Introduction
The frequency and mortality rate of cancer are
increasing in South Korea (1–3). Gastric cancer is the fourth most common
type of cancer, and has the second highest mortality rate after
lung cancer worldwide (4).
Approximately 990,000 people are diagnosed with gastric cancer in
the world every year, of which approximately 738,000 succumb to
this disease. The incidence rate of gastric cancer is two to three
times higher in men than in women, with the rates varying by
country (5). North America and most
regions in Africa exhibit the lowest incidence rates of gastric
cancer, whereas East Asia, Eastern Europe, and South America have
the highest rates (6). The mortality
rate of gastric cancer is the highest among all types of cancer in
South Korea, China, and other East Asian countries, and is rapidly
increasing. Currently, surgery and chemotherapy are used to treat
gastric cancer alternating various types of therapies to raise the
effectiveness of treatment, however, the recurrence rates are high
even after surgery and the survival rate is low, leading to poor
prognosis. Therefore, new treatment methods are required to improve
the prognosis of gastric cancer patients (7–9).
Medications produced from various natural ingredients and resources
are being increasingly used as new methods of treatment, and some
studies have confirmed marked effects of some natural resources for
anticancer treatment and immunoregulation (10–12).
Flavonoids are widely distributed in plants, such as
fruits and vegetables, and have various structural properties. Many
plants have long been consumed to regulate various internal
biological activities (13,14). The roles of such natural products in
cancer prevention and chemotherapy have been widely studied to
examine their potential as anticancer agents mediated through
various effects, including antioxidation, inhibition of
vascularization, induction of apoptosis and differentiation, and
cell cycle arrest (15).
As a natural product acquired from the fruits and
seeds of the milk thistle plant (Silybum marianum L.),
silymarin is a compound that contains silibinin, isosoi slybin,
silydianin, and silychristin, which are flavonoids. For several
decades, it has been used as a functional food in liver protection
and in the treatment of chronic epilepsy (16,17).
Recent studies have revealed that it alters the expression of
proteins related to cell cycle regulation and apoptosis and thus
controls the balance between cell viability and apoptosis and
exhibits anti-inflammatory, vascularization inhibitory,
antioxidative, and anti-metastasis effects (18–20). It
has also been reported to exhibit anticancer effects in liver
(21), colorectal (22), breast (23), lung (24), and prostate cancer (25).
Apoptosis is an intracellular activity also known as
programmed cell death or cellular suicide, which plays central
roles in maintaining homeostasis by regulating the number of cells
and removing dysfunctional and unnecessary cells that cannot
recover from damage (26). Apoptosis
is mediated through two pathways: The extrinsic pathway mediated by
death receptors, and the intrinsic pathway mediated by
mitochondria. The extrinsic pathway works by activating caspase
with death receptors located on the cell membrane that forms a
complex with a death ligand (27,28). The
intrinsic pathway refers to apoptosis completed through the release
of apoptosis-related inducers in mitochondria through changes in
mitochondrial membrane permeability (MMP) (29). The Bcl-2 family is a major protein
type involved in the intrinsic pathway that regulates MMP according
to changes in its expression. Among these proteins, Bax forms the
apoptosome when its expression increases as a pro-apoptotic protein
and activates caspase-3 to induce apoptosis. Bcl-2 inhibits Bax as
an anti-apoptotic protein, and thus inhibits the induction of
apoptosis (30). In addition,
poly(ADP ribose) polymerase (PARP) is a regulator of apoptosis that
plays an important role in DNA repair in the nucleus (31).
The protein kinase, mitogen-activated protein
kinases (MAPK), plays an important role in cell death,
proliferation, and differentiation as a major signaling molecule
that that delivers extracellular stimuli to the nucleus. MAPK is
classified into extracellular signal-regulated protein kinase 1/2
(ERK1/2), c-Jun N-terminal kinase/stress-activated protein kinase
(JNK/SAPK), and p38. ERK1/2 mediates signal transmission of growth
hormone and thus plays important roles in cellular proliferation,
differentiation, and cell viability. JNK and p38 are activated by
stimuli, such as extracellular stress, and play essential roles in
inflammation and cell death (32).
The present study was performed to examine the
effects of the flavonoid, silymarin, on tumor growth inhibition and
induction of apoptosis in AGS human gastric cancer cells, and to
determine whether the induced apoptosis was mediated by the MAPK
pathway to confirm its tumor growth inhibitory effects in
vivo.
Materials and methods
Reagents
The AGS human gastric cancer cells used for this
study were purchased from the Korea Cell Line Bank (Seoul, Korea).
RPMI-1640, used for cell culture, was purchased from Welgene
(Gyeonsan, Korea), and fetal bovine serum (FBS) and
streptomycin/penicillin were purchased from Gibco BRL; Thermo
Fisher Scientific, Inc. Silymarin and general reagents used in this
study were purchased from Sigma-Aldrich; Merck KGaA. The primary
antibodies (anti-β-actin, anti-Bax, anti-Bcl-2, anti-PARP,
anti-Erk1/2, anti-phosphorylated (p)-Erk1/2, anti-JNK, anti-p-JNK,
anti-p38, anti-p-p38), and secondary antibody (anti-rabbit IgG)
were purchased from Cell Signaling Technology, Inc. Matrigel matrix
was purchased from Corning, Inc.
Cell culture
AGS human gastric cancer cells were cultured in the
incubator at 37°C and 5% CO2 in RPMI-1640 culture medium
supplemented with 1% streptomycin/penicillin and 5% FBS. When the
cell density reached ~80% in 175-cm2 flask, the cells
were washed with the phosphate-buffered saline (PBS; pH 7.4) and
treated with trypsin-EDTA for subculture. The culture medium was
replaced every ~2–3 days.
Cell viability assay
An MTT assay was performed to investigate the effect
of silymarin on proliferation of AGS human gastric cancer cells.
AGS cells were seeded in 96-well plate at a density of
2×104 cells/ml and cultured in the RPMI-1640 culture
medium for ~24 h in an incubator at 37°C and 5% CO2. The
cells were then treated with silymarin at concentrations of 0, 20,
40, 60, 80, 100 and 120 µg/ml. After 24 h, MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
solution was added to the 96-well plates containing AGS cells in a
volume of 40 µl/well and cultured for 2 h. After removing the MTT
solution, 100 µl/well of dimethyl sulfoxide (DMSO) was added to
dissolve all formazan formed in the well, and the absorbance was
measured at 595 nm with an ELISA-reader (Bio-Rad Laboratories
Inc.). The percentage of viable cells was estimated in comparison
to the untreated control cells.
Wound healing assay
AGS human gastric cancer cells were seeded in a
60-mm dish and cultured for 24 h. A uniform wound was created by
scratching cells with a sterile 1-ml blue-pipette tip. The culture
medium was replaced with that containing silymarin at
concentrations of 0, 40 and 80 µg/ml, followed by culture for 24 h.
After 24 h, the wound healing rates of cells treated with silymarin
at concentrations of 40 and 80 µg/ml and those without silymarin
were examined on images captured under a phase contrast microscope
(×200) at 0 and 24 h after wound incision.
DAPI staining
4′,6-Diamidino-2-phenylindole (DAPI) staining was
performed to examine the specific morphological changes in the
nuclei with induction of apoptosis. AGS human gastric cancer cells
were seeded in a 60-dish at 1×105 cells/ml, stabilized
for 24 h, treated with silymarin at 0, 40 and 80 µg/ml, and
cultured in an incubator for 24 h. The cells were then washed twice
with PBS and fixed with the 4% paraformaldehyde solution for 15
min. Subsequently, they were washed again with PBS, treated with
1:10 diluted DAPI solution (2 ml), and observed under a
fluorescence microscope (Zeiss AG) at an ×200 magnification in a
dark room.
Flow cytometric analysis
Apoptosis was measured using an FITC-Annexin V
apoptosis detection kit (BD Pharmingen). For Annexin V-propidium
iodide (PI) staining, AGS human gastric cancer cells were treated
with silymarin at concentrations of 0, 40 and 80 µg/ml. Cells
cultured for 24 h were washed with PBS, suspended in trypsin-EDTA,
and centrifuged (260 × g, 5 min, 4°C) to obtain the cell pellet.
They were then washed twice with cold PBS and centrifuged to obtain
the cell pellet. Next, they were suspended in 1X binding buffer at
a concentration of 1×106 cells/ml. Fluorescein
isothiocyanate (FITC)-conjugated Annexin V and phycoerythrin
(PE)-conjugated PI were then added and reacted for 15 min followed
by flow cytometry.
Western blot analysis
Western blot analysis was performed to determine the
changes in protein expression associated with silymarin treatment.
AGS human gastric cancer cells cultured in 175-cm2
flasks in an incubator at 37°C and 5% CO2 were treated
with silymarin at concentrations of 0, 40 and 80 µg/ml and cultured
for 24 h. Trypsin-EDTA was added to the cells, which were then
suspended and centrifuged (260 × g, 5 min, 4°C). Cell lysis buffer
(Invitrogen; Thermo Fisher Scientific, Inc.) was added to the cell
pellet, and allowed to react at 4°C for 20 min. The supernatant
obtained by centrifugation at 15,000 × g for 5 min was used as the
cell lysate. The concentration of the extracted protein was
determined by Bradford protein assay. Proteins were separated by
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto nitrocellulose membranes (Bio-Rad
Laboratories, Inc.). The membranes were blocked with 5% skim milk
for 2 h, followed by the addition of the primary antibodies
anti-β-actin (1:1,000; cat. no. 4967), anti-Bax (1:1,000; cat. no.
2772), anti-Bcl-2 (1:1,000; cat. no. 2876), anti-PARP (1:1,000;
cat. no. 9542), anti-Erk1/2 (1:1,000; cat. no. 9102), anti-p-Erk1/2
(1:1,000; cat. no. 9101), anti-JNK (1:1,000; cat. no. 9252),
anti-p-JNK (1:1,000; cat. no. 4668), anti-p38 (1:1,000; cat. no.
9212), and anti-p-p38 (1:1,000; cat. no. 4631), and left overnight
at 4°C. Anti-rabbit IgG (1:1,000; cat. no. 7074) (all from Cell
Signaling Technology, Inc.) was then added and allowed to react for
2 h. The results were obtained using the ECL detection reagent
(Pierce; Thermo Fisher Scientific, Inc.). The density of each band
was measured using the ImageJ Launcher imaging program (version
1.48; provided by NCBI).
In vivo xenograft tumor model
Ten BALB/c nude mice (Four-week-old, male, 20 g)
were purchased from the Nara-Biotec (Seoul, Korea). The mice were
housed in isolated and ventilated cages (≤3 mice per cage). Mice
were maintained under a 12-h light/dark cycle, and housed under
controlled temperature (23±3°C) and humidity (40±10%) conditions.
Mice were allowed access to laboratory pelleted food and water
ad libitum. Cervical dislocation was used to sacrifice the
mice. AGS human gastric cancer cells were cultured in an incubator
at 37°C and 5% CO2 in RPMI-1640 culture medium
containing 5% FBS. When the cell density reached approximately
80–90%, they were transferred into 175-cm2 flasks and
suspended by addition of trypsin-EDTA, followed by centrifugation
(260 × g, 5 min, 4°C). They were then washed with PBS and
centrifuged again (260 × g, 3 min, 4°C) to obtain the cell pellet,
which was divided into aliquots in culture medium at a
concentration of 1×107 cells/ml. AGS cells were injected
in a volume of 200 µl (1:1 Matrigel mixture) into the backs of male
BALB/c nude mice. One week later, after tumors had formed, the mice
were anesthetized with diethyl ether and the tumor tissue was
extracted, cut into blocks ~1 mm3, and then reinjected
into nude mice. Diethyl ether was provided as inhalant. They were
grouped according to uniform tumor size. The injected group
received oral administration of 100 mg/kg of silymarin diluted in
ethanol five times per week, at the same time of day in each
session, for 2 weeks. The control group received oral
administration of a mixture of ethanol and distilled water
according to the same schedule for 2 weeks. During the
administration period, the general conditions of the mice were
examined, and tumor size was measured twice a week with Vernier
calipers (Mitutoyo Corporation) and calculated as follows: Size
(mm3) = [0.5 × (length + width)]3. The animal
experiments were conducted in accordance with the regulations of
the Institutional Animal Care and Use Committee with the approval
of the Ethics Committee in the Kongju National University.
TUNEL assay
TUNEL assays were performed to identify apoptotic
cells using the Dead End Colorimetric TUNEL System (Promega
Corportaion). Paraffin-embedded tissue sections 5-µm thick were
deparaffinized with xylene, followed by rehydration using a graded
series of 100, 95, 85, 70 and 50% ethanol. After washing with PBS,
proteinase K was added to each slide and allowed to react at room
temperature for 15 min. The equilibration buffer, biotinylated
nucleotide mixture, and recombinant terminal deoxynucleotidyl
transferase (rTdT) were mixed, added to each slide, and allowed to
react at 37°C for 1 h. Then, 0.3% hydrogen peroxide
(H2O2) in PBS was added and allowed to react
for 5 min. Streptavidin-HRP was added to each slide, on which
3,3′-diaminobenzidine tetrahydrochloride (DAB) solution was reacted
for 10 min and positive cells were observed under a light
microscope (×200).
Immunohistochemistry
After examination of apoptotic cells by TUNEL assay,
immunohistochemistry (IHC) was performed to investigate for target
proteins related to apoptosis. Paraffin-embedded tissue sections
5-µm thick were deparaffinized with xylene, followed by rehydration
using a graded series of 100, 90, 80 and 70% ethanol. After washing
with PBS, intrinsic peroxidase was deactivated with 0.3%
H2O2. After washing with PBS, intrinsic
biotin was deactivated with skim milk, and sections were reacted
with the primary antibody. After washing, sections were reacted
with the secondary antibody, and H2O2 was
added to DAB to undergo reaction. Sections were then stained with
methyl green, and the target proteins were observed under a light
microscope (×200).
Histological examination
To investigate organ homogeneity by silymarin, test
animals were sacrificed, and their livers and kidneys were
extracted. Paraffin-embedded tissue sections 5-µm thick were
deparaffinized with xylene, and rehydrated with 100 and 95%
ethanol. They were then stained with hematoxylin and eosin
(H&E) and observed under a light microscope (×200) after
hydration and transparency processes.
Statistical analysis
The results are expressed as the means ± standard
deviation (SD). Differences between the mean values for the groups
were assessed by a one-way analysis of variance (ANOVA) and
Dunnett's t-tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
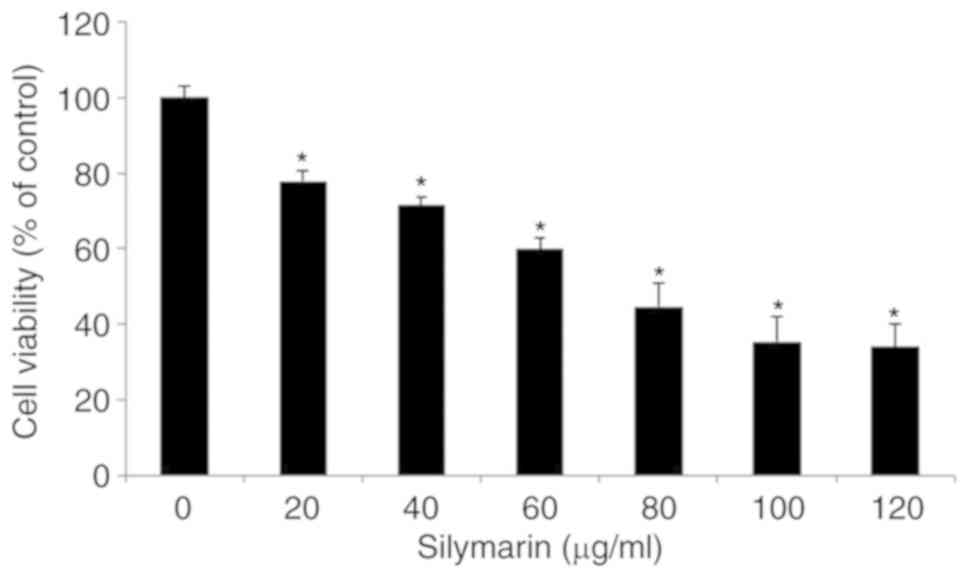
Effects of silymarin on the viability
of AGS human gastric cancer cells
To evaluate its effects on the viability of AGS
human gastric cancer cells, silymarin was applied at different
concentrations and the results were examined by MTT assay. Cancer
cells were divided into aliquots of 2×104 cells/ml in
96-well plates, cultured for 24 h, and treated with silymarin at 0,
20, 40, 60, 80, 100 and 120 µg/ml for 24 h. The viability of AGS
cells was 77.9% in the presence of 20 µg/ml silymarin, 71.5% at 40
µg/ml, 59.8% at 60 µg/ml, 44.5% at 80 µg/ml, 35.3% at 100 µg/ml and
33.9% at 120 µg/ml. These results indicated a significant
concentration-dependent inhibitory effect on the viability of AGS
cells starting at 20 µg/ml (Fig.
1).
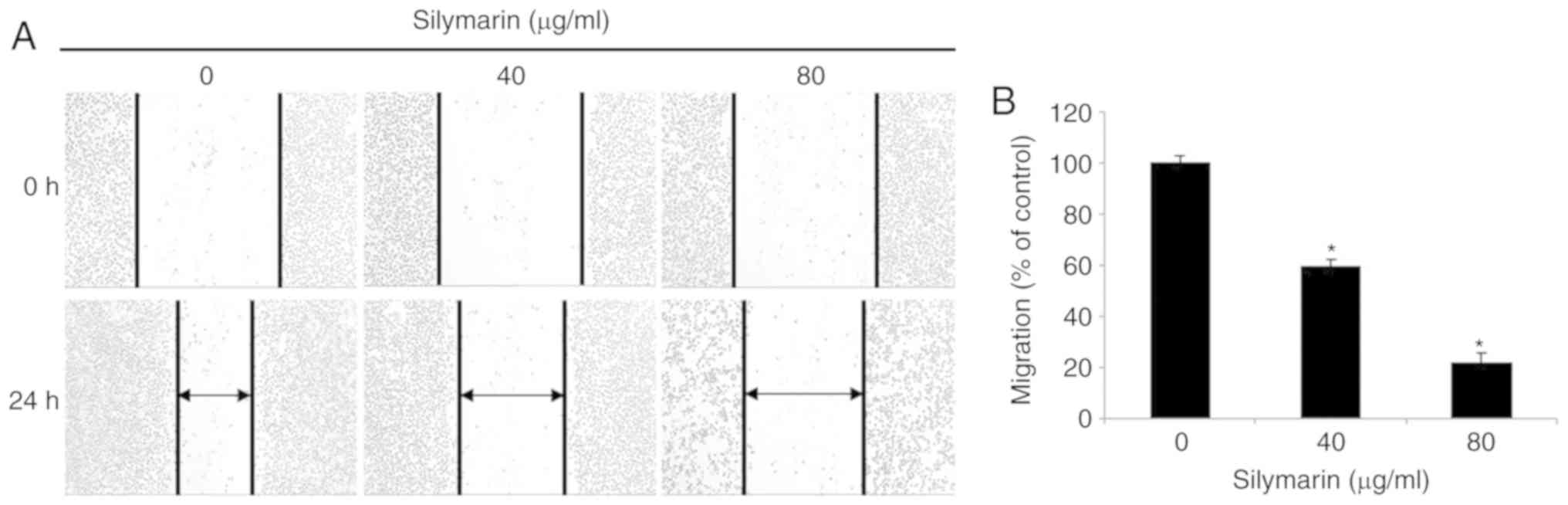
Effects of silymarin on the migration
of AGS human gastric cancer cells
A wound healing assay was performed to examine the
effects of silymarin on the migration of the AGS human gastric
cancer cells. AGS gastric cancer cell cultures with a wound of a
specific size were treated with silymarin at 0, 40 and 80 µg/ml,
and cultured for 24 h. The effects of silymarin on cell migration
were examined by comparing the degree of wound healing at specified
intervals. Silymarin was revealed to inhibit the migration of the
AGS cells in a concentration-dependent manner (Fig. 2A). The numbers of cells present at
various time-points were counted. In comparison with the control
group, silymarin was confirmed to inhibit migration of AGS cells in
a concentration-dependent manner (59.4% at 40 µg/ml and 21.7% at 80
µg/ml) (Fig. 2B).
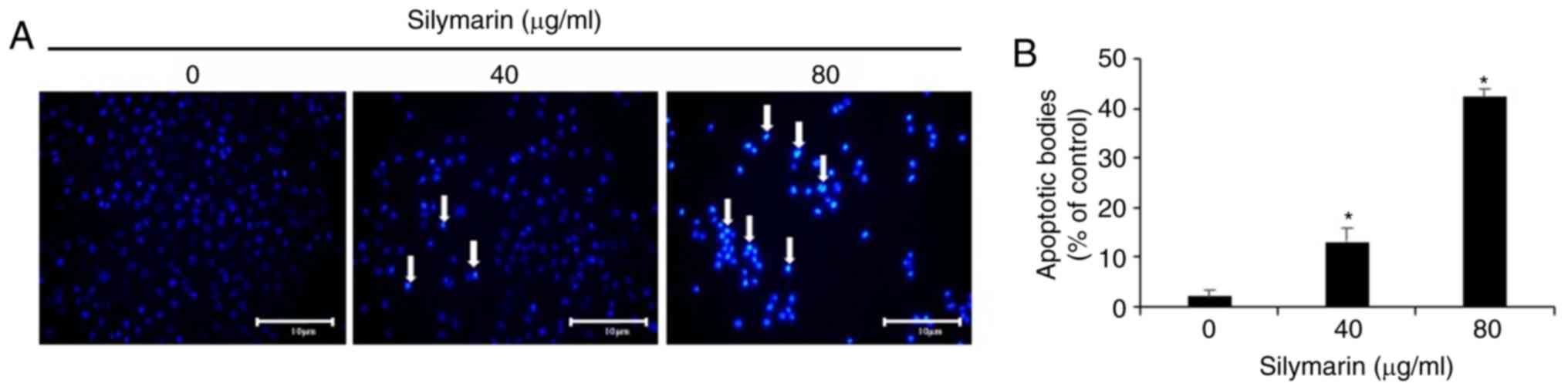
Morphological changes in AGS human
gastric cancer cells induced by silymarin
The experiments outlined above confirmed the
inhibitory effect of silymarin on the viability and migration of
AGS human gastric cancer cells. To examine whether these effects
were related to apoptosis, AGS cells were treated with silymarin at
concentrations of 40 and 80 µg/ml. DAPI staining was then performed
to examine morphological changes in the nucleus and the phenomenon
of chromatin condensation by fluorescence microscopy (Fig. 3A). The proportion of cells exhibiting
positive DAPI staining increased, while the cell count decreased.
The growth of AGS cells was inhibited in a concentration-dependent
manner similar to the results of the MTT and wound healing assays.
One hundred cells from five random fields at ×200 magnification
under a fluorescence microscope were quantified to analyze the
degree of apoptosis induction. The proportion of cells exhibiting
chromatin and nuclear condensation as well as morphological changes
associated with apoptosis increased in the silymarin-treated group
in a concentration-dependent manner (2% at 0 µg/ml, 13% at 40 µg/ml
and 42.2% at 80 µg/ml) in comparison with the controls (Fig. 3B).
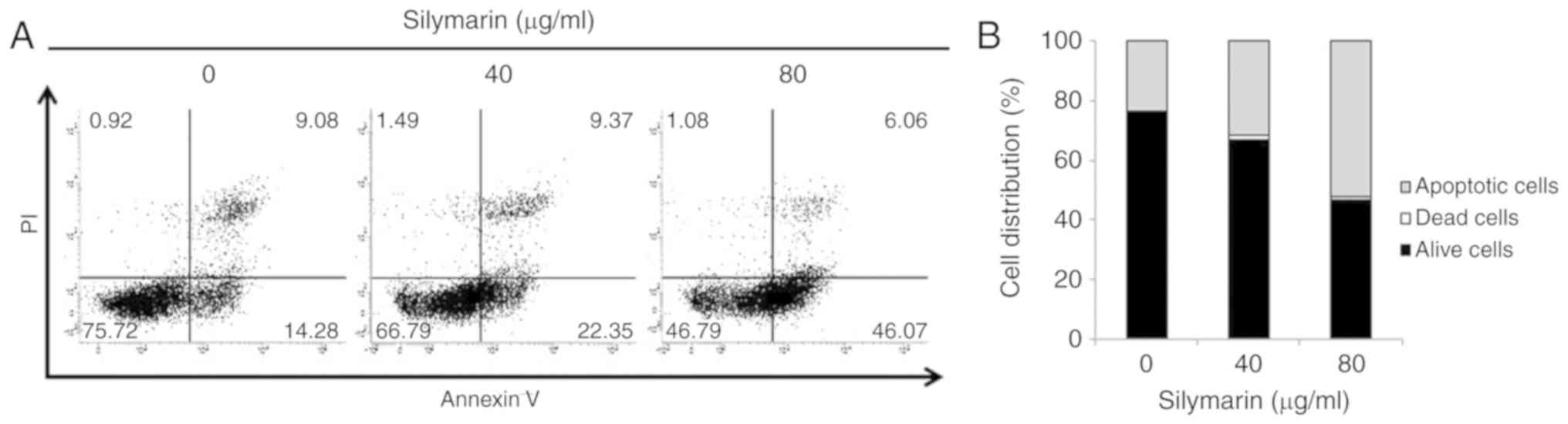
Effects of silymarin on apoptosis in
AGS human gastric cancer cells
DAPI staining indicated a close relationship between
silymarin and apoptosis in reduction of AGS human gastric cancer
cell viability and proliferation. To examine the degree of
apoptosis induction, Annexin V/PI staining was applied to AGS cells
treated with silymarin at concentrations of 0, 40 and 80 µg/ml and
cultured for 24 h, after which apoptotic cells were determined by
flow cytometry (Fig. 4A). Comparison
of the total ratio of early to late apoptosis cells indicated
silymarin concentration-dependent increases of 23.36% at 0 µg/ml,
31.72% at 40 µg/ml and 52.13% at 80 µg/ml (Fig. 4B).
Effects of silymarin on
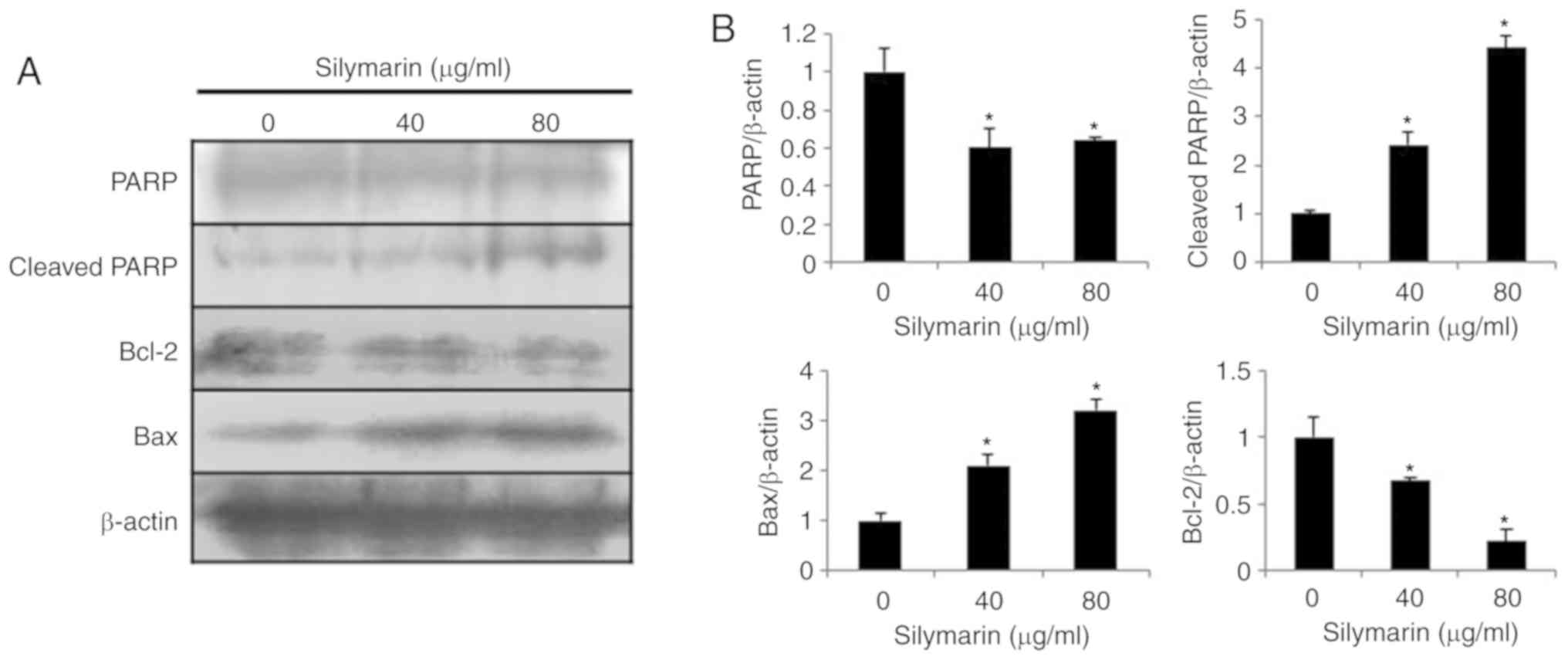
apoptosis-related proteins in AGS human gastric cancer cells
Western blotting was performed to examine the
changes in the expression levels of apoptosis-related proteins
following treatment of AGS human gastric cancer cells with
silymarin at concentrations of 0, 40 and 80 µg/ml (Fig. 5A). The results indicated
concentration-dependent increases in the expression levels of the
pro-apoptotic proteins, Bax and cleaved PARP, while expression of
the anti-apoptotic protein, Bcl-2, was decreased in AGS cells
treated with silymarin at 40 and 80 µg/ml (Fig. 5B).
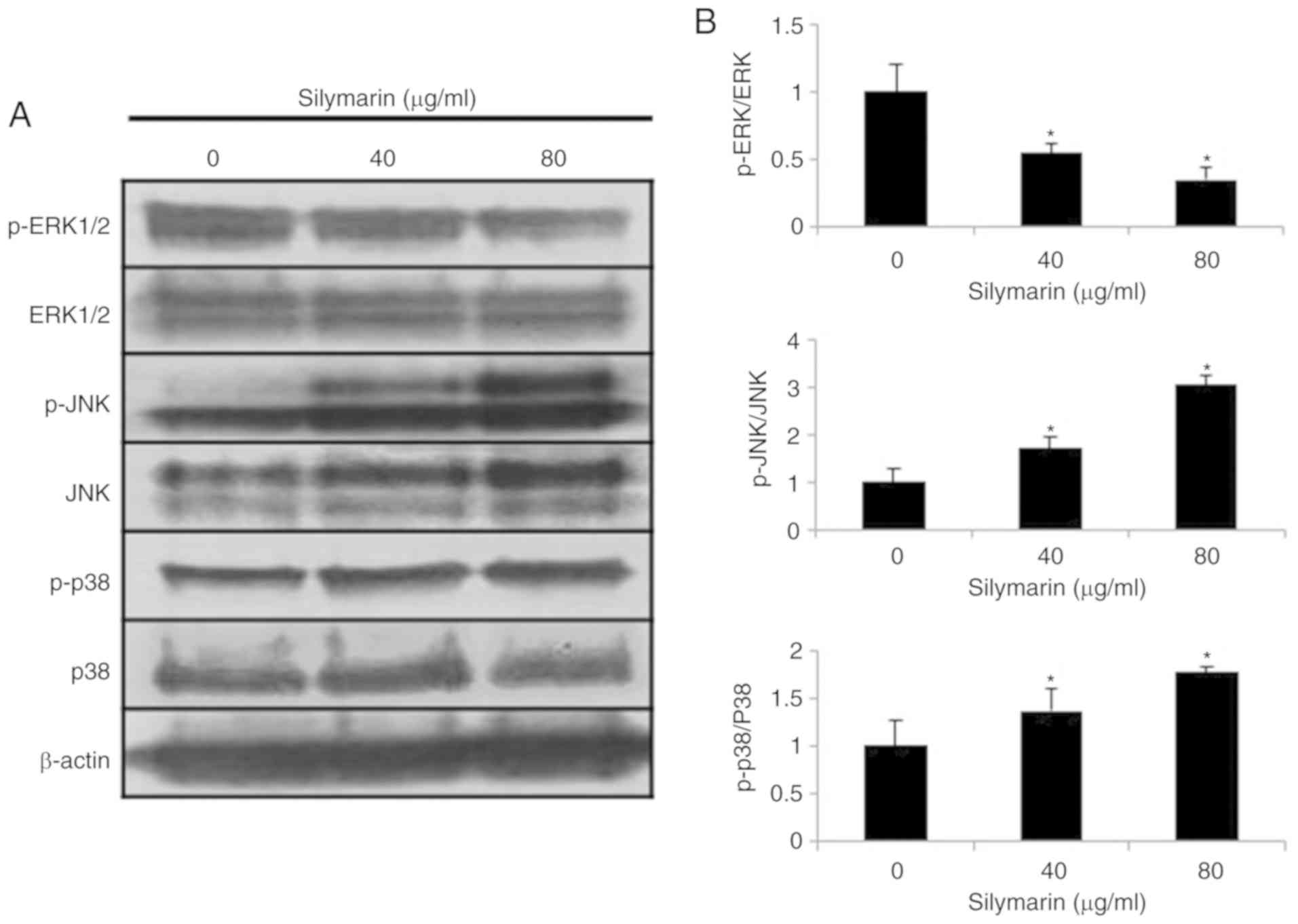
Effects of silymarin on the MAPK
pathway in AGS human gastric cancer cells
Changes in proteins involved in the MAPK signaling
pathway were examined in AGS human gastric cancer cells treated
with silymarin at concentrations of 0, 40 and 80 µg/ml for 24 h
(Fig. 6A). The results indicated
concentration- dependent decreases in the expression of p-ERK1/2
and increases in the expression of p-JNK and p-p38 (Fig. 6B).
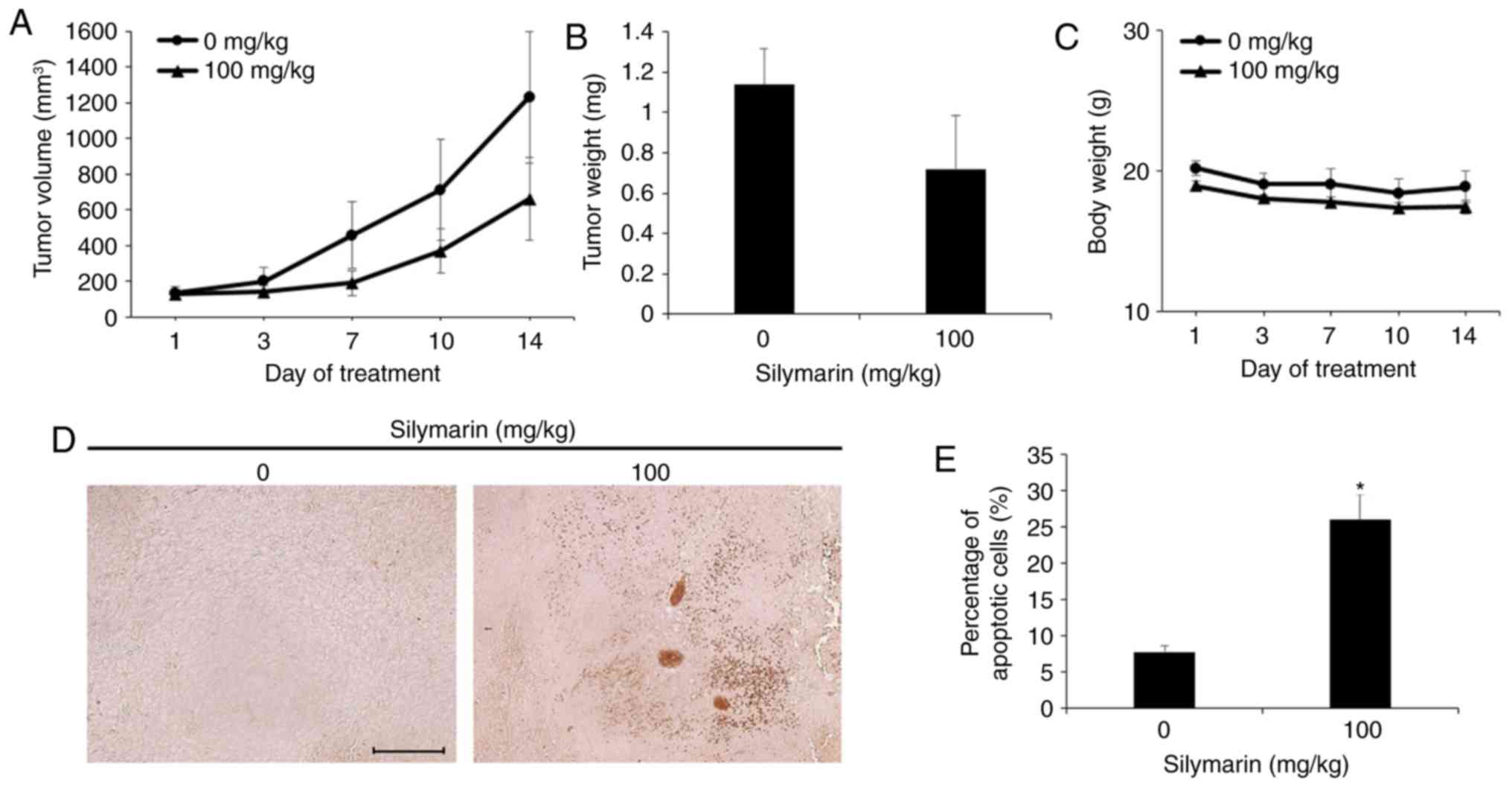
Effects of silymarin on tumor growth
in vivo in an animal model
In an in vivo experiment with reference to
the in vitro findings, xenografting was performed in
4-week-old male BALB/c nude mice to examine the effects of
silymarin injection on AGS human gastric cancer cell-derived
tumors. The tumor size and body weight of the animals were measured
twice per week. Silymarin was diluted in ethanol and orally
administered five times per week at 0 or 100 mg/kg for 2 weeks. The
control group received oral administration of ethanol and distilled
water according to the same schedule for 2 weeks. The results
indicated that the tumor size decreased in the silymarin injection
group from 7 days after commencement of administration. The degree
of decrease in tumor size was higher in the group administered 100
mg/kg silymarin (Fig. 7A). At 14
days, the 100 mg/kg silymarin injection group exhibited a 46.2%
decrease in tumor size in comparison with the control group
(Table I). The final tumor size was
1,230 mm3 in the control group and 661 mm3 in
the 100 mg/kg silymarin group. At the end of the experimental
period, the measured tumor weights were 1.14±0.17 g in the control
group and 0.72±0.26 g in the 100 mg/kg silymarin group (Fig. 7B). The body weights of
silymarin-treated and control mice remained similar throughout the
experimental period (Fig. 7C).
 | Table I.Tumor inhibition rate of mice
implanted with AGS gastric cancer cells tumor-treated with
silymarin. |
Table I.
Tumor inhibition rate of mice
implanted with AGS gastric cancer cells tumor-treated with
silymarin.
| Silymarin
(mg/kg) | Pre-experiment size
(mm3) | Post-experiment
size (mm3) | Inhibition
rateb (%) |
|---|
| AGS |
|
|
|
| 0a | 131.8 | 1,230.7 |
|
| 100 | 125.5 | 661.7 | 46.2 |
Effects of silymarin on apoptosis
induction of gastric tumor tissue
The tumors formed by subcutaneous transplantation of
AGS human gastric cancer cells into 4-week-old male BALB/c nude
mice, as aforementioned, were extracted and examined by TUNEL assay
to confirm the anticancer effect of silymarin in tumor tissue
through the induction of apoptosis. AGS tumor tissue extracted from
nude mice administered silymarin exhibited a significant increase
in the proportion of TUNEL-positive cells compared to those without
silymarin administration (Fig. 7D).
The proportion of apoptotic cells revealed a significant increase
of 26% in the 100 mg/kg silymarin group compared to the controls
(Fig. 7E).
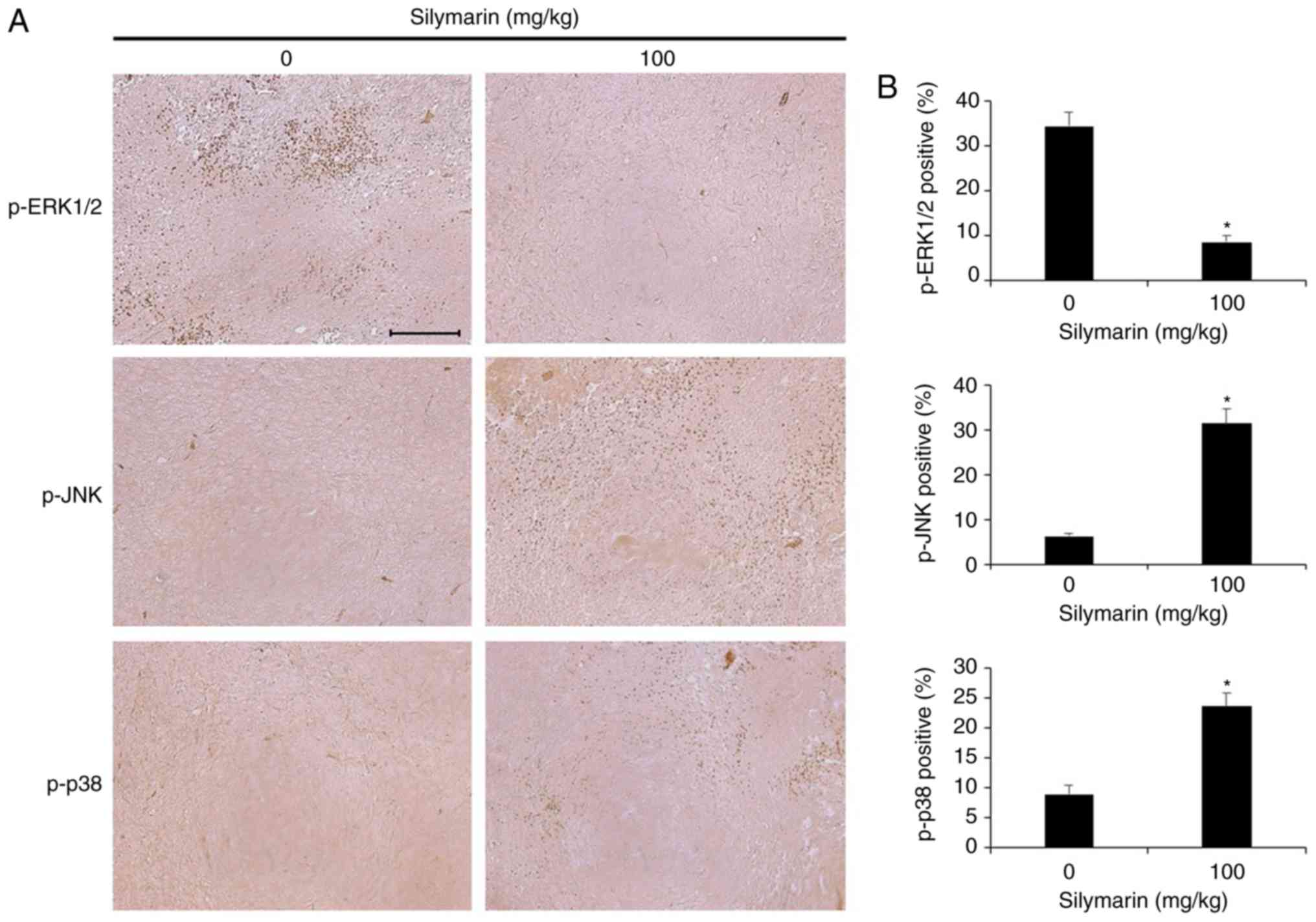
Effects of silymarin on the MAPK
pathway in AGS gastric tumor tissue
Silymarin was administered to xenografted nude mice,
and immunohistochemistry was performed to examine p-ERK1/2, p-JNK,
and p-p38 expression (Fig. 8A). The
group receiving 100 mg/kg silymarin exhibited a decrease in
expression of p-ERK1/2 and increases in the expression of p-JNK and
p-p38 in comparison with the control group. Quantitative comparison
of the expression of each protein resulted in a decrease of
p-ERK1/2 from 34.4 to 8.6% in the silymarin-treated group, compared
with the control group. Conversely, p-JNK increased from 6.2 to
31.4%, and p-p38 from 9 to 23.6% (Fig.
8B).
Toxicity evaluation of silymarin in
liver and kidney tissues
After the experimental period, the nude mice with
transplanted AGS tumors were sacrificed to investigate for organ
toxicity caused by administration of silymarin. The liver and
kidneys were stained with H&E and examined under an optical
microscope (Fig. 9). The results
indicated no histopathological abnormalities in the silymarin
injection group or the non-injected control group.
Discussion
Infinite proliferation through acquisition of growth
signaling and deviation of growth inhibition signals is one of the
best-known features of cancer cells (33). To examine the viability and migration
inhibitory effects in AGS human gastric cancer cells, an MTT assay
was performed following silymarin treatment at concentrations of 0,
20, 40, 60, 80, 100 and 120 µg/ml for 24 h, and a wound healing
assay was performed following silymarin treatment at 0, 40 and 80
µg/ml for 24 h. The results of the MTT assay revealed a
concentration-dependent decrease in cell viability starting at 20
µg/ml. In the wound healing assay, the groups treated with
silymarin exhibited concentration-dependent inhibition of cell
migration in comparison with the control group. Ramakrishnan et
al (34) also demonstrated
concentration-dependent inhibition of cancer cell viability
beginning at a concentration of 50 µg/ml when liver cancer cells
were treated with silymarin at concentrations of 50, 75, 100 and
200 µg/ml for 24 h. Zhong et al (35) also treated leukemic cells with
silymarin at 10, 50 and 100 µg/ml, and demonstrated a significant
decrease in viability beginning at 50 µg/ml. Fan et al
(36) treated ovarian cancer cells
with 25, 50, 100, 150 and 200 µg/ml silymarin and demonstrated a
concentration-dependent decrease in viability from 50 µg/ml.
Significant decreases in viability were also observed with
silymarin treatment at 100 µg/ml for 24, 48 and 72 h. Vaid et
al (37) treated human melanoma
cells with 10, 20 and 40 µg/ml silymarin and reported that the
wound healing assay revealed significant inhibition of cell
migration at concentrations of 20 and 40 µg/ml. These findings
indicated that silymarin decreased the viability and inhibited the
migration of AGS human gastric cancer cells in this study.
When apoptosis occurs, apoptotic bodies are observed
accompanied by cell and nuclear condensation and division, as well
as dissolution of chromosomal DNA (38,39). DAPI
staining and flow cytometric analysis were conducted to confirm
whether the viability decrease and inhibition of proliferation by
silymarin in gastric cancer cells are caused by apoptosis. AGS
cells were treated with silymarin at 0, 40 and 80 µg/ml for 24 h,
and then subjected to staining with DAPI to identify apoptotic
cells. DAPI-stained cells were counted to quantify the degree of
apoptosis induction. The results indicated a dose-dependent
increase in the number of DAPI-stained cells (2% at 0 µg/ml, 13% at
40 µg/ml and 42.2% at 80 µg/ml) in comparison with the control
group. Fan et al (36)
reported the occurrence of apoptosis in ovarian cancer cells
following treatment with silymarin, while Katiyar et al
(40) reported a
concentration-dependent increase in apoptotic bodies with treatment
of skin epidermal cancer cells with silymarin. The results of flow
cytometric analysis confirmed the concentration-dependent increase
in apoptotic cells by silymarin treatment (23.26% at 0 µg/ml,
31.72% at 40 µg/ml and 52.13% at 80 µg/ml). Zhong et al
(35) also performed flow cytometric
analysis of leukemic cells treated with silymarin at concentrations
of 10, 50 and 100 µg/ml, and revealed a concentration-dependent
increase in the proportion of apoptotic cells (3.24, 6.06, 17.92
and 67.83%, respectively). Vaid et al (41) treated melanoma cells with silymarin at
concentrations of 0, 10, 20, 30, 40 and 60 µg/ml and performed flow
cytometric analysis to confirm the concentration-dependent increase
in the proportion of apoptotic cells. Therefore, the decreases in
survival rate and cell proliferation of AGS human gastric cancer
cells associated with silymarin treatment in the present study were
determined to be due to the induction of apoptosis.
Apoptosis induction can be divided into the
intrinsic pathway mediated by mitochondria and the extrinsic
pathway mediated by death receptors (27,28). The
Bcl-2 family major proteins involved in the intrinsic pathway,
among which Bax forms apoptosomes and activates caspase-3 to induce
apoptosis as a pro-apoptotic protein when its expression level is
increased. On the other hand, Bcl-2 inhibits Bax as an
anti-apoptotic protein to inhibit the induction of apoptosis
(30). PARP, which plays an important
role in the DNA repair, also regulates apoptosis (31). Therefore, western blotting analysis
was performed to examine the changes in protein expression when
apoptosis was induced by silymarin in AGS human gastric cancer
cells. The results indicated concentration-dependent increases in
expression levels of the pro-apoptosis proteins, cleaved PARP and
Bax. By contrast, the expression level of the anti-apoptosis
protein, Bcl-2, was decreased by silymarin in a
concentration-dependent manner. Katiyar et al (40) reported concentration-dependent
increases in the expression of Bax and cleaved PARP and a
concentration-dependent decrease in Bcl-2 expression in skin
epidermal cancer cells following treatment with silymarin. Fan
et al (36) also reported a
concentration-dependent increase in Bax expression and decrease in
Bcl-2 expression in silymarin-treated ovarian cancer cells.
Ramakrishnan et al (34)
reported a concentration-dependent increase in Bax expression
following treatment of liver cancer cells with silymarin. These
results indicated that silymarin altered the expression of
apoptosis-related proteins to induce apoptosis in AGS human gastric
cancer cells in a concentration-dependent manner.
As one of the protein kinase, mitogen-activated
protein kinases (MAPK) is a major signaling pathway involved in
delivering extracellular stimuli to the nucleus. MAPK can be
divided into ERK1/2, which plays an important role in regulating
cell proliferation, division, and viability, JNK/SAPK, and p38,
which play essential roles in inflammation and cell death by
reacting to various stimuli, such as extracellular stress (32). Therefore, silymarin-treated AGS human
gastric cancer cells were subjected to western blotting to
investigate the expression of ERK1/2, p38, and JNK, which are
involved in MAPK signaling. Increases in the expression levels of
p-p38 and p-JNK were observed, while p-ERK1/2 expression decreased
in a concentration-dependent manner in AGS cells treated with
silymarin at 0, 40 and 80 µg/ml for 24 h. Yan et al
(42) observed a
concentration-dependent decrease in p-ERK1/2 expression in A431
human epidermal cancer cells treated with silymarin. Huang et
al (43) reported the
concentration-dependent regulation of p-p38 and p-JNK expression in
silymarin-treated HeLa ovarian cancer cells. In summary, silymarin
appears to inhibit the growth and proliferation of cells by
regulating MAPK signaling and inducing apoptosis in
vitro.
AGS cells were injected into nude mice, and
silymarin was orally administered for 2 weeks at concentrations of
0 and 100 mg/kg to examine its effects on the tumor xenograft. The
tumor size began to vary in the silymarin injection group on day 7
after commencement of administration. On day 14, the tumor size was
inhibited by 46.2% in the silymarin injection group in comparison
with the control group. The tumor weights were 1.14 g in the
control group and 0.72 g in the injection group, indicating the
inhibition of tumor growth in the silymarin injection group. Won
et al (44) orally
administered silymarin at the concentration of 200 mg/kg to animals
with HSC-4 oral cancer tumor xenografts five times a week for 5
weeks, and reported the inhibition of tumor growth in the silymarin
injection group in comparison with the control. These findings
suggest that silymarin also inhibited the growth of AGS human
gastric cancer cells.
To examine whether the inhibition of tumor growth
was mediated by apoptosis, the extracted tumors were subjected to
TUNEL assay. The results indicated a 26% increase in TUNEL-positive
apoptotic cells in the 100 mg/kg silymarin injection group compared
to the control group. Singh et al (45) also reported an increase in
TUNEL-positive apoptotic cells in skin cancer tumors extracted from
mice treated with silymarin. Therefore, silymarin was suggested to
inhibit tumor growth by inducing apoptosis in the AGS tumor
xenografts in the present study.
ERK1/2 plays an important role in cell proliferation
and division, while JNK and p38 are major factors involved in the
MAPK signaling pathway, which is essential for cell death in
response to extracellular stimuli (32). Therefore, immunohistochemical analyses
were performed to examine the expression of proteins involved in
the MAPK signaling pathway in extracted tumor tissue in relation to
silymarin treatment. The results indicated decreased expression of
p-ERK1/2 and increased p-JNK and p-p38 expression in the silymarin
injection group (100 mg/kg) compared with the control group. These
observations were consistent with the effects of silymarin on
regulation of MAPK signaling pathway-related factors, p-ERK1/2,
p-JNK, and p-p38 in vitro as determined by western blotting
analyses.
Therefore, the present study indicated that
silymarin is highly promising for the development of gastric cancer
medications as it inhibits cell growth and tumor generation by
inducing apoptosis in AGS human gastric cancer cells both in
vitro and in vivo.
Acknowledgements
Not applicable.
Funding
The present research was supported by the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (NRF 2017R1A2B4005516).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SHK performed the experiments, analyzed the data and
wrote the manuscript. GSC, ESY, JSW, SHH and JHL helped perform the
experiments and assembled the data. SHK and JSW performed the
statistical analysis. SHK and ESY analyzed and interpreted the
results. JYJ designed the experiments and contributed to the
drafting of manuscript as a supervisor. All authors read and
approved the final manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The animal experiments were conducted in accordance
with the regulations of the Institutional Animal Care and Use
Committee with the approval of the Ethics Committee of Kongju
National University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jung KW, Won YJ, Kong HJ and Lee ES:
Prediction of cancer incidence and mortality in Korea, 2019. Cancer
Res Treat. 51:431–437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Won YJ, Sung J, Jung KW, Kong HJ, Park S,
Shin HR, Park EC, Ahn YO, Hwang IK, Lee DH, et al: Nationwide
cancer incidence in Korea, 2003–2005. Cancer Res Treat. 41:122–131.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH
and Lee JS: Cancer statistics in Korea: Incidence, mortality,
survival, and prevalence in 2011. Cancer Res Treat. 46:109–123.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forman D and Burley VJ: Gastric cancer:
Global pattern of the disease and an overview of environmental risk
factors. Best Pract Res Clin Gastroenterol. 20:633–649. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee MS, Cha EY, Thuong PT, Kim JY, Ahn MS
and Sul JY: Down-regulation of human epidermal growth factor
receptor 2/neu oncogene by corosolic acid induces cell cycle arrest
and apoptosis in NCI-N87 human gastric cancer cells. Biol Pharm
Bull. 33:931–937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li N, Fan LL, Sun GP, Wan XA, Wang ZG, Wu
Q and Wang H: Paeonol inhibits tumor growth in gastric cancer in
vitro and in vivo. World J Gastroenterol. 16:4483–4490. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Hou YH, Wu K, Zhai JS and Lin N:
Proteomic analysis reveals molecular biological details in
varioliform gastritis without helicobacter pylori infection. World
J Gastroenterol. 16:3664–3673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seeram NP: Berry fruits for cancer
prevention: Current status and future prospects. J Agric Food Chem.
56:630–635. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
An IJ, Kwon JK, Lee JS, Park HS, Kim DC,
Choi BJ, Lee KM, Park YJ and Jung JY: Induction of apoptosis in
human cancer cells with compositae extracts. J Korean Soc Food Sci
Nutr. 41:584–590. 2012. View Article : Google Scholar
|
|
12
|
Kwon MJ and Nam TJ: A polysaccharide of
the marine alga capsosiphon fulvescens induces apoptosis in AGS
gastric cancer cells via an IGF-IR-mediated PI3K/Akt pathway. Cell
Biol Int. 31:768–775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schuier M, Sies H, Illek B and Fischer H:
Cocoa-related flavonoids inhibit CFTR-mediated chloride transport
across T84 human colon epithelia. J Nutr. 135:2320–2325. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murakami A, Ashida H and Terao J:
Multitargeted cancer prevention by quercetin. Cancer Lett.
269:315–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren W, Qiao Z, Wang H, Zhu L and Zhang L:
Flavonoids: Promising anticancer agents. Med Res Rev. 23:519–534.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hackett ES, Twedt DC and Gustafson DL:
Milk thistle and its derivative compounds: A review of
opportunities for treatment of liver disease. J Vet Intern Med.
27:10–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen CH, Huang TS, Wong CH, Hong CL, Tsai
YH, Liang CC, Lu FJ and Chang WH: Synergistic anti-cancer effect of
baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem
Toxicol. 47:638–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ramasamy K and Agarwal R: Multitargeted
therapy of cancer by silymarin. Cancer Lett. 269:352–362. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Surai PF: Silymarin as a natural
antioxidant: An overview of the current evidence and perspectives.
Antioxidants (Basel). 4:204–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katiyar SK: Silymarin and skin cancer
prevention: Anti-inflammatory, antioxidant and immunomodulatory
effects (Review). Int J Oncol. 26:169–176. 2005.PubMed/NCBI
|
|
21
|
Féher J and Lengyel G: Silymarin in the
prevention and treatment of liver diseases and primary liver
cancer. Curr Pharm Biotechnol. 13:210–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eo HJ, Park GH and Jeong JB: Inhibition of
Wnt signaling by silymarin in human colorectal cancer cells. Biomol
Ther (Seoul). 24:380–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hajighasemlou S, Farajollahi M, Alebouyeh
M, Rastegar H, Manazi MT, Mirmoghtadaei M, Moayedi B, Ahmadzadeh M,
Kazemi M, Parvizpour F and Gharibzadeh S: Study of the effect of
silymarin on viability of breast cancer cell lines. Adv Breast
Cancer Res. 3:100–105. 2014. View Article : Google Scholar
|
|
24
|
Wu T, Liu W, Guo W and Zhu X: Silymarin
suppressed lung cancer growth in mice via inhibiting
myeloid-derived suppressor cells. Biomed Pharmacother. 81:460–467.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deep G, Singh RP, Agarwal C, Kroll DJ and
Agarwal R: Silymarin and silibinin cause G1 and G2-M cell cycle
arrest via distinct circuitries in human prostate cancer PC3 cells:
A comparison of flavanone silibinin with flavanolignan mixture
silymarin. Oncogene. 25:1053–1069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu HF, Houng JY, Kuo CF, Tsao N and Wu
YC: Glossogin, a novel phenylpropanoid from glossogyne tenuifolia,
induced apoptosis in A549 lung cancer cells. Food Chem Toxicol.
46:3785–3791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaufmann T, Strasser A and Jost PJ: Fas
death receptor signalling: Roles of Bid and XIAP. Cell Death
Differ. 19:42–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tummers B and Green DR: Caspase-8:
Regulating life and death. Immunol Rev. 277:76–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim D and Chung J: Akt: Versatile mediator
of cell survival and beyond. J Biochem Mol Biol. 35:106–115.
2002.PubMed/NCBI
|
|
30
|
Burz C, Berindan-Neagoe I, Balacescu O and
Irimie A: Apoptosis in cancer: Key molecular signaling pathways and
therapy targets. Acta Oncol. 48:811–821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soldani C and Scovassi AI: Poly
(ADP-ribose) polymerase-1 cleavage during apoptosis: An update.
Apoptosis. 7:321–328. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ramakrishnan G, Lo Muzio L, Elinos-Báez
CM, Jagan S, Augustine TA, Kamaraj S, Anandakumar P and Devaki T:
Silymarin inhibited proliferation and induced apoptosis in hepatic
cancer cells. Cell Prolif. 42:229–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhong X, Zhu Y, Lu Q, Zhang J, Ge Z and
Zheng S: Silymarin causes caspases activation and apoptosis in K562
leukemia cells through inactivation of Akt pathway. Toxicology.
227:211–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan L, Ma Y, Liu Y, Zheng D and Huang G:
Silymarin induces cell cycle arrest and apoptosis in ovarian cancer
cells. Eur J Pharmacol. 743:79–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vaid M, Prasad R, Sun Q and Katiyar SK:
Silymarin targets β-catenin signaling in blocking
migration/invasion of human melanoma cells. PLoS One. 6:e230002011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Halicka HD, Bedner E and Darzynkiewicz Z:
Segregation of RNA and separate packaging of DNA and RNA in
apoptotic bodies during apoptosis. Exp Cell Res. 260:248–256. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Katiyar SK, Roy AM and Baliga MS:
Silymarin induces apoptosis primarily through a p53-dependent
pathway involving Bcl-2/Bax, cytochrome c release, and caspase
activation. Mol Cancer Ther. 4:207–216. 2005.PubMed/NCBI
|
|
41
|
Vaid M, Singh T, Prasad R and Katiyar SK:
Silymarin inhibits melanoma cell growth both in vitro and in vivo
by targeting cell cycle regulators, angiogenic biomarkers and
induction of apoptosis. Mol Carcinog. 54:1328–1339. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan C, Yahua WU, Wang Z, Yin C and Yin M:
Inhibitory effect of silymarin on human osteosarcoma Saos-2 cells
and its mechanism. Chin Pharmacol Bull. 32:966–969. 2016.(In
Chinese).
|
|
43
|
Huang Q, Wu LJ, Tashiro S, Onodera S, Li
LH and Ikejima T: Silymarin augments human cervical cancer HeLa
cell apoptosis via P38/JNK MAPK pathways in serum-free medium. J
Asian Nat Prod Res. 7:701–709. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Won DH, Kim LH, Jang B, Yang IH, Kwon HJ,
Jin B, Oh SH, Kang JH, Hong SD, Shin JA and Cho SD: In vitro and in
vivo anti-cancer activity of silymarin on oral cancer. Tumor Biol.
40:10104283187761702018. View Article : Google Scholar
|
|
45
|
Singh RP, Tyagi AK, Zhao J and Agarwal R:
Silymarin inhibits growth and causes regression of established skin
tumors in SENCAR mice via modulation of mitogen-activated protein
kinases and induction of apoptosis. Carcinogenesis. 23:499–510.
2002. View Article : Google Scholar : PubMed/NCBI
|