Introduction
Ovarian cancer is the most life-threatening form of
gynecological cancer and epithelial ovarian cancer (EOC), the most
frequent type of ovarian cancer, is a serious threat to female
health (1). Due to a lack of
pathognomonic clinical symptoms and effective early detection
markers, up to 80% of patients with EOC with widespread metastases
are diagnosed in the advanced stages (2). Clinically, conventional treatments
mainly include surgery, chemotherapy and/or radiotherapy. These
therapeutic approaches can elevate progression-free survival rates
to 50%, however, the associated prognosis remains poor (3). Cell invasion and migration are the
main factors contributing to the poor prognosis in EOC, whereas the
underlying mechanisms driving the biological processes are complex
and remain to be fully elucidated (4). It has been suggested that
epithelial-mesenchymal transition (EMT) serves a critical role in
the tumor invasion-metastasis cascade (5). EMT, a reversible cellular process that
converts cells from the epithelial polarized phenotype to the
mesenchymal fibroblastoid phenotype, is primarily associated with
epithelial cell depolarization, loss of cell-to-cell contacts, and
an increase in migratory motility and capacity (6). Unfortunately, tumor cells usually
undergo EMT, which induces the invasive growth and metastasis of
ovarian cancer (7). Therefore, the
inhibition of EMT may be an effective strategy with the greatest
potential for improving outcomes in patients with EOC.
Natural products have contributed to the development
of a number of anticancer agents in recent decades. Due to their
structural diversity, several agents used clinically are natural
products or derivatives (8),
including paclitaxel and vincristine (9,10).
Associated studies have also shown that alkaloids are widely
distributed in nature (11,12), with notable pharmacological
activities in eliminating cancer, and inflammatory and oxidant
activities (13–15). Furthermore, >450 alkaloids are
present in Aconitum species, which have been widely used in
China, Japan and other regions for treating a number of disorders
(16). The main alkaloid in
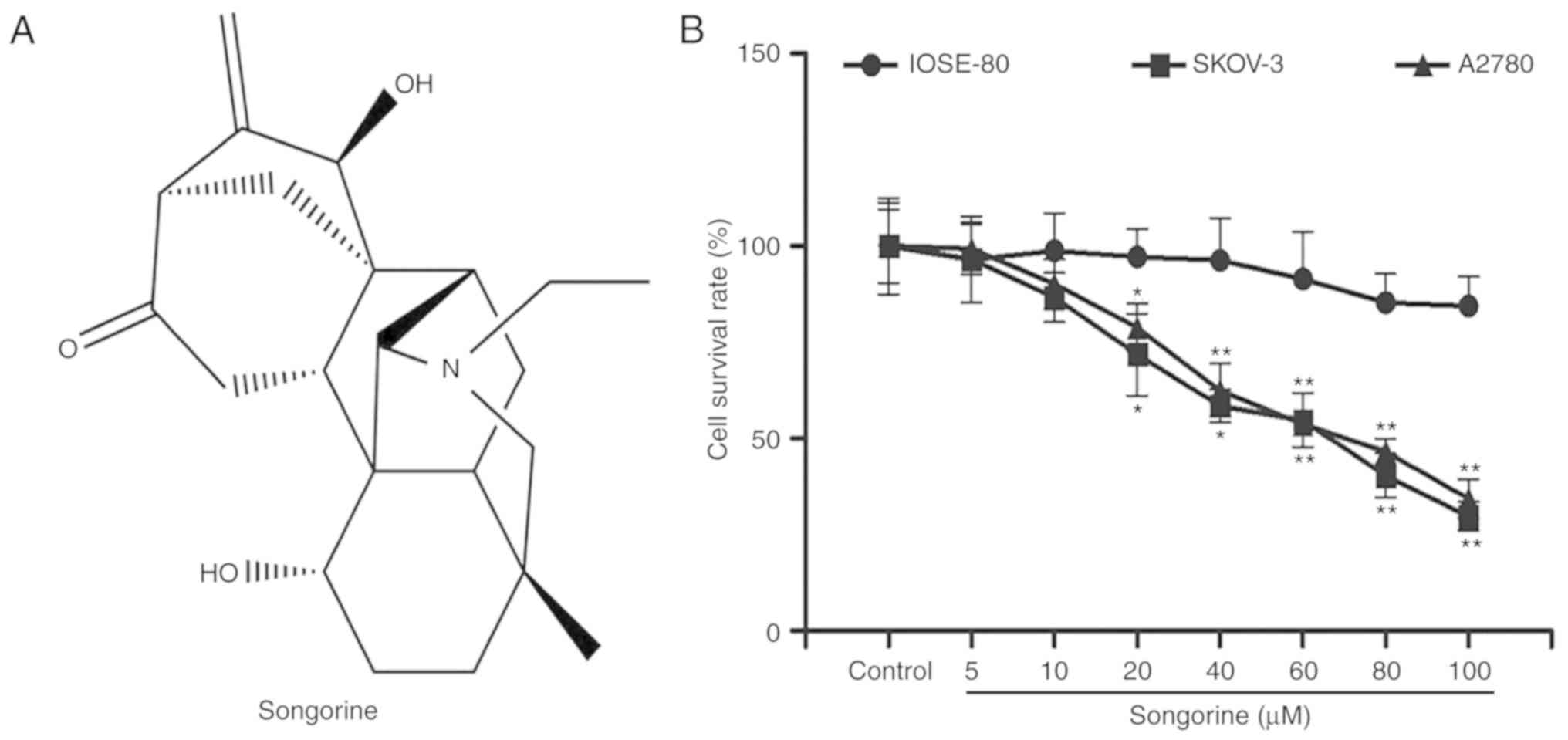
Aconitum soongaricum Stapf is songorine (Fig. 1A), a
C20-diterpenoidaconitum alkaloid (17,18).
Songorine possesses several properties, including
anti-inflammatory, anti-arrhythmic and anti-central nervous system
disorder properties (19). A recent
study (20) revealed that songorine
can significantly inhibit the survival of human liver cancer cells,
which is suggestive of its potential activities in treating cancer.
Based on this pharmacological study, it was hypothesized that
songorine may maintain its therapeutic action in EOC. The aim of
the present study was to evaluate the efficacy of songorine in
regulating EOC cell proliferation, migration, invasion and
apoptosis. The mechanisms underlying songorine in treating EOC were
also discussed. Furthermore, the present study aimed to verify the
pharmacological activity of songorine in murine xenograft models.
Overall, the results may provide valuable information for drug
development or potential strategies against EOC.
Materials and methods
Cell lines and experimental
animals
The EOC cells (SKOV3 and A2780) and normal ovarian
epithelial cells (IOSE-80) were obtained from the Shanghai Cell
Bank of China Academy of Sciences (Shanghai, China). The SKOV3,
A2780 and IOSE-80 cells were cultured in Dulbecco's modified
Eagle's medium (DMEM), RPMI-1640 and DMEM (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), respectively, and then
maintained in a humidified incubator with 5% CO2 at
37°C. In addition, 4-week old female BALB/C nude mice (n=24, 20±2
g) were kindly provided by Shanghai SLAC Experimental Animal
Company (Shanghai, China) and housed in a pathogen-free environment
at a constant temperature (23±2°C) and humidity under a 12-h
light/dark cycle on a full-balanced diet with free access to water
and food.
Main reagents and instruments
The primary reagents used in the present study
included the following: Songorine (Baoji Herbest Biotech Co., Ltd.,
Baoji, China); cisplatin (Haosen Pharmaceutical Co., Ltd.,
Lianyungang, China); SB216763, MTT and dimethyl sulfoxide (DMSO)
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany); an Enhanced
Chemiluminescence (ECL) Western Blotting Kit (Beyotime Institute of
Biotechnology, Beijing, China); Matrigel, crystal violet and a
bicinchoninic acid kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China); a small Transwell chamber (Corning, Inc., Somerset, NJ,
USA); alanine aminotransferase (ALT), aspartate aminotransferase
(AST), blood urea nitrogen (BUN), creatinine (CREA) and creatine
kinase (CK) kits (Jiancheng Bioengineering Institute, Nanjing,
China); phosphorylated (p)-glycogen synthase kinase (GSK)-3β (cat.
no. 5558S), GSK-3β (cat. no. 12456S), β-catenin (cat. no. 8480S),
Ki67 (cat. no. 9449S), N-cadherin (cat. no. 13116S), vimentin (cat.
no. 5741S), matrix metalloproteinase (MMP)-2 (cat. no. 40994S),
MMP-9 (cat. no. 13667S), B-cell lymphoma 2 (Bcl-2; cat. no.
15071S), E-cadherin (cat. no. 14472S), Bcl-2-associated X (Bax;
cat. no. 5023S), cleaved caspase-3 (c-caspase-3; cat. no. 9664S),
caspase-3 (9662S), c-caspase-9 (cat. no. 9505S), caspase-9 (cat.
no. 9504S) and β-tubulin (cat. no. 2128S) primary antibodies (Cell
Signaling Technology, Inc., Danvers, MA, USA); goat anti-rabbit
immunoglobulin G (IgG) secondary antibody (cat. no. A0208; Bioworld
Technology, Inc., St. Louis Park, MN, USA); a protein gel
electrophoresis system (Tanon Science & Technology Co., Ltd.,
Shanghai, China); a light microscope (Olympus Optical Co., Ltd.,
Tokyo, Japan); an inverted fluorescence microscope (Nikon Instech
Co., Ltd., Tokyo, Japan); and image Quant LAS 4000 (Ge Healthcare,
Chicago, IL, USA).
Cell survival assay
Cell survival was measured using the MTT
colorimetric method. Briefly, logarithmic phase cells were seeded
into 96-well plates (5×104/ml) and incubated with
different concentrations of songorine (0, 20, 40, 60, 80 or 100 µM)
for 24 h at 37°C. Subsequently, 20 µl MTT (5 mg/ml) was added into
each well. Following 4 h of incubation at 37°C, 150 µl DMSO was
added to dissolve the formazan prior to measurements using a plate
reader. Finally, the optical density value was measured at 490 nm.
Six independent experiments were performed.
Wound scratch and Transwell chamber
assays
The logarithmic phase cells were seeded in 6-well
plates (1×105/ml) and incubated overnight to allow cells
to attach. Scratches were created using a 200-µl pipette tip,
creating a scratch along a marked line, and floating cells were
washed away using PBS. Subsequently, fresh medium with different
concentrations of songorine (0, 20, 40 or 80 µM) was added.
Following culture (5% CO2, 37°C) for 24 h, the migrated
cells were observed and the cell migration area was calculated.
The invasion assay was performed using Transwell
invasion chambers (Costar; Corning, Inc.) pre-coated with Matrigel
(BD Biosciences, San Jose, CA, USA). Firstly, 100 µl serum-free
medium with logarithmic phase cells (1×105/ml) and
different concentrations of songorine (0, 20, 40 or 80 µM) were
added into the upper chamber. Following this, 600 µl medium
containing 10% (v/v) fetal bovine serum (Gemini Bio-Products, West
Sacramento, CA, USA) was loaded into the lower chamber. After 24 h,
the non-invading cells were gently removed, and the invading cells
on the bottom inserts were fixed with 4% paraformaldehyde (20 min)
and stained with 0.1% crystal violet (12 min), successively. The
number of cells was then counted per five fields of view under a
light microscope.
Annexin V/propidium iodide (PI)
staining assay
The logarithmic phase cells were seeded in 6-well
plates (1×105/ml) and incubated overnight. Following
treatment with different concentrations of songorine (0, 20, 40 or
80 µM) for 24 h, the cells were harvested and re-suspended
(1×106 cells/ml) in binding buffer. The Annexin
V-fluorescein isothiocyanate and PI were added into the cell
suspension according to the instructions provided by the
manufacturer of the apoptosis detection kit. Finally, cell
apoptosis was detected by flow cytometry (BD Biosciences).
In vivo experiments
The in vivo investigation of songorine was
performed using a xenograft model of ovarian cancer cells. Briefly,
200 µl resuspended serum-free PBS with 1×107 viable
SKOV3 cells was injected subcutaneously into the right axillary
region of the nude mice. When the tumor volume [volume = (length ×
width2)/2] reached 100 mm3, this was
indicative of successful establishment of the ovarian cancer model.
According to a previous study (21), the same administration dosages of
songorine (0.25 and 2.5 mg/kg) were used in the present study.
Subsequently, the mice were randomized into four groups (n=6): Mice
were intraperitoneally injected with saline, songorine (0.25 or 2.5
mg/kg/day) and cisplatin (2 mg/kg/day) for 3 weeks. All animals
were sacrificed under anesthesia following blood collection from
the eye socket vein, and the subcutaneous tumors were then
harvested. The blood supernatants were separated to detect the
indicators of liver, renal and cardiac functions, whereas tumors
were obtained for histomorphological observations and
immunoblotting assays. All animal experimental procedures were
performed in accordance with the International Standards of Animal
Welfare and were approved by the Institute of Animal Care and Use
Committee of Qingdao University (Qingdao, China).
Spectrophotometer detection
The levels of ALT, AST, BUN, CREA and CK in the
blood supernatants of each group were measured according to the
operating steps described in the instructions of the commercial
assay kits.
Hematoxylin and eosin (H&E)
staining
The tumor samples were collected and dehydrated in a
graded ethanol series prior to paraffin embedding. For
histopathological analysis, serial paraffin sections (5-µm) were
stained with H&E. The morphological changes were observed under
a light microscope (Eclipse TE200; Nikon Instech Co., Ltd.) by
investigators blinded to the experimental groups.
Immunohistochemistry
The sections were deparaffinized, rehydrated and
then immersed in 100 µl 3% hydrogen peroxide for 15 min at room
temperature. Following antigen retrieval in boiled sodium citrate
buffer, the sections were successively incubated with the rabbit
anti-Ki67 antibody (dilution 1:500) at 4°C overnight and goat
anti-rabbit IgG (dilution 1:500) for 30 min at room temperature.
Subsequently, the sections were visualized by incubating with DAB
solution. The expression level of Ki67 was assessed under a light
microscope (magnification, ×400) and presented as the average
staining intensity of five fields.
Immunoblotting assay
Each group of cells or tumor tissues were washed
with PBS three times, and proteins were then extracted using a
total protein assay kit. A total of 30 mg of denatured protein was
separated by 10% SDS-PAGE and electrotransferred onto PVDF
membranes (Merck KGaA Co., Ltd., Darmstadt, Hessen, Germany), and
then incubated with specific antibodies of p-GSK-3β, GSK-3β,
β-catenin, N-cadherin, vimentin, MMP-2, MMP-9, Bcl-2, E-cadherin,
Bax, c-caspase-3, caspase-3, c-caspase-9, caspase-9 and β-tubulin
(dilution 1:1,000) overnight at 4°C. The PVDF membranes were washed
three times in PBST, and then incubated with horseradish
peroxidase-linked secondary antibody (dilution 1:5,000; cat. no.
BA1054; Wuhan Boster Biological Technology, Ltd., Wuhan, China) for
1 h at room temperature. The specific proteins on the blots were
visualized using ECL and quantified using the Image Quant LAS 4000
system. β-tubulin was used as the loading control.
Statistical analysis
Quantitative data are expressed as the mean ±
standard deviation. Data were analyzed using one-way analysis of
variance (ANOVA), followed by Dunnett's post hoc test using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Songorine suppresses the survival of
EOC cells
To preliminarily examine the effect of songorine on
EOC, human EOC cells were treated with a range of concentrations of
songorine. An MTT assay was used to measure the cell viabilities of
SKOV-3 and A2780 cells. As expected, songorine inhibited SKOV-3 and
A2780 cell viability in a concentration-dependent manner (Fig. 1B). In addition, the half-maximal
inhibitory concentration values of songorine were 55.63 µM for
SKOV-3 cells and 64.42 µM for A2780 cells. To verify whether
songorine was hypotoxic to normal ovarian cells, IOSE-80 cells
subsequently underwent songorine treatment. The results
demonstrated that songorine exerted no significant inhibitory
effects on the normal ovarian cell survival (Fig. 1B). Based on the above results, it
was hypothesized that songorine is effective for the treatment of
EOC and provides a favorable safety profile. To confirm this
hypothesis, the underlying pharmaco-mechanisms of songorine were
determined in vivo and in vitro.
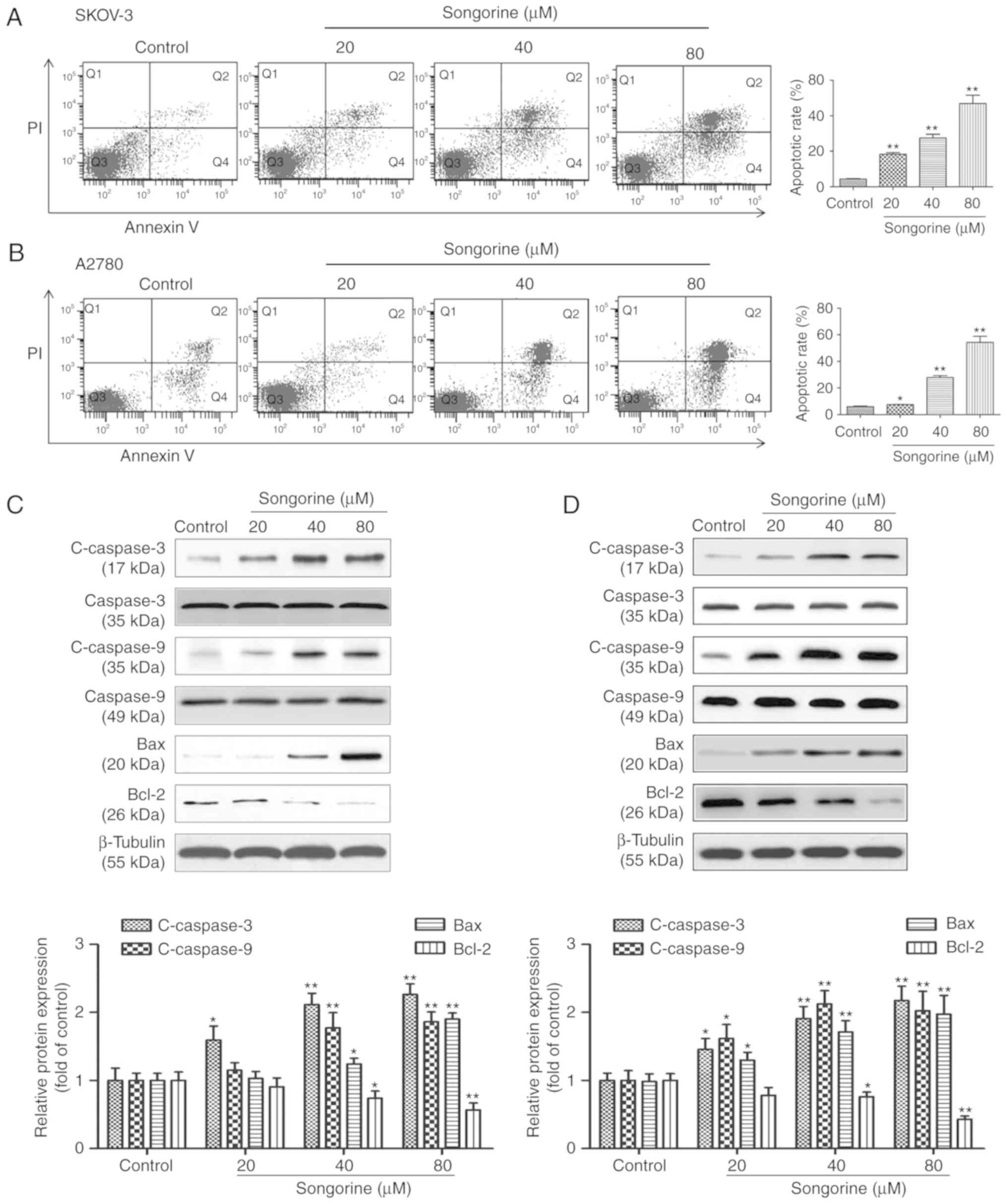
Songorine promotes the apoptosis of
EOC cells via the Bcl-2/Bax signaling pathway
To further verify whether the cytotoxic properties
of songorine were generated by apoptosis, the degree of cell
apoptosis and associated mechanisms were visualized using flow
cytometric analysis and immunoblotting assays, respectively. As
shown in Fig. 2A and B, the flow
cytometry results revealed that treatment of the SKOV-3 and A2780
cells with songorine dose-dependently increased the numbers of
early and late apoptotic cells. The percentages of apoptotic cells
were 18.4, 27.5 and 46.9% in total SKOV-3 cells treated with 20, 40
and 80 µM songorine, respectively. The apoptotic rates of the A2780
cells were 7.5, 27.8 and 54.4%, respectively. Apoptosis is a type
of programmed cell death which is caspase-dependent and mainly
regulated by the Bcl-2/Bax signaling pathway. The results of the
present study demonstrated that songorine treatment decreased the
expression of Bcl-2, but increased the expression levels of
c-caspase-3, c-caspase-9 and Bax in a dose-dependent manner
(Fig. 2C and D). Therefore,
regulation of the Bcl-2/Bax signaling pathway by songorine
represents a strategy to influence the apoptotic mechanism in
SKOV-3 and A2780 cells.
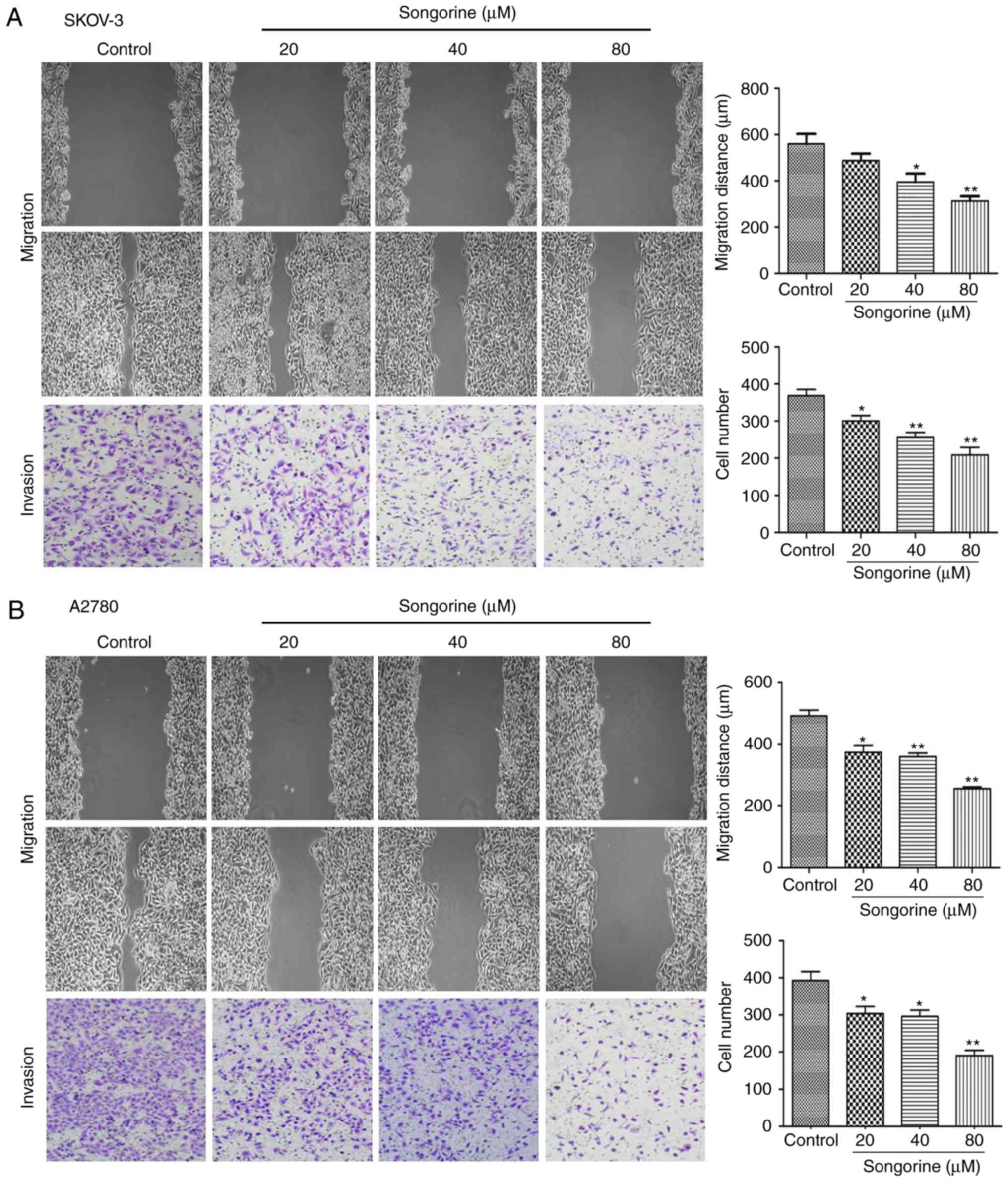
Songorine attenuates the migration and
invasion of EOC cells via the GSK3β/β-catenin signaling
pathway
To investigate the underlying antimetastatic effect
of songorine on EOC cells, wound healing and Transwell chamber
assays were conducted. The results demonstrated that the migration
distances of the SKOV-3 and A2780 cells treated with songorine were
dose-dependently decreased (Fig. 3A and
B). In addition, the number of SKOV3 and A2780 cells that
invaded the Transwell chamber were markedly reduced by songorine
(Fig. 3A and B). Therefore, as
songorine effectively inhibited the migration and invasion
abilities of the EOC cells, the present study subsequently measured
metastasis-associated protein expression by western blot analysis.
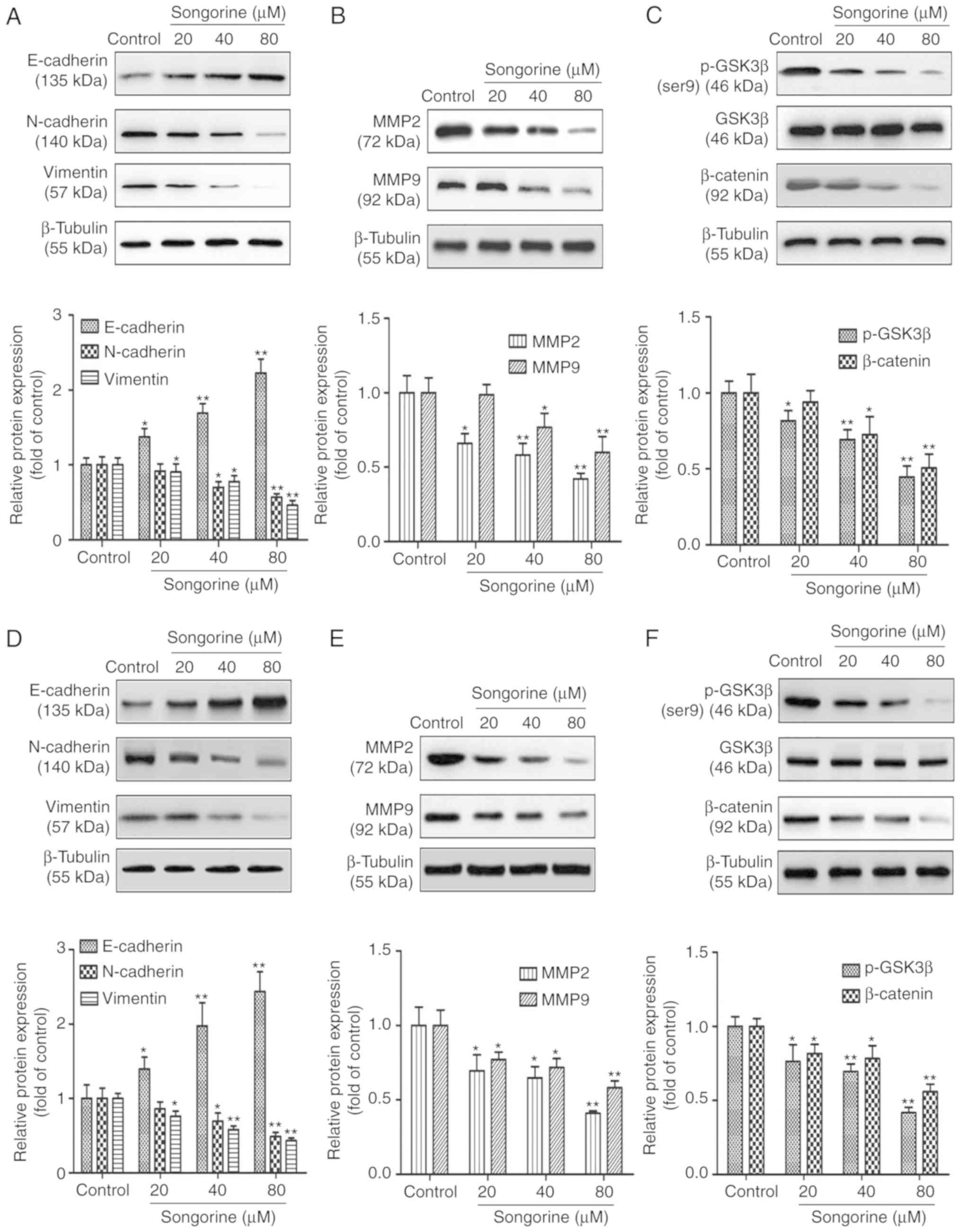
As shown in Fig. 4A and D, the
mesenchymal markers of SKOV3 and A2780 cells, including vimentin
and N-cadherin, were significantly downregulated, whereas the
epithelial marker E-cadherin was markedly upregulated. The results
also indicated that songorine markedly reduced the levels of MMP-2
and MMP-9 in the SKOV3 and A2780 cells (Fig. 4B and E). Taken together, the results
suggested that songorine exerted an antimetastatic effect by
interfering with the EMT process and secretion of MMPs in the two
cancer cell lines. To further elucidate the possible regulatory
mechanisms, the present study investigated the GSK3β/β-catenin
signaling pathway. The results of the western blot analysis
demonstrated that songorine suppressed the phosphorylation of
GSK3β, and thus induced the degradation of β-catenin in a
time-dependent manner (Fig. 4C and
F), indicating the involvement of the GSK3β/β-catenin pathway
in the songorine-induced reduction in cell metastatic abilities.
The results revealed significant differences in cell migration and
invasion, which suggest that the songorine-mediated inhibition of
the EMT processes and secretion of MMPs in EOC cells may be closely
associated with downregulation of the GSK3β/β-catenin signaling
pathway.
Songorine inhibits EMT processes and
the secretion of MMP potentially by targeting GSK3β
To verify whether songorine suppressed the EMT
processes and the secretion of MMPs in EOC cells by targeting
GSK3β, the present study subsequently used SB216763 (a GSK3β
inhibitor) for SKOV-3 cell pretreatment. The results demonstrated
that SB216763 significantly reversed the effects of songorine by
upregulating the expression of β-catenin (Fig. 5). In particular, in the
songorine-treated SKOV-3 cells, SB216763 markedly reduced the
levels of the epithelial marker E-cadherin but increased the levels
of the mesenchymal markers N-cadherin and vimentin, and MMP-2 and
MMP-9 (Fig. 5). These results
suggest that songorine inhibited the migration and invasion
abilities of EOC cells by alleviating EMT processes and levels of
MMPs, potentially via targeting GSK3β.
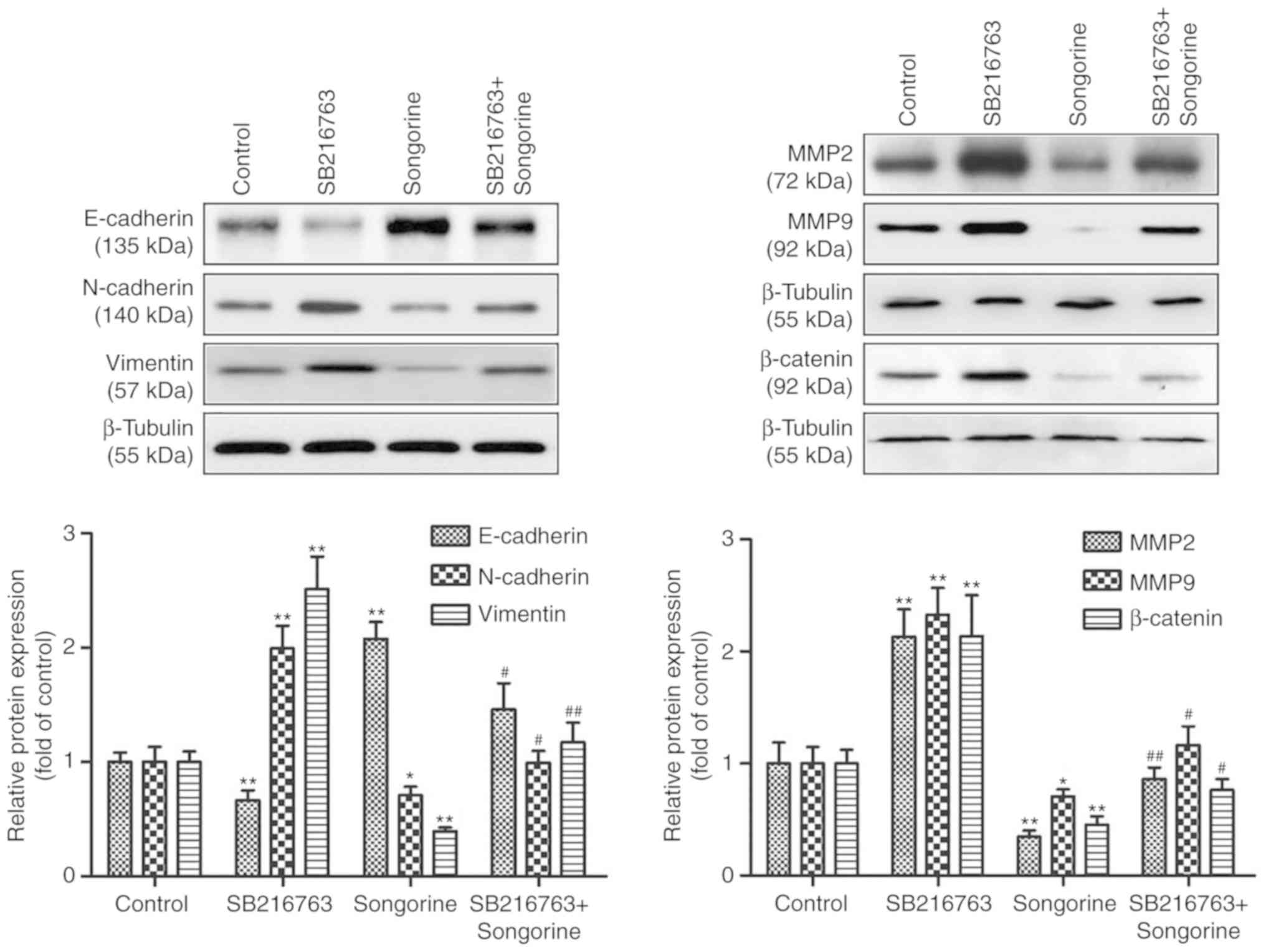 | Figure 5.Songorine represses the EMT process
and secretion of MMPs potentially by targeting GSK3β. The SKOV-3
cells were treated with 10 µM SB216763, with or without 40 µM
songorine, following which cell lysates were subjected to western
blotting and probed for the expression levels of E-cadherin,
N-cadherin, vimentin, MMP-2, MMP-9 and β-catenin; β-tubulin served
as the loading control. Representative blots are presented with the
densitometry results. Values are presented as the mean ± standard
deviation of three independent experiments. *P<0.05 and
**P<0.01, vs. control group; #P<0.05 and
##P<0.01, vs. songorine group. EMT,
epithelial-mesenchymal transition; MMP, matrix metalloproteinase;
GSK3β, glycogen synthase kinase 3β. |
Songorine attenuates tumorigenic
activity in a xenograft model of ovarian cancer
To further investigate the associated
pharmacodynamic activities in vivo, SKOV-3 tumor-bearing
BALB/c nude mice were injected with songorine. Reduced tumor size
and tumor weight were observed following treatment (Fig. 6A). Pathological analysis of the
H&E-stained samples indicated the typical pathological
characteristics of tumors, including large and irregularly shaped
nuclei, closely packed together. However, songorine effectively
improved pathological conditions by inducing cell shrinkage,
fragmentation, sparse arrangement and chromatin disappearance
(Fig. 6B). In addition, the
immunohistochemical assay revealed that songorine at different
concentrations effectively decreased the expression of the
proliferation marker Ki67 in tumor tissues (Fig. 6C). These morphological changes were
indicative of the positive role of songorine in suppressing tumor
growth, and promoting tumor necrosis to varying degrees. As
detected by western blotting, the protein expression levels of
N-cadherin, vimentin, MMP-2, MMP-9, p-GSK3β, β-catenin and Bcl-2
were significantly decreased, whereas the protein expression levels
of E-cadherin, c-caspase-3, c-caspase-9 and Bax were markedly
increased in the songorine-treated nude mice (Fig. 6D-F). Therefore, the results
suggested that songorine inhibited EOC tumorigenic activities in
vivo by reducing cell migration and invasion via the
GSK3β/β-catenin signaling pathway, and inducing cell apoptosis
through the Bcl-2/Bax signaling pathway. Finally, blood biochemical
analysis revealed that all biochemical parameters of the mice
treated with songorine were within ranges similar to those of the
mice in the control group (Fig.
6G). These results suggested that songorine induced no
significant systemic toxicity in vivo at the dose used.
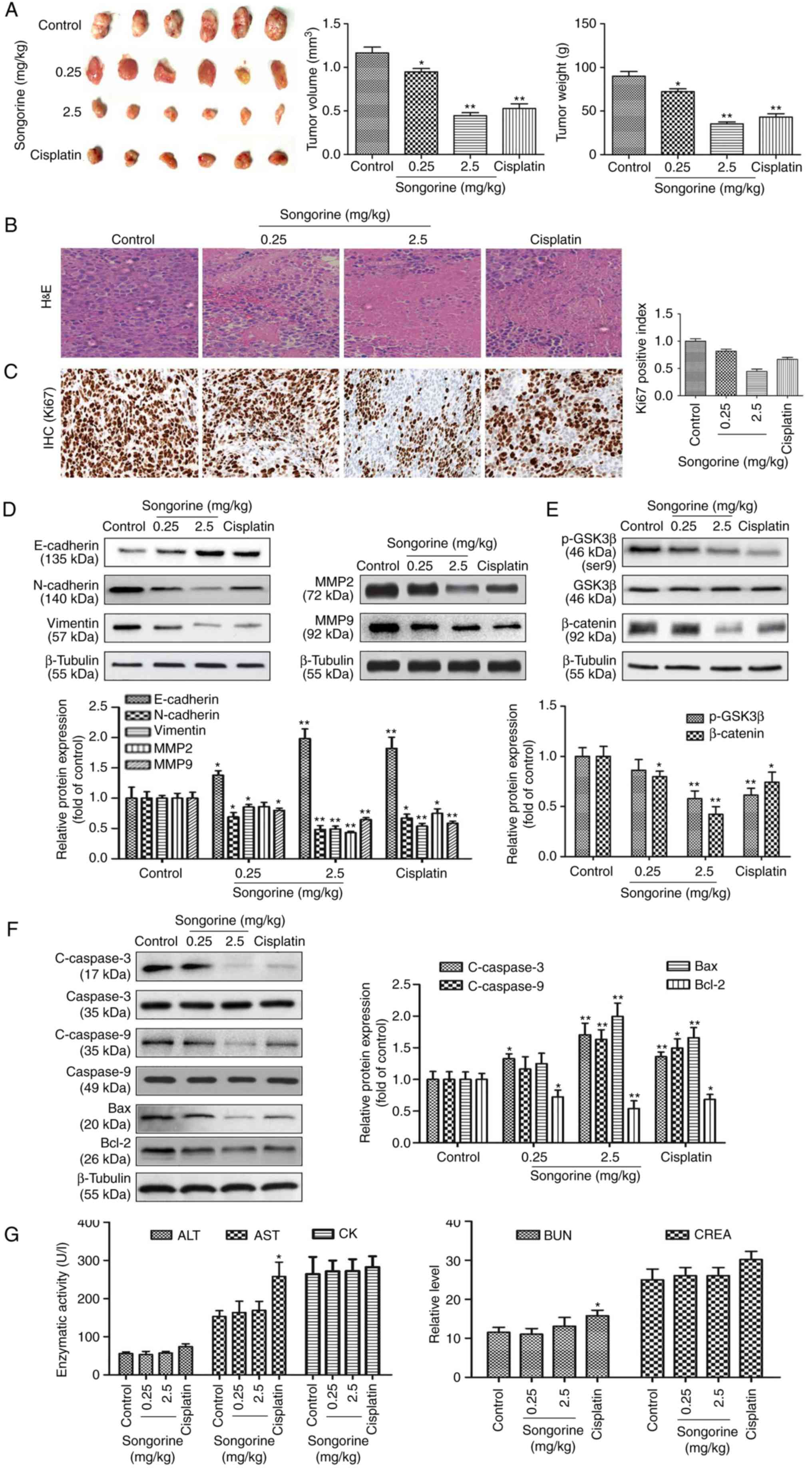 | Figure 6.Songorine attenuates tumorigenic
activity in vivo. (A) Tumors extracted following
subcutaneous growth. The tumor volume and weight were measured in
each group. (B) H&E staining of the tumor tissues of SKOV-3
tumor-bearing BALB/c nude mice following songorine treatment. (C)
An IHC assay was performed to detect the expression of the
proliferation marker Ki67 in tissue sections. (D) Expression levels
of E-cadherin, N-cadherin, vimentin, MMP-2 and MMP-9 in tumor
tissues were analyzed via western blotting with β-tubulin serving
as the internal control. (E) Expression levels of GSK3β, p-GSK3β
and β-catenin in tumor tissues were analyzed via western blotting
with β-tubulin serving as the internal control. (F) Expression
levels of caspase-3, c-caspase-3, caspase-9, c-caspase-9, Bax and
Bcl-2 in tumor tissues were analyzed via western blotting with
β-tubulin serving as the internal control. Representative blots are
presented with the densitometry results. (G) Cardiac, liver and
renal toxicities of songorine were evaluated by measuring the CK,
ALT, AST, BUN and CREA levels. Values are presented as the mean ±
standard deviation of three independent experiments. *P<0.05 and
**P<0.01, vs. control group. MMP, matrix metalloproteinase;
GSK3β, glycogen synthase kinase 3β; p-, phosphorylated; c-,
cleaved; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X; CK,
creatine kinase; ALT, alanine aminotransferase; AST, aspartate
aminotransferase; BUN, blood urea nitrogen; CREA, creatinine;
H&E, hematoxylin and eosin; IHC, immunohistochemistry. |
Discussion
EOC accounts for ~90% of all ovarian tumors, making
it one of the major gynecological malignancies worldwide (22). In the last 50 years, the lack of
improvement in the rates of mortality highlight the requirement for
novel chemotherapy strategies. The Aconitum genus is a
member of the Ranunculaceae family and is important in traditional
medicine in general and in traditional Chinese medicine in
particular (16). Notably,
songorine is one of the main aconitum alkaloids extracted from
Aconitum soongaricum L, with notable pharmacological effects
and promising preclinical potential (19). In the present study, different
concentrations of songorine significantly suppressed the
proliferation of EOC cells, but exerted hypotoxicity towards normal
ovarian epithelial cells. The results demonstrated the potential
therapeutic values of songorine in treating EOC, and the associated
pharmacological mechanisms require further investigation.
In clinical practice, the invasion and migration of
ovarian cancer are the most difficult and important concerns, as
they frequently cause EOC-associated mortality (23). EMT is a necessary physical function
of the mammalian embryonic development process, however, studies
have indicated that EMT induces cancer cell invasion and migration
by accelerating cellular and micro-environmental changes (24–26).
Following EMT, cancer cells lose normal epithelial polarity and
gain mesenchymal traits, which is accompanied by the downregulation
of epithelial markers, including E-cadherin, and upregulation of
interstitial markers, including N-cadherin and vimentin (24,27,28).
Subsequently, the cell-cell connections and cell-matrix contacts
are destroyed, and the malignant cells migrate and invade the
surrounding matrix to exacerbate tumor progression and invasion
(29). In the present study, when
EOC cells were exposed to songorine, their relative migration and
invasion abilities were markedly reduced. In addition, songorine
induced the upregulation of E-cadherin, which was accompanied by
the downregulation of N-cadherin and vimentin. Accordingly, these
results indicated that songorine suppressed EOC cell migration and
invasion abilities by attenuating EMT. By contrast, MMP-2 and MMP-9
are expressed in cancer cells during malignant invasion and
migration (30). They are important
proteolytic enzymes for the degradation of extracellular matrix,
and can induce cancer cell penetration into the basement membrane
(31). A previous study
demonstrated that reductions in the levels of MMP-2 and MMP-9 in
ovarian cancer alleviated cell metastatic activity (32). This is consistent with the results
of the present study, as MMP-2 and MMP-9 were significantly
decreased when cell metastasis was suppressed in songorine-treated
EOC cells. Collectively, these results suggest that songorine has
the potential to repress EOC cell migration and invasion by
restraining the EMT progress and secretion of MMPs.
Previous studies have demonstrated that the
Wnt/β-catenin signaling pathway serves a crucial role in cellular
differentiation, migration, innovation and proliferation.
Additionally, stimulation of the Wnt/β-catenin signaling pathway is
closely associated with cancer development (33). Following activation of the
Wnt/β-catenin signaling pathway during neoplastic transformation,
cell migration and invasion are subsequently exacerbated (34). Increased GSK3β protein stability may
accelerate the degradation of β-catenin (35) and interfere with the occurrence of
EMT, which indirectly causes the upregulation of E-cadherin, and
the downregulation of N-cadherin and vimentin (36,37).
Furthermore, the degradation of β-catenin results in the
downregulation of MMP secretion, which in turn causes the
inhibition of tumor invasion and migration (38). Subsequently, western blotting
analysis confirmed that the expression levels of p-GSK3β and
β-catenin were downregulated by songorine. Therefore, it was
hypothesized that inhibition of the GSK3β/β-catenin signaling
pathway induced by songorine may contribute to the disturbance in
malignant cell migration and invasion. To investigate whether the
songorine-mediated alteration of EOC cell migration and invasion is
associated with the phosphorylation of GSK3β, the present study
performed measurements following SB216763 (GSK3β inhibitor)
treatment. As the xenograft models were established using SKOV-3
cells in follow-up research. SB216763 was provisionally used for
incubation with SKOV-3 cells. As a result, SB216763 significantly
increased the levels of p-GSK3β, β-catenin, N-cadherin, Vimentin,
MMP-2 and MMP-9, and decreased the expression of E-cadherin in EOC
cells. Specifically, GSK3β inhibitor treatment restored the
songorine-induced regulative effects. Taken together, it was
concluded that songorine inhibits EOC cell invasion and migration
by targeting GSK3β. However, SB216763 acted as a competitive
inhibitor of GSK3β, it partially verified the songorine as a
potential inhibitor of GSK3β. To confirm GSK3β as the target of
songorine, gene-silencing and biolayer interferometry technologies
may be applied in further investigations.
An increasing body of evidence has revealed that
apoptosis is an essential target of cancer chemotherapy (39,40).
Apoptosis, a type of programmed cell death, serves a crucial role
in the normal physiological activity of the body (41). During the apoptotic process, the
caspase protein family is mainly involved in the cleavage and
activation of each other. Active caspase-9 triggers the activation
of downstream ‘executioner’ caspase-3, which facilitates cell death
(42). Bcl-2 is an integral
membrane protein that is mainly located on the outer membrane of
mitochondria and induces the release of caspase-3/-9. Proteins in
this family exhibit either pro-apoptotic or anti-apoptotic
activities, with the Bcl-2/Bax ratio serving as a marker to
determine cell susceptibility to apoptosis (43). Notably, the results of the present
study showed that songorine increased the expression levels of Bax,
caspase-3 and caspase-9, but downregulated the expression of Bcl-2.
Furthermore, the results revealed that the number of EOC cells
undergoing apoptosis was significantly increased by songorine.
These novel findings indicated that songorine induced Bax/Bcl-2
hyperfunctioning in EOC cells, which suggested that songorine
promoted cell apoptosis via Bcl-2/Bax signaling.
Upon confirming that songorine significantly
inhibits EOC cell migration and invasion, and induces cell
apoptosis in vitro, the present study further examined the
therapeutic effects of songorine in vivo. For the xenografts
in nude mice, SKOV3 cells were inoculated to form subcutaneous
transplant tumors, as these cells germinate as moderately
well-differentiated adenocarcinoma similar to primary ovarian
cancer (44). Treatment with
songorine in the xenografted nude mice led to notably reduced tumor
size, and it also caused the inactivity or regression of tumor
cells. These results indicate that songorine serves a potential
role in the treatment of EOC. Following further analysis, the
promotion of Bax/Bcl-2 hyperfunctioning was accompanied by
increased levels of c-caspase-3 and c-caspase-9 following songorine
treatment. In addition, the expression of E-cadherin in the tumors
was upregulated, whereas the expression levels of p-GSK3β,
β-catenin, MMP-2, MMP-9, N-cadherin and vimentin were downregulated
following songorine treatment. Taken together, the results of the
present study highlight the positive effects of songorine in
treating EOC by influencing cell survival, apoptosis, migration and
invasion. Additionally, hematologic toxicity analysis revealed the
hypotoxicity of songorine in vivo, which support the
potential clinical value of songorine as a novel antitumor agent
for EOC in the future.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate the potential role of
songorine in treating EOC. Treatment with songorine suppressed the
migration and invasion properties of EOC cells, and the underlying
mechanism may be associated with inhibition of the EMT process and
secretion of MMP via the GSK3β/β-catenin signaling pathway. The
results also indicated that the Bcl2/Bax signaling pathway may be a
critical target of songorine-mediated apoptosis in anti-EOC
activities. Taken together, these results are valuable for
understanding the pharmacodynamic actions of songorine in treating
EOC. However, this mechanism requires additional elucidation and
confirmation in future studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medicine and
Health Guidance Program Project of Qingdao (grant no.
2015-WJZD015).
Availability of data and materials
The datasets used in the present study are available
from the corresponding author upon reasonable request.
Authors' contributions
HZ and YW conceived and designed the study, and
wrote the manuscript. HZ, RD and PZ performed the experiments. RD
reviewed and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research.
Ethics approval and consent to
participate
All experimental protocols in the present study were
approved by the Institutional Animal Care and Use Committee of
Qingdao University (Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Willmott LJ and Fruehauf JP: Targeted
therapy in ovarian cancer. J Oncol. 2010:7404722010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gadducci A, Sartori E, Maggino T, Zola P,
Landoni F, Fanucchi A, Palai N, Alessi C, Ferrero AM, Cosio S, et
al: Analysis of failures after negative second-look in patients
with advanced ovarian cancer: An italian multicenter study. Gynecol
Oncol. 68:150–155. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kleppe M, Wang T, Van Gorp T, Slangen BF,
Kruse AJ and Kruitwagen RF: Lymph node metastasis in stages I and
II ovarian cancer: A review. Gynecol Oncol. 123:610–614. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takai M, Terai Y, Kawaguchi H, Ashihara K,
Fujiwara S, Tanaka T, Tsunetoh S, Tanaka Y, Sasaki H, Kanemura M,
et al: The EMT (epithelial-mesenchymal-transition)-related protein
expression indicates the metastatic status and prognosis in
patients with ovarian cancer. J Ovarian Res. 7:762014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bagnato A and Rosano L:
Epithelial-mesenchymal transition in ovarian cancer progression: A
crucial role for the endothelin axis. Cells Tissues Organs.
185:85–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao B, Lin D and Zhang X, Zhang M and
Zhang X: TTF1, in the form of nanoparticles, inhibits angiogenesis,
cell migration and cell invasion in vitro and in vivo in human
hepatoma through STAT3 regulation. Molecules. 21:E15072016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Fátima A, Terra BS, da Silva CM, da
Silva DL, Araujo DP, da Silva Neto L and Nascimento de Aquino RA:
From nature to market: Examples of natural products that became
drugs. Recent Pat Biotechnol. 8:76–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsubaki M, Takeda T, Ogawa N, Sakamoto K,
Shimaoka H, Fujita A, Itoh T, Imano M, Ishizaka T, Satou T, et al:
Overexpression of survivin via activation of ERK1/2, Akt, and NF-κB
plays a central role in vincristine resistance in multiple myeloma
cells. Leuk Res. 39:445–452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amirkia V and Heinrich M: Alkaloids as
drug leads-a predictive structural and biodiversity-based analysis.
Phytochem Lett. 10:xlviii–liii. 2014. View Article : Google Scholar
|
|
12
|
Khan H: Alkaloids: Potential therapeutic
modality in the management of asthma. J Ayurvedic Herb Med.
1:32015.
|
|
13
|
Khattak S and Khan H: Anti-cancer
potential of phyto-alkaloids: A prospective review. Curr Cancer
Ther Rev. 12:66–75. 2016. View Article : Google Scholar
|
|
14
|
Marya and Khan H: Anti-inflammatory
potential of alkaloids as a promising therapeutic modality. Lett
Drug Des Discov. 14:240–249. 2017. View Article : Google Scholar
|
|
15
|
Rehman S and Khan H: Advances in
antioxidant potential of natural alkaloids. Curr Bio Comp.
13:101–108. 2017. View Article : Google Scholar
|
|
16
|
Nyirimigabo E, Xu Y, Li Y, Wang Y,
Agyemang K and Zhang Y: A review on phytochemistry, pharmacology
and toxicology studies of aconitum. J Pharm Pharmacol. 67:1–19.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ameri A: Effects of the aconitum alkaloid
songorine on synaptic transmission and paired-pulse facilitation of
CA1 pyramidal cells in rat hippocampal slices. Br J Pharmacol.
125:461–468. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okamoto T, Natsume M, Iitaka Y, Yoshino A
and Amiya T: The structure of lucidusculine and the absolute
configuration of songorine. Chem Pharm Bull. 13:1270–1272. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khan H, Nabavi SM, Sureda A, Mehterov N,
Gulei D, Berindan-Neagoe I, Taniguchi H and Atanasov AG:
Therapeutic potential of songorine, a diterpenoid alkaloid of the
genus aconitum. Eur J Med Chem. 153:29–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun JR, Qiu ZJ, Wang DH, Zhang B and Yuan
JF: Anti-tumor activity of 3-acetylaconitine and songorine from
Aconitum szechenyianum gay. Fine Chemicals. 35:1163–1169.
2018.
|
|
21
|
Nesterova YV, Povet'eva TN, Suslov NI,
Shults EE, Ziuz'kov GN, Aksinenko SG, Afanas'eva OG, Krapivin AV
and Kharina TG: Anxiolytic activity of diterpene alkaloid
songorine. Bull Exp Biol Med. 159:620–622. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grassi ML, Palma CS, Thomé CH, Lanfredi
GP, Poersch A and Faça VM: Proteomic analysis of ovarian cancer
cells during epithelial-mesenchymal transition (EMT) induced by
epidermal growth factor (EGF) reveals mechanisms of cell cycle
control. J Proteomics. 151:2–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bernaudo S, Salem M, Qi X, Zhou W, Zhang
C, Yang W, Rosman D, Deng Z, Ye G, Yang BB, et al: Cyclin g2
inhibits epithelial-to-mesenchymal transition by disrupting
Wnt/β-catenin signalling. Oncogene. 35:48282016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gelfand R, Vernet D, Bruhn K, Vadgama J
and Gonzalez-Cadavid NF: Long-term exposure of MCF-12A normal human
breast epithelial cells to ethanol induces epithelial mesenchymal
transition and oncogenic features. Int J Oncol. 48:2399–2414. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Powell CD, Paullin TR, Aoisa C, Menzie CJ,
Ubaldini A and Westerheide SD: The heat shock transcription factor
HSF1 induces ovarian cancer epithelial-mesenchymal transition in a
3D spheroid growth model. PLoS One. 11:e01683892016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bian Y, Chang X, Liao Y, Wang J, Li Y,
Wang K and Wan X: Promotion of epithelial-mesenchymal transition by
Frizzled2 is involved in the metastasis of endometrial cancer.
Oncol Rep. 36:803–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garg M: Epithelial, mesenchymal and hybrid
epithelial/mesenchymal phenotypes and their clinical relevance in
cancer metastasis. Expert Rev Mol Med. 19:e32017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang LL, Wang Z, Cao CJ, Ke ZF, Wang F,
Wang R, Luo CQ, Lu X and Wang LT: AEG-1 associates with metastasis
in papillary thyroid cancer through upregulation of MMP2/9. Int J
Oncol. 51:812–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng TC, Din ZH, Su JH, Wu YJ and Liu CI:
Sinulariolide suppresses cell migration and invasion by inhibiting
matrix metalloproteinase-2/-9 and urokinase through the
PI3K/AKT/mTOR signaling pathway in human bladder cancer cells. Mar
Drugs. 15:E2382017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pei S, Yang X, Wang H, Zhang H, Zhou B,
Zhang D and Lin D: Plantamajoside, a potential anti-tumor herbal
medicine inhibits breast cancer growth and pulmonary metastasis by
decreasing the activity of matrix metalloproteinase-9 and −2. BMC
Cancer. 15:9652015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Serman L, Nikuseva Martic T, Serman A and
Vranic S: Epigenetic alterations of the Wnt signaling pathway in
cancer: A mini review. Bosn J Basic Med Sci. 14:191–194. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma Y, Zhu B, Liu X, Yu H, Yong L, Liu X,
Shao J and Liu Z: Inhibition of oleandrin on the proliferation show
and invasion of osteosarcoma cells in vitro by suppressing
Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res. 34:1152015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luu HH, Zhang R, Haydon RC, Rayburn E,
Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W and He TC:
Wnt/beta-catenin signaling pathway as a novel cancer drug target.
Curr Cancer Drug Targets. 4:653–671. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gilles C, Polette M, Mestdagt M,
Nawrocki-Raby B, Ruggeri P, Birembaut P and Foidart JM:
Transactivation of vimentin by beta-catenin in human breast cancer
cells. Cancer Res. 63:2658–2664. 2003.PubMed/NCBI
|
|
38
|
Wu B, Crampton SP and Hughes CC: Wnt
signaling induces matrix metalloproteinase expression and regulates
T cell transmigration. Immunity. 26:227–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Doughan AK and Dikalov SI: Mitochondrial
redox cycling of mitoquinone leads to superoxide production and
cellular apoptosis. Antioxid Redox Signal. 9:1825–1836. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Ma H, Lu Y, Tan BJ, Xu L, Lawal TO,
Mahady GB and Liu D: Menoprogen, a TCM herbal formula for
menopause, increases endogenous E2 in an aged rat model of
menopause by reducing ovarian granulosa cell apoptosis. Biomed Res
Int. 2016:25746372016.PubMed/NCBI
|
|
41
|
Day TW, Huang S and Safa AR: c-FLIP
knockdown induces ligand-independent DR5-, FADD-, caspase-8-, and
caspase-9-dependent apoptosis in breast cancer cells. Biochem
Pharmacol. 76:1694–1704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bratton SB and Salvesen GS: Regulation of
the Apaf-1-caspase-9 apoptosome. J Cell Sci. 123:3209–3214. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gu A, Jie Y, Yao Q, Zhang Y and Mingyan E:
Slug is associated with tumor metastasis and angiogenesis in
ovarian cancer. Reprod Sci. 24:291–299. 2016. View Article : Google Scholar : PubMed/NCBI
|