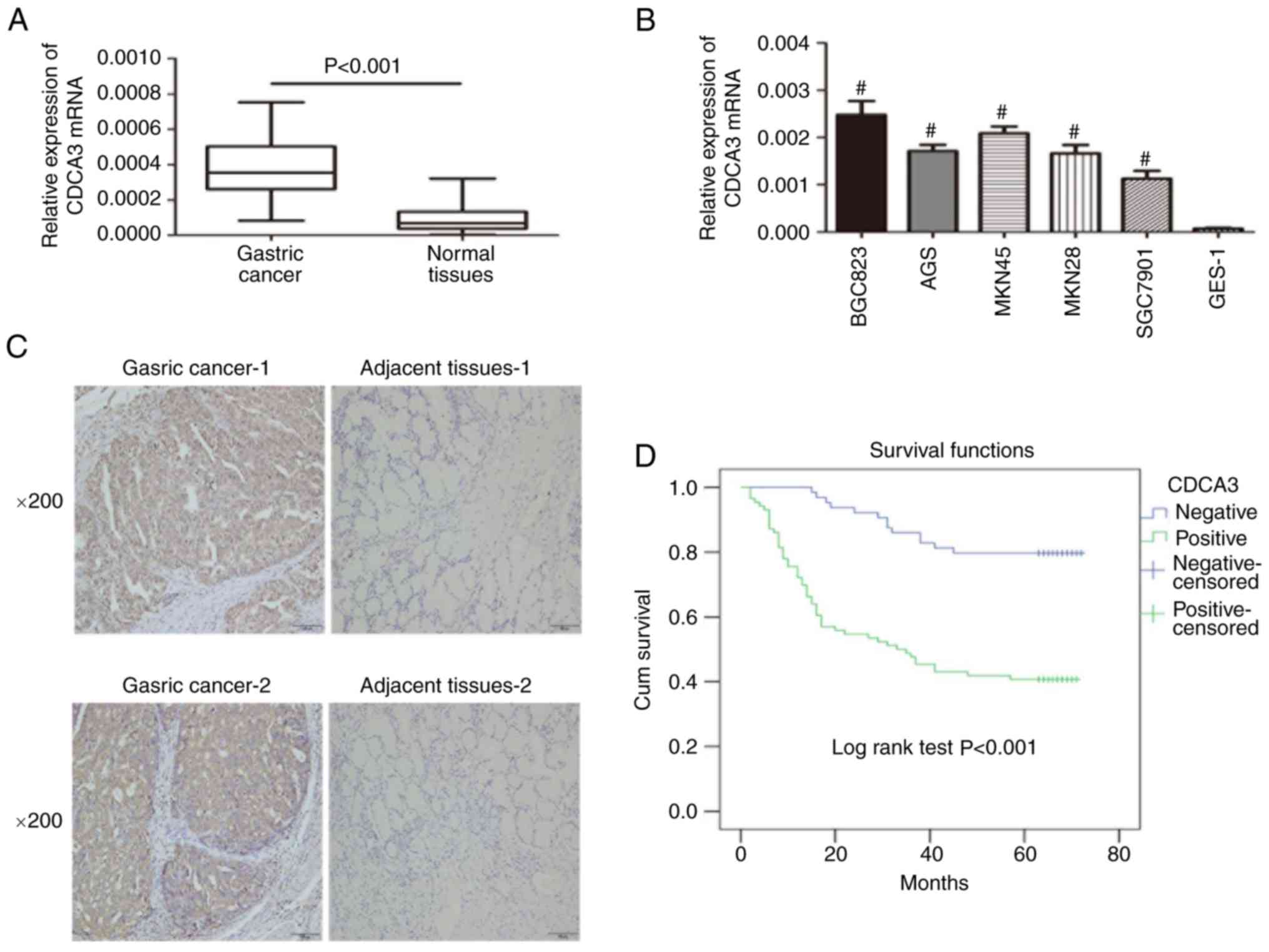
CDCA3 expression in GC tissues and GC
cell lines
RT-qPCR was performed to assess the CDCA3 mRNA
expression levels in GC and adjacent non-cancerous tissues from 150
patients. The results indicated that CDCA3 mRNA was increased in
106 (70.7%) of the 150 GC specimens compared with the matched
normal gastric tissues (Fig. 1A).
Furthermore, the expression of CDCA3 in a normal human gastric
epithelial cell line (GES-1) and diverse human GC cell lines was
also detected by qRT-PCR analysis. Our results indicated that CDCA3
expression was markedly upregulated in all GC-derived cell lines
(BGC823, AGS, MKN45, MKN28 and SGC7901) compared with GES-1
(Fig. 1B). IHC analysis was
performed to evaluate CDCA3 protein expression in GC specimens and
paired normal gastric tissues in the same 150 matched samples. Of
these specimens, 86/150 (57.3%) of cancerous specimens exhibited
high CDCA3 expression, whereas 43/150 (28.7%) of normal gastric
tissues exhibited high CDCA3 expression (Fig. 1C). The collective results indicated
an aberrant upregulation of CDCA3 in GC.
Clinical significance of CDCA3 in GC
patients
To access the clinical significance of ectopic CDCA3
expression in GC progression, the relationship between CDCA3
expression and clinicopathological features were evaluated. The
patients with GC were split into 2 groups according to the results
obtained by the results of IHC analysis for CDCA3: A high-CDCA3
expression group and a low-CDCA3 expression group, and associations
between high CDCA3 expression and tumor size (P=0.008),
differentiation (P=0.005), primary tumor (P=0.024), lymph node
metastasis (P=0.001) and TNM stage (P=0.001) were identified
(Table I). Univariate analysis was
applied to investigate all relevant features, and high CDCA3
expression (P<0.001), along with tumor size (P=0.003), tumor
differentiation (P=0.005), primary tumor (P<0.001), lymph node
metastasis (P<0.001) and tumor TNM stage (P<0.001), were
identified to be significantly associated with patient survival.
Multivariate regression analysis was subsequently employed to
further confirm that CDCA3 expression (P=0.003) and TNM stage
(P=0.008) were independent prognostic indicators in GC (Table II).
 | Table II.Univariate and multivariate analysis
of prognostic factors of the 5-year overall survival in GC
patients. |
Table II.
Univariate and multivariate analysis
of prognostic factors of the 5-year overall survival in GC
patients.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR | P-value | 95% CI | HR | P-value | 95% CI |
|---|
| CDCA3
expression | 4.265 |
<0.00a | 2.316–7.857 | 2.719 | 0.003a | 1.396–5.296 |
| High
vs. low and none |
| Sex | 0.823 | 0.447 | 0.523–1.250 |
|
|
|
| Male
vs. female |
| Age (years) | 0.883 | 0.619 | 0.498–1.360 |
|
|
|
| ≤60 vs.
>60 |
| Location | 0.946 | 0.748 | 0.673–1.329 |
|
|
|
| Lower
vs. middle vs. upper |
| Tumor size
(cm) | 2.686 | 0.003a | 1.402–5.148 | 1.044 | 0.910 | 0.496–2.198 |
| <3
vs. ≥3 |
|
Differentiation | 0.416 | 0.005a | 0.225–0.767 | 0.830 | 0.572 | 0.435–1.584 |
| Low vs.
middle and high grade vs. others |
| Primary tumor | 2.606 |
<0.001a | 1.693–4.010 | 0.799 | 0.504 | 0.413–1.544 |
| T1 vs.
T2 vs. T3+T4 |
| Lauren type |
|
Intestinal vs.
diffuse/other | 0.955 | 0.872 | 0.542–1.681 |
|
|
|
| Lymph node
metastasis | 2.035 |
<0.001a | 1.649–2.510 | 1.248 | 0.126 | 0.939–1.658 |
| N0 vs.
N1 vs. N2+N3 |
| TNM stage | 3.716 |
<0.001a | 2.460–5.614 | 2.462 | 0.008a | 1.263–4.800 |
| I vs.
II vs. III+IV |
Increased expression of CDCA3 predicts
poor prognosis in patients with GC
A total of 150 patients were enrolled in the present
study. At the last point of follow-up 64 patients had died, of whom
51 exhibited high CDCA3 expression, and 13 exhibited low CDCA3
expression. Among the survivors, the data indicated that 35
patients exhibited high expression levels of CDCA3 and 51 exhibited
low CDCA3 expression. Furthermore, the results indicated that the
overall survival rate over 5 years for the high CDCA3 group was
40.7%, whereas it was 79.7% for the low CDCA3 group (Fig. 1D).
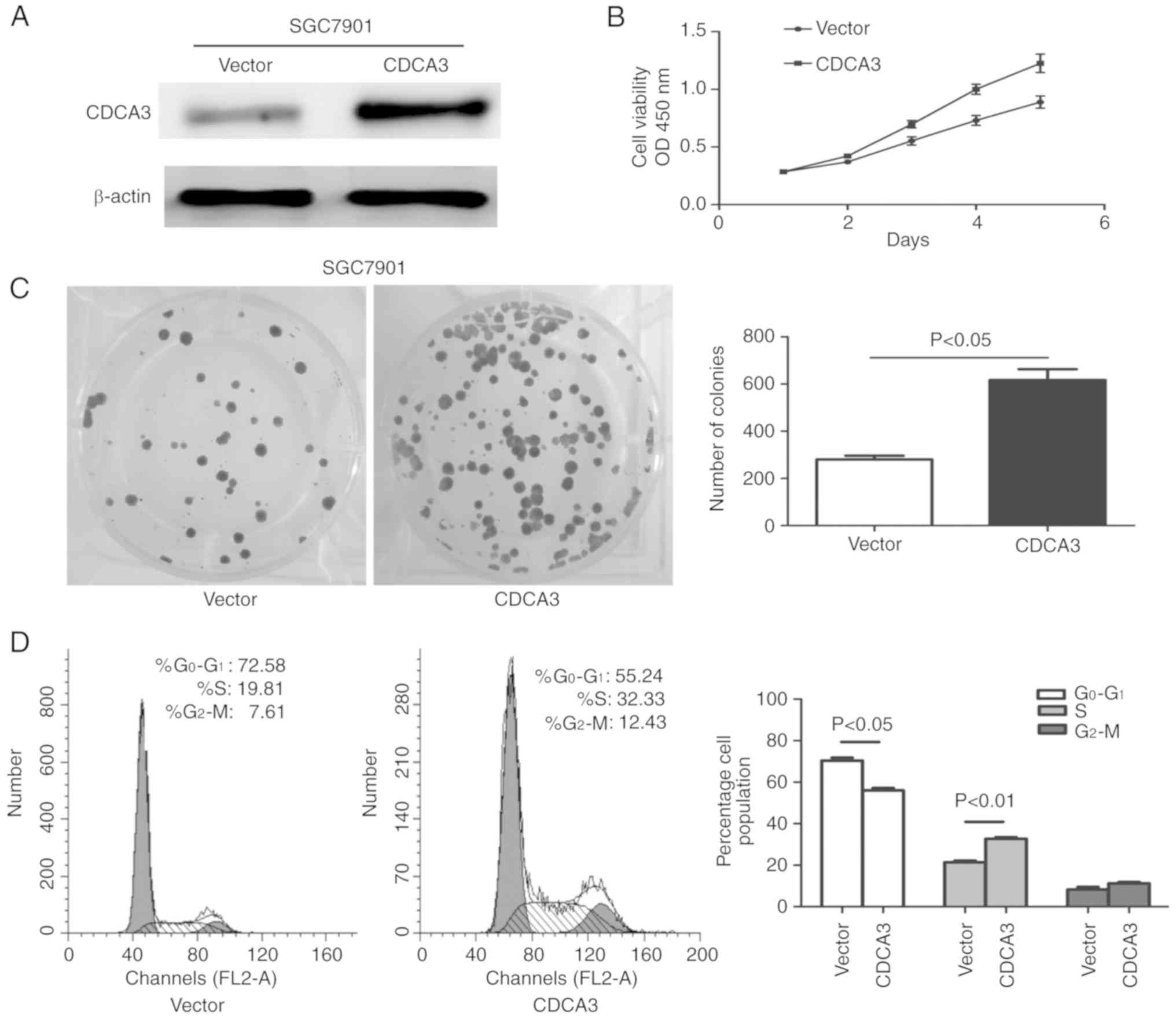
Overexpression of CDCA3 promotes cell
proliferation and induces the cell cycle transition from the
G0/G1 to the S phase
To explore the role of CDCA3 in the tumor growth of
GC, CDCA3 overexpression was established, employing pcDNA3.1B-CDCA3
in the GC cell line SGC7901. Western blotting was used to determine
the transfection efficiency of CDCA3 protein expression in the
SGC7901 cell line at 48 h following transfection. The results
indicated that pcDNA3.1B-CDCA3 effectively upregulated CDCA3
expression in the SGC7901 cell line (Fig. 2A). The ectopic expression of CDCA3
significantly promoted cell growth compared with SGC7901 cells that
were transfected with an empty vector (Fig. 2B). In conjunction with this, the
number of colonies that formed in CDCA3-transfected SGC7901 cells
were significantly increased compared with the empty
vector-transfected SGC7901 cells (P<0.05) (Fig. 2C). To further evaluate the mechanism
by which CDCA3 promotes cell proliferation, flow cytometry was used
to determine the effect of CDCA3 on cell cycle distribution at 24 h
following transfection. As illustrated in Fig. 2D the ectopic expression of CDCA3 in
SGC7901 cells successfully accomplished cycle transition from the
G0/G1 to the S phase compared with the empty
vector-transfected SGC7901 and the percentage of cells in the S
phase increased by 12.52% in the CDCA3-transfected SGC7901 cells
(P<0.05) at 24 h. These results demonstrated that CDCA3 is
important for the promotion of GC cell growth in vitro.
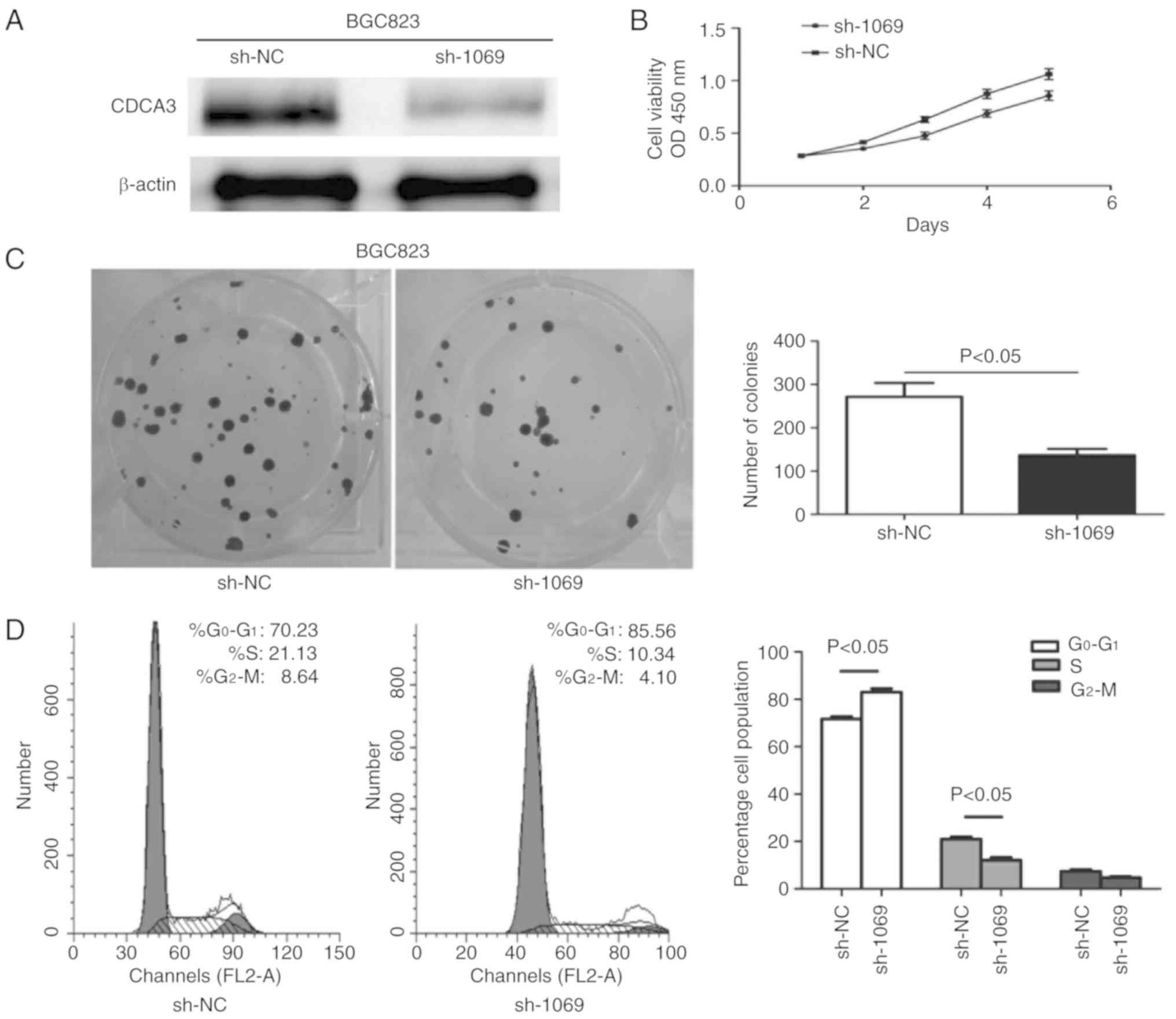
CDCA3 knockdown inhibits cell
proliferation and induces cell cycle arrest in the
G0/G1 phase
shRNA-1069 derived from recombinant pSUPER was
chemically synthesized to knockdown endogenous CDCA3, concurrently
as sh-NC in the BGC823 cell line. Western blotting was used to
determine the transfection efficiency of CDCA3 protein expression
in the BGC823 cell line at 48 h following transfection. The results
indicated that p-SUPER-sh-1069 effectively downregulated CDCA3
expression in the BGC823 cell line (Fig. 3A). The downregulated expression of
CDCA3 significantly suppressed cell growth compared with sh-NC
transfected cells (P<0.05) (Fig.
3B). In keeping with this, the number of colonies that formed
in BGC823 cells transfected with p-SUPER-shRNA-CDCA3 were
significantly inhibited in comparison with BGC823 cells transfected
with sh-NC (P<0.05) (Fig. 3C).
To further evaluate the effect of downregulation of CDCA3 on cell
proliferation, flow cytometry was used to determine cell cycle
distribution 24 h following transfection. As illustrated in
Fig. 3D, knockdown of CDCA3 was
associated with cell cycle arrest at the G0/G1 stage, as there was
an increase of the percentage of cells in the
G0/G1 phase in the p-SUPER-sh-CDCA3
transfected group by 15.33% compared to the sh-NC group (P<0.05)
at 24 h.
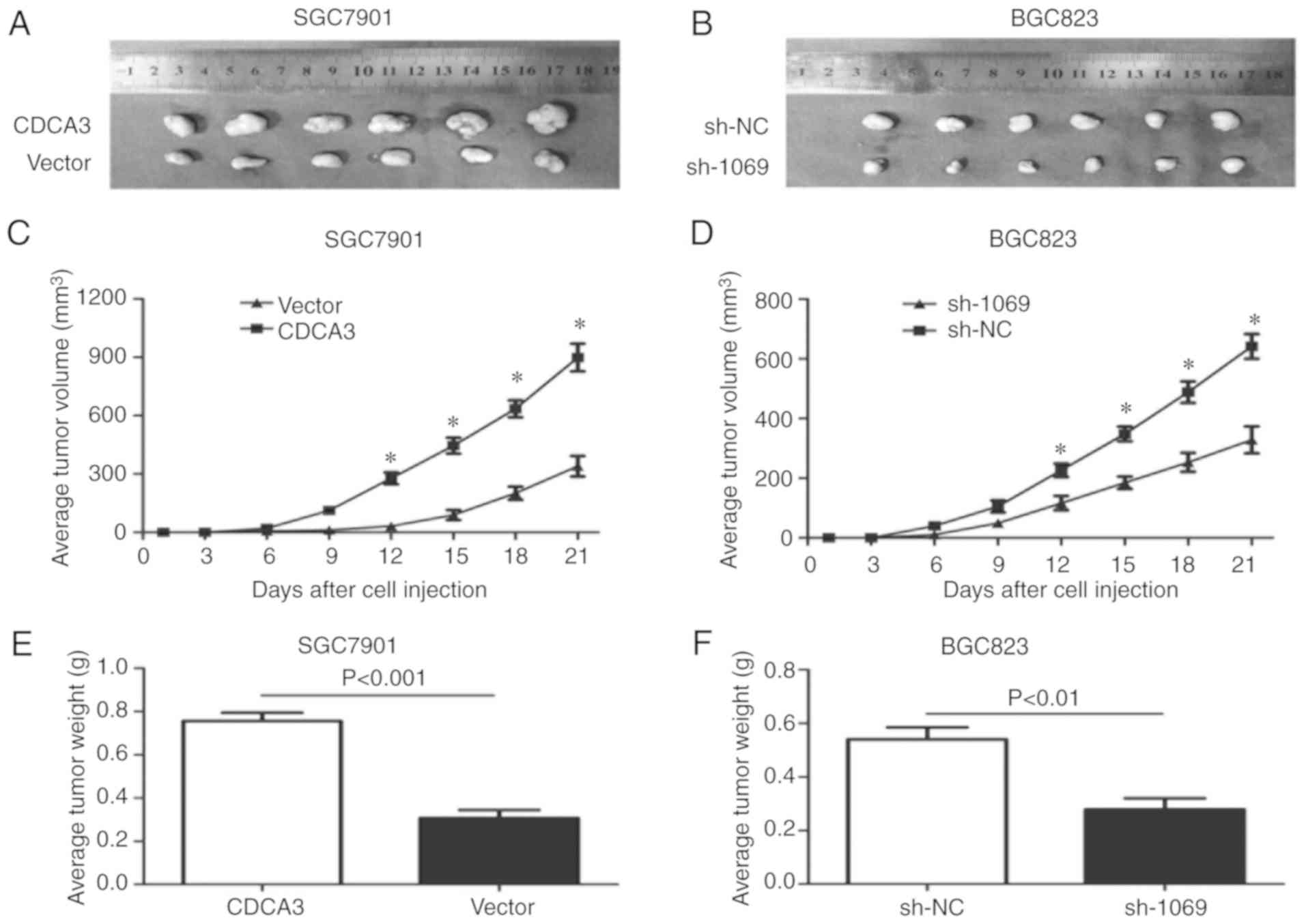
Differential expression of CDCA3
affects tumorigenesis and tumor burden
To explore the effect of CDCA3 expression on
tumorigenic potential of GC cell lines in vivo, the present
study performed a nude mouse xenograft assay. SGC7901 cells
overexpressing CDCA3 and BGC823 cells with downregulated CDCA3
expression were injected subcutaneously into the flank of nude
mice. CDCA3-overexpressing cells significantly promoted tumor
growth in terms of increased capacity for tumorigenesis compared
with the control group (Fig. 4A, C and
E). Furthermore, the present study also demonstrated that CDCA3
silencing substantially inhibited tumor growth in terms of
decreased capacity for tumorigenesis in comparison with the
negative controls (Fig. 4B, D and
F). These data indicated that the in vivo and in
vitro investigations had the same varying trend regarding cell
proliferation, and indicated that CDCA3 may function as an
accelerator of tumorigenicity.
CDCA3 promotes GC progression by
activating the Ras signaling pathway
To further explore the signaling pathway by which
CDCA3 enhances the progression of GC, western blotting was
performed to determine potential CDCA3-regulated molecules. As
illustrated in Fig. 5, the levels
of p-Ras, p-MEK, p-ERK, cyclin D1 and cyclin E were increased;
however, CDKI (p21Cip1) was decreased in the CDCA3-overexpressing
SGC7901 cells when compared with the control group. However, no
difference in the t-Ras, t-MEK and t-ERK protein levels were
observed between the 2 groups.
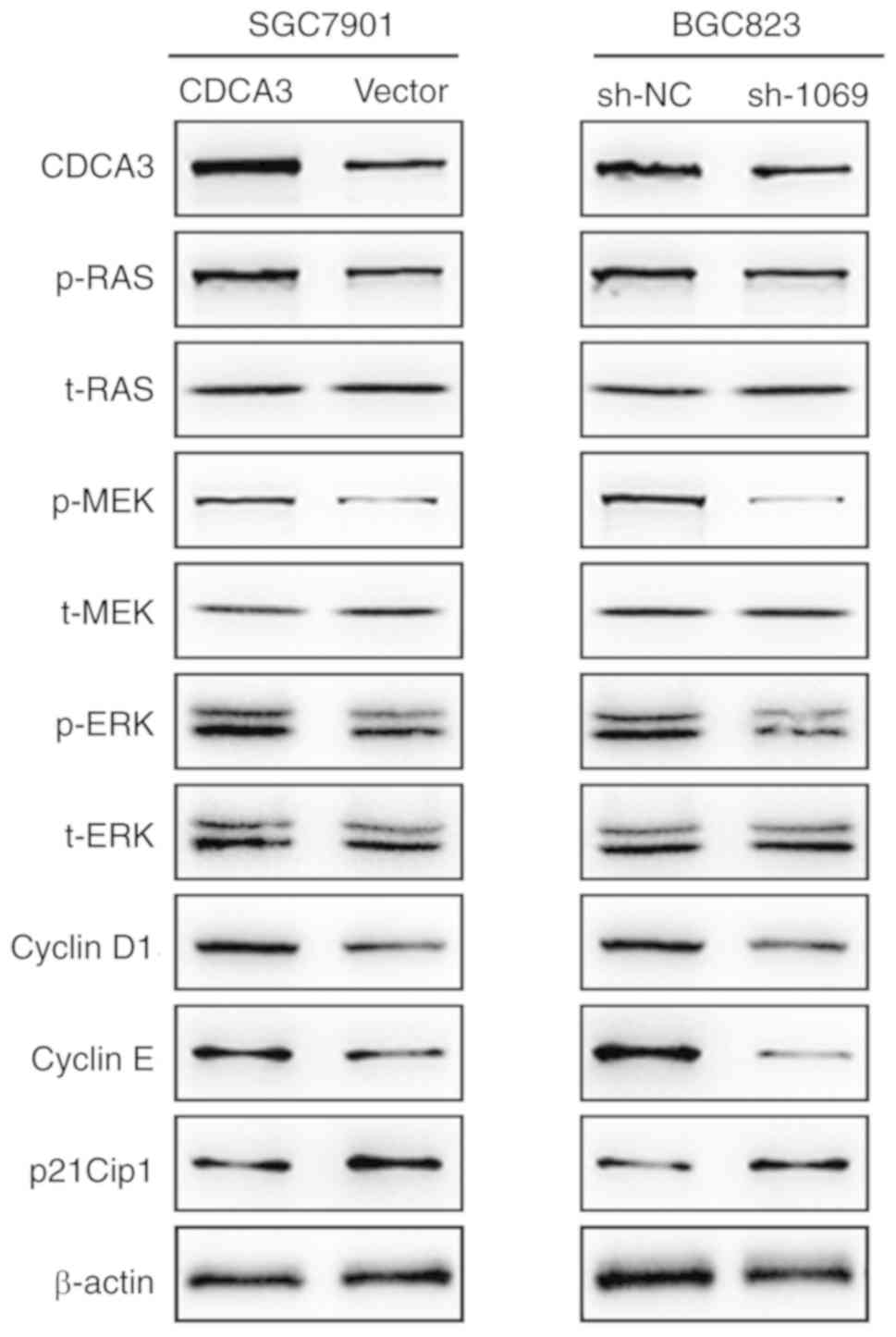 | Figure 5.CDCA3 exerts a proliferative effect
via the Ras signaling pathway. The protein expression levels of
CDCA3, cyclin D1, cyclin E, p-Ras, t-Ras, p-MEK, t-MEK, p-ERK,
t-ERK, and CDKI (p21Cip1) were determined in the indicated cells.
β-actin was used as the loading control. CDCA3, cell division
cycle-associated 3. |
The levels of p-Ras, p-MEK, p-ERK, cyclin D1 and
cyclin E were decreased, however CDKI (p21Cip1) was increased in
CDCA3-downregulated BGC823 cells compared with the control group.
Similarly, the expression of t-Ras, t-MEK and t-ERK protein levels
were significantly unvaried between the 2 groups (Fig. 5). Collectively, this data
demonstrated that the Ras signaling pathway may participate in
CDCA3-mediated GC progression.
Discussion
The present study demonstrated that CDCA3 was highly
expressed in GC tissues compared with peritumoral gastric tissue by
RT-qPCR and IHC analysis. In addition, high levels of CDCA3
expression were associated with tumor size, differentiation and TNM
stage in GC. Notably, Cox proportional hazard regression analysis
revealed that CDCA3 could act as an independent unfavorable
biomarker to predict the outcome of patients with GC. Through in
vitro and in vivo analyses, the present study also
demonstrated that CDCA3 could markedly influence the proliferation
of GC cells. Collectively, these results indicated that CDCA3 had a
critical role in GC development and progression.
Recently, it has been revealed that the upregulation
of CDCA3 is closely linked to human cancers, such as oral and
prostate cancer, which strengthens the significance of CDCA3 as a
valuable prognostic marker in clinical treatment (17,20).
However, the precise molecular mechanisms of CDCA3 in GC
development are still unclear. Previous studies have demonstrated
that the RAS-GTP enzyme-activated protein DAB2IP can combine with
DAB2 to produce a specific protein complex, which can exert a
negative regulatory effect on the ERK/MAPK signaling pathway
(23–25). Research has been performed in recent
years demonstrating that there is a low expression of DAB2IP in
numerous malignant tumors, including prostate and breast cancer
(26,27). Furthermore, additional studies have
demonstrated that the downregulation of endogenous DAB2IP
expression can enhance the proliferation of prostate and breast
cancer (28,29). Such downregulation can promote the
transfer of epithelial cells from epithelium to mesenchymal, a
critical step of tumor metastasis (30–32).
The ERK/MAPK signaling pathway is a cascade process composed of
receptor tyrosine kinase activated by small GTP protein as well as
plasmosin (33,34). The crux of activation is to make RAS
experience guanine-nucleotide exchange and further progress into
the activated form RAS-GTP (35,36).
Located in the downstream of RAS, ERK is principally activated by
growth factor receptor with the involvement of the RAS protein
(37). Typically, ERK is located
within the cytoplasm (37,38). Upon being activated by
phosphorylation, ERK will rapidly pass through the nuclear
membrane, and regulate the activation of some intra-nuclear
transcription factors, which may further regulate the transcription
of their respective targeted genes, trigger the alteration of
specific protein expression and activation, eventually regulate
cell metabolism and function, and influence the specific biological
function of cells (35).
A train of biological functional experiments of
CDCA3 in vitro and in vivo, in the present study,
indicated that CDCA3 possesses a tumor-stimulative function in GC.
The overexpression of CDCA3 could promote cell proliferation in the
SGC7901 cell line in vitro and function as a promotor of
tumorigenicity in vivo; while the opposite phenomenon
occured with knockdown of CDCA3. The data obtained by the present
study strongly suggests that CDCA3 functions as an oncogene in GC.
To address the underlying molecular mechanism by which CDCA3
regulates cell proliferation, the effect of differential CDCA3
expression on the variation in the protein expression profile and
cell cycle progression was explored. The results of the present
study indicated that the changes in cell proliferation were due to
the blocking of the cell cycle at the G0/G1
phase or entering the S phase from the G0/G1
phase with overexpression as a result of the knockdown of CDCA3
expression-mediated Ras signaling pathway-related proteins.
Therefore, we hypothesize that, in GC cells,
overexpression of CDCA3 resulted in the continuous activation of
Ras, and eventually induced the aberrant activation of the ERK/MAPK
signaling pathway, which resulted in the development of GC
progression. However, the detailed interactions between CDCA3 and
the ERK/MAPK signaling pathway-related proteins remain to be
evaluated.
In summary, to the best of our knowledge, the
present study is the first to confirm that CDCA3 was upregulated in
GC tissues and cell lines and was associated with an unfavorable
prognosis in patients with GC. CDCA3 expression was associated with
the promotion of cell proliferation in GC cell lines. Furthermore,
evidence was presented to suggest that CDCA3 mediated the
Ras/ERK/MAPK axis to promote GC cell proliferation. Collectively,
these findings indicate that the ectopic expression of CDCA3 may
serve as a potential diagnostic and prognostic marker in the
treatment of GC.
Acknowledgements
Not applicable.
Funding
This study were supported by the National Natural
Science Foundations for Young Scientists of China (grant no.
81502053).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
QN conceived and designed the experiments. YZ, WY
and WC carried out the experiments. PC and LB participated in the
statistical analysis and interpretation of data. QN and YZ wrote
this manuscript. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Nantong University. All
procedures performed in this study were in accordance with the 1964
Helsinki Declaration and its later amendments. Written informed
consent was obtained from all patients included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Tao J, Zhi X, Zhang X, Fu M, Huang H, Fan
Y, Guan W and Zou C: miR-27b-3p suppresses cell proliferation
through targeting receptor tyrosine kinase like orphan receptor 1
in gastric cancer. J Exp Clin Cancer Res. 34:1392015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee YY and Derakhshan MH: Environmental
and lifestyle risk factors of gastric cancer. Arch Iran Med.
16:358–365. 2013.PubMed/NCBI
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng XJ, Lin JC and Tu SP: Etiology and
prevention of gastric cancer. Gastrointest Tumors. 3:25–36. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pennathur A, Farkas A, Krasinskas AM,
Ferson PF, Gooding WE, Gibson MK, Schuchert MJ, Landreneau RJ and
Luketich JD: Esophagectomy for T1 esophageal cancer: Outcomes in
100 patients and implications for endoscopic therapy. Ann Thorac
Surg. 87:1048–1055. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
GASTRIC (Global Advanced/Adjuvant Stomach
Tumor Research International Collaboration) Group, ; Paoletti X,
Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P,
Sakamoto J, Sargent D, et al: Benefit of adjuvant chemotherapy for
resectable gastric cancer: A meta-analysis. JAMA. 303:1729–1737.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahn JY, Hwang HS, Park YS, Kim HR, Jung
HY, Kim JH, Lee SE and Kim MA: Endoscopic and pathologic findings
associated with clinical outcomes of melanoma in the upper
gastrointestinal tract. Ann Surg Oncol. 21:2532–2539. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith A, Simanski S, Fallahi M and Ayad
NG: Redundant ubiquitin ligase activities regulate wee1 degradation
and mitotic entry. Cell Cycle. 6:2795–2759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshida K: Cell-cycle-dependent regulation
of the human and mouse Tome-1 promoters. FEBS Lett. 579:1488–1492.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim HH and Surana U: Tome-1, wee1, and the
onset of mitosis: Coupled destruction for timely entry. Mol Cell.
11:845–546. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YJ and Bahk YY: A study of substrate
specificity for a CTD phosphatase, SCP1, by proteomic screening of
binding partners. Biochem Biophys Res Commun. 448:189–194. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng N, Schulman BA, Song L, Miller JJ,
Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, et al:
Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin
ligase complex. Nature. 416:703–709. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Itzel T, Scholz P, Maass T, Krupp M,
Marquardt JU, Strand S, Becker D, Staib F, Binder H, Roessler S, et
al: Translating bioinformatics in oncology: Guilt-by-profiling
analysis and identification of KIF18B and CDCA3 as novel driver
genes in carcinogenesis. Bioinformatics. 31:216–224. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adams MN, Burgess JT, He Y, Gately K,
Snell C, Zhang SD, Hooper JD, Richard DJ and O'Byrne KJ: Expression
of CDCA3 is a prognostic biomarker and potential therapeutic target
in non-small cell lung cancer. J Thorac Oncol. 12:1071–1084. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen J, Zhu S, Jiang N, Shang Z, Quan C
and Niu Y: HoxB3 promotes prostate cancer cell progression by
transactivating CDCA3. Cancer Lett. 330:217–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Q, Fu J, Luo B, Huang M, Guo W, Lin Y,
Xie X and Xiao S: OY-TES-1 may regulate the malignant behavior of
liver cancer via NANOG, CD9, CCND2 and CDCA3: A bioinformatic
analysis combine with RNAi and oligonucleotide microarray. Oncol
Rep. 33:1965–1975. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pérez-Peña J, Alcaraz-Sanabria A,
Nieto-Jiménez C, Páez R, Corrales-Sánchez V, Serrano-Oviedo L, Wali
VB, Patwardhan GA, Amir E, Győrffy B, et al: Mitotic read-out genes
confer poor outcome in luminal A breast cancer tumors. Oncotarget.
8:21733–21740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uchida F, Uzawa K, Kasamatsu A, Takatori
H, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H and Bukawa H:
Overexpression of cell cycle regulator CDCA3 promotes oral cancer
progression by enhancing cell proliferation with prevention of G1
phase arrest. BMC Cancer. 12:3212012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Byrne K, Adams M, Burgess J and Richard
D: 24P CDCA3 regulates the cell cycle and modulates cisplatin
sensitivity in non-small cell lung cancer. J Thorac Oncol. 11
(Suppl):S652016. View Article : Google Scholar
|
|
22
|
Su P, Wen S, Zhang Y, Li Y, Xu Y, Zhu Y,
Lv H, Zhang F, Wang M and Tian Z: Identification of the key genes
and pathways in esophageal carcinoma. Gastroenterol Res Pract.
2016:29681062016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou J and Hsieh JT: The inhibitory role
of DOC- 2/DAB2 in growth factor receptor-mediated signal cascade.
DOC-2/DAB2-mediated inhibition of ERK phosphorylation via binding
to Grb2. J Biol Chem. 276:27793–27798. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Tseng CP, Pong RC, Chen H,
McConnell JD, Navone N and Hsieh JT: The mechanism of
growth-inhibitory effect of DOC-2/DAB2 in prostate cancer.
Characterization of a novel GTPase-activating protein associated
with N-terminal domain of DOC-2/DAB2. J Biol Chem. 277:12622–12631.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen H, Pong RC, Wang Z and Hsieh JT:
Differential regulation of the human gene DAB2IP in normal
and malignant prostatic epithelia: Cloning and characterization.
Genomics. 79:573–581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu K, Liu J, Tseng SF, Gore C, Ning Z,
Sharifi N, Fazli L, Gleave M, Kapur P, Xiao G, et al: The role of
DAB2IP in androgen receptor activation during prostate cancer
progression. Oncogene. 33:1954–1963. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Valentino E, Bellazzo A, Di Minin G,
Sicari D, Apollonio M, Scognamiglio G, Di Bonito M, Botti G, Del
Sal G and Collavin L: Mutant p53 potentiates the oncogenic effects
of insulin by inhibiting the tumor suppressor DAB2IP. Proc Natl
Acad Sci USA. 114:7623–7628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren G, Baritaki S, Marathe H, Feng J, Park
S, Beach S, Bazeley PS, Beshir AB, Fenteany G, Mehra R, et al:
Polycomb protein EZH2 regulates tumor invasion via the
transcriptional repression of the metastasis suppressor RKIP in
breast and prostate cancer. Cancer Res. 72:3091–3104. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsieh JT, Karam JA and Min W: Genetic and
biologic evidence that implicates a gene in aggressive prostate
cancer. J Natl Cancer Inst. 99:1823–1824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dote H, Toyooka S, Tsukuda K, Yano M,
Ouchida M, Doihara H, Suzuki M, Chen H, Hsieh JT, Gazdar AF and
Shimizu N: Aberrant promoter methylation in human DAB2 interactive
protein (hDAB2IP) gene in breast cancer. Clin Cancer Res.
10:2082–2089. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie D, Gore C, Liu J, Pong RC, Mason R,
Hao G, Long M, Kabbani W, Yu L, Zhang H, et al: Role of DAB2IP in
modulating epithelial-to-mesenchymal transition and prostate cancer
metastasis. Proc Natl Acad Sci USA. 107:2485–2490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie D, Gore C, Zhou J, Pong RC, Zhang H,
Yu L, Vessella RL, Min W and Hsieh JT: DAB2IP coordinates both
PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc
Natl Acad Sci USA. 106:19878–19883. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ryan MB, Finn AJ, Pedone KH, Thomas NE,
Der CJ and Cox AD: ERK/MAPK signaling drives overexpression of the
Rac-GEF, PREX1, in BRAF- and NRAS-mutant melanoma. Mol Cancer Res.
14:1009–1018. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vial E, Sahai E and Marshall CJ: ERK-MAPK
signaling coordinately regulates activity of Rac1 and RhoA for
tumor cell motility. Cancer Cell. 4:67–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Widmann C, Gibson S, Jarpe MB and Johnson
GL: Mitogen-activated protein kinase: Conservation of a
three-kinase module from yeast to human. Physiol Rev. 79:143–180.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Robinson MJ and Cobb MH: Mitogen-activated
protein kinase pathways. Curr Opin Cell Biol. 9:180–186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Datta A, Kim H, Lal M, McGee L, Johnson A,
Moustafa AA, Jones JC, Mondal D, Ferrer M and Abdel-Mageed AB:
Manumycin A suppresses exosome biogenesis and secretion via
targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in
castration-resistant prostate cancer cells. Cancer Lett. 408:73–81.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Liu G, Ding L, Jiang T, Shao S,
Gao Y and Lu Y: HOXA3 promotes tumor growth of human colon cancer
through activating EGFR/Ras/Raf/MEK/ERK signaling pathway. J Cell
Biochem. 119:2864–2874. 2018. View Article : Google Scholar : PubMed/NCBI
|