Introduction
Colon carcinoma is one of the most common types of
malignant tumours, with a high mortality rate due to early
metastases. On the one hand, metastases of colon carcinoma are
connected with the metastatic potential of tumour cells, but on the
other hand, it is also closely related to the body's immune
response (1). Many tumours are
associated with immune dysfunction. T lymphocytes are generally
considered the core of tumour immunity (2). CD4+T cells mainly comprise
auxiliary T cells that contain T helper 0 (Th0), T helper 1 (Th1),
and T helper 2 (Th2) subsets. Th0 cells differentiate into Th1
cells that could stimulate the immune response and Th2 cells that
can induce peripheral immune tolerance by the stimulation of
various cytokines. Additionally, some CD4+T cells act as
regulatory T cells (T-reg) that induce peripheral immune tolerance.
CD8+T cells are mainly cytotoxic T cells, and some
CD8+T cells are T-reg cells that not only induce
cytotoxic effects on target cells, but also inhibit the
activation/proliferation of CD4+T cells. Maintaining the
dynamic balance of the CD4+/CD8+ ratio serves
an important role in the stability of immune function. It has been
identified that a patient's immune function is reduced when the
CD4+/CD8+ ratio decreases, resulting in
tumour proliferation (3).
As the most powerful antigen-presenting cells
(APCs), dendritic cells (DCs) can directly activate naïve T cells
to differentiate into auxiliary and cytotoxic T cells, thereby
serving an important role in the immune response system (4). DCs originate from bone marrow
precursors and include two distinct lineages, myeloid DCs (or DC1),
which express myeloid markers (such as CD11c and CD11b), and
plasmacytoid DCs (or DC2), which lack myeloid markers and express
lymphoid markers CD123 (or B220) and DEC205 (3–6). DCs
affect the differentiation and proliferation of CD4+T
cells and CD8+T cells. DC1 cells mainly induce the
differentiation of CD4+T cells into Th1 to stimulate the
immune response, whereas DC2 cells mainly promote the
differentiation of Th2 or T-reg and are dedicated to the
progression of peripheral immune tolerance (7). A previous study reported that the
number of DCs in the peripheral blood of patients with colon
carcinoma is significantly reduced and that the number of
CD11c+DCs is markedly lower than that of
CD123+DCs when there is distant metastasis (8). In mammary carcinomas, purified
CD11b+CD11c+ myeloid cells can enhance
inducible nitric oxide synthase (iNOS) expression in tumour cells
to promote tumour cell apoptosis (9). Our previous study suggested that
B220+DEC205+DCs induced from the mouse liver
can promote liver metastasis of colon carcinoma (10). All these results suggest that DCs in
the tumour microenvironment have intimate relationships with tumour
immunity and that DCs can induce different tumour immunity
according to the DC phenotype to further affect the growth and
metastasis of tumours.
Poly(ADP-ribose) glycohydrolase (PARG) and poly(ADP-
ribose) polymerase (PARP) are closely related to the occurrence and
development of inflammation, ischaemia-reperfusion injury, cancer
and other diseases. Silencing PARG can reduce cell proliferation
and increase the sensitivity of cells to toxicity, thus inhibiting
embryonic development (11).
Knocking out the PARG gene in the mouse enteritis model inhibits
the progression of bowel tissue inflammation (12). Our previous research findings
demonstrated that PARG silenced in the colon carcinoma mouse model
can suppress the matrix adhesion, movement and attack ability of
human colon carcinoma LoVo cells (13) so that the growing, invasion and
metastasis of LoVo cells were effectively inhibited (14). Nevertheless, the role of PARG in
immune function and how it affects the invasion and metastasis of
tumours has not been investigated.
In the present study, a liver metastasis model of
colon carcinoma was established in mice with the inoculation of
PARG-silenced CT26 cell lines obtained by transfecting CT26 cell
lines with a lentivirus vector containing PARG-short hairpin RNA
(shRNA) to observe tumour metastases and the survival times of
tumour-bearing mice, to determine the effect of silencing PARG on
the proliferation and differentiation of local DCs and T cells and
the possible underlying mechanisms, and to explore the impact of
PARG on immune function in invasion and metastasis of colon
carcinoma to identify a new target in the clinical treatment of
anti-colon carcinoma.
Materials and methods
Mice
A total of 48 female BALB/c mice were purchased from
the Animal Experimental Center of Chongqing Medical University
(Chongqing, China; certification no. 20020001). All mice were
maintained in the specific pathogen-free facility (SPF) room
(20–26°C, 12-h light/12-h dark cycle) and were inoculated with
tumour cells at a weight of 18–20 g and 6–8 weeks of age.
Experiments were performed at the SPF Animal Laboratory.
Cell transfection
The colon carcinoma CT26 cell line was a gift from
Professor Wei (Sichuan University, Chengdu, China). According to
the specification of the lentivirus transfection reagent (Sigma;
Merck KGaA, Darmstadt, Germany), lentivirus PARG-shRNA was
transfected into CT26 cells and screened to obtain stable
PARG-silenced CT26 cells by Dr SWeiqiang Wu in our laboratory
(15). Untreated CT26 cells served
as the non-transfected group, CT26 cells treated with empty vector
served as the empty vector control group, and CT26 cells
transfected with lentivirus PARG-shRNA served as the PARG-silenced
group. Routine culture for each CT26 cell group was performed, and
the culture supernatant was collected.
Establishment of mouse models for
liver metastases of colon carcinoma
According to the method of Liu et al
(16), 48 BALB/c mice were randomly
divided into the non-transfected group, empty vector control group
and PARG-silenced group. Each CT26 cell group in exponential growth
was collected and mixed with PBS into a suspension
(1×107 cells/ml). The mice were anaesthetized with 2%
chloral hydrate (16 ml/kg) by injection into the abdominal cavity,
followed by an abdominal wall incision parallel to the left
subcostal margin. Laparotomy was performed, and then, splenic
subcapsular inoculation of 0.05 ml of each CT26 cell suspension was
performed. The incision was then sewn with a #1 suture. After 14
days, 10 mice were randomly chosen and assigned to each group. The
abdominal cavity was opened to observe and record the number of
liver metastases of colon carcinoma nodules and whether the nodules
were fused. Liver metastasis was divided into 4 grades according to
the following description: grade 0, no liver metastases; grade I,
1–5 liver metastasis nodules; grade II, 6–10 liver metastasis
nodules; grade III, >10 liver metastasis nodules (14). The spleen and liver tissues were
preserved in liquid nitrogen, and the supernatant of the blood was
stored at −80°C. The survival time of the remaining tumour-bearing
mice were recorded daily.
Detection of liver metastases of colon
carcinoma by haematoxylin and eosin (H&E) staining
Liver metastases of colon carcinoma were frozen at
−20°C for 30 sec and then sliced into 8-µm thick sections at −20°C
using a freezing microtome (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), fixed with methanol and successively immersed in
different levels of ethanol for hydration. Following immersion of
the specimens in xylene, the specimens were washed with water. The
specimens were stained with haematoxylin for 5 sec at room
temperature, dipped in Neurolithium for 2 sec and then stained with
eosin. Each coverslip was detected by microscopy at ×400
magnification (Olympus Corporation, Tokyo, Japan).
Double-label immunofluorescence
assay
Spleen or liver frozen sections were fixed with
acetone for 5 min and were incubated with bovine serum albumin
(BSA) for 30 min at 37°C. The spleen or liver sections were first
incubated with anti-DEC205 antibody (cat. no. ab51820; 1:50 diluted
in PBS; Abcam, Cambridge, MA, USA) or anti-CD11b antibody (cat. no.
sc-1186; 1:50 diluted in PBS; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), followed by the addition of anti-B220 antibody
(cat. no. bs-10599R; 1:100 diluted in PBS; BIOSS, Beijing, China)
or anti-CD11c antibody (cat. no. bs-2508R; 1:50 diluted in PBS;
BIOSS) at 4°C overnight. Phycoerythrin (PE)-conjugated streptavidin
anti-biotin (cat. no. bs-0437P-PE; 1:50 diluted in PBS; BIOSS),
cyanine 3 (Cy3)-conjugated donkey anti-goat (cat. no. A0502; 1:50
diluted in PBS; Beyotime Institute of Biotechnology, Haimen,
China), fluorescein isothiocyanate (FITC)-conjugated goat
anti-rabbit (cat. no. bs-0295G-FITC; 1:100 diluted in PBS; BIOSS)
and FITC-conjugated mouse anti-rabbit (cat. no. bs-0293M-FITC; 1:50
diluted in PBS; BIOSS) were used as secondary antibodies (1 h,
37°C, incubated in the dark). Negative controls were performed with
PBS instead of primary antibody. Images of the sections were
captured and analysed by confocal laser-scanning microscopy (Leica
TCS SP2; Leica Microsystems GmbH, Wetzlar, Germany) at ×400
magnification.
Immunofluorescence assay
Spleen or liver frozen sections were fixed with
acetone for 5 min and incubated with goat serum for 30 min at 37°C.
Spleen or liver sections were incubated with anti-CD4 antibody
(cat. no. bs-0766R; 1:50 diluted in PBS; BIOSS) or anti-CD8
antibody (cat. no. bs-0648R; 1:50 diluted in PBS; BIOSS) at 4°C
overnight. FITC-conjugated goat anti-rabbit IgG (cat. no.
bs-0295G-FITC; 1:50 diluted in PBS; BIOSS) was used as the
secondary antibody (1 h, 37°C, incubated in the dark). Negative
controls were performed with PBS instead of primary antibody.
Images of the sections were captured and analysed by confocal
laser-scanning microscopy at ×200 magnification.
Western blotting
The tumour tissues of the spleen or liver preserved
in liquid nitrogen were lysed with radioimmunoprecipitation buffer
(cat. no. P0013K; Beyotime Institute of Biotechnology) and the
supernatant was collected by centrifugation at 22,351 × g for 20
min at 4°C. The total protein or nucleoprotein concentration was
determined using a bicinchoninic acid assay kit (Biomed Gene
Technology Co., Ltd., Beijing, China), according to the
manufacturer's protocol. The total proteins using specific
antibodies against PARG (cat. no. ab169639; 1:1,000 dilution;
Abcam) and nucleoproteins using NF-κB (cat. no. sc-1190; 1:1,000
dilution; Santa Cruz Biotechnology, Inc.) and PARP (cat. no.
sc-136208; 1:1,000 dilution; Santa Cruz Biotechnology, Inc.) were
analysed by western blotting. Using vertical slab PAGE, the protein
was electrically transferred onto polyvinylidene fluoride (PVDF)
membranes. The membranes were then blocked with TBST (10 mM
Tris/HCl, pH 7.4, 150 mM NaCl and 0.1% Tween-20) containing 5%
non-fat dry milk and were incubated with the primary antibody at
4°C overnight. Following washing with PBS, the membranes were
incubated with the secondary antibody for 2 h at room temperature
and then dipped into enhanced chemiluminescent substrates (GE
Healthcare, Chicago, IL, USA) and images were captured using a
gel-imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Finally, Quantity One software (version 4.5; Bio-Rad Laboratories,
Inc.) was used for the densitometric analysis of proteins.
ELISA
IL-10 and TGF-β in the serum of tumour-bearing mice
and supernatant of tumour cells were assayed using ELISA kits (cat.
nos. DY417 and DY1679; R&D Systems, Inc., Minneapolis, MN, USA)
according to the manufacturer's protocol. The optical density (OD)
at 450 nm was measured using an automated reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA). A standard curve was
plotted, and a formula was used to fit the OD of the standard
samples. The lower limits of detection of these assays were as
follows: IL-10, 30–700 pg/ml; and TGF-α, 10–200 ng/ml.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (SPSS, Chicago, IL, USA). The data were expressed as the
means ± standard error of the mean. The grade of liver metastasis
carcinoma nodules was analysed by the Kruskal-Wallis and Nemenyi
methods. The survival data were estimated by the Kaplan-Meier
method and were compared by the log-rank test. One-way ANOVA and
the least significant difference (LSD) test were used for many sets
of the data. P<0.05 was considered to indicate a statistically
significant difference.
Results
Number and grade of liver metastases
of colon carcinoma nodules
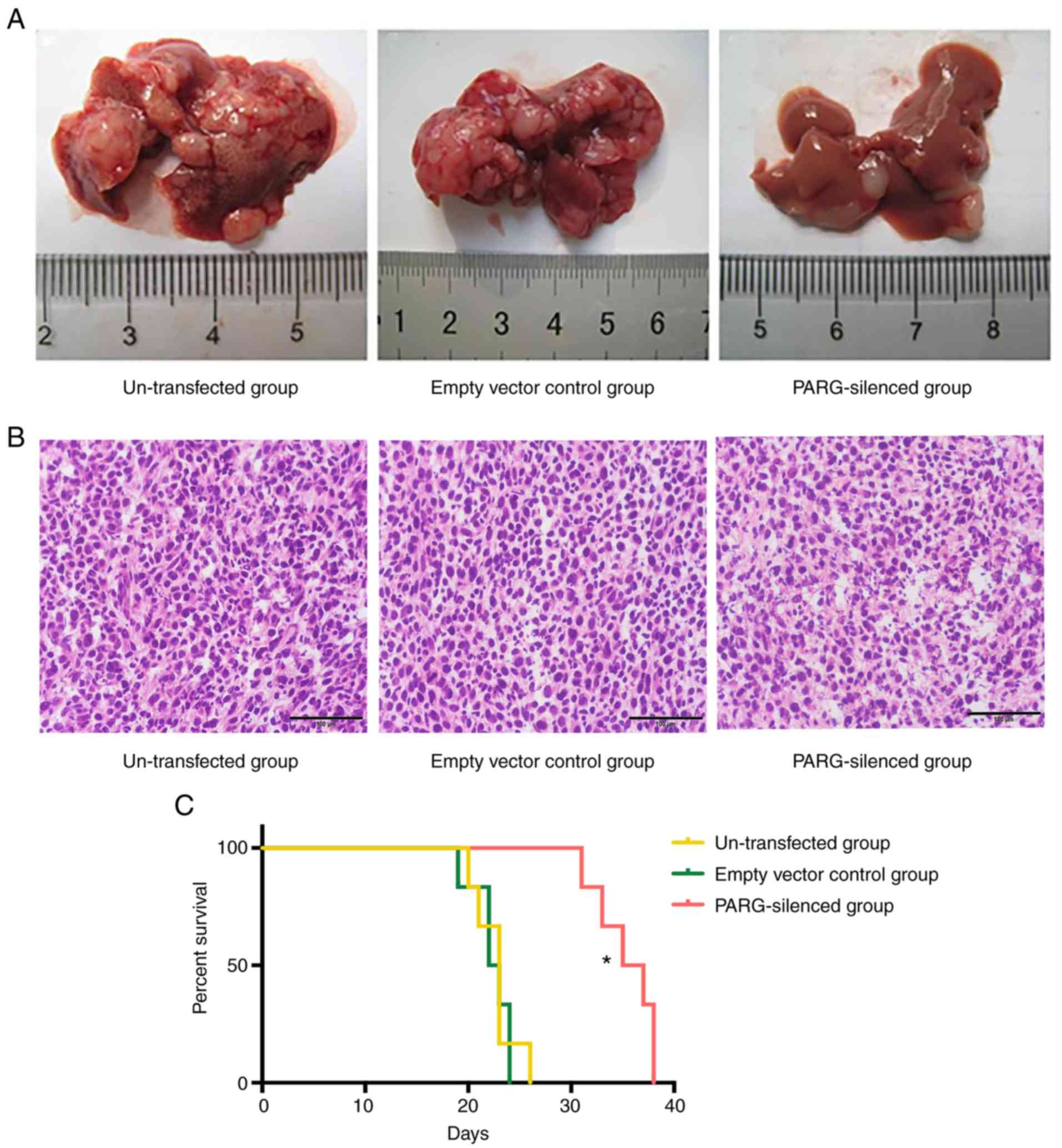
No mouse succumbed during the 2-week experimental
period. There were carcinoma nodules on the inoculated parts of the
spleen in all mice, many of them single nodules. The liver
metastatic rates of the non-transfected group, empty vector group
and PARG-silenced group were 100, 100, and 30%, respectively. The
number and grade of liver metastases in the PARG-silenced group
were significantly less than those in the control groups
(P<0.05; Table I; Fig. 1A). There was no significant
difference between the non-transfected group and empty vector
control group (P>0.05). The liver metastasis carcinoma nodules
were staining with H&E (Fig.
1B).
 | Table I.The numbers and grading of liver
metastases carcinoma nodules in various groups. |
Table I.
The numbers and grading of liver
metastases carcinoma nodules in various groups.
|
|
| The liver
metastatic nodules grading |
|---|
|
|
|
|
|---|
| Group | The numbers of
liver metastatic nodules | 0 | I | II | III |
|---|
| Un-transfected | 32.50±13.69 | 0 | 0 | 0 | 10 |
| Empty vector
control | 31.17±16.98 | 0 | 0 | 0 | 10 |
| PARG-silenced |
1.83±1.72a | 7 | 1 | 1 | 1a |
Survival time
The survival time of the remaining tumour- bearing
mice in multiple groups was calculated from the day that the mice
were inoculated with CT26 cells to their natural death. The
survival ratio was represented as the vertical axis and time
following inoculation as the horizontal axis to draw the survival
curves. The survival time of the PARG-silenced group was remarkably
longer than that in the control groups by the log-rank test
(P<0.05) (Fig. 1B). There was no
significant difference between the non-transfected group and the
empty vector control group (P>0.05).
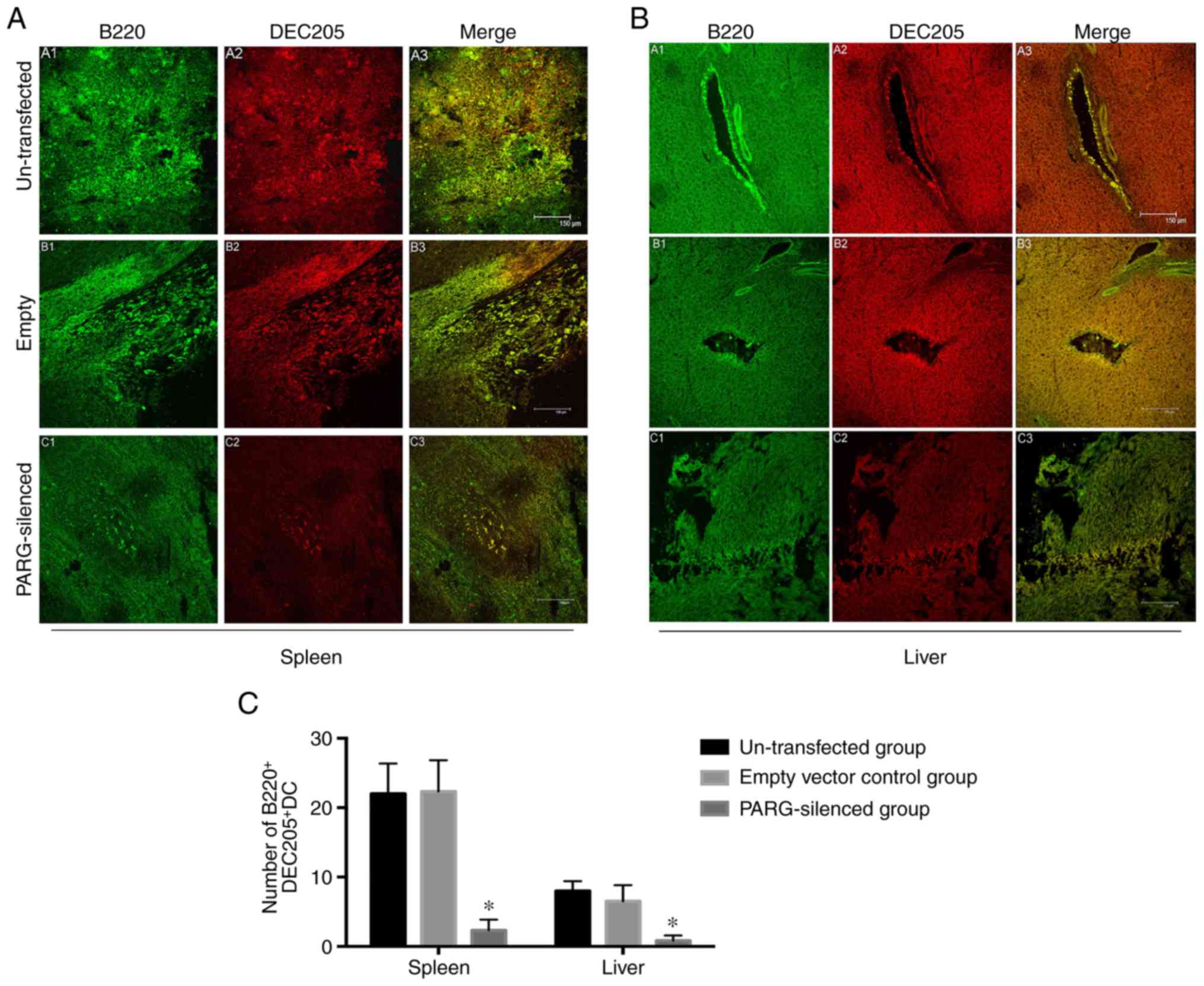
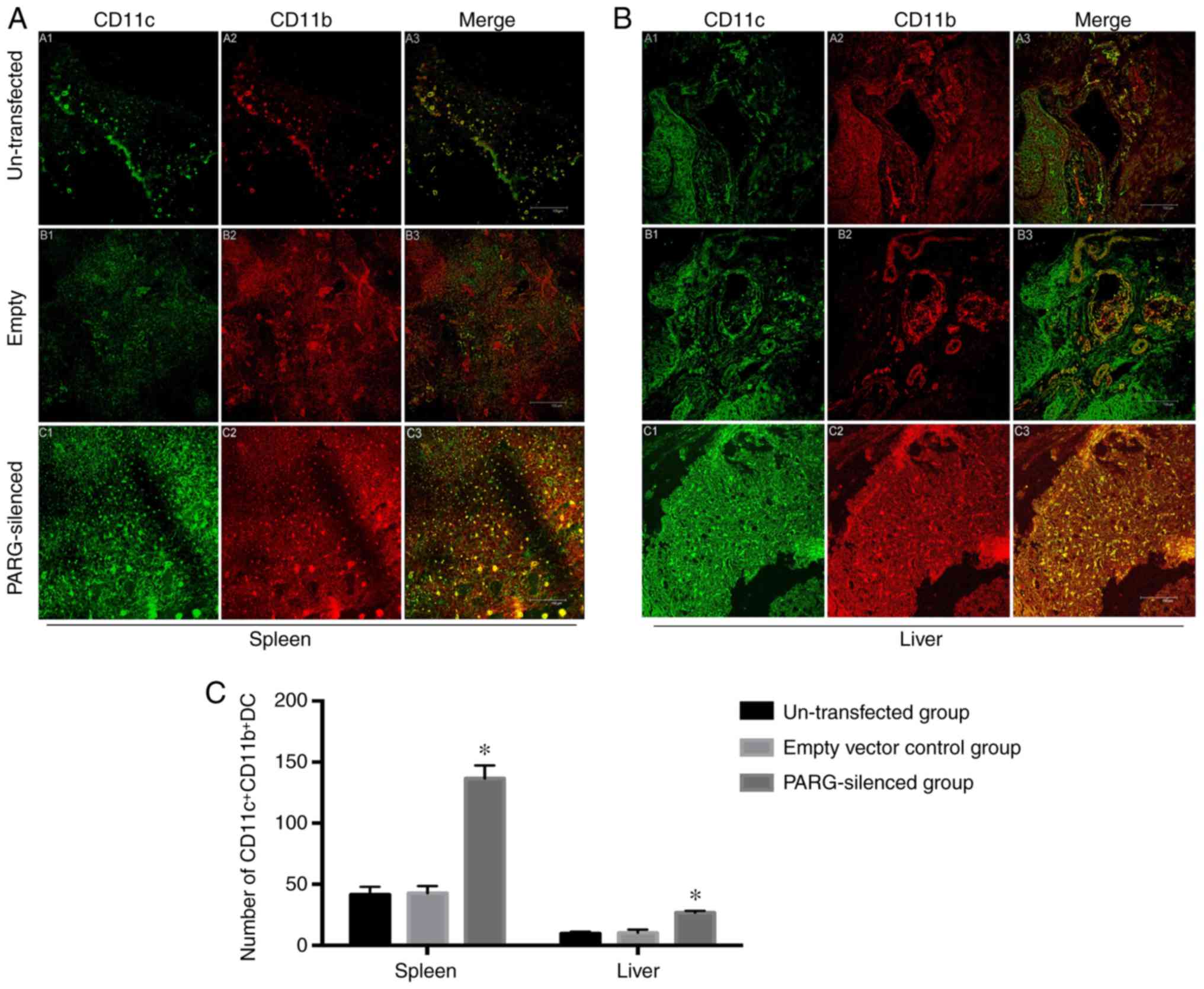
Numbers of
B220+DEC205+DCs and
CD11c+CD11b+DCs in the spleen and liver
Double-label immunofluorescence staining for B220
and DEC205 or CD11c and CD11b was performed on DCs within the
spleen and liver frozen sections to determine the change in the
immune status following PARG silencing. The numbers of
B220+DEC205+DCs in the spleen and liver were
significantly fewer in the PARG-silenced group than in the control
groups (P<0.05; Fig. 2A-C), but
an obviously increased amount of
CD11c+CD11b+DCs was seen in the spleen and
liver of the PARG-silenced group compared to that of the control
(P<0.05; Fig. 3A-C). There was
no significant difference between the non-transfected group and the
empty vector control group (P>0.05).
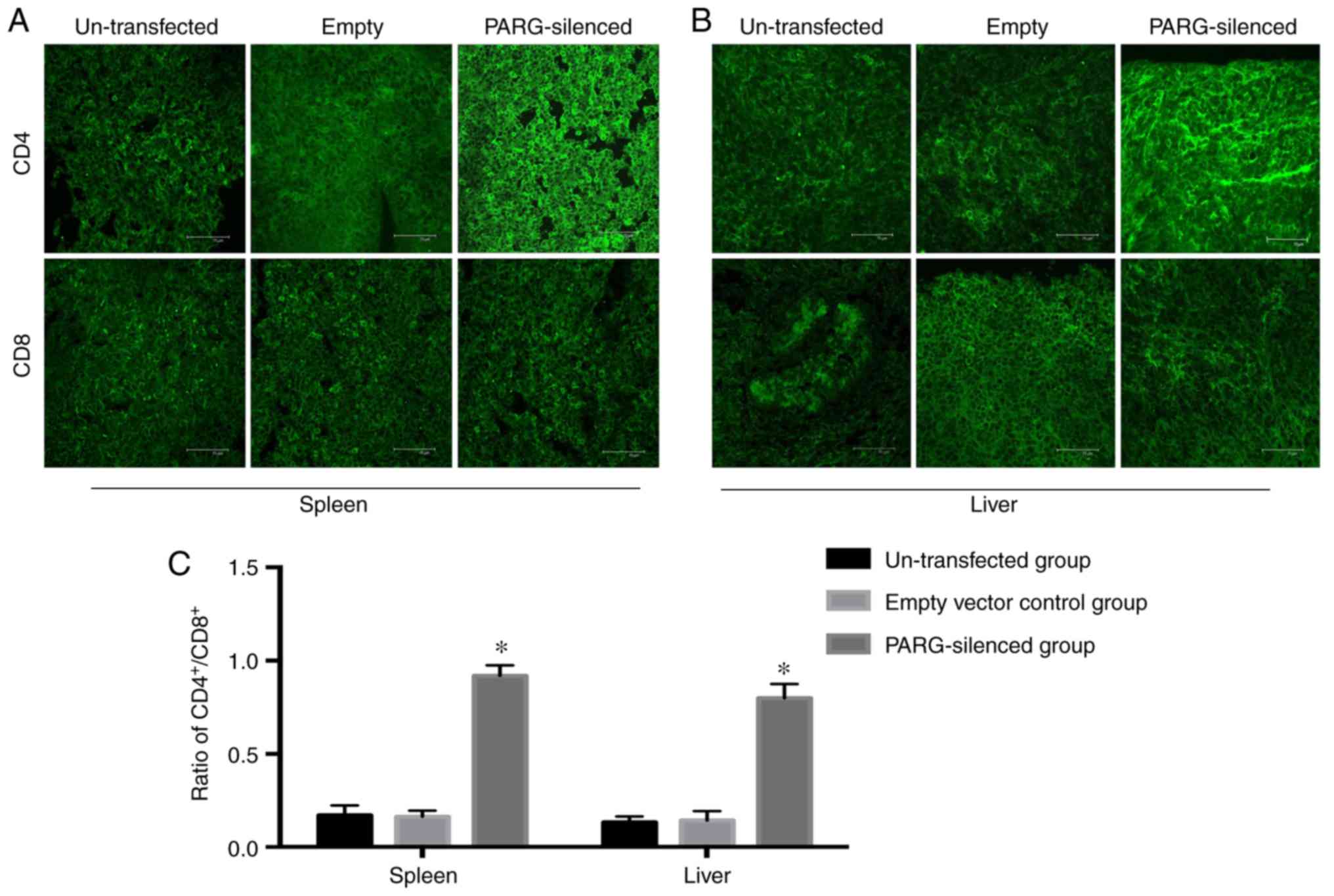
Ratio of
CD4+/CD8+ in the spleen and liver
Single-staining immunofluorescence was employed to
detect CD4 or CD8 cells in T cells within the spleen and liver and
determine the change in immune function following PARG was
silenced. The number of CD4+T cells in the spleen and
liver of the PARG-silenced group was more than that in the control
group, but the number of CD8+T cells in the
PARG-silenced group and control group exhibited no difference.
Therefore, the ratio of CD4+/CD8+ in the
spleen and liver was increased in the PARG-silenced group compared
with that in the control group (P<0.05; Fig. 4A-C). There was no significant
difference between the non-transfected group and empty vector
control group (P>0.05).
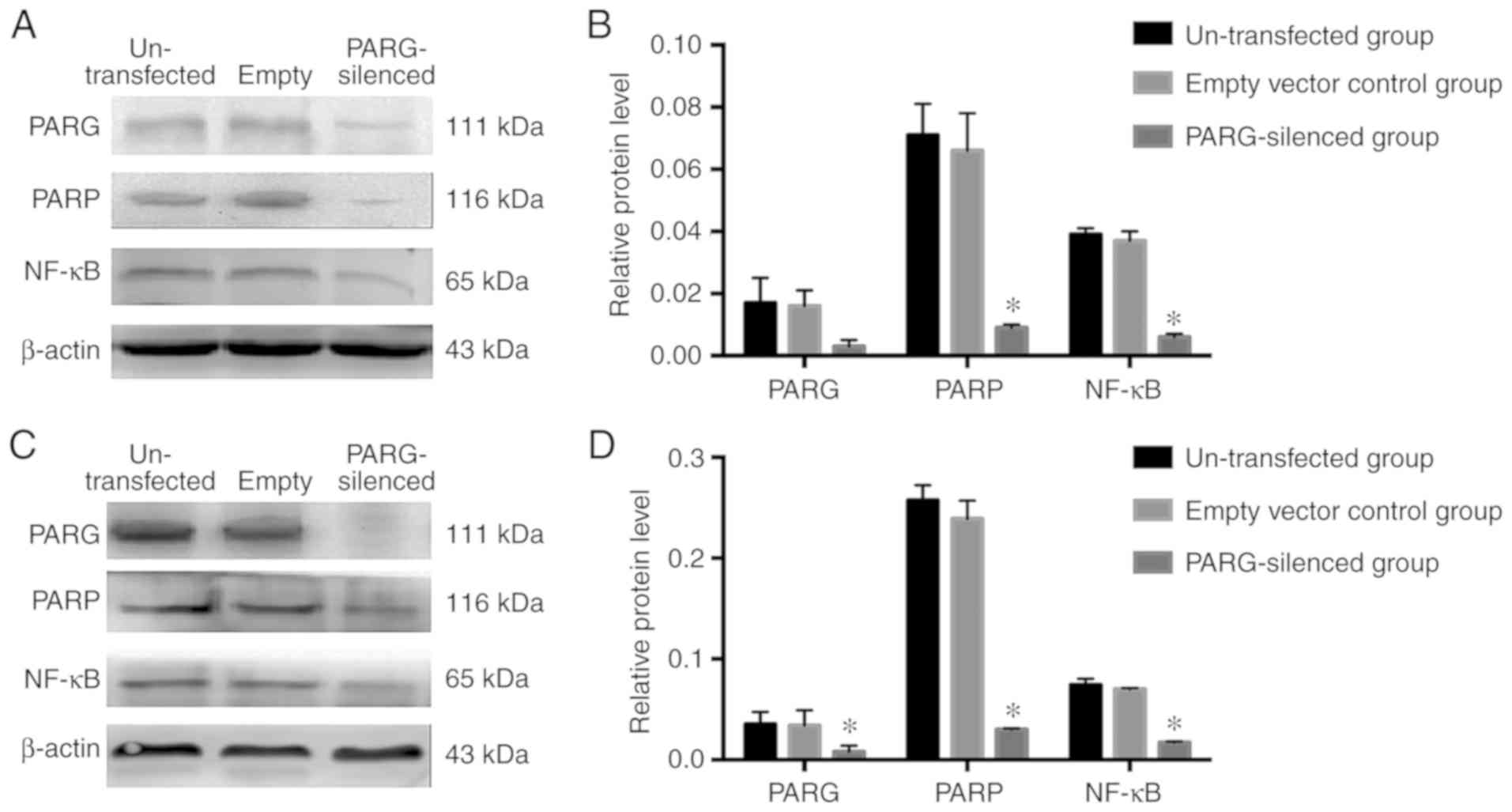
Expression of PARG, PARP and NF-κB of
the spleen transplant tumour and liver metastases of colon
carcinoma
The expression levels of PARG, PARP and NF-κB
proteins in spleen transplant tumours were decreased in the
PARG-silenced group than in the control groups (P<0.05; Fig. 5A and B). In liver metastases of
colon carcinoma, the expression levels of PARG, PARP and NF-κB were
decreased compared with those in the control groups (P<0.05;
Fig. 5C and D). There was no
significant difference between the non-transfected group and empty
vector control group (P>0.05).
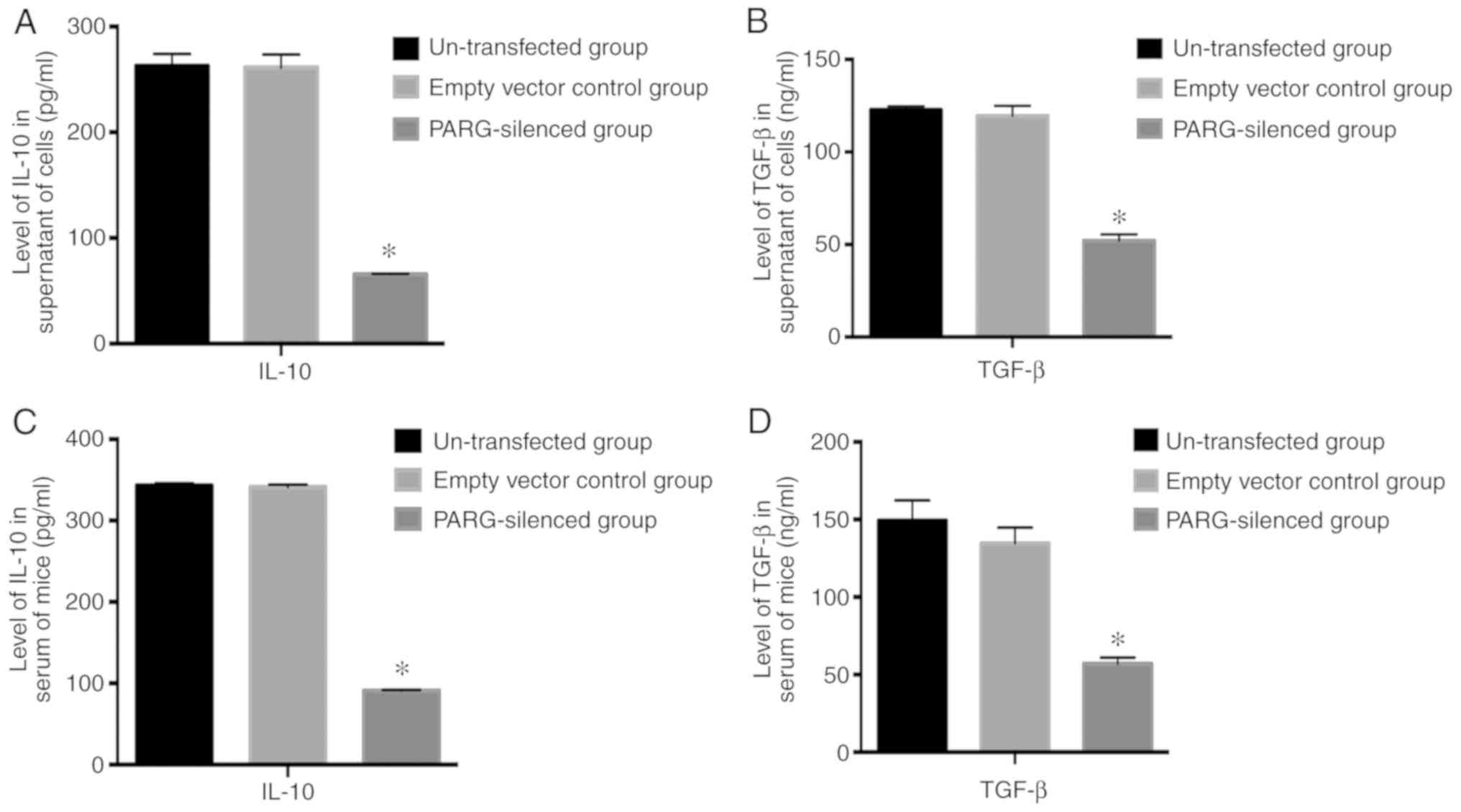
Influence of silencing PARG on IL-10
and TGF-β
The secretion of IL-10 and TGF-β in the supernatant
of PARG-silenced CT26 cells was significantly decreased compared
with non-transfected CT26 cells and empty vector control CT26 cells
(P<0.05; Fig. 6A and B). The
levels of IL-10 and TGF-β in serum were also reduced in the
PARG-silenced group compared with those in the non-transfected
group and empty vector control group (P<0.05; Fig. 6C and D). There was no significant
difference between the non-transfected group and empty vector
control group (P>0.05).
Discussion
Proper regulation of PARPs1/2 and PARG is required
for the cellular responses to genotoxic stress, and PARG serves an
important role in regulating the activity of PARP (17). The poly(ADP-ribose) (PAR) degrading
enzyme PARG could catalyse PAR turnover via hydrolysis of the α(1″
→ 2′) and α(1″′ → 2″) ribosyl-ribose linkages to produce free
ADP-ribose (ADPR), and then restore the activity of the
poly(ADP-ribosyl)ated PARP-1 by detaching PAR to be recycled for
poly(ADP-ribosyl)ation (18,19).
Frizzell et al (20)
demonstrated that PARG serves a vital role not only in the
regulation of PARP but also in adjusting the expression of
downstream genes by collaborating with PARP. Therefore, we
hypothesize that PARG repression served as an effective method to
inhibit PARP. Chandak et al (21) indicated that the expression of PARP
was reduced when the expression of PARG was inhibited by a specific
inhibitor of PARG tannins. In our previous in vitro studies,
silencing PARG in colon carcinoma cells reduces the expression of
PARP (13,14). In the present study, a liver
metastasis model of colon carcinoma was established in mice with
inoculation of PARG-silenced CT26 cell lines. The expression of
PARG was revealed to be reduced, and the expression of PARP was
also decreased in spleen transplant tumours and liver metastases of
colon carcinoma.
As a pleiotropic transcription factor, NF-κB
activates the transcription of >500 genes and participates in
the regulation of the expression of many genes related to
inflammation, cellular transformation, tumour cell survival,
proliferation, invasion, angiogenesis and metastasis (22). The most common NF-κB dimer (p50/p65)
is found in all the members of NF-κB family. The change in the
content of NF-κB to speculate the variation of NF-κB activity has
been reported in several studies (23–25).
Our previous study indicated that PARP-1 could regulate the
activity of NF-κB by which PARP-1 influences the activation of
TLR4-associated signal transduction, leading to NF-κB activation
and subsequent nuclear retention (26). Suppressing the expression of PARP
could further control the activity of NF-κB through the
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signalling
pathway in several diseases, including tumours or non-tumours
(27–29). Our previous in vitro study
indicated that PARG silencing could reduce the expression of PARP
and inhibit the activity of NF-κB at the same time (13,14).
In our in vivo experiment, the levels of PARP and NF-κB in
spleen transplant tumours and liver metastases of colon carcinoma
are reduced following PARG silencing.
As the downstream factor of NF-κB, IL-10 and TGF-β
are considered cytokines with immunosuppressive activity. They have
a wide range of biological effects, including regulating immunocyte
and non-immune cell activation, proliferation and differentiation
(30–34). Our previous studies have indicated
that PARG silencing in colon carcinoma cells could reduce the
expression of PARP as well as inhibit the transcription of NF-κB,
thereby inhibiting tumour metastasis (13,14).
This conclusion is supported by the results that the expression
levels of PARP and NF-κB in the spleen transplant tumour and liver
metastases of colon carcinoma were depressed following PARG
silencing, the secretion of IL-10 and TGF-β in the supernatant of
PARG-silenced CT26 cells was decreased, and the levels of IL-10 and
TGF-β in the serum of PARG-silenced tumour-bearing mice were also
reduced. These results suggest that PARG silencing in colon
carcinoma can regulate the secretion of IL-10 and TGF-β through
adjusting the activity of NF-κB. Further studies are needed to
uncover the underlying mechanism.
Accumulating evidence has suggested that DCs may
serve an important role in tumour immunity (35). The number and function of DCs in
tumour tissue are closely related to the occurrence, development,
transfer and prognosis of cancer (36). Initially,
DEC205+B220+CD19−DCs were induced
from the liver in vitro by Lu et al (37). That this subtype of DCs could
prolong the cardiac allograft survival time suggests that they may
serve an active role in tolerance induction and maintenance in this
model (37).
B220+DEC205+DCs were induced from mice and it
was identified that they are associated with the immune tolerance
of liver transplantation through regulating the proliferation and
differentiation of lymphocytes (38). It was also identified that
B220+DEC205+DCs can promote the liver
metastasis of colon carcinoma and speculated that they seem closely
linked to the regulation of DCs to T-subset differentiation
(10). Other studies have also
reported that CD11b+CD11c+DCs, which can
induce specific immune activity, could promote tumour cell
apoptosis to inhibit tumour metastasis (9).
CD4+T cells have a major role in
secondary immune response via recognizing the exogenous antigen
peptide presented by the MHC class II molecules, while
CD8+T cells participate in the primary immune response
via recognizing the endogenous antigen peptide presented by MHC
class I molecules. A steady ratio of
CD4+/CD8+ is important in maintaining the
normal immune response (39).
Studies have demonstrated that the proportion of CD4+T
cells and the ratio of CD4+/CD8+ are reduced
with an increase in TNM staging (40,41).
The change in DCs and T cells in the local area of
tumours is related to the effect on their proliferation and
differentiation by both IL-10 and TGF-β secreted by the tumour
cells. TGF-β is a potent regulatory cytokine that not only can
regulate the proliferation and differentiation of APCs, mast cells,
NK cells, NKT cells, CD4+T cells and CD8+T
cells but can also regulate the expression of DCs at the
transcription and translation levels by the activation of NF-κB via
PI3K/Akt phosphorylation or the noncanonical pathway (42–44).
IL-10, as an important immunomodulatory and anti-inflammatory
cytokine, can adjust the proliferation and differentiation of DCs
as well as directly affect the function of T cells (45,46).
However, no data have indicated whether PARG silencing in colon
carcinoma can inhibit the expression of PARP and, thus, restrain
the activity of NF-κB, downregulate the secretion of downstream
factor IL-10 and TGF-β, and then influence the proliferation and
differentiation of DCs and T cells, finally inhibiting the
metastases of colon carcinoma by the change in immunity
function.
In the present study, the effect of PARG on DCs and
T cells was observed in the spleen and liver of tumour-bearing
mice. The results indicated that in the PARG-silenced group, the
number of B220+DEC205+DCs was reduced, but
the level of CD11c+CD11b+DCs was increased in
the spleen and liver of tumour-bearing mice, with an increased
ratio of CD4+/CD8+ cells. Thus, IL-10 and
TGF-β regulated by PARG could promote the differentiation of DCs
into the immunoreactive subset and further influence the
differentiation of T cells. It is concluded that silencing the PARG
gene in CT26 cells could suppress the metastasis of tumour cells to
the liver in colon carcinoma and prolong the survival time of the
tumour-bearing mice. This effect is probably related to the
downregulation of PARP by PARG silencing and inhibition of NF-κB
activity, further reducing the secretion of IL-10 and TGF-β and
influencing the proliferation and differentiation of DCs and T
cells. Briefly, the results of the present study imply that PARG
could serve an important role in the growth of tumours and
metastasis to the liver of colon carcinoma and may serve as a new
target for clinical colon carcinoma therapy. The underlying
mechanism concerning the immune function requires further
research.
Acknowledgements
The authors thank Professor YQ Wei (Sichuan
University, Chengdu, China) for providing the CT26 cell line.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant no. 30870946).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JQW performed the experiments and wrote the first
draft of the paper. YT analyzed the data and edited the manuscript
prior to acceptance. QSL produced the figures, organized the
references and performed the H&E staining to detect the liver
metastasis of colon carcinoma. MX performed the double-label
immunofluorescence assay. ML performed the western blot analysis.
YY and YTS participated in the construction of the transplanted
tumour model in BALB/c mice. YLW designed and performed the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Chongqing Medical University (Chongqing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kubota Y, Sunouchi K, Ono M, Sawada T and
Muto T: Local immunity and metastasis of colorectal carcinoma. Dis
Colon Rectum. 35:645–650. 1922. View Article : Google Scholar
|
|
2
|
Yasutomo K: The cellular and molecular
mechanism of CD4/CD8 lineage commitment. J Med Invest. 49:1–6.
2002.PubMed/NCBI
|
|
3
|
Lonial S, Torre C, David E, Harris W,
Arellano M and Waller EK: Regulation of alloimmune responses by
dendritic cell subsets. Exp Hematol. 36:1309–1317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fassbender M, Herter S, Holtappels R and
Schild H: Correlation of dendritic cell maturation and the
formation of aggregates of poly-ubiquitinated proteins in the
cytosol. Med Microbiol Immunol. 197:185–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zarnani AH, Moazzeni SM, Shokri F,
Salehnia M and Jeddi-Tehrani M: Kinetics of murine decidual
dendritic cells. Reproduction. 133:275–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sato K and Fujita S: Dendritic
cells-nature and classification. Allergol Int. 56:183–191. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reid SD, Penna G and Adorini L: The
control of T cell responses by dendritic cell subsets. Curr Opin
Immunol. 12:114–121. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Della Porta M, Danova M, Rigolin GM,
Brugnatelli S, Rovati B, Tronconi C, Fraulini C, Russo Rossi A,
Riccardi A and Castoldi G: Dendritic cells and vascular endothelial
growth factor in colorectal cancer: Correlations with
clinicobiological findings. Oncology. 68:276–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parajuli N, Müller-Holzner E, Böck G,
Werner ER, Villunger A and Doppler W: Infiltrating
CD11b+CD11c+ cells have the potential to
mediate inducible nitric oxide synthase-dependent cell death in
mammary carcinomas of HER-2/neu transgenic mice. Int J Cancer.
126:896–908. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ke Q, Wang Y and You H: Effect of mouse
liver-derived B220+/DEC205+ dendritic cells
on hepatic metastases from colonic cancer in vivo. Chin J Anatomy.
32:724–727. 2009.(In Chinese).
|
|
11
|
Min W, Cortes U, Herceg Z, Tong WM and
Wang ZQ: Deletion of the nuclear isoform of poly(ADP-ribose)
glycohydrolase (PARG) reveals its function in DNA repair, genomic
stability and tumorigenesis. Carcinogenesis. 31:2058–2065. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cuzzocrea S, Mazzon E, Genovese T,
Crisafulli C, Min WK, Di Paola R, Muià C, Li JH, Malleo G, Xu W, et
al: Role of poly(ADP-ribose) glycohydrolase in the development of
inflammatory bowel disease in mice. Free Radic Biol Med. 42:90–105.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li QZ, Wang YL and Li X: Lentivirus
PARG-shRNA transfection decreases matrix adhesion, migration
and invasion potencies of colon carcinoma lovo cells. Basic Clin
Med. 30:237–241. 2010.(In Chinese).
|
|
14
|
Fauzee NJ, Li Q, Wang YL and Pan J:
Silencing poly (ADP-Ribose) glycohydrolase (PARG) expression
inhibits growth of human colon cancer cells in vitro via
PI3K/Akt/NFκ-B pathway. Pathol Oncol Res. 18:191–199. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu WQ, Wang YL, Pan J and Yan JX:
PARG silencing inhibits lymphangiogenesis in mouse colonal
carcinoma in vitro. Basic Clin Med. 31:625–629. 2011.(In
Chinese).
|
|
16
|
Liu HY, Huang ZL, Yang GH, Lu WQ and Yu
NR: Inhibitory effect of modified citrus pectin on liver metastases
in a mouse colon cancer model. World J Gastroenterol. 14:7386–7391.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Botta D and Jacobson MK: Identification of
a regulatory segment of poly(ADP-ribose) glycohydrolase.
Biochemistry. 49:7674–7682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miwa M, Tanaka M, Matsushima T and
Sugimura T: Purification and properties of glycohydrolase from calf
thymus splitting ribose-ribose linkages of poly(adenosine
diphosphate ribose). J Biol Chem. 249:3475–3482. 1974.PubMed/NCBI
|
|
19
|
Blenn C, Wyrsch P, Bader J, Bollhalder M
and Althaus FR: Poly(ADP-ribose)glycohydrolase is an upstream
regulator of Ca2+ fluxes in oxidative cell death. Cell
Mol Life Sci. 68:1455–1466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frizzell KM, Gamble MJ, Berrocal JG, Zhang
T, Krishnakumar R, Cen Y, Sauve AA and Kraus WL: Global analysis of
transcriptional regulation by poly(ADP-ribose) polymerase-1 and
poly(ADP-ribose) glycohydrolase in MCF-7 human breast cancer cells.
J Biol Chem. 284:33926–33938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chandak PG, Gaikwad AB and Tikoo K:
Gallotannin ameliorates the development of streptozotocin-induced
diabetic nephropathy by preventing the activation of PARP.
Phytother Res. 23:72–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bannwart CF, Nakaira-Takahagi E, Golim MA,
de Medeiros LT, Romao M, Weel IC and Peracoli MT: Downregulation of
nuclear factor-kappa B (NF-kappaB) pathway by silibinin in human
monocytes challenged with Paracoccidioides brasiliensis. Life Sci.
86:880–886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bar-Shai M, Carmeli E and Reznick AZ: The
role of NF-kappaB in protein breakdown in immobilization, aging,
and exercise: From basic processes to promotion of health. Ann NY
Acad Sci. 1057:431–447. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai D, Frantz JD, Tawa NE Jr, Melendez PA,
Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, et
al: IKKbeta/NF-kappaB activation causes severe muscle wasting in
mice. Cell. 119:285–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayden MS, West AP and Ghosh S: NF-kappaB
and the immune response. Oncogene. 25:6758–6780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zerfaoui M, Errami Y, Naura AS, Suzuki Y,
Kim H, Ju J, Liu T, Hans CP, Kim JG, Abd Elmageed ZY, et al:
Poly(ADP-ribose) polymerase-1 is a determining factor in
Crm1-mediated nuclear export and retention of p65 NF-kappa B upon
TLR4 stimulation. J Immunol. 185:1894–1902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bartha E, Solti I, Kereskai L, Lantos J,
Plozer E, Magyar K, Szabados E, Kálai T, Hideg K, Halmosi R, et al:
PARP inhibition delays transition of hypertensive cardiopathy to
heart failure in spontaneously hypertensive rats. Cardiovasc Res.
83:501–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Williams AC, Smartt H, HZadeh AM,
MacFarlane M, Paraskeva C and Collard TJ: Insulin-like growth
factor binding protein 3 (IGFBP-3) potentiates TRAIL-induced
apoptosis of human colorectal carcinoma cells through inhibition of
NF-kappa B. Cell Death Differ. 14:137–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang K, Chen SP, Lin CW, Cheng WC and
Huang HT: Ellipticine-induced apoptosis depends on Akt
translocation and signaling in lung epithelial cancer cells. Lung
Cancer. 63:227–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yadav VR, Prasad S, Gupta SC, Sung B,
Phatak SS, Zhang S and Aggarwal BB: 3-Formylchromone interacts with
cysteine residue 38 in p65 and with cysteine residue 179 in IκBα
kinase, leading to downregulation of NF-κB-regulated gene products
and sensitization of tumor cells. J Biol Chem. 2011:
|
|
31
|
Baugé C, Attia J, Leclercq S, Pujol JP,
Galéra P and Boumédiene K: Interleukin-1beta up-regulation of Smad7
via NF-kappaB activation in human chondrocytes. Arthritis Rheum.
58:221–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frangogiannis NG: The immune system and
cardiac repair. Pharmacol Res. 58:88–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J and Liu XS: Development and
function of IL-10 IFN- gamma-secreting CD4+ T cells. J
Leukoc Biol. 86:1305–1310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schmidt-Weber CB and Blaser K: Regulation
and role of transforming growth factor-beta in immune tolerance
induction and inflammation. Curr Opin Immunol. 16:709–716. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bellik L, Gerlini G, Parenti A, Ledda F,
Pimpinelli N, Neri B and Pantalone D: Role of conventional
treatments on circulating and monocyte-derived dendritic cells in
colorectal cancer. Clin Immunol. 121:74–80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schröder S, Schwarz W, Rehpenning W,
Löning T and Böcker W: Dendritic/Langerhans cells and prognosis in
patients with papillary thyroid carcinomas. Immunocytochemical
study of 106 thyroid neoplasms correlated to follow-up data. Am J
Clin Pathol. 89:295–300. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu L, Bonham CA, Liang X, Chen Z, Li W,
Wang L, Watkins SC, Nalesnik MA, Schlissel MS, Demestris AJ, et al:
Liver-derived DEC205+B220+CD19−
dendritic cells regulate T cell responses. J Immunol.
166:7042–7052. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Zheng N, Lu Z, Wu W, Wang L, Nakao
A, Lotze MT, Langer CE, Fung JJ, Qian S, et al: In vivo expansion
of two distinct dendritic cells in mouse livers and its impact on
liver immune regulation. Liver Transpl. 12:1850–1861. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rosenberg SA: Progress in human tumour
immunology. Nature. 411:380–384. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bai DJ, Yang GL and Yuan HY: The study of
T lymphocyte and NK cell immune function in the patients with
gastrointestinal cancer. Med J Wuhan University. 22:142–144.
2001.(In Chinese).
|
|
41
|
Yang Z Y.L.J and Song XY: The clinical
study of immune function in lung cancer, liver cancer, colon
carcinoma and breast cancer patients. Sichuan Med J.
26:7542005.
|
|
42
|
Rubtsov YP and Rudensky AY: TGFbeta
signalling in control of T-cell-mediated self-reactivity. Nat Rev
Immunol. 7:443–453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li MO, Wan YY, Sanjabi S, Robertson AK and
Flavell RA: Transforming growth factor-β regulation of immune
responses. Annu Rev Immunol. 24:99–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Belladonna ML, Volpi C, Bianchi R, Vacca
C, Orabona C, Pallotta MT, Boon L, Gizzi S, Fioretti MC, Grohmann U
and Puccetti P: Cutting edge: Autocrine TGF-beta sustains default
tolerogenesis by IDO-competent dendritic cells. J Immunol.
181:5194–5198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Corinti S, Albanesi C, la Sala A, Pastore
S and Girolomoni G: Regulatory activity of autocrine IL-10 on
dendritic cell functions. J Immunol. 166:4312–4318. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rojas RE, Balaji KN, Subramanian A and
Boom WH: Regulation of human CD4+ αβ
T-cell-receptor-positive (TCR+) and γδ TCR+
T-cell responses to Mycobacterium tuberculosis by
interleukin-10 and transforming growth factor β. Infect Immun.
67:6461–6472. 1999.PubMed/NCBI
|