Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors globally, with >1 million new cases diagnosed
annually (1). The 5-year survival
rate for patients at the early stage of CRC is 90.3%, but the
survival rate drops to 50–70.4% once metastasis occurs (2). Various genetic alterations have been
reported to promote the initiation and progression of CRC; however,
the molecular mechanisms leading to CRC development and progression
remain unclear (3). Exploring
molecular and genetic changes in CRC and the underlying oncogenic
mechanisms has attracted increasing attention in tumor research.
Genomic instability is widely regarded as the hallmark of cancer,
and is considered to decrease the viability of cells, permit
genetic changes and lead to cancer cells evading immune
surveillance (4,5). Genomic instability has various causes,
of which chromosomal instability (CIN) and microsatellite
instability have received the most focus (6). In CRC, CIN is the most common form of
genomic instability, which occurs in nearly 80–85% of patients with
CRC (7). Current research in the
field is focused on elucidating the molecular basis of CIN,
including the possible roles of defects in the spindle checkpoint
and other regulators of mitosis. CIN has been reported to be
crucial in precancerous development as well as cancer evolution
(8). It is also associated with the
prognosis of CRC (9). A recent study
demonstrated that CIN affected the efficacy of chemotherapy and
immunotherapy in CRC (10); however,
the mechanisms underlying CIN in CRC are yet to be elucidated.
Therefore, exploring the molecular mechanisms of CIN for the
development of novel tumor markers and therapeutic targets is a
high priority.
The structural maintenance of chromosomes 1 (SMC1)
gene is a member of the SMC family that serves critical roles in
organizing and stabilizing chromosomal segregation during mitosis,
and is considered to be a component of the signaling network
involved in the maintenance of genome stability (11). The SMC1 protein is an
evolutionarily-conserved multifunctional protein known for its role
in sister chromatid cohesion (12),
DNA recombination and repair (13),
and cell cycle checkpoint activation by ionizing radiation
(14), ultraviolet light and other
genotoxic agents (15). SMC1 forms a
heterodimeric cohesion complex with SMC3 that encircles and
mediates sister chromatid cohesion DNA replication in S phase until
chromosome separation, which occurs in anaphase (16). Cohesin-associated genes have been
reported to be potential drivers of tumor genomic instability;
progression and mutations in various subunits of cohesin have been
identified in sarcoma, melanoma, colon and glioblastoma tumors
(17). Kitagawa et al
(18) demonstrated that the
expression of SMC1 was significantly increased in triple-negative
breast cancer, and SMC1 binding with BRCA1 is proposed to be
important for genomic stability, regulating tumor development and
progression; however, the significance and the underlying
mechanisms responsible for the aberrant expression of SMC1 in CRC
remain unknown.
In the present study, it was demonstrated that SMC1
was significantly upregulated in CRC cell lines. The role of
knocking down or overexpressing SMC1 was tested by cell
proliferation and apoptosis assays in CRC cells. The present
results provided evidence that abnormal SMC1 expression may serve a
direct role in carcinoma progression and could be used for
predicting therapeutic outcomes of CRC.
Materials and methods
Cell culture
The colon cancer cell lines, SW480, SW620 and
HCT116, the human normal colonic epithelial cells NCM460, and 293T
cells were obtained from The Cell Bank of Shanghai Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences. The
cells were routinely maintained in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin, and incubated at
37°C in an atmosphere of 95% air and 5% CO2.
Cell viability assay
Cell viability was measured by an MTT assay. Cells
were seeded in 96-well plates at a density of 3,000-5,000
cells/well and cultured overnight. For the assay, 20 µl MTT
solution (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added to each
well and incubated for a further 2–4 h at 37°C. Then, the medium
was discarded and 100 µl DMSO was added to dissolve the resulting
formazan crystals. For the colorimetric analysis, the optical
density (OD) value at 490 nm was measured using a Multiskan
Spectrum UV/visible Microplate Reader (Thermo Fisher Scientific,
Inc.).
Lentiviral vector construction and
transfection
The SMC1 short hairpin RNA (shRNA; shSMC1) and the
negative control shRNA (shCont) were synthesized (Shanghai GeneChem
Co., Ltd.): shSMC1 sequence, 5′-TAGGAGGTTCTTCTGAGTACA-3′; shCont
sequence, 5′-GGAGGTTCTTCTGAGTACA-3′. They were inserted into a
pGCSIL-GFP vector (Shanghai GeneChem Co., Ltd.) using AgeI
and EcoRI restriction sites, and then transfected into 293T
cells (30–50% confluence) together with lentiviral helper plasmid
pHelper1.0 and pHelper2.0 using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Recombinant lentiviruses containing shSMC1
or the shCont were prepared and titrated to 1×107 TU/ml
for transfection. SW620 cells (60% confluence) were plated and
infected with lentiviruses expressing shSMC1/shCont for 48 h using
Lipofectamine 2000, followed by puromycin selection (6 µg/ml).
Fluorescence microscopy (Eclipse E600; Nikon Corporation), reverse
transcription-quantitative PCR (RT-qPCR) and western blot analyses
were performed to verify knockdown efficiency, and cells were
allocated for different assays.
For SMC1 overexpression, a pTango-SMC1 plasmid was
purchased from Synbio Technologies LLC. Then, primers targeting
SMC1 fragments for PCR amplification were designed, and NheI
and SwaI restriction sites were added to the 5′ and 3′ ends
of the primers, respectively. The primer sequences for PCR were
forward, 5′-AGGCTAGCGGAGCAGCAGCAGATTGAG-3′ and reverse,
5′-GGATTTAAATTCTCTTCTTCCATCCGTTCTTC-3′, and PCR amplification was
performed using pTango-SMC1 plasmid (200 ng) as a PCR template and
Taq DNA polymerase (Vazyme Biotech) under the following
conditions: 94°C for 3 min, then 34 cycles of 94°C for 15 sec, 56°C
for 30 sec and 72°C for 90 sec, with a final elongation at 72°C for
5 min. The amplification products were visualized by 2% agarose gel
electrophoresis and purified using a gel extraction kit (Omega
Bio-Tek, Inc.), then digested by NheI and SwaI
restriction enzymes (Fermentas Inc.) and cloned by T4 DNA ligase
(Takara Biotechnology Co., Ltd.) into a pCDH-puro lentiviral vector
(Shanghai GeneChem Co., Ltd.). Then, the pCDH-puro-SMC1 plasmid was
packaged into lentivirus via the same method as described for the
shRNA vectors. SW680 cells (~50% confluence) were plated and
infected with pCDH-puro (negative control) or pCDH-puro-SMC1
(titrated to 1×107 TU/ml) for 48 h, followed by
puromycin selection (6 µg/ml).
Small interfering RNA (siRNA) and
plasmid construction and transfection
siRNAs [siSMC1-1 sequence:
5′-CCAACATTGATGAGATCTATA-3′; siSMC1-2 sequence:
5′-CGGCGTATTGATGAAATCAAT-3′; si-negative control (si-NC) sequence:
5′-TTCTCCGAACGTGTCACGT-3′] were provided by Shanghai GenePharma
Co., Ltd. After reaching 30% confluence, SW620 cells were
transfected with siRNAs at a final concentration of 50 nM, and
Lipofectamine 2000 reagent according to the manufacturer's
protocol. Knockdown efficiency was tested via western blotting
after 48 h transfection.
An pcDNA3.1-NF-κB p65 plasmid was obtained from
Synbio Technologies LLC. The plasmid vectors were prepared for
transfection using DNA Midiprep kits (E.Z.N.A Endo-Free Plasmid
Mini kit II; Omega Bio-Tek, Inc.). After reaching >70-80%
confluence, the SW620 cells were transfected with 2 µg plasmid and
Lipofectamine 2000 reagent according to the manufacturer's
protocol. Empty pcDNA3.1 vector was used as the negative
control.
RT-qPCR
Total RNA was extracted from cultured cells with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. RT reactions were
performed using a Super cDNA First-Strand Synthesis kit (Beijing
CoWin Biotech Co., Ltd.). The 15 µl reaction mixtures were
incubated in a 96-well Thermal Cycler (Applied Biosystems; Thermo
Fisher Scientific, Inc.) for 40 min at 42°C and 5 min at 85°C. The
resulting cDNA was used for RT-PCR using SYBR Green Master PCR Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) in triplicates
and a TP800 Thermal Cycler Dice™ Real Time System (Takara
Biotechnology Co., Ltd.). The primer sequences for qPCR for SMC1
were forward, 5′-GGAGCAGCAGCAGATTGAG-3′ and reverse,
5′-TCTCTTCTTCCATCCGTTCTTC-3′. Primers for the control GAPDH were
forward, 5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-ACCCTGTTGCTGTAGCCAAA-3′. The 25 µl reactions were incubated at
94°C for 1 min, followed by 30 cycles at 94°C for 30 sec, annealing
at 50°C for 30 sec, and elongation at 72°C for 45 sec, followed by
a final elongation step at 72°C for 5 min. All PCR reactions were
run in triplicate. The relative mRNA levels of SMC1 were compared
with those of GAPDH, which was amplified as an internal control and
calculated using the comparative 2−ΔΔCq method (19).
Western blot analysis
The expression levels of various proteins were
detected via western blot analysis, as previously described
(20–22). Additionally, cytoplasmic and nuclear
extracts were isolated with a nuclear extraction kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. Western blotting assays were conducted using antibodies
against SMC1 (1:1,000; cat. no. ab9262; Abcam), GAPDH (1:5,000;
cat. no. AP0063; Bioworld Technology, Inc.), caspase-3 (1:1,000;
cat. no. 9662; Cell Signaling Technology, Inc.), β-actin (1:5,000;
cat. no. A5441; Sigma-Aldrich; Merck KGaA), cleaved caspase-3
(1:1,000; cat. no. 9664; Cell Signaling Technology, Inc.), Bcl-2
(1:1,000; cat. no. 4223; Cell Signaling Technology, Inc.), Bax
(1:1,000; cat. no. 5023; Cell Signaling Technology, Inc.),
inhibitor of nuclear factor-κB subunit β (IKKβ; 1:1,000; cat. no.
ab124957; Abcam), phosphorylated (p)IKKβ (Ser177; 1:1,000; cat. no.
ab194528; Abcam), inhibitor of nuclear factor-κB subunit α (IκBα;
1:1,000; cat. no. ab32518; Abcam), pIκBα (Ser32; 1:1,000; cat. no.
ab92700; Abcam), NF-κB p65 (1:10,000; cat. no. ab16502; Abcam),
pNF-κB p65 (Ser536; 1:1,000; cat. no. ab86299; Abcam) and Lamin B1
(1:10,000; cat. no. ab133741; Abcam). Horseradish peroxidase
(HRP)-conjugated anti-rabbit IgG (1:5,000; cat. no. 7074; Cell
Signaling Technology, Inc.) or anti-mouse IgG (1:5,000; cat. no.
7076; Cell Signaling Technology, Inc.) was used as secondary
antibody.
Colony formation assay
Colony formation assays were performed to evaluate
the long-term proliferative potential of SW620 and SW480 cells.
SW620 cells were transfected with LV-shCont or LV-shSMC1 and SW480
cells were transfected with LV-SMC1 or empty overexpression vector
(LV-NC). Cells (800 cells/well) were seeded into 6-well plates and
incubated at 37°C with 5% CO2 for 10 days. The cells
were fixed with 4% paraformaldehyde at 4°C for 15 min and stained
using Giemsa at room temperature for 30 min. Then, the number of
stained colonies that contained ≥50 cells was manually counted
under a light microscope (magnification, ×4).
Cell viability assay
Cell viability was measured via an MTT assay. To
examine the effects of SMC1 knockdown on SW620 cell viability,
SW620 cells infected with LV-Cont or LV-shSMC1 were plated into
96-well plates at a density of 2×103 cells/well and
cultured overnight at 37°C. Thereafter, 20 µl MTT solution (5
mg/ml; Sigma-Aldrich; Merck KGaA) was added to each well to
incubate for 2–4 h at 37°C. Then, the medium was discarded and 200
µl DMSO was added to dissolve the resulting formazan crystals. For
colorimetric analysis, the OD value at 490 nm was measured by a
Multiskan Spectrum UV/visible Microplate Reader (Thermo Fisher
Scientific, Inc.). Data from three independent experiments were
analyzed.
Flow cytometric analysis of cell
cycle
The effects of SMC1 knockdown in SW620 cells and
SMC1 overexpression in SW480 cells on the cell cycle were
determined by flow cytometry analysis. For cell cycle analysis,
transfected cells were harvested and fixed with ice-cold methanol
at 4°C for a minimum of 30 min, and then washed twice with ice-cold
0.01 M PBS (pH 7.2). Then, the cells were resuspended in PBS
containing propidium iodide (PI) solution (Sigma-Aldrich; Merck
KGaA) at a final concentration of 10 µg/ml at room temperature for
20 min. The cell samples were subjected to flow cytometric analysis
on a BD FACScan flow cytometer (BD Biosciences). Cells in each
phase of the cell cycle were analyzed using FlowJo 10.0 (FlowJo
LLC). The ratio of cells in the G0/G1, S and M phases of the cell
cycle was determined by their DNA content.
Cell invasion and migration assay
Cell invasive and migratory abilities were detected
with a Transwell system using Transwell plates from Costar
(Corning, Inc.) with 8.0-µm diameter pores. For invasion assays,
plates were coated with 40 µl diluted Matrigel before assays. In
total, 1×104 cells in 300 µl serum-free medium were
added into the upper chamber of Transwell plates, and 10%
FBS-containing medium was added to the lower chamber. Following
incubation for 24 or 48 h (for the migration and invasion assays,
respectively), the number of cells that had migrated were counted
after removing the cells on the upper side of the filter. The cells
were fixed with 4% paraformaldehyde at 4°C for 20 min and stained
using hexamethylpararosaniline at room temperature for 15 min. The
number of cells were counted using an IX70 inverted fluorescence
microscope (magnification, ×400; Olympus Corporation), and cells
were scored in four randomly selected fields per sample. All the
experiments were performed in triplicate.
Tumorigenicity assay in vivo
Male BALB/c nude mice (4 weeks old; 17–20 g; n=24)
were ordered from Beijing Vital River Laboratory Animal Technology,
Co., Ltd., and housed in a specific pathogen-free environment (12-h
light/dark cycle at 25°C and 60% relative humidity; the mice were
provided with food and water ad libitum in the animal
research center of Nanjing Medical University). Mice were randomly
divided into two groups (6 mice/group), and the previously
established LV-SMC1 SW480 cells (1×106) or LV-shSMC1
SW620 cells (1×106) were suspended in 0.1 ml serum-free
DMEM and subcutaneously injected into the right axillary fossa of
each nude mouse for the experimental group. The same vector control
cells (LV-NC and LV-shCont, respectively) were used as the blank
control. When palpable tumors arose, the tumor sizes were measured
using vernier calipers every 3 days. The mice were monitored daily
for health and weighed twice weekly. After 21 days (the diameter of
the largest tumor in the control mice reached ~1.0 cm), mice were
euthanized by CO2 asphyxiation with a 25% volume/min gas
displacement flow rate until all animals stopped breathing, then
the tumors were dissected and weighed. The tumor size was
calculated using the formula V = (width2xlength/2). The
tumors were fixed at 4°C for 24 h in 4% paraformaldehyde and were
evaluated by immunohistochemistry, and apoptosis in
paraffin-embedded tumor sections was detected using a TUNEL assay
kit, according to the manufacturer's protocol. All animal
experiments were performed following the guidelines of The
Institutional Animal Care and Use Committee of The Affiliated
Huai'an No. 1 People's Hospital of Nanjing Medical University,
which approved the present study (approval no. IACUC-1810008).
Cell apoptosis assay
SW620 cells infected with LV-shControl or LV-shSMC1
and SW480 cells infected with LV-NC or LV-SMC1 (1×105)
were cultured, and an Alexa Fluor® 488 Annexin V/Dead
Cell Apoptosis kit (Invitrogen; Thermo Fisher Scientific, Inc.) was
used for apoptosis analysis, according to the manufacturer's
protocol. Cells were collected and resuspended in staining solution
at room temperature for 15 min in the dark, and the samples were
then subjected to flow cytometric analysis and analyzed using
FlowJo 10.0. The percentage of early + late apoptotic cells was
analyzed to calculate the apoptotic rate.
TUNEL analysis
An In Situ Apoptosis Detection kit (Abcam)
was used to detect apoptosis in tumor tissues. Briefly, sections (4
µm) were deparaffinized in xylene and hydrated using a graded
alcohol series. Sections were treated with 0.3%
H2O2 peroxidase for 15 min. Then, apoptotic
cells were labeled with terminal deoxynucleotidyl transferase at
4°C overnight, followed by incubation with streptavidin-HRP
conjugate at room temperature for 2 h. The signal was detected
using 3,3′-diaminobenzidine (DAB) substrate at room temperature for
15 min, and the nuclei were stained by hematoxylin for 15 sec at
room temperature. The number of apoptotic cells was counted using
an IX70 inverted fluorescence microscope (magnification, ×200) and
cells were scored in eight randomly selected fields per sample.
Patients and human tissue
specimens
A total of 51 samples (tumors and adjacent normal
tissues) were collected from January 2010 to March 2013 at the
Affiliated Huai'an No. 1 People's Hospital of Nanjing Medical
University. The CRC diagnosis was confirmed by at least two
pathologists. All patients were classified according to a TNM
staging system using the Union for International Cancer Control
(UICC) (23). A total of 51 patients
with CRC were enrolled (35 male and 16 female) with a median age of
63 years (range, 37–87 years). None of the 51 patients received any
preoperative anticancer treatments. Informed consent was obtained
from every patient, and the use of the specimens was approved by
the Ethics Committee of The Affiliated Huai'an No. 1 People's
Hospital of Nanjing Medical University.
Immunohistochemistry analysis
Human tissues were fixed in 4% paraformaldehyde at
4°C for 48 h and then embedded in paraffin. Sections (4 µm) were
deparaffinized in xylene and hydrated using a graded alcohol
series. Antigen retrieval was performed in antigen unmasking
solution at 100°C for 15 min. Then, sections were treated with 0.3%
H2O2 peroxidase for 15 min and blocked with
5% BSA (Sigma-Aldrich; Merck KGaA) for 1 h at room temperature.
Sections were incubated with an anti-SMC1 antibody (1:200; cat. no.
ab9262; Abcam) overnight at 4°C and then incubated with
SignalStain® HRP-conjugated Rabbit IHC detection reagent
(1:5,000; cat. no. 8114; Cell Signaling Technology, Inc.) for 1 h
at 37°C. The immunoreactive cells were visualized using DAB, and
the nuclei were stained by hematoxylin for 15 sec at room
temperature. The cells were counted using an IX70 inverted
fluorescence microscope (magnification, ×200) and cells were scored
in eight randomly selected fields per sample. The staining
intensity was graded as follows: i) Negative - (0–15% positive);
ii) positive + (16–50%); and iii) positive ++ (51–100%). Patients
with ‘negative’ expression were classed as exhibiting low
expression, whereas those with ‘positive’ expression were classed
as exhibiting high expression.
Mouse tumor tissues were embedded in paraffin, and
immunohistochemistry was performed as previously described for
human tissues. Following 0.3% H2O2 treatment,
the sections (4 µm) were incubated with primary antibodies against
SMC1 (1:200; cat. no. ab9262; Abcam) and NF-κB p65 (1:2,000; cat.
no. ab16502; Abcam), followed by incubation with HRP-conjugated
detection reagent (1:200; cat. no. 8114; Cell Signaling Technology,
Inc.) for 1 h at 37°C. The number of positive cells were counted
using an IX70 inverted fluorescence microscope (magnification,
×400), and cells were scored in eight randomly selected fields per
sample.
Statistical analysis
All data in this study are presented as means ± SD.
All statistical analyses were performed using SPSS 17.0 software
(SPSS, Inc.). Pearson's χ2 test was used to compare
qualitative variables, and comparisons among different groups were
performed using one-way ANOVA followed by Bonferroni test.
Student's t-test was used to analyze the statistical significance
between two groups. Kaplan-Meier survival analysis was used to
assess the association between SMC1 expression and CRC prognosis.
The Wilcoxon signed-rank test was used to analyze differences in
expression between CRC tissue and adjacent normal tissue. P<0.05
was considered to indicate a statistically significant difference.
All experiments were performed at least three independent
times.
Results
SMC1 is upregulated in CRC cell
lines
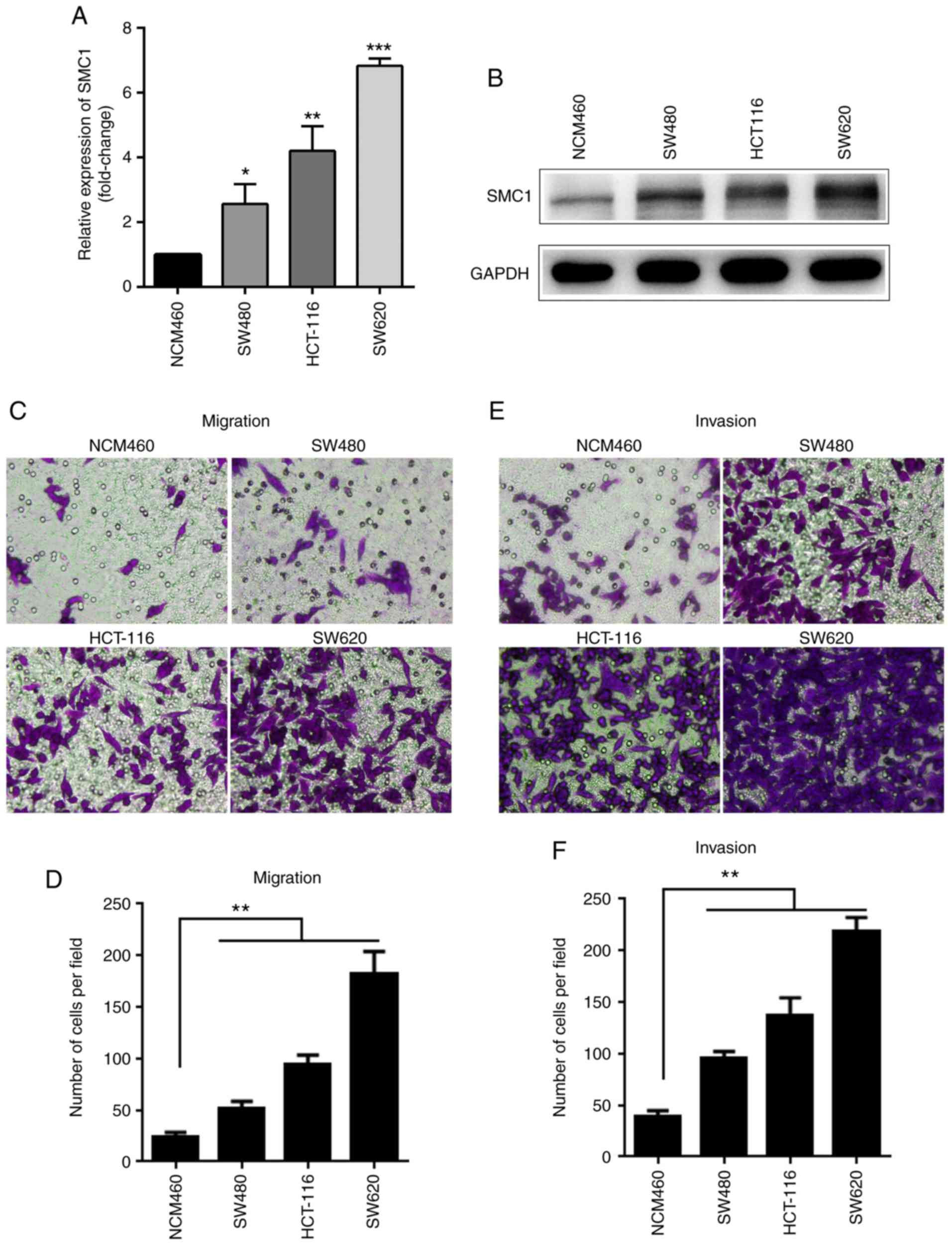
RT-qPCR analysis was used to detect the mRNA
expression of SMC1 in colon cancer cell lines and normal cells. As
presented in Fig. 1A, the expression
of SMC1 in the CRC cell lines (SW480, HCT-116 and SW620) was
2.56±0.50, 4.21±0.62 and 6.83±0.19 times higher, respectively,
compared with in the normal NCM460 colonic epithelial cells
(P<0.05). Western blot analysis also showed that protein
expression of SMC1 in the CRC cell lines (SW480, HCT-116 and SW620)
was higher than NCM460 cells (Fig.
1B); the highest SMC1 expression levels for both mRNA and
protein were observed in the SW620 cells among the three CRC cells
(Fig. 1A and B). SW620 cells also
exhibited the highest migratory and invasive abilities in Transwell
assays compared with the other cancer and normal cells (Fig. 1C-F).
Knockdown of SMC1 inhibits malignant phenotypes and
induces apoptosis in SW620 cells. As is shown in Fig 1A-B, the SMC1 expression is highest in
SW620 cells. To determine whether SMC1 expression is associated
with malignant phenotypes of SW620 cells, LV-shSMC1 was used to
knockdown SMC1 expression. As presented in Fig. 2A, the efficacy of transfection was
≤90%. After 48 h transfection with LV-shSMC1, RT-qPCR and western
blot analysis were used to detect SMC1 expression. The present data
revealed that SMC1 expression was not significantly different
between the LV-shCont and blank groups (P>0.05; Fig. 2B). In the LV-shSMC1 group, the mRNA
and protein expression levels of SMC1 were 0.26±0.06 and 0.45±0.01,
respectively, compared with the LV-shCont group, (P<0.05;
Fig. 2B and C). To determine the
potential involvement of SMC1 in the proliferation of CRC cells,
MTT and colony formation assays were conducted. As presented in
Fig. 2D and E, cell viability and
colony formation were markedly reduced in the LV-shSMC1 group
compared with the LV-shCont and blank groups (P<0.05). Transwell
assays revealed that the numbers of migrating cells were 93±12,
190±9 and 169±10 in the LV-shSMC1, LV-shCont and blank groups,
respectively; meanwhile, Matrigel invasion assays revealed that the
numbers of invading cells were 251±33, 419±25 and 448±28 in the
LV-shSMC1, LV-shCont and blank groups, respectively (P<0.01;
Fig. 2F and G); indicating that
knocking down SMC1 reduced the invasive and migratory abilities of
SW620 cells.
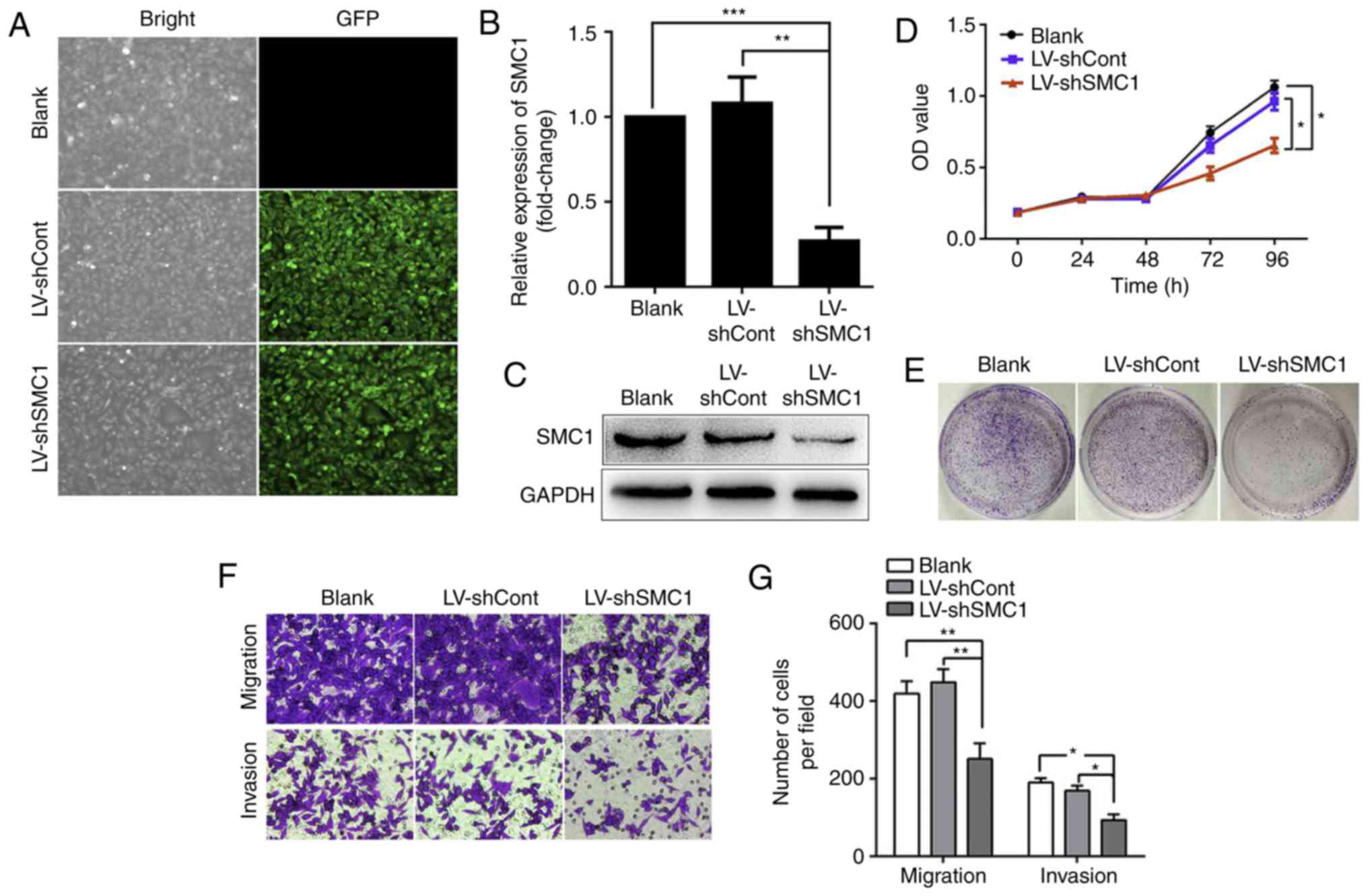 | Figure 2.Knockdown of SMC1 inhibits malignant
phenotypes and induces apoptosis in SW620 cells. (A) LV infection
efficiency in SW620 cells. (B) Reverse transcription-quantitative
PCR analysis of SMC1 mRNA levels in SW620 cells. (C) Western blot
analysis of SMC1 protein levels in SW620 cells. (D) In vitro
growth curves of SW620 cells transfected with lentivirus. Cell
viability was determined by MTT assays, and the OD was detected at
450 nm. (E) Colony formation of SW620 cells following lentiviral
transduction. (F and G) Migratory and invasive abilities of SW620
cells following SMC1 knockdown were determined by Transwell assays.
Cells were counted in 4 random fields (magnification, ×400). (H)
Knockdown of SMC1 induced cell cycle arrest in the G2/M phase in
SW620 cells. (I) Quantitative analysis of the cell cycle
distribution of SW620 cells. (J and K) Apoptosis of SW620 cells
following SMC1 knockdown was measured via flow cytometry. (L and M)
Western blot analysis of the expression of apoptosis-associated
molecules, including caspase-3, cleaved caspase-3 and Bcl-2/Bax.
Data are presented as the mean ± SD of three independent
experiments. *P<0.05, **P<0.01, ***P<0.001. Cont, control;
LV, lentivirus; OD, optical density; PI, propidium iodide; sh,
short hairpin (RNA); SMC1, structural maintenance of chromosomes
1. |
In a cell cycle assay, as presented in Fig. 2H and I, the percentage of S phase
cells (33.73±3.21%) was reduced, whereas that of G2/M phase cells
(17.81±1.99%) was increased in the LV-shSMC1 group compared with
the LV-shCont and blank groups (P<0.05). In addition, an Annexin
V-PI assay revealed that the apoptotic rate was significantly
increased in the LV-shSMC1 group (18.9±2.9%) compared with in the
LV-shCont and blank groups (5.89±1.42 and 5.43±2.01%, respectively;
P<0.01; Fig. 2J and K). Western
blot analysis revealed that the expression levels of cleaved
caspase-3 and Bax were increased, whereas the expression of Bcl-2
was reduced in LV-shSMC1 SW620 cells compared with the control
(Fig. 2L and M).
Different siRNAs (si-NC, siSMC1-1 and siSMC1-2) were
used to knockdown SMC1 expression in SW620 cells (Fig. S1A). As with the lentiviral knockdown,
siRNA-mediated downregulation of SMC1 significantly inhibited SW620
cell proliferation, as determined by an MTT assay (Fig. S1B), suppressed cell migration and
invasion, observed with Transwell and Matrigel assays (Fig. S1C and D), and promoted apoptosis
(Fig. S1E and F).
SMC1 overexpression promotes the proliferation,
migration and invasion of SW480 cells. To further
investigate the effects of SMC1 expression on malignant phenotypes
in CRC cells, an SMC1 overexpression lentivirus, LV-SMC1, was
transfected into SW480 cells, which exhibited the lowest SMC1
expression out of the three CRC cell lines tested in the present
study. As hypothesized, the mRNA and protein expression levels of
SMC1 were notably increased in the LV-SMC1 group after transfection
of pCDH-puro-SMC1 plasmids compared with the control and blank
groups (P<0.01; Fig. 3A and B). An
MTT assay revealed significantly increased viability of SW480 cells
following overexpression of SMC1 compared with the vector control
cells (P<0.05; Fig. 3C). A colony
formation assay also showed markedly increased colony numbers in
the LV-SMC1 group (Fig. 3D). In
addition, a Transwell assay showed that the number of migratory
cells (251±3) was increased in the LV-SMC1 compared with the LV-NC
and blank groups (111±9 and 122±16, respectively); meanwhile, a
Matrigel assay demonstrated significantly increased numbers of
invasive cells (109±16) in the LV-SMC1 group compared with the
LV-NC and blank groups (56±12 and 64±11, respectively; P<0.01;
Fig. 3E and F). A cell cycle assay
indicated a significantly increased percentage of S phase cells
(33.83±2.11%) in the LV-SMC1 group compared with in both the LV-NC
and blank groups (28.14±5.35, 29.41±4.12%); with a lower ratio of
G2/M phase cells (9.63±1.79%) compared with the blank and negative
controls (17.59±1.38 and 16.36±3.42%, respectively; P<0.05;
Fig. 3G and H). Of note, there was no
difference in the rate of apoptosis between the SMC1-overexpressing
and control SW480 cells (Fig. 3I and
J). These results further demonstrated that overexpression of
SMC1 promoted the proliferation, migration and invasion of CRC
cells.
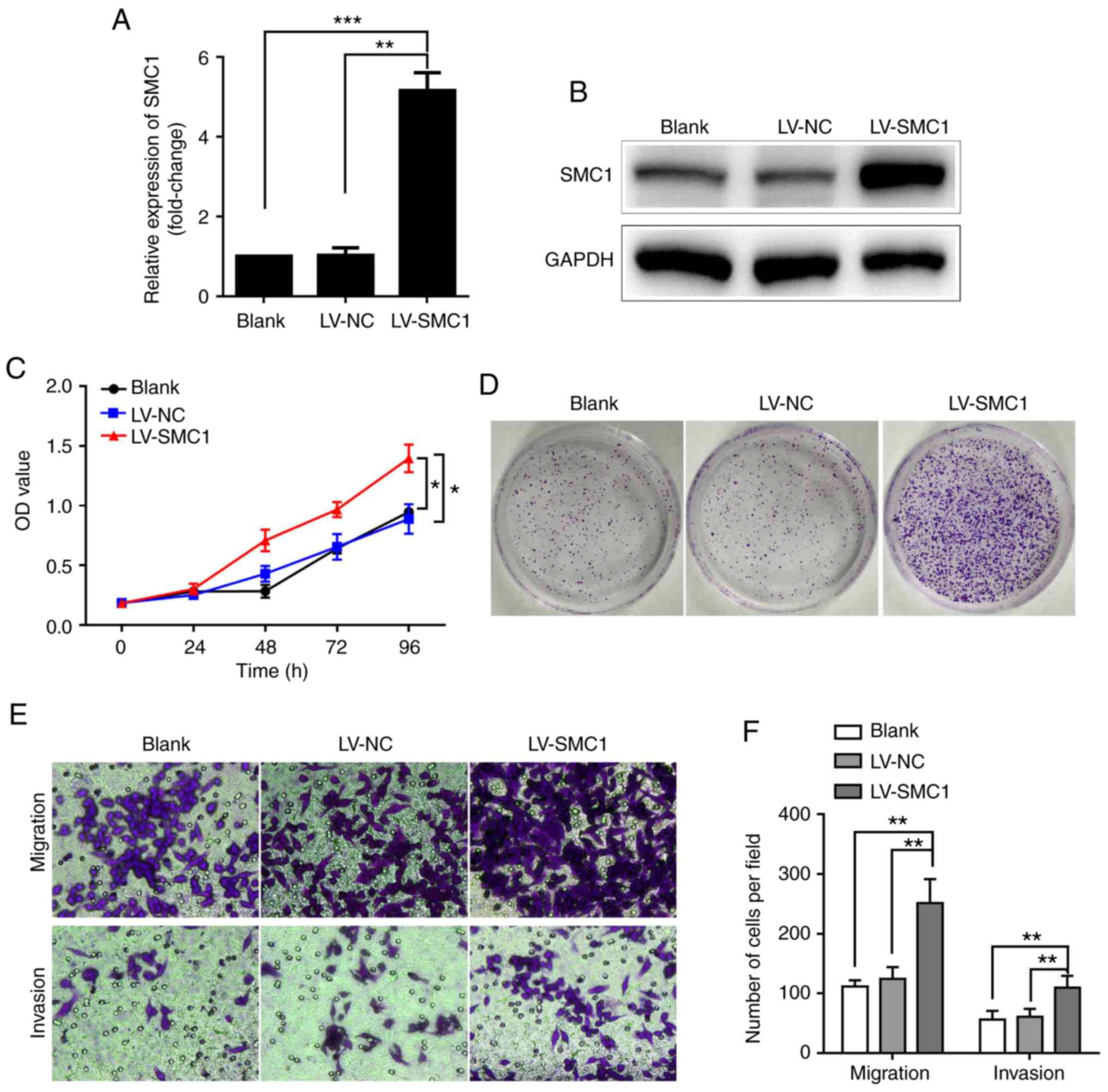 | Figure 3.Upregulation of SMC1 promotes the
proliferation, migration and invasion of SW480 cells. (A) SW480
cell lines were transfected with LV-SMC1, and SMC1 mRNA levels were
detected via reverse transcription-quantitative PCR analysis. (B)
Western blot analysis of SMC1 protein levels in SW480 cells. (C)
In vitro growth curves of SW480 cells following
overexpression of SMC1. Cell viability was determined using an MTT
assay. (D) Colony formation of SW480 cells following overexpression
of SMC1. (E and F) Migratory and invasive abilities of SW480 cells
as determined by Transwell assays. Cells were counted in 4 random
fields (magnification, ×400). (G and H) Cell cycle distribution of
SW480 cells following overexpression of SMC1. (I and J) Apoptosis
of SW480 cells following SMC1 overexpression as determined via flow
cytometry. Data are presented as the mean ± SD of three independent
experiments. *P<0.05, **P<0.01, ***P<0.001. LV,
lentivirus; NC, negative control; NS, not significant; OD, optical
density; PI, propidium iodide; SMC1, structural maintenance of
chromosomes 1. |
SMC1 promotes CRC cell proliferation
and apoptosis in vivo
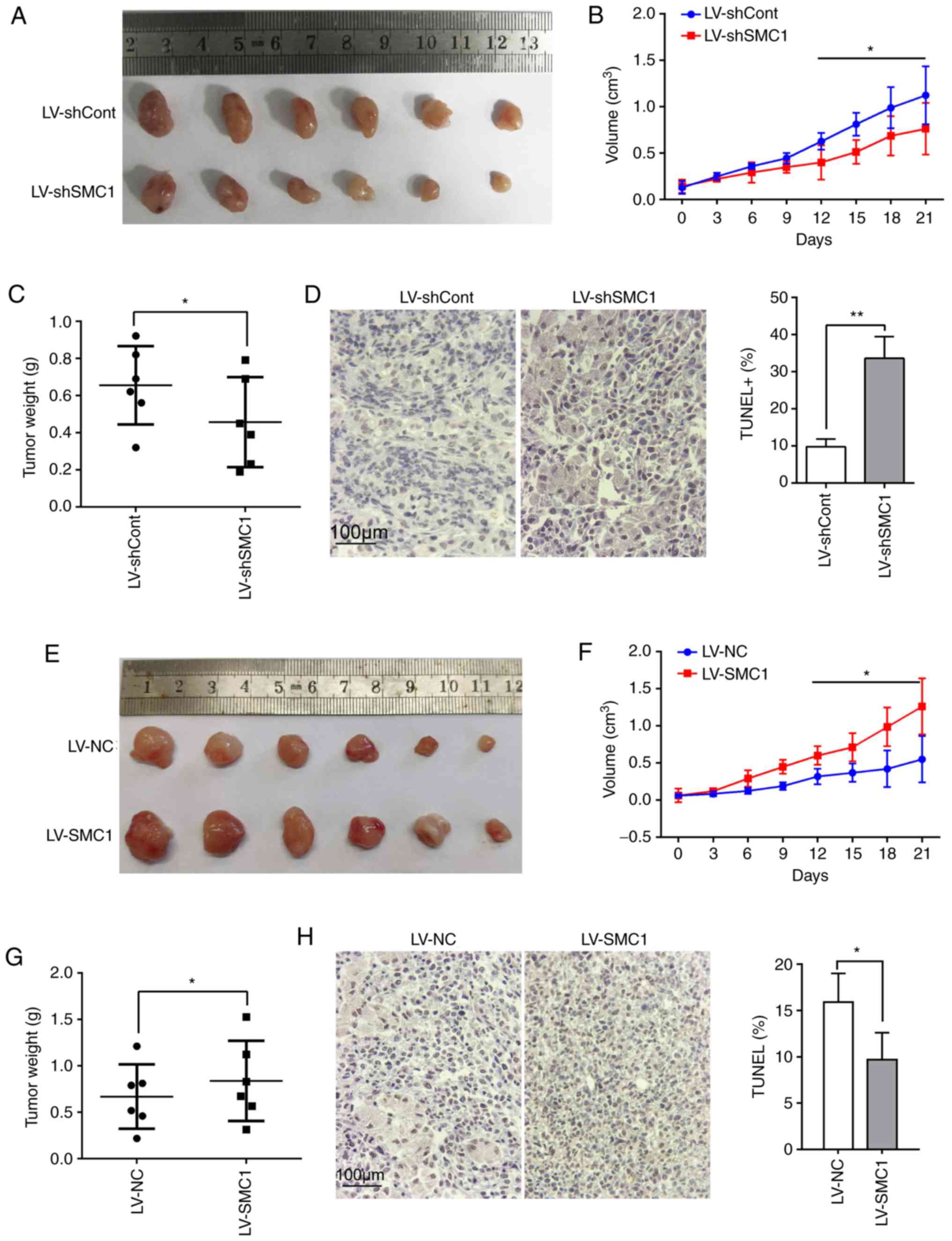
To investigate whether SMC1 has functional effects
on CRC progression in vivo, a SW620 CRC cell xenograft mouse
model was established. It was observed that tumor growth in the
LV-shSMC1-infected group was reduced compared with the LV-shCont
group at 21 days after subcutaneous inoculation (Fig. S2A; Fig. 4A
and B). As presented in Fig. 4C,
the weight of the tumor mass from the LV-shSMC1 group was
significantly decreased compared with the LV-shCont group
(P<0.05). A TUNEL assay revealed that apoptotic cell numbers
were significantly higher in the LV-shSMC1 group (33.59±5.90)
compared with in the LV-shCont group (9.80±2.10; P<0.05;
Fig. 4D).
To support these observations, another xenograft
mouse model was established using SMC1-overexpressing SW480 CRC
cells. It was revealed that tumor growth in mice inoculated with
LV-SMC1-infected SW480 CRC cells was increased compared with those
inoculated with LV-NC-infected SW480 cells (Fig. S2B; Fig. 4E
and F). The weight of the tumor mass from the LV-SMC1 group was
also significantly increased compared with the LV-NC group
(P<0.05; Fig. 4G). The rate of
apoptosis in tumor tissue was lower in the LV-SMC1 group
(9.7±2.90%) compared with the LV-NC group [15.90±3.10%; P<0.05;
Fig. 4H]. Collectively, these results
indicated that SMC1 promoted CRC cell proliferation and inhibited
apoptosis in vivo.
SMC1 is associated with NF-κB
signaling in CRC cells
NF-κB is a nuclear transcription factor that
regulates the expression of a large number of genes that are
critical for the regulation of various biological processes,
including cell apoptosis, viral replication, tumorigenesis,
inflammation and various autoimmune diseases (24). To determine whether NF-κB is involved
in the SMC1-mediated regulation of proliferation and apoptosis in
CRC cells, the phosphorylation of IKKβ (at Ser177), IκBα (at Ser32)
and NF-κB p65 (at Ser536) were determined by western blotting after
knocking down SMC1. A marked decrease in the phosphorylation of
IKKβ and IκBα was observed (Fig. 5A).
Conversely, an increased level of phosphorylation of NF-κB p65 was
observed (Fig. 5A). In addition, cell
localization of NF-κB p65 altered after knocking down SMC1; the
protein accumulated from the cytoplasm to the nucleus (Fig. 5B). Similarly, it was identified that
knocking down SMC1 promoted NF-κB p65 translocation from the
cytoplasm to the nucleus in vivo (Fig. 5C and D). Subsequently, the expression
of p65 was ectopically reversed in SMC1-knocked down SW620 cells.
It was revealed that in p65-overexpressing SW620 cells (Fig. 5E), the reduced cell proliferation
(Fig. 5F) and increased apoptosis
(Fig. 5G and H) induced by knockdown
of SMC1 were partly reversed. This suggested that the effects of
SMC1 on the proliferation and apoptosis of CRC cells are mediated
at least partially via the NF-κB signaling pathway.
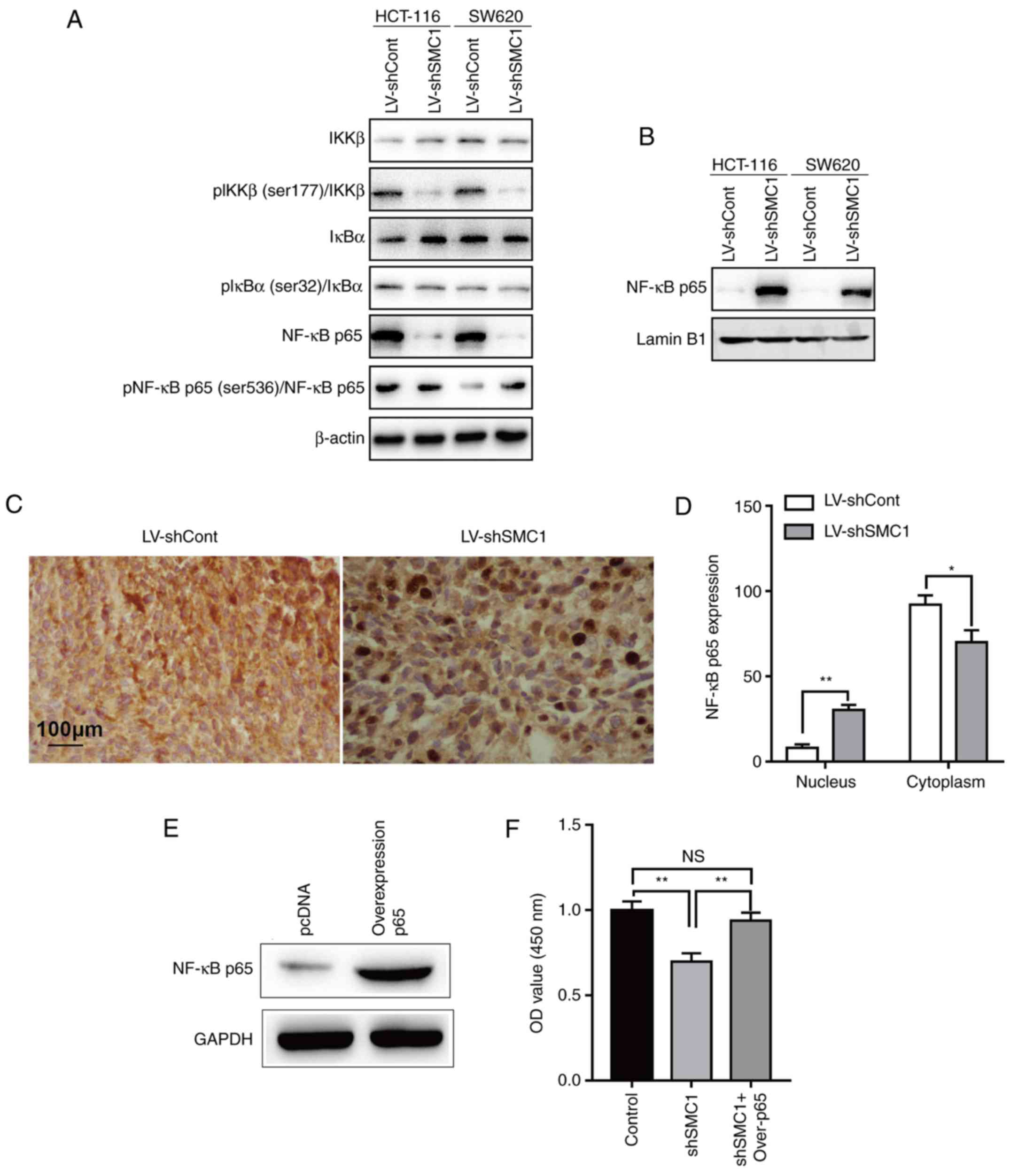 | Figure 5.Effects of SMC1 expression on the
activity of the NF-κB signaling pathway in colorectal cancer cells.
(A) Protein expression was examined in cytoplasmic cells lysates
via western blotting. (B) Protein expression was examined in
nuclear cells lysates by western blotting. (C and D) NF-κB p65
expression in xenograft tissue was determined via
immunohistochemistry analysis. (E) SW620 cells were transfected
with p65-overexpression plasmids, and NF-κB p65 protein levels were
detected via western blotting. (F) Viability of SW620 cells at 96 h
after knockdown of SMC1 and overexpression of p65. Cell viability
was determined using an MTT assay. (G and H) Apoptosis was
determined via flow cytometry in SW620 cells at 48 h following SMC1
knockdown and p65 overexpression. *P<0.05, **P<0.01. Cont,
control; IκBα, inhibitor of nuclear factor-κB subunit α; IKKβ,
inhibitor of nuclear factor-κB subunit β; LV, lentivirus; NC,
negative control; NS, not significant; OD, optical density;
Over-p65, overexpression of p65; p, phosphorylated; PI, propidium
iodide; sh, short hairpin (RNA); SMC1, structural maintenance of
chromosomes 1. |
SMC1 expression and pathologic
parameters in patients with CRC
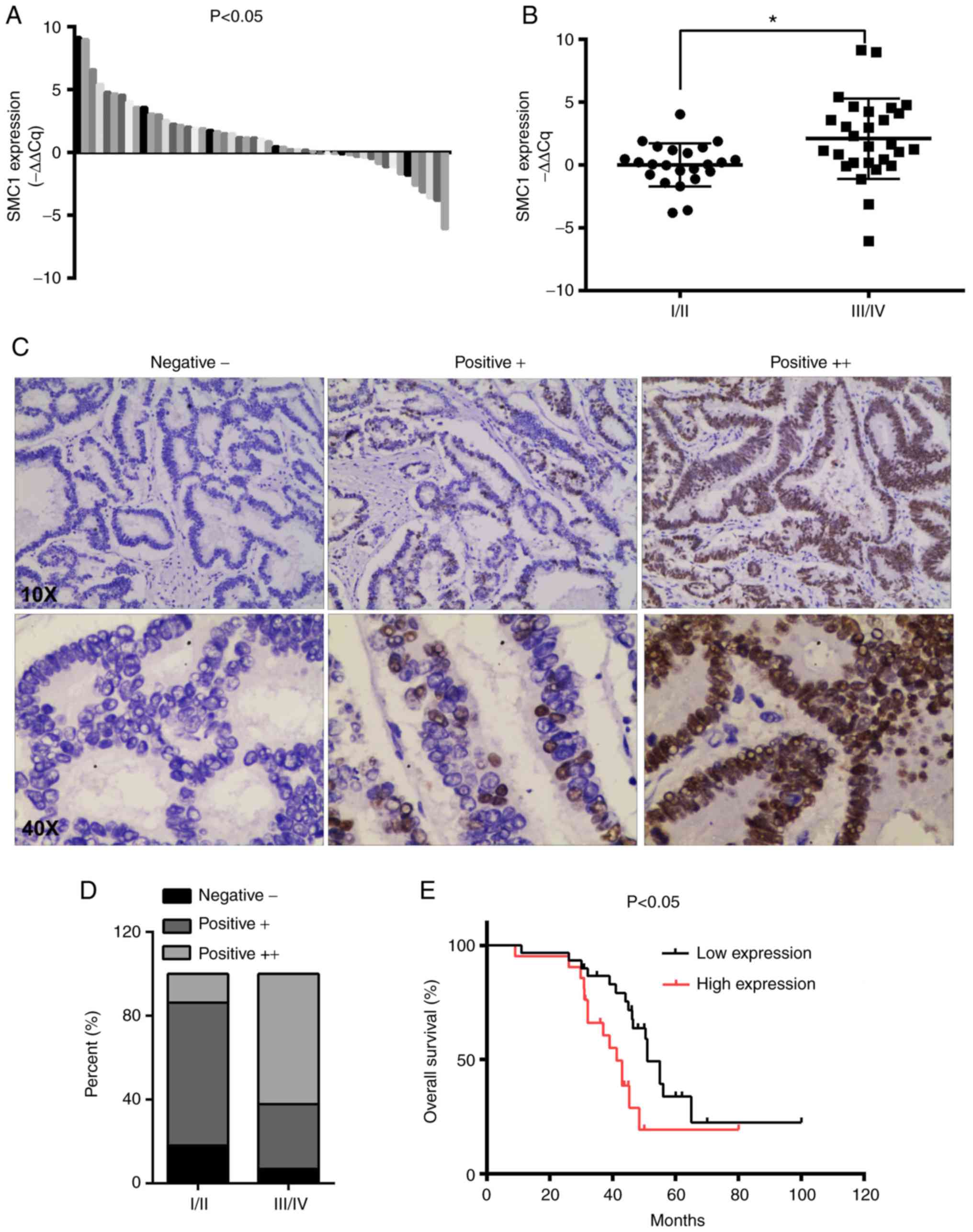
Potential associations between SMC1 expression
levels in CRC tumor tissues and clinicopathological characteristics
were analyzed in patients. The collected clinical data indicated
that SMC1 was upregulated in CRC tissues compared with matched
adjacent normal tissues, as determined via RT-qPCR analysis
(Fig. 6A). Furthermore, increased
expression of SMC1 was positively associated with advanced TNM
stage (P<0.05; Fig. 6B). The
associations between SMC1 expression and clinicopathological
parameters are presented in Table I.
Higher expression of SMC1 was significantly associated with
advanced TNM stage (P=0.007), primary tumor size (P=0.035) and
lymph node metastasis (P=0.017). In the present study, no
significant associations were observed between SMC1 expression and
sex, age, tumor location, tumor differentiation and vascular
invasion (P>0.05). Representative images of SMC1 expression in
tissue are presented in Fig. 6C.
There were more strongly positive (++) SMC1 cells in advanced-stage
(III/IV) compared with early-stage tissue (I/II; Fig. 6D). As presented in Fig. 6E, high SMC1 expression was
significantly associated with worse overall survival in patients
with CRC (P<0.05; Fig. 6E).
 | Table I.Associations between SMC1 expression
in cancer tissue and clinicopathological characteristics in
patients with colorectal cancer. |
Table I.
Associations between SMC1 expression
in cancer tissue and clinicopathological characteristics in
patients with colorectal cancer.
|
|
| SMC1 expression [N
(%)] |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | N | Low expression | High
expression | χ2 | P-value |
|---|
| Total number | 51 | 22 | 29 |
|
|
| Sex |
|
|
| 0.448 | 0.503 |
|
Male | 35 | 14 (63.64) | 21 (72.41) |
|
|
|
Female | 16 | 8 (36.36) | 8 (27.59) |
|
|
| Age (years) |
|
|
| 0.015 | 0.903 |
|
<65 | 25 | 11 (50.00) | 14 (48.28) |
|
|
|
≥65 | 26 | 11 (50.00) | 15 (51.72) |
|
|
| Location |
|
|
| 0.019 | 0.889 |
| Rectal
cancer | 18 | 8 (36.36) | 10 (34.48) |
|
|
| Colon
cancer | 33 | 14 (63.64) | 19 (65.52) |
|
|
| Cell
differentiation |
|
|
| 3.960 | 0.138 |
|
Well | 5 | 2 (9.09) | 3 (10.34) |
|
|
|
Moderate | 20 | 12 (54.55) | 8 (27.59) |
|
|
|
Poor | 26 | 8 (36.36) | 18 (62.07) |
|
|
| TNM |
|
|
| 7.322 | 0.007b |
|
I/II | 26 | 16 (72.73) | 10 (34.48) |
|
|
|
III/IV | 25 | 6 (27.27) | 19 (65.52) |
|
|
| Primary tumor
size |
|
|
| 4.469 | 0.035a |
|
T1-2 | 23 | 14 (63.64) | 9 (31.03) |
|
|
|
T3-4 | 28 | 8 (36.36) | 20 (68.97) |
|
|
| Lymph node
metastasis |
|
|
| 5.685 | 0.017a |
| N0 | 25 | 15 (68.18) | 10 (34.48) |
|
|
|
N1-2 | 26 | 7 (31.82) | 19 (65.52) |
|
|
| Vascular
invasion |
|
|
| 0.292 | 0.589 |
| No | 30 | 12 (54.55) | 18 (62.07) |
|
|
|
Yes | 21 | 10 (45.45) | 11 (37.93) |
|
|
Discussion
CRC is one of the most common malignant diseases
globally (1). Recently, the function
of SMC1 in CRC has attracted increasing attention, with evidence
suggesting that the cohesin multiprotein complex is implicated in
several diseases, including colorectal cancer (25–27). The
cohesin multiprotein complex includes four major subunits: SMC1,
SMC3, sister chromatid cohesion (SCC) protein 1 and SCC3. The
cohesin multiprotein complex plays an important role in the
regulation of transcription and development (28,29). SMC1
is an X-linked gene that can escape X-inactivation in humans, but
is subject to X-inactivation in mice (30). Several mutations have been identified
in the SMC1 gene, all of which are missense or small deletion
mutations (12,20). Although SMC1 mutations have been
reported in CRC (21,22), the role of SMC1 in CRC remains
unclear. Therefore, elucidating how SMC1 is involved in CRC is of
great importance.
In the present study, it was demonstrated that SMC1
was significantly upregulated in CRC cell lines compared with
colonic epithelial cells. SMC1 overexpression contributed to an
increase in the proliferation, and a reduction in the apoptosis of
CRC cells in vitro and in vivo. Further evidence
indicated that the effects of SMC1 on cell proliferation involved
the regulation of the cell cycle. Additionally, it was demonstrated
that SMC1 knockdown affected the balance of Bcl-2/Bax, indicating
that SMC1 serves an antiapoptotic role in CRC.
NF-κB is a nuclear transcription factor involved in
various biological events (31). The
activation of NF-κB is considered to be part of a stress response,
as it is usually activated by a variety of stimuli that include
growth factors, cytokines, lymphokines, UV, pharmacological agents
and stress (32). In its inactive
form, NF-κB is sequestered in the cytoplasm and bound by members of
the IκB family of inhibitor proteins (33). In the present study, it was
demonstrated that knocking down SMC1 promoted NF-κB p65
translocation from the cytoplasm to the nucleus in vitro and
in vivo. The present data demonstrated that SMC1 knockdown
promoted cell apoptosis, which could be reversed by overexpressing
p65, suggesting that the roles of SMC1 in cell proliferation and
apoptosis are mediated by the NF-κB signaling pathway. However,
details regarding the mechanisms of action require further
investigation.
The significance of SMC1 in CRC was also supported
by clinical evidence. In the present study, it was demonstrated
that the expression of SMC1 in CRC tissues was higher compared with
adjacent normal tissues. Patients with high SMC1 expression had
larger tumors, and increased incidence of distant and local
metastasis; it was suggested that high SMC1 expression was an
independent prognostic predictor for patients with advanced CRC
stage, and was associated with overall survival. These results
suggested that SMC1 may have an important role in the development
of CRC and be a predictive biomarker in patients with CRC. The
inhibition of SMC1 may serve as a promising therapeutic strategy
for human CRC.
In conclusion, the present data demonstrated a role
of SMC1 as a tumor-promoting biomarker in CRC. The present data
also demonstrated a novel mechanism for the regulation of CRC
cells, as SMC1 promoted proliferation and inhibited apoptosis,
potentially via the NF-κB signaling pathway. To the best of our
knowledge, this is the first study to demonstrate that targeting
SMC1 may be a potential therapeutic strategy in CRC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Nanjing Medical
University Foundation of Jiangsu Province, China (grant no. NMUB
2018149), the Science and Technology Bureau project of Huai'an
(grant no. HAS2015001) and the National Natural Science Foundation
of China (grant no. 91229125).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article and its supplementary
information files.
Authors' contributions
JL, YS and JZ designed the study. JL, JH and YW
performed the experiments. JL and JH analyzed the data. JL and JZ
discussed the project. JL and JH drafted, and JZ proofread and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal treatment protocols were approved by the
Institutional Animal Care and Use Committee of The Affiliated
Huai'an No. 1 People's Hospital of Nanjing Medical University.
Informed consent was obtained from every patient, and the use of
human specimens was approved by the Ethics Committee of The
Affiliated Huai'an No. 1 People's Hospital of Nanjing Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RG, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Ward EM, Johnson CJ, Cronin KA,
Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, et al:
Annual report to the nation on the status of cancer, 1975–2014,
featuring survival. J Natl Cancer Inst. 109:2017. View Article : Google Scholar
|
|
3
|
Harrison S and Benziger H: The molecular
biology of colorectal carcinoma and its implications: A review.
Surgeon. 9:200–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peters U, Bien S and Zubair N: Genetic
architecture of colorectal cancer. Gut. 64:1623–1636. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fiedler D, Heselmeyer-Haddad K, Hirsch D,
Hernandez LS, Torres I, Wangsa D, Hu Y, Zapata L, Rueschoff J,
Belle S, et al: Single-cell genetic analysis of clonal dynamics in
colorectal adenomas indicates CDX2 gain as a predictor of
recurrence. Int J Cancer. 144:1561–1573. 2019.PubMed/NCBI
|
|
6
|
Barresi V, Castorina S, Musso N, Capizzi
C, Luca T, Privitera G and Condorelli DF: Chromosomal instability
analysis and regional tumor heterogeneity in colon cancer. Cancer
Genet. 210:9–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Librelotto CS, Simon D, de Souza AP,
Alvares-da-Silva MR and Dihl RR: Chromosomal instability and
cytotoxicity induced by ribavirin: Comparative analysis in cell
lines with different drug-metabolizing profiles. Drug Chem Toxicol.
42:343–348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McClelland SE: Role of chromosomal
instability in cancer progression. Endocrine-Related Cancer.
24:T23–T31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sideris M and Papagrigoriadis S: Molecular
biomarkers and classification models in the evaluation of the
prognosis of colorectal cancer. Anticancer Res. 34:2061–2068.
2014.PubMed/NCBI
|
|
10
|
Marginean EC and Melosky B: Is there a
role for programmed death ligand-1 testing and immunotherapy in
colorectal cancer with microsatellite instability? Part
I-colorectal cancer: Microsatellite instability, testing, and
clinical implications. Arch Pathol Lab Med. 142:17–25. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirano T: At the heart of the chromosome:
SMC proteins in action. Nat Rev Mol Cell Biol. 7:311–322. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huber RG, Kulemzina I, Ang K, Chavda AP,
Suranthran S, Teh JT, Kenanov D, Liu G, Rancati G, Szmyd R, et al:
Impairing cohesin Smc1/3 head engagement compensates for the lack
of Eco1 function. Structure. 24:1991–1999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schar P, Fasi M and Jessberger R: SMC1
coordinates DNA double-strand break repair pathways. Nucleic Acids
Res. 32:3921–3929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bauerschmidt C, Woodcock M, Stevens DL,
Hill MA, Rothkamm K and Helleday T: Cohesin phosphorylation and
mobility of SMC1 at ionizing radiation-induced DNA double-strand
breaks in human cells. Exp Cell Res. 317:330–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo Y, Deng X, Cheng F, Li Y and Qiu J:
SMC1-mediated intra-S-phase arrest facilitates bocavirus DNA
replication. J Virol. 87:4017–4032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Laugsch M, Seebach J, Schnittler H and
Jessberger R: Imbalance of SMC1 and SMC3 cohesins causes specific
and distinct effects. PLoS One. 8:e651492013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi F, Wang Z, Liu J, Zhang Y, Wang Z, Xu
H, Li X, Bai N, Cao L and Song X: Structural maintenance of
chromosomes protein 1: Role in genome stability and tumorigenesis.
Int J Biol Sci. 13:1092–1099. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitagawa R, Bakkenist CJ, McKinnon PJ and
Kastan MB: Phosphorylation of SMC1 is a critical downstream event
in the ATM-NBS1-BRCA1 pathway. Genes Dev. 18:1423–1438. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Musio A, Selicorni A, Focarelli ML,
Gervasini C, Milani D, Russo S, Vezzoni P and Larizza L: X-linked
Cornelia de lange syndrome owing to SMC1L1 mutations. Nat Genet.
38:528–530. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Yu S, Cui L, Wang W, Li J, Wang K
and Lao X: Role of SMC1A overexpression as a predictor of poor
prognosis in late stage colorectal cancer. BMC Cancer. 15:902015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Feng W, Chen L and He J:
Downregulation of SMC1A inhibits growth and increases apoptosis and
chemosensitivity of colorectal cancer cells. J Int Med Res.
44:67–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours, 8th edition.
Hoboken. Wiley–Blackwell. 2016.
|
|
24
|
Bharti AC and Aggarwal BB: Chemopreventive
agents induce suppression of nuclear factor-κB leading to
chemosensitization. Ann NY Acad Sci. 973:392–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Ruiten MS and Rowland BD: SMC
Complexes: Universal DNA looping machines with distinct regulators.
Trends Genet. 34:477–487. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alt A, Dang HQ, Wells OS, Polo LM, Smith
MA, McGregor GA, Welte T, Lehmann AR, Pearl LH, Murray JM and
Oliver AW: Specialized interfaces of Smc5/6 control hinge stability
and DNA association. Nat Commun. 8:140112017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mannini L, Liu J, Krantz ID and Musio A:
Spectrum and consequences of SMC1A mutations: The unexpected
involvement of a core component of cohesin in human disease. Hum
Mutat. 31:5–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Misulovin Z, Pherson M, Gause M and
Dorsett D: Brca2, Pds5 and Wapl differentially control cohesin
chromosome association and function. PLoS Genet. 14:e10072252018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kakui Y and Uhlmann F: SMC complexes
orchestrate the mitotic chromatin interaction landscape. Curr
Genet. 64:335–339. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gdula MR, Nesterova TB, Pintacuda G,
Godwin J, Zhan Y, Ozadam H, McClellan M, Moralli D, Krueger F,
Green CM, et al: The non-canonical SMC protein SmcHD1 antagonises
TAD formation and compartmentalisation on the inactive X
chromosome. Nat Commun. 10:302019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mussbacher M, Salzmann M, Brostjan C,
Hoesel B, Schoergenhofer C, Datler H, Hohensinner P, Basílio J,
Petzelbauer P, Assinger A and Schmid JA: Cell type-specific roles
of NF-κB linking inflammation and thrombosis. Front Immunol.
10:852019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang I, Yoon DW, Braun KR and Wight TN:
Expression of versican V3 by arterial smooth muscle cells alters
tumor growth factor β (TGFβ)-, epidermal growth factor (EGF)-, and
nuclear factor κB (NFκB)-dependent signaling pathways, creating a
microenvironment that resists monocyte adhesion. J Biol Chem.
289:15393–15404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meng F, Zhang S, Song R, Liu Y, Wang J,
Liang Y, Wang J, Han J, Song X, Lu Z, et al: NCAPG2 overexpression
promotes hepatocellular carcinoma proliferation and metastasis
through activating the STAT3 and NF-κB/miR-188-3p pathways.
EBioMedicine. 44:237–249. 2019. View Article : Google Scholar : PubMed/NCBI
|