Introduction
Traditional Chinese medicines, which are widely used
in certain cultures and traditions worldwide, have been recommended
as effective complementary and alternative medicines for numerous
different types of disease by the World Health Organization
(1). Berberine (BBR), which is the
main active ingredient derived from Traditional Chinese medicinal
herbs belonging to the Berberidaceae, Ranunculaceae and
Papaveraceae families, has been reported to possess multiple
biological and medicinal properties, including
cholesterol-reducing, antioxidant, antibacterial, anti-inflammatory
and immunomodulatory effects (2–4). To
date, BBR has also received significant attention due to its
observed therapeutic potential in different types of cancer.
Several research groups have reported that BBR exerted
antineoplastic effects by inhibiting proliferation, migration and
invasion, and inducing apoptosis in various cancer types, including
lung (5), breast (6,7),
tongue (8) and esophageal
(9) cancer, as well as
hepatocellular carcinoma (10),
melanoma (11), glioblastoma
(12) and pancreatic cancer
(13). Numerous studies have also
identified telomerase, DNA topoisomerase I, p53, NF-κB,
Wnt/β-catenin, AMP-activated protein kinase (AMPK) and estrogen
receptors as molecular targets through which BBR exerts its
antitumor effects (4,8,11,12).
However, although the beneficial effects of BBR appear to be
partially mediated by these aforementioned targets, few studies
have focused on the effects of BBR on the glucose metabolism
process and associated proteins, which may represent an additional
possible mechanism underlying the antineoplastic effects of
BBR.
The Warburg effect is a common hallmark of cancer
cells, which have an increased energy metabolic demand, but
preferentially undergo glycolysis (14). Numerous studies have revealed that
glycolysis levels increase in different human malignancies despite
the presence of an adequate oxygen supply to support aerobic
respiration (15–17). Notably, our preliminary experiments
identified that BBR strongly decreased the glucose uptake ability
of HepG2 and MCF7 cell lines, therefore, it was hypothesized that
BBR may interfere with tumor progression by inhibiting
glycolysis.
Glucose transmembrane transport is the first step of
glucose metabolism and it is also the rate-limiting step of
glycolysis (18). As a member of
the glucose transporter family, glucose transporter 1 (GLUT1)
regulates glucose transport across the cell membrane (19). Multiple studies have demonstrated
that GLUT1 expression levels were upregulated and associated with a
shorter overall survival in prostate, lung and pancreatic cancer
(20,21). Furthermore, GLUT1 was discovered to
play a key role in various types of cancer, such as hepatocellular
carcinoma, and breast and renal cancer, by promoting cell
proliferation, migration and invasion, and inhibiting apoptosis
(22–24). However, whether GLUT1 mediates the
antineoplastic effects of BBR via regulating glucose metabolism is
yet to be elucidated, to the best of our knowledge.
The present study used HepG2 liver and MCF7 breast
cancer cell lines, and the normal fibroblastic
epithelial/myoepithelial cell line, Hs 578Bst (derived from the
human breast) to investigate the biological effects of BBR. In
addition, the study also determined the potential of BBR to reverse
the Warburg effect and whether the underlying antineoplastic
mechanism of BBR was mediated by GLUT1.
Materials and methods
Cell lines and culture
MCF7 (HTB-22), HepG2 (HB-8065) and Hs 578Bst
(HTB-125) cell lines were purchased from the American Type Culture
Collection and cultured routinely at 37°C in a humidified 5%
CO2 atmosphere in DMEM containing 10% fetal bovine serum
(FBS), 100 U/ml penicillin and 100 mg/ml streptomycin. All
constituents used were purchased from Sigma-Aldrich; Merck
KGaA.
Cell Counting Kit-8 (CCK-8) assay
The antiproliferative effect of BBR was determined
using CCK-8 reagent (GLPBIO Technology, Inc.) according to the
manufacturer's protocol. Briefly, cells were seeded into 96-well
plates at a density of 5×103 cells/well in DMEM
supplemented with 10% FBS, and allowed to adhere for 24 h.
Following the incubation, the cells were cultured in medium
supplemented with 1% FBS in the presence or absence of different
concentrations of BBR (10, 25, 50, 75 or 100 µM) (Sigma-Aldrich;
Merck KGaA) for a further 24 or 48 h. The negative control group
was treated with DMSO and the positive control was exposed to
glucose deprivation. After the treatment, 10 µl CCK-8 reagent was
added into each well and incubated at 37°C for another 3 h. The
optical densities were measured at wavelengths of 450 and 630 nm
using a microplate reader (Multiskan Mk3; Thermo Fisher Scientific,
Inc.).
Colony formation assay
A total of 3×102 cells/well were plated
into 6-well plates and cultured for 2 days. Following the initial
culture, cells were treated with BBR (50 µM for HepG2 and Hs 578Bst
cells; and 25 µM for MCF7 cells). Following 14 days of incubation,
the cells were fixed with 4% paraformaldehyde and stained with 5%
Giemsa solution (Beijing Leagene Biotech Co., Ltd.) at room
temperature (RT) for 15 min. After removing the staining solution,
the cells were thoroughly washed in distilled water three times and
air-dried. Only the clones with >10 cells were counted under
×40-magnified visual fields with an inverted light microscope
(IX71; Olympus Corporation), and images of the 6-well plate were
captured with a Nikon DX digital camera (D5000; Nikon
Corporation).
Flow cytometric analysis of the cell
cycle and apoptosis
For the cell cycle analysis, cells were seeded into
a 6-well plate at a density of 1×106 cells/well and
incubated with BBR at 37°C for 24 h. The cells were then
trypsinized and harvested by centrifugation (200 × g) at RT for 10
min, washed with cold PBS and fixed with 70% ethanol at 4°C
overnight. Following the incubation, cells were resuspended and
incubated with 50 µg/ml RNase A for 30 min at RT. After
centrifugation (200 × g), the cells were resuspended in PBS and
intracellular DNA was labeled with 50 µg/ml PI (Invitrogen; Thermo
Fisher Scientific, Inc.) by incubation in the dark for 15 min at
RT. Cell cycle analysis was performed using a flow cytometer
(FACScan; BD Biosciences).
Annexin V/PI double staining was performed for
apoptosis analysis. Briefly, after treatment with BBR for 24 h,
1×106 cells were trypsinized, washed with cold PBS and
resuspended in 200 µl binding buffer. The cells were subsequently
stained with 0.5 µg/ml Annexin V-FITC and 50 µg/ml PI in the dark
for 15 min, then analyzed using a FACScan flow cytometer. The
apoptotic rate was calculated as the sum of the cell proportion
undergoing early apoptosis (lower right quadrant) and the cell
proportion undergoing late-stage apoptosis (upper right quadrant),
and then the differences in the cell apoptotic rate between the BBR
and control groups were compared.
The cell cycle and apoptosis data were analysed
using FlowJo V10 software (Tree Star, Inc.).
Luminescence ATP detection assay
ATP levels were determined using an ATPlite
Luminescence ATP assay kit (cat. no. 6016736; PerkinElmer, Inc.)
according to the manufacturer's protocol. In total,
5×103 cells/well were plated into white opaque 96-well
CulturPlates (PerkinElmer, Inc.) and after 24 h of incubation at
37°C, ATP levels were determined. Briefly, 50 µl mammalian cell
lysis solution (provided in the kit) was added to each well of the
microplate and the plates were mixed on an orbital shaker (100 × g)
for 5 min at RT to lyse the cells and stabilize the ATP. Then, 50
µl Luciferase/Luciferin substrate solution was added to each well
of the microplate and mixed on the orbital shaker for 5 min. The
plate was subsequently incubated in the dark for 10 min and the
luminescence of each well was measured using a spectrophotometric
microplate reader (EnVision™; PerkinElmer, Inc.).
Glucose uptake assay
The cell medium was harvested after BBR treatment
(50 µM for HepG2 cells and 25 µM for MCF7 cells) for 24 h, and the
concentration of glucose in the media was measured using a Glucose
Uptake Colorimetric assay kit (cat. no. K676-100; BioVision, Inc.)
according to the manufacturer's protocol. The optical density was
measured at a wavelength of 412 nm using a microplate reader
(Infinite F50; Tecan Group, Ltd.).
Immunofluorescence staining of
GLUT1
In total, 6×104 cells were plated onto
glass coverslips. Following treatment with BBR (50 µM for HepG2
cells and 25 µM for MCF7 cells) for 24 h, the cells were washed
three times with PBS, then fixed with 4% paraformaldehyde and
permeabilizated with 0.1% Triton X-100 at RT. Non-specific binding
was blocked by incubation with 5% BSA (Beijing Solarbio Science
& Technology Co., Ltd.) for 30 min at RT and the cells were
subsequently incubated with an anti-rabbit polyclonal GLUT1
antibody (1:1,000; cat. no. 21829-1-AP; ProteinTech Group, Ltd.)
overnight at 4°C. Following the primary antibody incubation, the
cells were incubated with an Alexa Fluor 488-conjugated secondary
antibody (1:200; product code ab150077; Abcam) at 37°C for 1 h.
Nuclei were stained with 10 µM Hoechst 33258 (ADooQ Bioscience) for
1 min. Stained cells were visualized using an inverted fluorescence
microscope (IX71; Olympus Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Takara Bio, Inc.). Total RNA (2 µg) was
reverse transcribed into cDNA using a RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.) for 15 min at 30°C,
42°C for 60 min and 72°C for 10 min using a 10 µl reaction volume.
qPCR was subsequently performed using SYBR Premix Ex Taq (Takara
Bio, Inc.) according to the manufacturer's protocol, on a 7500
Real-Time PCR Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The qPCR reaction mixture (20 µl volume) was
measured in duplicate using the following thermocycling conditions:
Initial denaturation for 3 min at 95°C, followed by 45 cycles at
95°C for 15 sec, 60°C for 30 sec and 72°C for 15 min. The following
primer sequences were used for the qPCR: GLUT1 forward,
5′-TGGCATCAACGCTGTCTTCT-3′ and reverse, 5′-CTAGCGCGATGGTCATGAGT-3′;
and β-actin forward, 5′-TGACGTGGACATCCGCAAAG-3′ and reverse,
5′-CTGGAAGGTGGACAGCGAGG-3′. The mRNA relative expression levels of
GLUT1 were quantified using the 2−ΔΔCq method (25). β-actin was used as the internal
reference control.
Western blotting
Cells were seeded into a 6-well plate at a density
of 1×106 cells/well and cultured to 70–90% confluence,
and then incubated with BBR (50 µM for HepG2 cells and 25 µM for
MCF7 cells) at 37°C for 24 h. According to experimental
requirements, 8 µg/ml SC79 (cat. no. SF2730; Beyotime Institute of
Biotechnology), 50 µM MG-132 (cat. no. HY-13259; MedChemExpress) or
50 µM Leupeptin hemisulfate (cat. no. HY-18234A; MedChemExpress)
was pretreated at 37°C for 8 h before BBR treatment. Subsequently,
total protein was extracted from cells using RIPA lysis buffer
(cat. no. C1053; Applygen Technologies, Inc.) supplemented with
protease inhibitor cocktail (Roche Diagnostics). The total protein
concentration was determined using the bicinchoninic acid (BCA)
reaction, and 20 µg/lane of protein samples were separated via 8%
SDS-PAGE and transferred onto PVDF membranes, which were blocked
with 5% non-fat milk at RT for 90 min. Membranes were subsequently
incubated with the following primary antibodies at 4°C overnight:
Rabbit anti-GLUT1 (1:1,000), rabbit anti-phosphorylated
(p)-Akt-S473 (1:1,000; cat. no. AP0140; ABclonal Biotech Co.,
Ltd.), rabbit anti-Akt (1:1,000; cat. no. A11016; ABclonal Biotech
Co., Ltd.), rabbit anti-p-mTOR-S2448 (1:1,000; cat. no. AP0094;
ABclonal Biotech Co., Ltd.), rabbit anti-mTOR (1:1,000; cat. no.
20657-1-AP; ProteinTech Group, Inc.) and rabbit anti-GAPDH
(1:1,000; product no. 2118S; Cell Signaling Technology, Inc.).
Following the primary antibody incubation, the membranes were
washed and incubated at RT for 90 min with HRP-conjugated goat
anti-rabbit secondary antibody (1:3,000; cat. no. AS014; ABclonal
Biotech Co., Ltd.). Protein bands were visualized using a
SuperSignal™ West Pico PLUS Chemiluminescent substrate (Thermo
Fisher Scientific, Inc.) on a gel imaging system (GE Healthcare),
and the band density was quantified by densitometric analysis using
ImageJ V1.8.0 software (NIH).
Generation of Flag- or histidine
(His)-tagged proteins
The open reading frames (ORFs) of GLUT1, ubiquitin
conjugating enzyme E2 I (Ubc9) and Gα-interacting
protein-interacting protein at the C-terminus (GIPC) were amplified
with PrimerSTAR® Max DNA Polymerase (cat. no. R045A;
Takara Bio, Inc.) using cDNA as a template. The primers are as
follow: Flag-GLUT1 forward,
5′-CCCAAGCTTATGGATTACAAGGACGACGATGACAAGATGGAGCCCAGCAGCAAGAAGCTGA-3′
and reverse, 5′-CCGCTCGAGTCACACTTGGGAATCAGCCCCCAGG-3′; His-Ubc9
forward,
5′-CCCAAGCTTATGCATCATCACCATCACCATATGAGTAGTTTGTGTCTACAGCGTC-3′ and
reverse, 5′-CCGCTCGAGCTATTTAGAGTACTGTTTAGCTTG-3′; and His-GIPC
forward,
5′-CCCAAGCTTATGCATCATCACCATCACCATATGCCGCTGGGACTGGGGCGGCGGA-3′ and
reverse, 5′-CCGCTCGAGCTAGTAGCGGCCGACCTTGGCGTCC-3′. The cycling
conditions comprised initial 5-min polymerase activation at 95°C,
followed by 30 cycles at 95°C for 30 sec, 57°C for 30 sec, and 72°C
for 1 min, and ultimately 72°C for 5 min. Following the
amplification, the ORF with either the Flag- or His-tag was
inserted between the HindIII and XhoI sites of the
pcDNA3.0 plasmid (Bioeagle Biotech Company, Ltd.). The resulting
constructs were sequenced to verify the insertion of the desired
tags.
Co-immunoprecipitation assay
Cells were seeded into 6-well plates, cultured to
70–90% confluence and then co-transfected with 2 µg Flag-GLUT1
alongside 2 µg empty, His-Ubc9 or His-GIPC plasmid into MCF7 cells
using TurboFect™ DNA transfection reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Cells
were subsequently treated with 25 µM BBR or DMSO for 48 h.
Following the treatment, the cells were collected and centrifuged
at 200 × g for 10 min at 4°C, washed twice with PBS and dissolved
in weak RIPA lysis buffer (Beyotime Institute of Biotechnology)
supplemented with 1X EDTA-free complete protease inhibitor (Roche
Diagnostics). The lysates (100 µl) were pre-cleared with 20 µl
Protein A/G PLUS-Agarose beads (cat. no. sc-2003; Santa Cruz
Biotechnology, Inc.) at 4°C for 2 h. The precleared lysates were
subsequently incubated with anti-Flag antibody (1:50; cat. no.
8146) or anti-His antibody (1:50; cat. no. 2365; both from Cell
Signaling Technology, Inc.) at 4°C for 4 h, then with 50 µl Protein
A/G PLUS-Agarose beads at 4°C overnight. After the incubation, the
Protein A/G PLUS-Agarose beads with bound proteins were washed with
pre-cooled PBS containing 1X EDTA-free complete protease inhibitor
four times and boiled with 60 µl 1X protein loading buffer for 10
min at 95°C to elute the bound proteins. The co-immunoprecipitation
products were detected using western blotting and anti-Flag
(1:1,000) and anti-His (1:1,000) antibodies, as described in the
western blotting section.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 software (GraphPad Software, Inc.) and data are presented
as the mean ± SD. Statistical differences between two groups were
determined using unpaired Student's t-test, while a one-way ANOVA
followed by a Bonferroni's post hoc test was used for multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Antineoplastic activity of BBR on
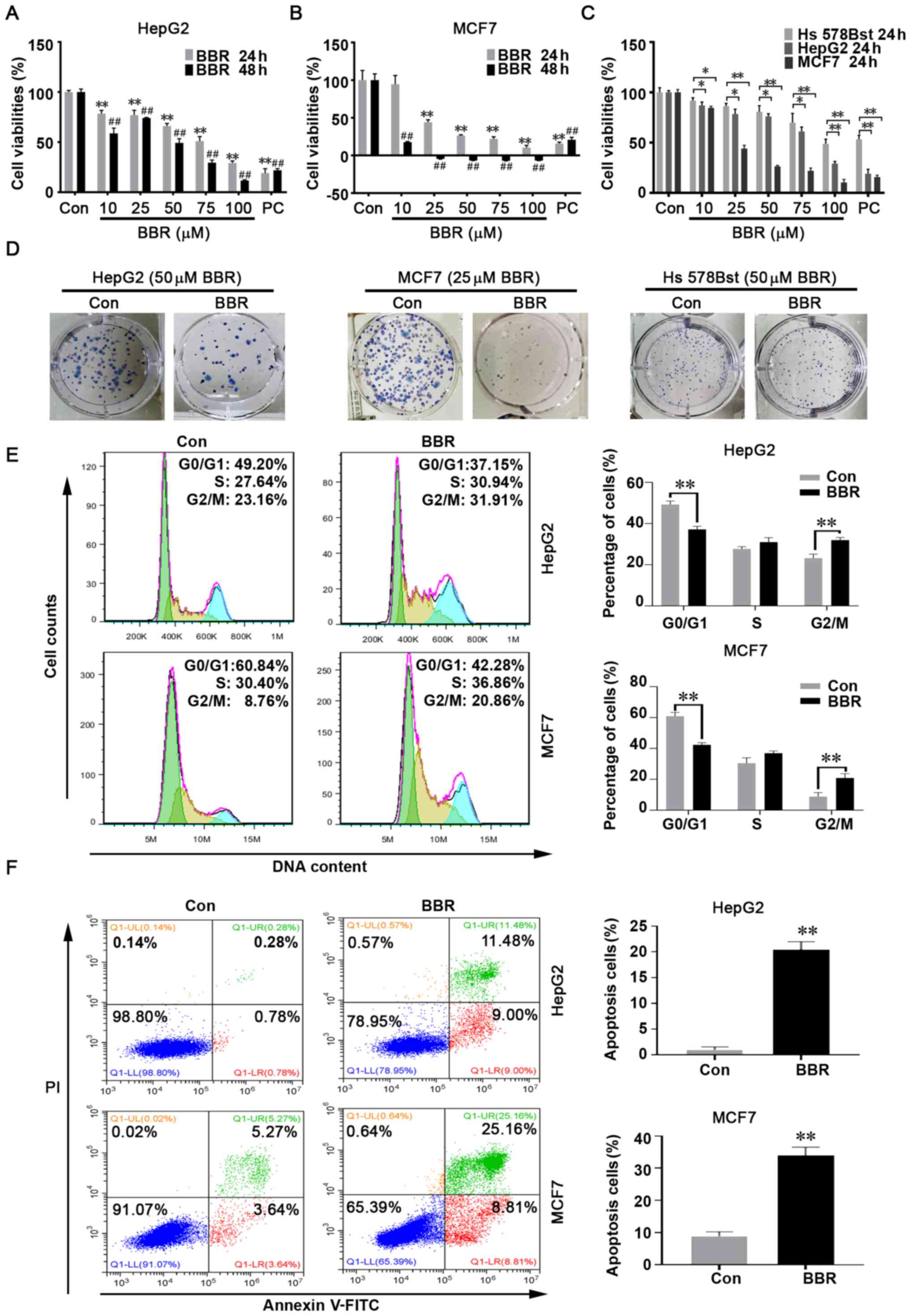
cancer cells
To determine how BBR affected the biological traits
of cancer cells, HepG2 and MCF7 cells, and normal breast cells, Hs
578Bst, were treated with different concentrations of BBR (10, 25,
50, 75 or 100 µM) and cell viability was analyzed using a CCK-8
assay. The results revealed that the viability was significantly
suppressed in both cancer cell lines following BBR treatment
compared with the DMSO treatment group, with the greatest reduction
in viability observed in MCF7 cells, in which BBR exerted a
significant cytotoxic effect at concentrations >25 µM after 48 h
of treatment (Fig. 1A and B).
Moreover, the antiproliferative effect of BBR on the two cancer
cell lines was significantly higher compared with that on Hs 578Bst
cells (Fig. 1C).
As another indicator of cell proliferation, colony
formation assays were performed. The results demonstrated that the
colony-forming capacity was slightly inhibited in Hs 578Bst normal
breast cells following BBR treatment, but significantly inhibited
in both cancer cell lines. In particular, MCF7 cells were unable to
form colonies following 50 µM BBR treatment (data not shown) and
only small colonies formed after 25 µM BBR treatment (Fig. 1D). Therefore, follow-up experiments
were performed using 50 µM BBR to treat HepG2 cells and 25 µM BBR
to treat MCF7 cells; the negative control group was treated with
DMSO and the positive control was exposed to glucose
deprivation.
To further determine whether BBR affected cell
proliferation, flow cytometry was used to analyze the cell cycle
distribution of HepG2 and MCF7 cells. The results revealed that BBR
effectively induced cell cycle arrest at the G2M phase
(Figs. 1E and S1).
When proliferation is blocked, cells may initiate
the apoptotic program (26).
Therefore, Annexin V/PI double staining was performed and the
results revealed that BBR treatment reduced the percentage of live
cells and increased the percentage of cells in the middle and late
apoptotic stages (Fig. 1F).
These findings indicated that BBR may exert
extensive and effective antitumor activity, which was demonstrated
by its ability to inhibit cell proliferation and colony formation,
induce cell cycle arrest and promote apoptosis.
Antineoplastic effects of BBR are
associated with the reversal of the Warburg effect
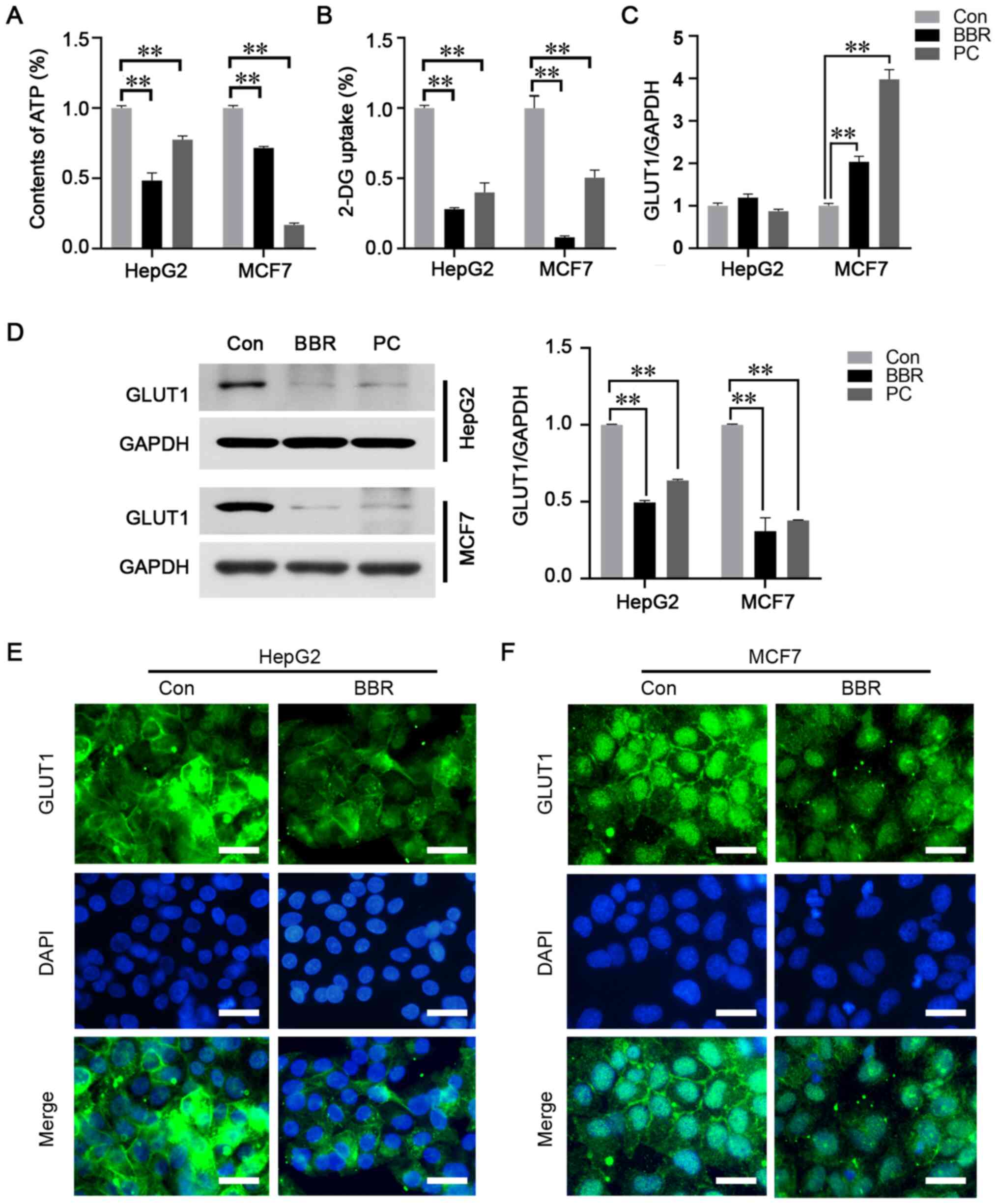
To investigate whether the Warburg effect was a key
modulator on the antineoplastic effects of BBR, ATP content and
glucose uptake were analyzed using luminescence ATP detection and
glucose uptake assays, respectively. The results revealed that both
the ATP levels and glucose uptake capacity were significantly
reduced in the cell lines following BBR treatment (Fig. 2A and B).
GLUT1 is a key regulatory component that mediates
glucose transmembrane transport (20). To determine whether GLUT1 mediated
the effects of BBR on glucose uptake, the expression and
distribution of GLUT1 was detected. RT-qPCR analysis revealed that
the mRNA expression levels of GLUT1 were not altered in HepG2
cells, but were upregulated in MCF7 cells following BBR treatment
(Fig. 2C). However, western blot
analysis revealed that GLUT1 expression levels were significantly
downregulated in both cancer cell lines (Fig. 2D). Immunofluorescence analysis
demonstrated that the green fluorescence intensity of GLUT1 was
reduced in BBR-treated cells. In addition, GLUT1 was found to be
located within the cell membrane in the control group, whereas
following BBR treatment, the membrane distribution was diminished
in HepG2 cells and was absent in MCF7 cells (Fig. 2E and F). It is worth noting that
further to the significant membranous distribution of GLUT1 in
BBR-untreated MCF7 cells, GLUT1 was also revealed to be located in
the nucleus in both BBR treated and untreated cells (Fig. 2F).
These findings indicated that the antineoplastic
effects of BBR may be associated with the regulation of GLUT1,
causing the subsequent reversal of the Warburg effect.
BBR-induced reversal of the Warburg
effect is mediated by the Akt/mTOR/GLUT1 signaling pathway
As it was revealed that BBR exerted a significant
effect over ATP synthesis and glucose metabolism to inhibit cancer
progression, the kinase activities of Akt and mTOR, classical
signaling molecules that have roles in glucose metabolism, cell
proliferation, survival and apoptosis (15), were analyzed. Western blot analysis
demonstrated that the phosphorylation levels of Akt and its
downstream signaling protein, mTOR, were significantly suppressed
in both cancer cell lines (Fig. 3A and
B). These findings suggested that BBR may exert its
antineoplastic effect via regulating the Akt/mTOR signaling
pathway. Cells were subsequently treated with 8 µg/ml SC79, a
unique specific activator of Akt, to augment the levels of Akt
phosphorylation. As anticipated, the results revealed that the
BBR-induced downregulation of p-Akt, p-mTOR and GLUT1 expression
levels (Fig. 3C), ATP synthesis
(Fig. 3D) and glucose uptake
(Fig. 3E) was abolished by SC79
pretreatment. Furthermore, the BBR-induced inhibition of viability
and induction of apoptosis in HepG2 and MCF7 cells was reversed
after pretreatment with SC79 (Fig.
3F-H).
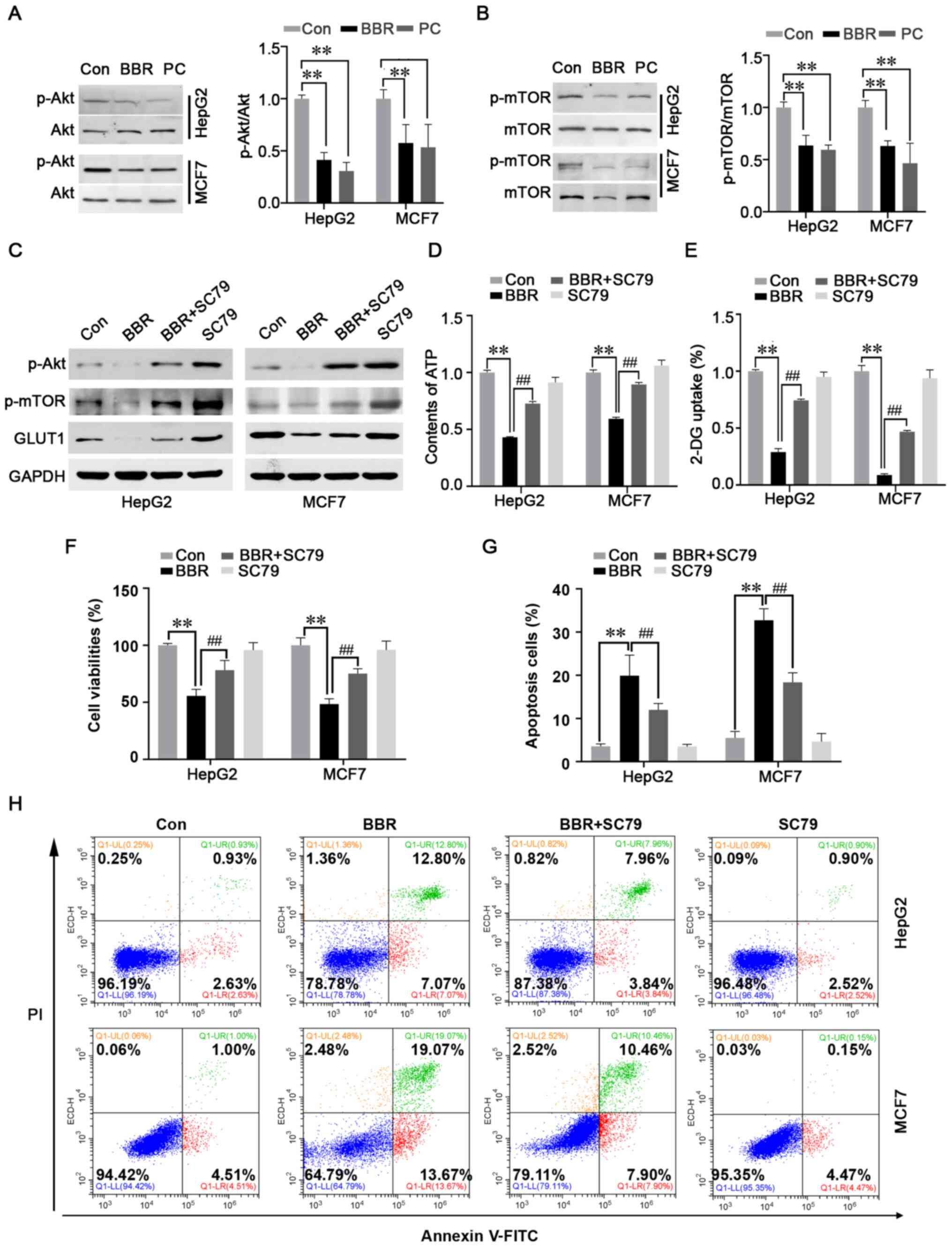 | Figure 3.BBR-induced reversal of the Warburg
effect is mediated by the Akt/mTOR/GLUT1 signaling pathway. Effect
of BBR on the expression levels of (A) p-Akt/Akt and (B)
p-mTOR/mTOR were analyzed using western blotting. Levels of
phosphorylated protein were normalized to the level of
corresponding total protein. Cells were pretreated with the Akt
activator, SC79, prior to BBR treatment. (C) Expression levels of
p-Akt, p-mTOR and GLUT1 were analyzed using western blotting. (D)
ATP content was detected using a luminescence ATP detection assay
kit. (E) 2-DG uptake was detected using a glucose uptake assay kit.
Cells were pretreated with SC79 before BBR treatment, then the (F)
viabilities of HepG2 and MCF7 cells were analyzed using a Cell
Counting Kit-8 assay and the (G and H) apoptosis rate was analyzed
by flow cytometry. Data are presented as the mean ± SD; n=3.
**P<0.01 and ##P<0.01. BBR, berberine; GLUT1,
glucose transporter 1; p-, phosphorylated; 2-DG, 2-deoxy-D-glucose;
Con, control; PC, positive control. |
These findings indicated that the BBR-induced
reversal of the Warburg effect may be mediated via the
Akt/mTOR/GLUT1 signaling pathway.
Downregulation of GLUT1 expression may
be due to proteasomal degradation
BBR was discovered to downregulate the protein
expression levels of GLUT1 and reduce the membranous distribution,
which would induce GLUT1 dysfunction and prevent the transport of
glucose. The most common cause of downregulated protein expression
is the reduction in transcription or increase in post-translational
degradation (27). As BBR did not
downregulate the mRNA expression levels of GLUT1 in both HepG2 and
MCF7 cells (Fig. 2C), it was
hypothesized that BBR may degrade GLUT1. To investigate this
hypothesis, MCF7 cells were used, as the MCF7 cell line was more
sensitive to BBR than the HepG2 cells. MCF7 cells were pretreated
with the proteasome inhibitor, MG-132, or lysosomal inhibitor,
leupeptin, to inhibit the proteasomal or lysosomal degradation
pathways, respectively, prior to BBR treatment. The results
revealed that the BBR-induced downregulation of GLUT1 expression
levels was significantly inhibited by the pretreatment with MG-132,
but not leupeptin (Fig. 4A and B).
These findings indicated that the downregulated expression levels
of GLUT1 may be due to proteasomal degradation.
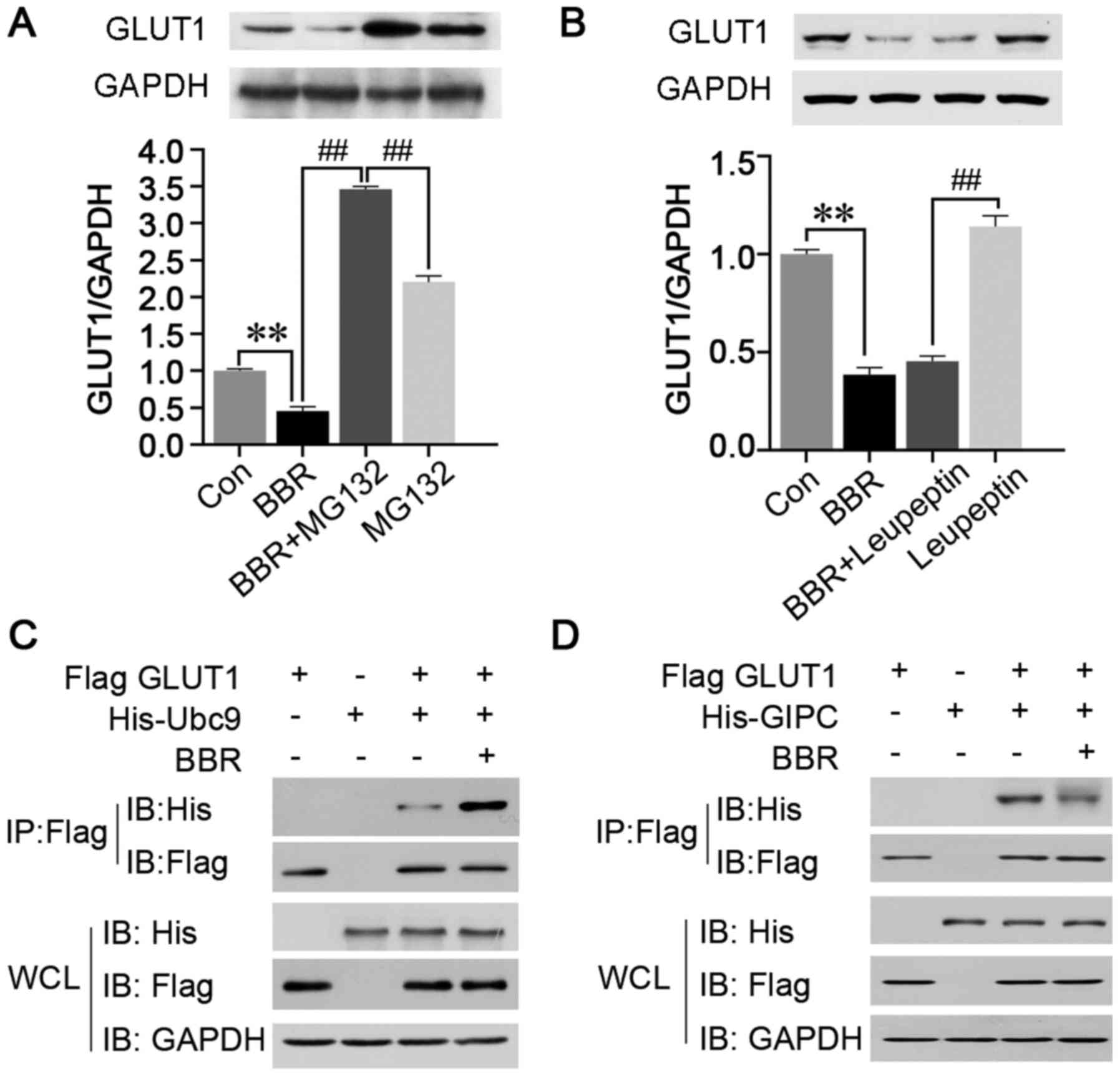 | Figure 4.Ubc9 and GIPC mediate the
post-translational modification and cytoplasmic retention of GLUT1,
respectively. MCF7 cells were pretreated with the (A) proteasome
inhibitor MG-132 or (B) lysosomal inhibitor, leupeptin, and the
expression levels of GLUT1 were analyzed using western blotting.
Flag-tagged GLUT1, His-tagged Ubc9 and His-tagged GIPC were
constructed, and the interactions between (C) Ubc9 and GLUT1 and
(D) GIPC and GLUT1 were analyzed using co-immunoprecipitation. Data
are presented as the mean ± SD; n=3. **P<0.01 and
##P<0.01. Ubc9, ubiquitin conjugating enzyme E2 I;
GIPC, Gα-interacting protein-interacting protein at the C-terminus;
GLUT1, glucose transporter 1; Con, control; His, histidine; WCL,
whole cell lysate. |
Ubc9 may mediate the
post-translational degradation of GLUT1
Ubc9, a structural homologue of the E2
ubiquitin-conjugating enzyme, was previously reported to be
involved in the post-translational degradation of GLUT1 (28). Thus, co-immunoprecipitation assays
using Flag-tagged GLUT1 and His-tagged Ubc9 were performed in MCF7
cells following BBR treatment. The results demonstrated that the
interaction between GLUT1 and Ubc9 was significantly increased,
which suggested that the BBR-induced post-translational degradation
of GLUT1 may be regulated by the post-translational modifications
mediated by Ubc9 (Fig. 4C).
Disruption of GIPC binding to GLUT1
may mediate the cytoplasmic retention of GLUT1
GIPC, a PDZ domain containing protein, was reported
to specifically interact with the PDZ binding motif, DSQV, at the
C-terminus of GLUT1, thereby promoting the stability of
intracellular GLUT1 and assisting in its return to the cell
membrane (29). Therefore,
co-immunoprecipitation of Flag-tagged GLUT1 and His-tagged GIPC was
performed in MCF7 cells following BBR treatment, and the results
revealed that the interaction between GLUT1 and GIPC was disrupted
(Fig. 4D). This finding indicated
that BBR may lead to the retention of GLUT1 in the cytoplasm by
inhibiting the binding between GLUT1 and GIPC.
The proposed mechanism of action for the
antineoplastic effects of BBR, which suggests that BBR can reverse
the Warburg effect via modulation of the Akt/mTOR/GLUT1 signaling
pathway, is presented in Fig.
5.
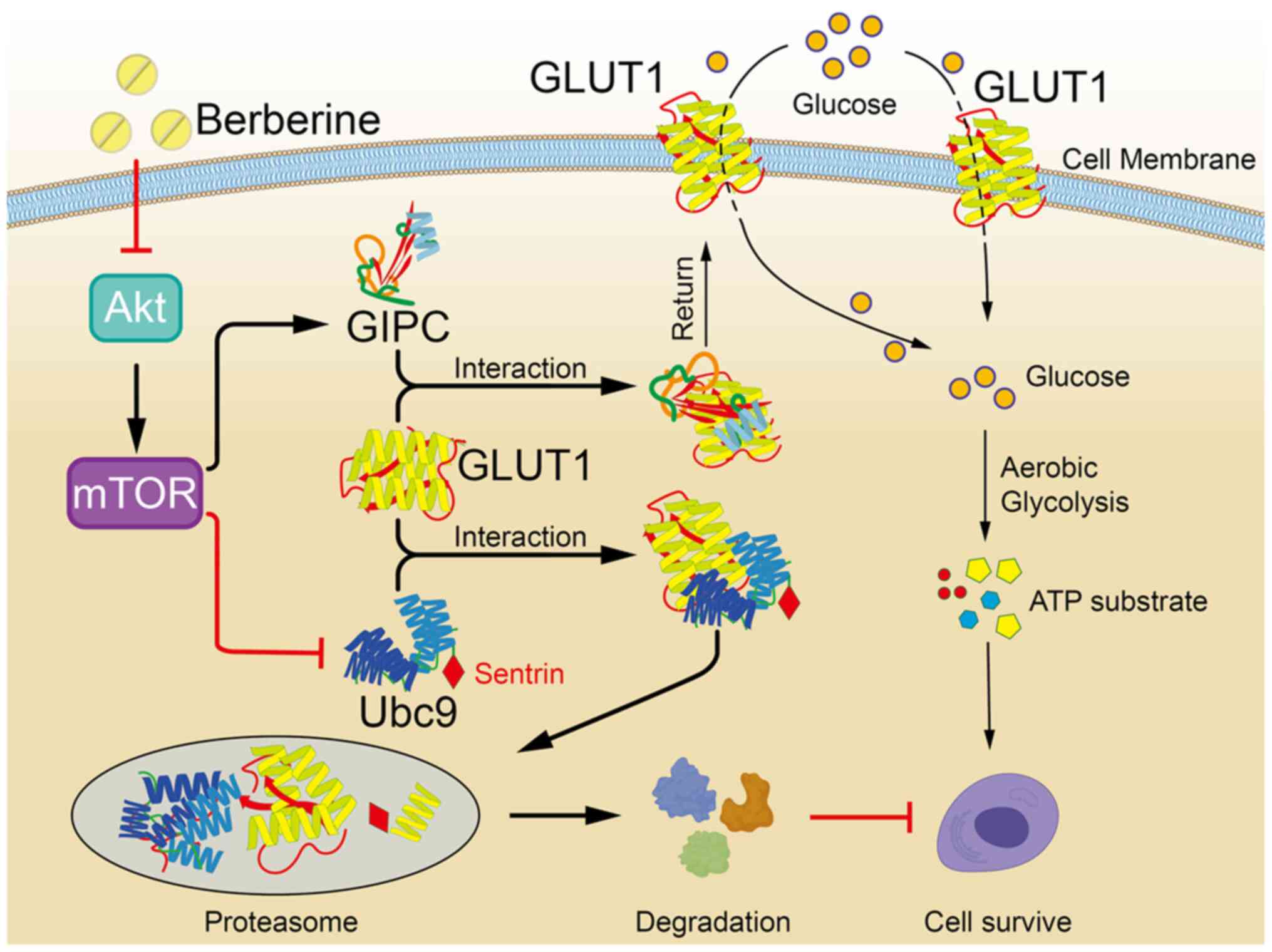 | Figure 5.Proposed underlying mechanism of the
antineoplastic effects of BBR, which involves the reversal of the
Warburg effect via downregulating the Akt/mTOR/GLUT1 signaling
pathway. Treatment with BBR downregulates the expression levels of
p-Akt in cancer cells, which in turn downregulates the levels of
its downstream signaling protein, p-mTOR. Subsequently, the binding
between GIPC and GLUT1 is weakened, which results in the
cytoplasmic retention of GLUT1. The weakened binding of GIPC with
GLUT1 also strengthens the binding between Ubc9 and GLUT1, which
leads to the post-translational degradation of GLUT1 and further
diminishes the glucose transport function of GLUT1. Consequently,
the glucose uptake capacity of cancer cells and ATP synthesis are
decreased, therefore the Warburg effect of cancer cells is
reversed, which contributes to the antineoplastic activity of BBR.
BBR, berberine; Ubc9, ubiquitin conjugating enzyme E2 I; GIPC,
Gα-interacting protein-interacting protein at the C-terminus;
GLUT1, glucose transporter 1; p-, phosphorylated. |
Discussion
The search for novel antineoplastic drugs that are
effective in tumors refractory to conventional therapy is crucial
for the development of efficient anticancer therapies. BBR is a
commonly used drug in Traditional Chinese medicine and recently,
its reported antineoplastic effects have attracted significant
attention. Previous studies have revealed that BBR inhibited
proliferation, invasion and metastasis, and induced cell cycle
arrest and apoptosis in multiple types of cancer (5–12).
It has been reported that 500 µg/ml BBR could inhibit cell
viability by 85 and 87% on HepG2 and MCF7 cells respectively, and
after treatment with BBR at a low concentration of 56 µg/ml, the
inhibitory rate was reduced to 19 and 11% on HepG2 and MCF7 cells
respectively. When the concentration of BBR was 19 µg/ml, no
inhibitory effect on HepG2 and MCF7 cells was observed (30). Another study also reported that
high-doses of BBR (50 and 100 µM) markedly inhibited HepG2 cell
survival by 41 and 36% respectively in 48 h, while a relative low
dose of BBR (10 µM) had almost no effects on HepG2 cell survival
(31). Consistent with these
previous studies, the results of the present study revealed that
BBR inhibited the proliferation and promoted the apoptosis of HepG2
and MCF7 cells, and also induced cell cycle arrest in the
G2M phase. Furthermore, the inhibitory rates of the
concentration of BBR in cell viability in our study were
approximate to or even higher than that in previous research for
the same cell line (30,31). The molecular weight of BBR used in
our study was 336.37 (C20H18NO4), 10 µM was ~3.36 µg/ml, and 100 µM
was ~33.6 µg/ml. In the present study, 10–100 µM BBR treatment was
conducted to investigate its effect on cell viability, and was
revealed to significantly suppress the cell viability in HepG2 and
MCF7 cells, especially in MCF7 cells, in which BBR exerted a
significant cytotoxic effect at concentrations >25 µM (8.4
µg/ml) after 48 h of treatment. Furthermore, 50 µM (16.8 µg/ml) BBR
was revealed to have 34% and 51% inhibitory rates within 24 and 48
h, respectively, for HepG2 cell viability, and 74% and 107%
inhibitory rates within 24 and 48 h, respectively, for MCF7 cell
viability, and 25 µM (8.4 µg/ml) BBR was found to have 56 and 105%
inhibitory rates within 24 and 48 h, respectively, for MCF7 cell
viability. Notably, the inhibitory effects of BBR on proliferation
and colony formation in the human normal breast cells, Hs 578Bst,
were not as significant as the effects on the cancer cell lines
used. These results indicated that BBR may represent a promising
antineoplastic drug that specifically targets tumor cells, while
exerting low toxicity to normal cells.
To rapidly proliferate and survive, cancer cells
have a high demand for ATP, utilize glucose more rapidly and
produce more lactate; however, they have a decreased demand for
oxygen, which is known as the Warburg effect (32–34).
It was previously reported that BBR inhibited glucose uptake and
metabolism in colon cancer cells (35). Consistent with the aforementioned
research (32–35), the findings of the present study
demonstrated that both ATP synthesis and the glucose uptake ability
were significantly decreased after BBR treatment in HepG2 and MCF7
cell lines. Furthermore, the protein expression levels of GLUT1,
the main transporter involved in glucose metabolism, were also
significantly downregulated, and GLUT1 was revealed to have
translocated from the membrane to the cytoplasm, which suggested
that the function of GLUT1 may be dysregulated in glucose
metabolism. These results further indicated that BBR may target the
Warburg effect and regulate glucose metabolism in cancer cells to
exert its effects.
Numerous studies have attempted to determine the
antineoplastic mechanisms of BBR by investigating its effects on
different signaling pathways. A large number of molecular targets
of BBR have been identified, including p53, NF-κB, β-catenin and
AMPK (4). BBR was previously
reported to suppress β-catenin signaling and cell proliferation via
binding to nuclear receptor retinoid X receptor α in colon cancer
(36), suppress the proliferation,
migration and invasion of endometrial cancer cells via regulating
the microRNA (miRNA/miR)-101/cyclooxygenase-2 axis (37) and reverse hypoxia-induced
chemoresistance in breast cancer through the inhibition of the
AMPK/hypoxia-inducible factor (HIF)-1α pathway (38). In addition, the combined treatment
of BBR and cisplatin exerted a significant inhibitory effect on
ovarian cancer cell proliferation, arrested the cell cycle at the
G0/G1 phase and markedly enhanced cancer cell
death by inducing caspase-dependent apoptosis and receptor
interacting serine/threonine kinase 3/mixed lineage kinase domain
like pseudokinase-dependent necroptosis (39). It was previously revealed that BBR
decreased the rate of glucose metabolism via suppression of
mTOR-dependent HIF-1α protein synthesis in colon cancer cells
(35). The results of the present
study discovered that BBR decreased the activity of the Akt/mTOR
signaling pathway in both HepG2 and MCF7 cell lines, which
suggested that Akt/mTOR may be a crucial downstream mediator of the
antineoplastic effects of BBR.
The Akt/mTOR signaling pathway plays important roles
in numerous biological functions of cancer cells, including cell
proliferation, viability, survival, glucose metabolism and protein
synthesis. Its activation has been associated with cancer
development and is frequently detected in malignancies (15). As important sources of anticancer
molecules, numerous different natural compounds have been
identified to inhibit Akt/mTOR signaling and have been associated
with a reduced risk of certain cancer types. For example, in a
previous study, apigenin, which is found in abundance in common
fruits and vegetables, was revealed to inhibit Akt function in
different cell types (40). In
addition, miR-171, a miRNA found in different types of plant, was
revealed to modulate the mTOR signaling pathway in 293 cells
(41). In the present study, it
was hypothesized that the antineoplastic activity of BBR may be due
to its ability to target the Warburg effect and regulate glucose
metabolism via the Akt/mTOR signaling pathway. By using a specific
activator of Akt, SC79, the findings of the present study revealed
that the activation of Akt diminished the inhibitory effects of BBR
on ATP synthesis and glucose uptake, as well as the downregulatory
effect on GLUT1 expression. Furthermore, the BBR-induced inhibition
of cell viability and induction of cell apoptosis were partially
abolished after pretreatment with SC79. Thus, these data indicated
that the antineoplastic effects of BBR may be associated with the
reversal of the Warburg effect via the downregulation of the
Akt/mTOR/GLUT1 signaling pathway.
The results of the present study also demonstrated
that the protein expression levels of GLUT1 were significantly
downregulated in BBR-treated cancer cells, while the mRNA
expression levels of GLUT1 were unaltered or in some cases,
upregulated. It was hypothesized that the downregulation of GLUT1
protein expression levels may occur due to post-translational
degradation. As anticipated, following the pretreatment of MCF7
cells with an inhibitor to inhibit the proteasomal degradation
pathway before BBR treatment, the BBR-induced downregulation of
GLUT1 expression levels was markedly abolished. In a previous
study, Ubc9 was revealed to interact with GLUT1 by binding to a
specific 11 amino acid sequence in the COOH terminus, and the
upregulation of Ubc9 expression in L6 myoblasts decreased the
cellular content of GLUT1 (28).
It was suggested that Ubc9 may direct GLUT1 towards proteasome- or
lysosome-mediated degradation by linking multiple residues of
single sentrin (a small ubiquitin-like protein) to GLUT1 (28). In the present study, the results
demonstrated that BBR promoted the interaction between Ubc9 and
GLUT1 in MCF7 cells, which suggested that Ubc9 may regulate the
post-translational modification of GLUT1. Concurrently, BBR also
led to the retention of GLUT1 in the cytoplasm by inhibiting the
binding between GLUT1 and GIPC. Therefore, it was hypothesized that
the binding between GLUT1 and Ubc9 may inhibit the GIPC-mediated
membrane translocation of GLUT1, resulting in the intracellular
retention of GLUT1, which would suppress the glucose transporter
function of GLUT1. These events would subsequently suppress the
proliferation of tumor cells by inhibiting access to the energy
source, glucose. The detailed mechanisms by which this mechanism
may occur should be explored in future studies.
There are some limitations in the present study.
Although it was demonstrated that the antineoplastic effects of BBR
were associated with the reversal of the Warburg effect which was
mediated by the Akt/mTOR/GLUT1 pathway in vitro, the effect
of BBR on solid tumors still requires further validation in in
vivo models. In addition, as one of the most used natural
products worldwide, BBR is limited to poor absorption when taken
orally, as well as intestinal side effects including cramping,
stomach upset, and shaping gut microbiota (42). Future experiments focusing on the
effects of BBR derivatives or designing BBR carriers, such as
silver nanoparticles, zinc oxide nanoparticles and nanostructured
lipids, should be implemented to overcome these limitations
(43). In conclusion, the findings
of the present study highlighted the antineoplastic activity of
BBR, which was evidenced through its ability to inhibit the
proliferation, induce cell cycle arrest at the G2M phase
and promote the apoptosis of cancer cells. The results of the
present study also indicated that Ubc9 and GIPC may mediate the
glucose transport function of GLUT1, and the antitumor effect of
BBR may be attributed to its ability to reverse the Warburg effect
via regulating the Akt/mTOR/GLUT1 signaling pathway. The proposed
underlying mechanism of action for the antineoplastic effects of
BBR is demonstrated in Fig. 5. In
addition, as a well-known plant alkaloid with a long history of
medicinal use in China, BBR was further demonstrated to exert low
toxicity and had a high safety profile in the present study. Hence,
the present results provided novel insight into the antineoplastic
mechanism of BBR and suggested that BBR may represent a potential
treatment strategy for cancer, highlighting the significance of
Traditional Chinese medicines in cancer treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81703015 and
31570353).
Availability of data and materials
All the datasets generated or analyzed during the
present study are included in this published article.
Authors' contributions
XHG and GHY designed the study, administrated the
research and were major contributors in writing the manuscript. SSJ
and LLZ performed the Cell Counting Kit-8, colony formation, flow
cytometry and Co-IP experiments, analyzed the data, helped to
interpret the data and revised the paper. JH, DE, YQF and ZXY
participated in the experiments of ATP detection, glucose uptake
assay, immunofluorescence staining and the acquisition of data. PCH
and HZ cultured cells, performed RT-qPCR and western blot analysis,
FY designed the study and administrated the research. PCH and FY
acquired the fundings. XHG, GHY and FY confirmed the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Efferth T, Xu AL and Lee DYW: Combining
the wisdoms of traditional medicine with cutting-edge science and
technology at the forefront of medical sciences. Phytomedicine.
64:1530782019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Yi X, Ghanam K, Zhang S, Zhao T
and Zhu X: Berberine decreases cholesterol levels in rats through
multiple mechanisms, including inhibition of cholesterol
absorption. Metabolism. 63:1167–1177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kong W, Wei J, Abidi P, Lin M, Inaba S, Li
C, Wang Y, Wang Z, Si S, Pan H, et al: Berberine is a novel
cholesterol-lowering drug working through a unique mechanism
distinct from statins. Nat Med. 10:1344–1351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hou Q, He WJ, Wu YS, Hao HJ, Xie XY and Fu
XB: Berberine: A Traditional Natural Product With Novel Biological
Activities. Altern Ther Health Med. 26S:20–27. 2020.PubMed/NCBI
|
|
5
|
Chen QQ, Shi JM, Ding Z, Xia Q, Zheng TS,
Ren YB, Li M and Fan LH: Berberine induces apoptosis in
non-small-cell lung cancer cells by upregulating miR-19a targeting
tissue factor. Cancer Manag Res. 11:9005–9015. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun Y, Wang W and Tong Y: Berberine
Inhibits Proliferative Ability of Breast Cancer Cells by Reducing
Metadherin. Med Sci Monit. 25:9058–9066. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Refaat A, Abdelhamed S, Yagita H, Inoue H,
Yokoyama S, Hayakawa Y and Saiki I: Berberine enhances tumor
necrosis factor-related apoptosis-inducing ligand-mediated
apoptosis in breast cancer. Oncol Lett. 6:840–844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JS, Oh D, Yim MJ, Park JJ, Kang KR,
Cho IA, Moon SM, Oh JS, You JS, Kim CS, et al: Berberine induces
FasL-related apoptosis through p38 activation in KB human oral
cancer cells. Oncol Rep. 33:1775–1782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang SX, Qi B, Yao WJ, Gu CW, Wei XF,
Zhao Y, Liu YZ and Zhao BS: Berberine displays antitumor activity
in esophageal cancer cells in vitro. World J Gastroenterol.
23:2511–2518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo Y, Tian G, Zhuang Z, Chen J, You N,
Zhuo L, Liang B, Song Y, Zang S, Liu J, et al: Berberine prevents
non-alcoholic steatohepatitis-derived hepatocellular carcinoma by
inhibiting inflammation and angiogenesis in mice. Am J Transl Res.
11:2668–2682. 2019.PubMed/NCBI
|
|
11
|
Kou Y, Li L, Li H, Tan Y, Li B, Wang K and
Du B: Berberine suppressed epithelial mesenchymal transition
through cross-talk regulation of PI3K/AKT and RARα/RARβ in melanoma
cells. Biochem Biophys Res Commun. 479:290–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maiti P, Plemmons A and Dunbar GL:
Combination treatment of berberine and solid lipid curcumin
particles increased cell death and inhibited PI3K/Akt/mTOR pathway
of human cultured glioblastoma cells more effectively than did
individual treatments. PLoS One. 14:e02256602019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abrams SL, Follo MY, Steelman LS,
Lertpiriyapong K, Cocco L, Ratti S, Martelli AM, Candido S, Libra
M, Murata RM, et al: Abilities of berberine and chemically modified
berberines to inhibit proliferation of pancreatic cancer cells. Adv
Biol Regul. 71:172–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Massari F, Ciccarese C, Santoni M,
Iacovelli R, Mazzucchelli R, Piva F, Scarpelli M, Berardi R,
Tortora G, Lopez-Beltran A, et al: Metabolic phenotype of bladder
cancer. Cancer Treat Rev. 45:46–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao H, Wang J, Yan W, Cui Y, Chen Z, Gao
X, Wen X and Chen J: GLUT1 regulates cell glycolysis and
proliferation in prostate cancer. Prostate. 78:86–94. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Powles T, Murray I, Brock C, Oliver T and
Avril N: Molecular positron emission tomography and PET/CT imaging
in urological malignancies. Eur Urol. 51:1511–1520; discussion
1520–1521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng D, Xu C, Sun P, Wu J, Yan C, Hu M and
Yan N: Crystal structure of the human glucose transporter GLUT1.
Nature. 510:121–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reinicke K, Sotomayor P, Cisterna P,
Delgado C, Nualart F and Godoy A: Cellular distribution of Glut-1
and Glut-5 in benign and malignant human prostate tissue. J Cell
Biochem. 113:553–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu M, Yongzhi H, Chen S, Luo X, Lin Y,
Zhou Y, Jin H, Hou B, Deng Y, Tu L, et al: The prognostic value of
GLUT1 in cancers: A systematic review and meta-analysis.
Oncotarget. 8:43356–43367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amann T, Maegdefrau U, Hartmann A, Agaimy
A, Marienhagen J, Weiss TS, Stoeltzing O, Warnecke C, Schölmerich
J, Oefner PJ, et al: GLUT1 expression is increased in
hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol.
174:1544–1552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oh S, Kim H, Nam K and Shin I: Glut1
promotes cell proliferation, migration and invasion by regulating
epidermal growth factor receptor and integrin signaling in
triple-negative breast cancer cells. BMB Rep. 50:132–137. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan DA, Sutphin PD, Nguyen P, Turcotte S,
Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, et al:
Targeting GLUT1 and the Warburg effect in renal cell carcinoma by
chemical synthetic lethality. Sci Transl Med. 3:94ra702011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fearnhead HO, Vandenabeele P and Vanden
Berghe T: How do we fit ferroptosis in the family of regulated cell
death? Cell Death Differ. 24:1991–1998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boado RJ: Post-transcription modulation of
the blood-brain barrier GLUT1 glucose transporter by brain-derived
factors. J Neural Transm Suppl. 59:255–261. 2000.PubMed/NCBI
|
|
28
|
Giorgino F, de Robertis O, Laviola L,
Montrone C, Perrini S, McCowen KC and Smith RJ: The
sentrin-conjugating enzyme mUbc9 interacts with GLUT4 and GLUT1
glucose transporters and regulates transporter levels in skeletal
muscle cells. Proc Natl Acad Sci USA. 97:1125–1130. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wieman HL, Horn SR, Jacobs SR, Altman BJ,
Kornbluth S and Rathmell JC: An essential role for the Glut1
PDZ-binding motif in growth factor regulation of Glut1 degradation
and trafficking. Biochem J. 418:345–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Balakrishna A and Kumar MH: Evaluation of
Synergetic Anticancer Activity of Berberine and Curcumin on
Different Models of A549, Hep-G2, MCF-7, Jurkat, and K562 Cell
Lines. BioMed Res Int. 2015:3546142015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu R, Zhang ZQ, Wang B, Jiang HX, Cheng L
and Shen LM: Berberine-induced apoptotic and autophagic death of
HepG2 cells requires AMPK activation. Cancer Cell Int. 14:492014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lebelo MT, Joubert AM and Visagie MH:
Warburg effect and its role in tumourigenesis. Arch Pharm Res.
42:833–847. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kennedy KM and Dewhirst MW: Tumor
metabolism of lactate: The influence and therapeutic potential for
MCT and CD147 regulation. Future Oncol. 6:127–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Semenza GL: Tumor metabolism: Cancer cells
give and take lactate. J Clin Invest. 118:3835–3837.
2008.PubMed/NCBI
|
|
35
|
Mao L, Chen Q, Gong K, Xu X, Xie Y, Zhang
W, Cao H, Hu T, Hong X and Zhan YY: Berberine decelerates glucose
metabolism via suppression of mTOR dependent HIF 1α protein
synthesis in colon cancer cells. Oncol Rep. 39:2436–2442.
2018.PubMed/NCBI
|
|
36
|
Ruan H, Zhan YY, Hou J, Xu B, Chen B, Tian
Y, Wu D, Zhao Y, Zhang Y, Chen X, et al: Berberine binds RXRα to
suppress β-catenin signaling in colon cancer cells. Oncogene.
36:6906–6918. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y and Zhang S: Berberine suppresses
growth and metastasis of endometrial cancer cells via
miR-101/COX-2. Biomed Pharmacother. 103:1287–1293. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pan Y, Shao D, Zhao Y, Zhang F, Zheng X,
Tan Y, He K, Li J and Chen L: Berberine Reverses Hypoxia-induced
Chemoresistance in Breast Cancer through the Inhibition of AMPK-
HIF-1α. Int J Biol Sci. 13:794–803. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu L, Fan J, Ai G, Liu J, Luo N, Li C and
Cheng Z: Berberine in combination with cisplatin induces
necroptosis and apoptosis in ovarian cancer cells. Biol Res.
52:372019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tong X and Pelling JC: Targeting the
PI3K/Akt/mTOR axis by apigenin for cancer prevention. Anticancer
Agents Med Chem. 13:971–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gismondi A, Nanni V, Monteleone V, Colao
C, Di Marco G and Canini A: Plant miR171 modulates mTOR pathway in
HEK293 cells by targeting GNA12. Mol Biol Rep. 48:435–449. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alolga RN, Fan Y, Chen Z, Liu LW, Zhao YJ,
Li J, Chen Y, Lai MD, Li P and Qi LW: Significant pharmacokinetic
differences of berberine are attributable to variations in gut
microbiota between Africans and Chinese. Sci Rep. 6:276712016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Farooqi AA, Qureshi MZ, Khalid S, Attar R,
Martinelli C, Sabitaliyevich UY, Nurmurzayevich SB, Taverna S,
Poltronieri P and Xu B: Regulation of cell signaling pathways by
berberine in different cancers: Searching for missing pieces of an
incomplete jig-saw puzzle for an effective cancer therapy. Cancers
(Basel). 11:4782019. View Article : Google Scholar : PubMed/NCBI
|