Introduction
According to the Anatomical Therapeutic Chemical
(ATC) classification system (1),
bendamustine belongs to the group of antineoplastic and
immunomodulating agents (L), antineoplastic agents (L01),
alkylating agents (L01A), nitrogen mustard analogues (L01AA) and is
given an ATC code L01AA09. Chemically, bendamustine is
4-{5-[bis(2-chloroethyl)amino]-1-methyl-2-bezimidazolyl} butyric
acid hydrochloride. It was first synthesized in the early 1960s at
the Institute for Microbiology and Experimental therapy in Jena, in
the former East German Democratic Republic (2). Nitrogen mustard, an alkylating drug
after which the L01AA ATC group was named, was used in the World
War I as chemical weapon causing skin lesions, blindness, lung
damage, nausea, and vomiting. After learning about mutagenic
properties of nitrogen mustard and its effects on lowering the
white blood cells count, many other alkylating agents had been
developed with a goal to treat malignant tumors (3). The synthesis of bendamustine was
based on the idea to produce a nitrogen mustard compound less toxic
and at least as effective as other alkylating agents. As observed
in Fig. 1, the specific mechanism
of action of bendamustine is related to its unique structure with
similarities to both alkylating agents and purine analogs.
Bendamustine was first registered in 1971 as a
treatment for both hematological malignancies and solid tumors.
Although not widely prescribed in East Germany, the interest for
this drug increased after the reunification of Germany in the
1990s, mostly as a treatment for lymphoid malignancies. The drug
gained US Food and Drug Administration (FDA) approval in 2008 and
European Medicines Agency approval in 2010. Bendamustine is also on
the World Health Organization list of essential medicines (4).
There are several excellent reviews regarding the
use of bendamustine in hematological and solid malignancies
(2,5–8).
Since the understanding of its role in hematology has changed
considerably in the past ten years, the present review focused on
more recent clinical trials investigating the efficacy and safety
of bendamustine in comparison or in combination with novel,
targeted agents. Additionally, more recent studies were reviewed
regarding its pharmacology, and finally, some light was shed on
bendamustine-mediated immunological effects, proving to be of great
importance in the current COVID-19 pandemic.
Bendamustine-mechanism of action, cell death
and cell cycle
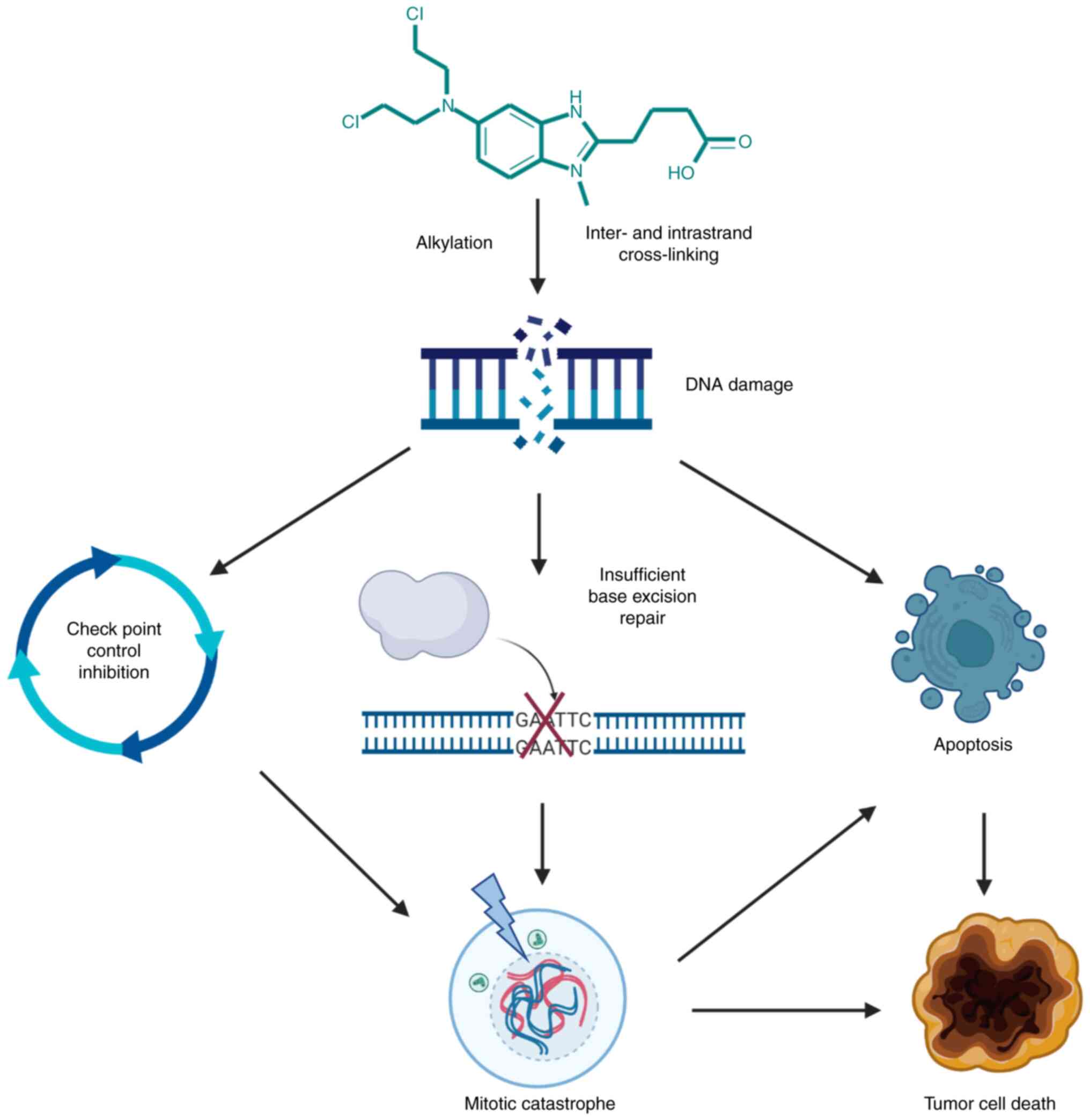
Cytotoxic effects of bendamustine primarily result
from alkylation-mediated DNA damage and possibly to a lesser extent
from antimetabolite properties of its benzimidazole ring.
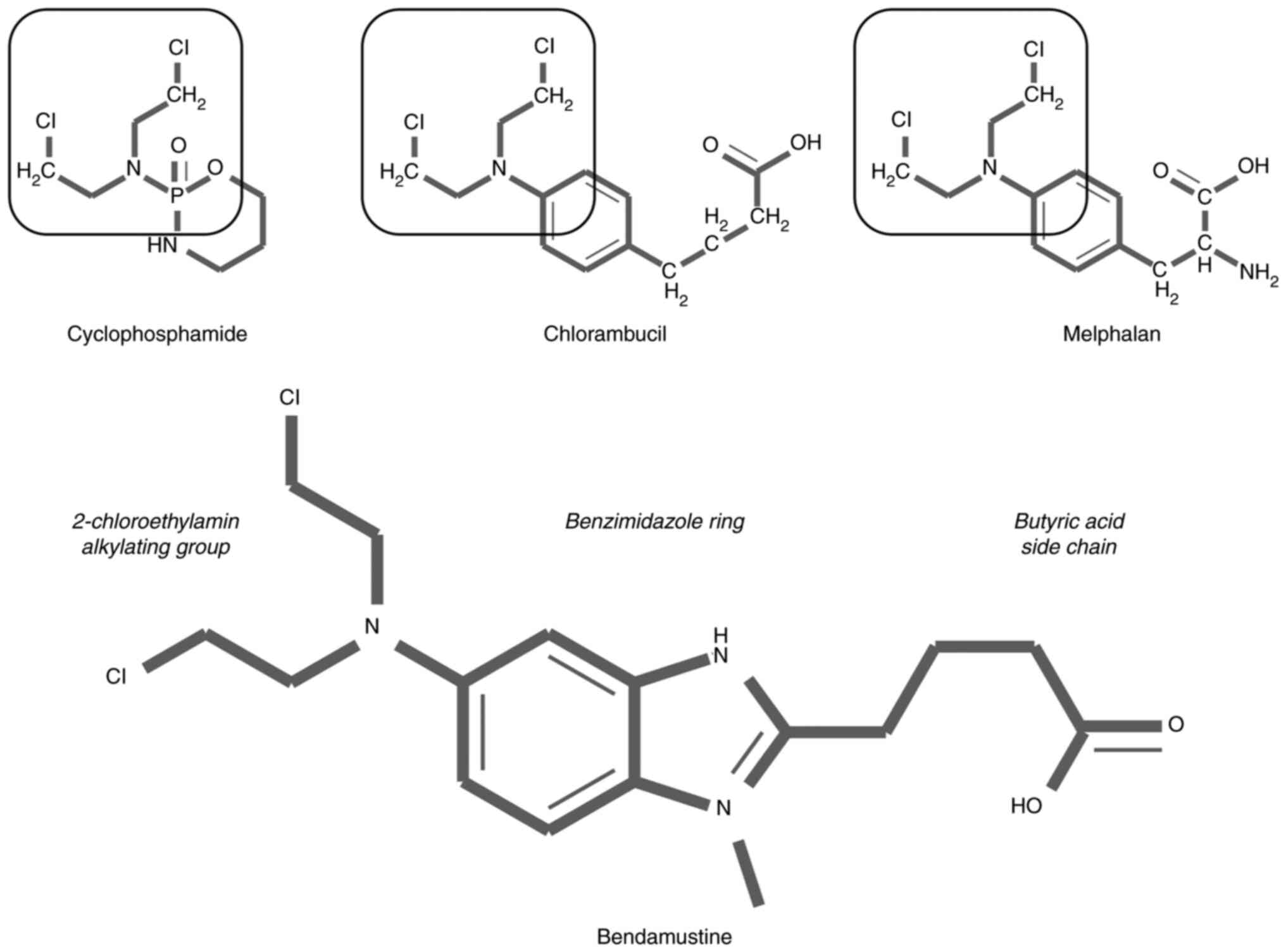
Bendamustine is a bifunctional alkylating agent containing two
reactive groups that can bond to separate DNA sites, a feature
characteristic of other nitrogen mustards-related agents such as
cyclophosphamide, chlorambucil and melphalan (9). Monofunctional alkylating agents, on
the other hand, contain a single active chemical moiety that is
able to modify only a single DNA site (9). In comparison with other alkylating
drugs, bendamustine shows a unique cytotoxicity profile such as
improved penetration and localization within DNA, and more DNA
double-strand breaks that persist longer (3). Upon alkylation, DNA damage induces
DNA damage repair signaling pathways (9). In contrast to other alkylating agents
that induce a repair of DNA by O-6-methylguanine-DNA
methyltransferase and base excision repair (BER), bendamustine
appears to preferably induce BER DNA repair (10). This mechanism could additionally be
an asset to its unique effects since this type of repair is more
complex, takes longer to perform and therefore further diminishes
the capacity of cells to repair the damage (3). Except for the mere addition of the
alkyl group, another mechanism of bendamustine cytotoxic properties
is cross-linking of DNA, creating links within strands (intrastrand
cross-linking) and between strands (interstrand cross-linking), the
latter caused by formation of covalent bonds of the electrophilic
alkyl group of bendamustine with electron-rich nucleophilic
moieties (3,8,9,11).
If the cell is unable to repair DNA damage, cell cycle progression
is inhibited and cell death via apoptotic mechanism occurs.
It has still not been fully elucidated to what
extent may the uniqueness of bendamustine-mediated effects be
related to its benzimidazole ring. Leoni hypothesized that two
mechanisms of action could be responsible for it (12). First, a direct antimetabolite
activity of bendamustine could be exerted by its incorporation into
newly synthesized DNA molecules or by inhibiting ribonucleotide
reductase or other enzymes involved in the generation of
deoxynucleoside triphosphates. Second, the benzimidazole ring may
enhance the alkylating activity of bendamustine, possibly by
facilitating nuclear transport and allowing the drug to reach
higher concentrations in the nucleus or by inhibiting DNA repair
(12). In mantle-cell lymphoma
(MCL), diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma and
multiple myeloma (MM) cell lines, bendamustine showed synergistic
effects with pyrimidine analogues and elicited DNA damage response
and subsequent apoptosis faster and with a shorter exposure time
than other alkylating agents examined (13). Although bendamustine cellular
uptake is debatable, Hiraoka et al (13) reported that it is at least partly
mediated through nucleoside transporters, suggesting its purine
analogue-like properties. On the other hand, Arimany-Nardi et
al (14) found no interaction
of bendamustine with hCNT and hENT proteins, known to mediate the
uptake of purine and pyrimidine drug analogs, suggesting a lack of
their role in cellular uptake of the drug and emphasizing the
importance of human organic cation transporter 1. The role of
organic transporters was further corroborated by the finding that
renal human organic anion transporter 3 increases the
susceptibility of lymphoma cells to bendamustine uptake (15).
Schwänen et al (16) first reported in vitro
efficacy of bendamustine alone or in combination with fludarabine
in inducing apoptosis in B-cell chronic lymphocytic leukemia (CLL)
cells (16). Notably, apoptosis is
not the only mechanism of bendamustine-mediated cytotoxic effects,
since it causes an alternative mechanism called mitotic catastrophe
that bypasses apoptosis which is often impaired in tumor cells
(11). Normally, when DNA damage
or DNA replication stress occurs, these changes are detected by
check points that arrest the cell cycle at either the G1-S (G1
check point) or the G2-M (G2 check point) transition to prevent the
accumulation and propagation of genetic errors during cell division
and to allow DNA repair to take place (9,17).
Occurrence of damaged DNA in form of double-strand breaks (DSBs)
triggers ataxia telangiectasia-mutated (ATM) check point protein
kinase and downstream targets, protein kinase called checkpoint
kinase (Chk)2 and transcription factor p53, most important for
prevention of cells to enter S phase (9,17).
Due to the repair mechanism of DSBs or due to replication stress
which arises during S phase, single-strand breaks are generated and
ataxia telangiectasia and Rad3-related protein and Chk1 signaling
pathways are activated. If no DNA repair is achieved, cells do not
enter mitosis but undergo apoptotic cell death or senescence, often
by TP53-dependent mechanisms (9,17).
In the case of mitotic catastrophe, if for example, TP53 is
mutated, there is an insufficient G2 check point regulation and
cells enter mitosis with significant DNA damage followed by
apoptosis, necrosis and senescence (17,18).
Gene expression profiling studies conducted by Leoni et al
(10) demonstrated
bendamustine-mediated inhibition of expression of genes involved in
DNA repair and mitotic checkpoints indicating that the assumed
intercalation of the drug into the DNA and downregulation of check
point inhibitors could be the mechanism behind mitotic
catastrophe.
Different experimental models have shown different
effects of bendamustine on the cell cycle. The drug causes
significantly more T-cell lymphoma cells to be arrested in the
S-phase than chlorambucil or phosphoramide (10), and similar effect was observed in
both MM (19,20) and MCL cell lines (21). However, in different experimental
models, bendamustine induced ATM-Chk2-Cdc2-mediated arrest in G2
phase of the cell cycle of MM cells and p53-mediated apoptosis, the
latter augmented by inhibition of p38 MAPK (22). In human DLBCL cell lines, the drug
increased the proportion of cells in G2-M and bendamustine-induced
activation of the ATM pathway and accumulation of surviving cells
at G2-M phase was inhibited by surviving suppressant (23). An explanation of these findings may
come from studies on HeLa cells suggesting a dose-dependent effect
on cell cycle checkpoints and DNA repair (24). Low concentrations of bendamustine
transiently arrested cells in G2, which then entered mitosis and
divided normally, while a 4-fold higher concentration arrested
cells in S phase resulting in aberrant mitosis and cell death
(24). Proposed mechanism of
action of bendamustine is shown in Fig. 2.
Bendamustine-pharmacokinetics
Pharmacokinetics of bendamustine has been studied,
in addition to humans, in mice, rats and dogs. The extent of
binding and formation of metabolites is different among species,
but while some authors considered this to be clinically relevant
(5), others suggested that the few
new metabolic products detected in the human mass balance study
that had not been observed in rats, largely represent adducts that
are formed by reaction of bendamustine with endogenous compounds in
the urine and conclude that the metabolic elimination of
bendamustine is qualitatively the same in humans and rats (25,26).
Although the pharmacokinetics of multiple-dose administration of
the drug have not been investigated, there is a significant
correlation between nausea and maximum drug concentration (Cmax)
observed in population pharmacokinetic (6,8,25).
Cmax of bendamustine depends on the dose. When administered at
doses of 30–200 mg/m2, Cmax varies between 0.1–30 µg/ml
and is reached after the mean time of 29.6 min (5,8).
Central volume of distribution following intravenous (IV)
administration is 8.6-19.3 l and steady-state volume of
distribution is 15.8-20.5 l (5,8). In
a previous study in which bendamustine, at a dose of 75
mg/m2 for two days, was a component of IV administered
salvage R-B(O)AD (rituximab, bendamustine, vincristine, cytarabine,
dexamethasone) regimen for the treatment of primary central nervous
system lymphoma, the Cmax mean for plasma and cerebrospinal fluid
were 2,669 and 0.397 ng/ml, respectively, and patients with
response at deep tumor sites displayed higher trends in peak
exposure (27).
After IV administration, high percentage (>95%)
of the drug is bound to proteins, mainly albumin, and is unlikely
to displace or be displaced by other highly protein-binding drugs
(5). Bendamustine is mainly
nonenzymatically hydrolyzed to the markedly less active metabolites
HP1 and HP2, and metabolized in the liver to active M3 and M4 via
CYP1A2 (25). Since their
concentrations in the plasma are significantly lower than those of
the parent drug, the main therapeutic effect is due to bendamustine
itself (25). Opinions on the
importance of renal excretion of the drug, and therefore its safety
in patients with renal failure, vary. Bendamustine is eliminated by
the kidneys with mean elimination half-life of 28.2 min and mean
total clearance of 639.4 ml/min (8). Peak metabolite concentrations are
found in the urine 1 h after administration, but only 3% of the
drug is excreted through kidneys unmetabolized (25,26).
Preclinical and the human mass balance study demonstrated 76–90%
recovery of radiolabeled bendamustine in the excreta, with varying
proportions being recovered in urine (20–50%) and in feces (25–70%)
(8,25,26).
Based on these data, Dubbelman et al (26) suggested that, in contrast to the
position of the majority of authors and the official FDA
prescribing information (28),
renal or hepatic impairment would not be expected to have an
important effect on the systemic exposure to bendamustine (25,26).
Bendamustine in hematological and solid
malignancies
The use of bendamustine in hematological
malignancies frequently deviates from its official labels, both in
terms of indications as well as dosage. Regarding the latter, doses
higher than 90 mg/m2 for 2 consecutive days are rarely
used, and are frequently reduced to 70 mg/m2 in
pretreated or unfit patients. A detailed list of all potential
clinical uses is beyond the scope of the present review. Main
indications for bendamustine are CLL, indolent non-Hodgkin
lymphomas (iNHL) and MCL. Other potential indications include MM,
DLBCL and Hodgkin lymphoma (HL) as well as lymphodepletion prior to
chimeric antigen receptor T-cell (CAR-T) infusion. Results of major
studies are presented in Table I.
The use of bendamustine has lately dwindled, due to the appearance
of more effective and less toxic agents, but also due to an
apparent increased risk of lethal outcome of COVID-19 in
bendamustine-treated patients (as seen below).
 | Table I.Bendamustine in hematological
malignancies. |
Table I.
Bendamustine in hematological
malignancies.
| Clinical trial | Disease
(status) | Objective | Phase | N | PFS | OS | (Refs.) |
|---|
|
| CLL (TN) | CLB vs. B | III | 319 | Median PFS 8.8
months vs. 21.2 months | Median OS 78.8
months vs. NR | Knauf et al
(29) |
| NCT00769522
CLL10 | CLL (TN) | FCR vs. BR | III | 561 | Median PFS 55.2
months vs. 41.7 months | 91 vs. 92% (3
years) | Eichhorst et
al (30) |
| NCT01886872
Alliance | CLL (TN) | BR vs. ibrutinib
vs. R + ibrutinib | III | 547 | 74 vs. 87 vs. 88%
(2 years) | 95 vs. 90 vs. 94%
(2 years) | Woyach et al
(31) |
| NCT03336333
SEQUOIA | CLL/SLL (TN) | Zanubrutinib vs.
BR | III | 479 | 85.5 vs. 69.5% (2
years) | 94.3 vs. 94.6% (2
years) | Tam CS et
al: Blood 138: 396, 2021 |
| NCT02970318
ASCEND | CLL (r/r) | BR/IdR vs.
acalabrutinib | III | 310 | Median PFS 16.5
months vs. NR | Median OS NR 91%
vs. 94% (1 year) | Ghia et al
(32) |
| NCT01611090
HELIOS | CLL (r/r) | Ibrutinib + BR vs.
BR | III | 578 | Median PFS 65.1
months vs. 14.3 months | Median OS NR 75.7
vs. 61.2 % (5 years) | Fraser et al
(33) |
| NCT02005471
MURANO | CLL (r/r) | VenR vs. BR | III | 389 | Median PFS NR vs.
17 months | Median OS NR 91.9
vs. 86.6% (2 years) | Seymour et
al (34) |
| NCT01332968
GALLIUM | FL (TN) | G + CHOP/CVP/B vs.
R + CHOP/CVP/B | III | 1202 | 80.0 vs. 73.3% (3
years) 82 vs. 75% (3 years) 70.5 vs. 63.2% (5 years) | 94 vs. 92.1% (3
years) 94 vs. 92.1% (3 years) 90.2 vs. 89.4% (5 years) | Marcus et al
(35) Hiddemann et al
(36) Townsend W et al: J
Clin Oncol 38: 8023, 2020 |
| NCT00991211 StiL
NHL 1-2003 | iNHL, MCL (TN) | BR vs. R-CHOP | III | 514 | Median PFS 69.5
months vs. 31.2 months | Median OS NR 84 vs.
82% | Rummel et al
(37) |
| NCT00877006
BRIGHT | iNHL, MCL (TN) | BR vs.
R-CHOP/R-CVP | III | 447 | 65.5 vs. 55.8% (5
years) | 81.7 vs. 85% (5
years) | Flinn et al
(38) |
| NCT01059630
GADOLIN | iNHL (R-r) | BG vs. B | III | 396 | Median PFS NR vs.
14.9 months | Median OS NE events
18 vs. 20% | Sehn et al
(39) |
|
|
|
|
| 413 | Median PFS 25.8
months vs. 14.1 months | Median OS NE events
25.5 vs. 34.9% | Cheson et al
(40) |
| NCT01456351 StiL
NHL 2-2003 | iNHL, MCL
(relapsed) | BR vs. FR | III | 230 | Median PFS 34.2
months vs. 11.7 months | Median OS 109.7
months vs. 49.1 months | Rummel et al
(41) |
| NCT01662050 | MCL (TN) | R-BAC | II | 57 | 76% (35
months) | 86% (2 years) | Visco et al
(42) |
|
| MCL (r/r) | R-BAC | retrospective
cohort study | 36 | Median PFS 10.1
months | Median OS 12.5
months | McCulloch et
al (43) |
| NCT01412879
S1106 | MCL (TN) | RH vs. BR | II | 52 | 62 vs. 66% (5
years) | 74 vs. 80% (5
years) | Kamdar et al
(44) |
| NCT01234467 | DLBCL (TN) | BR | II | 23 | Median PFS 5.4
months | Median OS 10.2
months | Park et al
(45) |
| NCT01626352 | DLBCL (TN) | OB | II | 21 | Median PFS 8.6
months | Median OS 12.
months | Flinn et al
(46) |
| NCT02257567 | DLBCL (r/r) | Pola-BR vs. BR | Ib/II | 192 | Median PFS 9.2
months vs. 3.7 months | Median OS 12.4
months vs. 4.7 months | Sehn et al
(47) |
| NCT01657331 | HL (r/r) | BV + B | I/II | 65 | Median PFS 7.5
months (phase I) and NR (phase II) | Median OS 43.3
months (phase I) and NR (phase II) | O'Connor et
al (48) |
| NCT02499627 | HL (r/r) | BV + B | II | 40 | 67.3% (3
years) | 88.1% (3
years) | Broccoli et
al (49) |
| NCT01874054 | HL (r/r) | BV + B | I/II | 55 | 60.3% (3
years) | 92% (3 years) | LaCasce et
al (50) |
| LYSA | MCL (TN) | BeEAM vs. BEAM | Multicenter | 168 | 84 vs. 63% (3
years) | 93 vs. 84% (3
years) | Hueso et al
(51) |
|
|
| (Conditioning prior
to ASCT) | retrospective |
|
|
|
|
|
| LBCL | FC vs. B
(Lympho-depletion) | Multicenter
retrospective | 113 | 22 vs. 27% (1
year) | 41 vs. 49% (2
years) | Ghilardi G et
al: Blood 138: 1438–1440, 2021 |
| NCT00916058 | MM (TN+r/r) | B + melphalan | II | 35 | Median PFS 47
months | Median OS NR | Gomez-Arteaga et
al (52) |
| NCT02095834 | MM (r/r) | B + Car + D | I | 17 | Median PFS 11.1
months | Median OS 56.3
months | Lee et al
(53) |
Bendamustine was approved for front-line treatment
of CLL at a dose of 100 mg/m2 on days 1 and 2 every 4
weeks for six cycles, after showing superiority to chlorambucil
monotherapy (29). A study of the
German CLL Group, comparing bendamustine plus rituximab (BR) to
fludarabine, cyclophosphamide and rituximab (FCR) showed that the
former resulted in decreased progression-free survival (PFS), while
overall survival (OS) remained similar (30). However, in elderly patients PFS was
not different between treatment groups, and BR caused significantly
fewer infectious complications (30). This rendered BR the treatment of
choice in elderly patients with CLL until the appearance of
targeted agents. All three approved Bruton's tyrosine kinase
inhibitors (BTKi), ibrutinib, acalabrutinib and zanubrutinib, have
been shown in randomized trials to improve PFS, but notably not OS
in comparison with BR (31,32)
(Table I). Ibrutinib + BR
demonstrated that both PFS and OS benefit over BR alone (33,54,55).
In patients with relapsed or refractory (r/r) CLL, the combination
of rituximab with venetoclax, a BCL2 inhibitor, was shown to be
superior to BR (34). Thus at
present, bendamustine is only rarely used in CLL; most patients
receive targeted agents, BTKi and venetoclax, and frail elderly
chlorambucil with or without anti-CD20 monoclonal antibodies
(MoAbs).
Bendamustine, first alone and later in combination
with anti-CD20 MoAbs has been widely investigated for treatment of
iNHL, including follicular lymphoma (FL), marginal-zone lymphoma
(MZL), and lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia
(LPL/WM) (2,56). StiL was the most important
randomized trial proving the efficacy of this drug in front-line
treatment of iNHL (37). Patients
with FL, MZL, MCL and LPL treated with BR had significantly longer
PFS compared with those treated with rituximab plus
cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP)
(median PFS 69.5 months vs. 31.2 months) (37). These findings were corroborated by
the BRIGHT study (38,57). In patients with relapsed iNHL, BR
was revealed to improve PFS and OS in comparison with fludarabine
plus rituximab (41). In patients
refractory to rituximab, the combination of bendamustine and
obinutuzumab, an alternative anti-CD20 MoAb, was identified to be
superior to bendamustine monotherapy (39,40).
Thus, bendamustine became the treatment of choice for front-line
treatment of iNHL as well as for patients who relapsed after R-CHOP
or rituximab plus cyclophosphamide, vincristine, and prednisone
(R-CVP). This position was recently challenged when some real-life
series and the GALLIUM suggested an increase in late
infection-related mortality in vulnerable patients treated with
bendamustine (35,36,58–60).
Thus currently, numerous centers that used to treat iNHL patients
with bendamustine only 2 or 3 years ago are switching back to
CHOP/CVP or even, particularly in the USA, to lenalidomide.
MCL is a type of B-NHL occurring preferentially in
elderly males and combining the aggressive clinical behavior of
large B-cell lymphoma with the continuous tendency for relapses of
iNHL. Young fit patients, typically treated with rituximab, a
high-dose cytarabine containing regimen (for instance
dexamethasone, high-dose cytarabine and cisplatin; DHAP), alone or
alternating with R-CHOP (R-CHOP/R-DHAP), are autografted in 1st
remission and then continue with rituximab maintenance.
Bendamustine is one of the most effective cytotoxic agents for MCL.
Randomized studies have shown BR to be superior to R-CHOP and R-CVP
(37,38,57).
The American S1106 study compared BR induction with R-hyper-CVAD
(RH, rituximab plus hyperfractionated cyclophosphamide,
vincristine, doxorubicin and dexamethasone, alternating with high
dose cytarabine and methotrexate) in patients with previously
untreated MCL and who were candidates for autologous stem-cell
transplantation (ASCT) (44,61).
Both regimens showed excellent antitumor activity, but BR was far
less toxic and had less stem cell mobilization failure rates
(44). Italian studies with the
combination of rituximab, bendamustine and high-dose cytarabine
produced excellent results with long-term PFS even without
rituximab maintenance (42,43).
Real-life series have confirmed the superiority of
bendamustine-based treatment in MCL over CHOP-like regimens
(62,63). BTKi, while very active in MCL, are
only approved for relapsed disease. Therefore, even in the current
situation, bendamustine-based regimens remain the mainstay of MCL
treatment, at least for patients unfit for aggressive chemotherapy,
such as DHAP. Notably, there are previous studies suggesting that
bendamustine-based regimens are less sensitive to p53 mutations
(3,64,65),
which are a negative prognostic factor in patients treated with
R-CHOP/R-DHAP (66). A possible
explanation for this observation may be the propensity of
bendamustine to eliminate cells via the mitotic catastrophe
mechanism.
BR is active in DLBCL (45), but the duration of remission is
short and is mainly used for palliation. Additionally, modest
efficacy was shown when bendamustine was combined with ofatumumab,
another anti-CD20 MoAb (46).
Recently, the combination of polatuzumab vedotin, a conjugated
anti-CD79b MoAb and BR was shown to significantly improve outcomes
in patients with r/r DLBCL unsuitable for intensive chemotherapy
and ASCT (47). This combination,
Pola-BR, has been registered and approved for this indication in
both USA (67), and EU (68) and is the current treatment of
choice for this group of patients. Bendamustine is active in HL
with median PFS 5.2 months, but again the duration of remission is
short (69). The combination of
bendamustine with brentuximab vedotin, an anti-CD30 conjugated MoAb
has been shown to be an effective salvage and is occasionally used
instead of more aggressive chemotherapy regimens prior to stem cell
transplantation (48–50). Bendamustine is also used instead of
carmustine in combination with etoposide, cytarabine and melphalan
for conditioning prior to ASCT (51), and as an alternative
lymphodepleting therapy prior to the infusion of tisagenlecleucel,
one of the registered CAR-T cell products (Ghilardi G et al:
Blood 138: 1438–1440, 2021). Although active in MM (52,53),
its use has all but disappeared even before the current COVID-19
pandemic, due to appearance of other, more effective and less toxic
agents: anti-CD38 monoclonal antibodies, proteasome inhibitors and
immunomodulators. Still, an occasional patient with MM, failing
these options or unsuitable for them, may benefit from
bendamustine.
There are several recent studies of bendamustine
effects in treating certain solid tumors (70–73).
In pretreated women with HER2-negative metastatic breast cancer in
combination with capecitabine OR was 46% and median PFS was 7.5
months (70). In a phase II study
of relapsed chemotherapy sensitive or resistant small-cell lung
cancer, median time to progression was 4.0 months, median OS was
4.8 months, and the response rate was 26% (71). In patients with refractory soft
tissue sarcoma who were treated with bendamustine, the estimated
3-month and 6-month PFS for all histological subtypes was 35.3 and
23.5%, respectively (72). In an
open trial including women with advanced ovarian cancer last
updates posted in 2018 reported on median OS of 393 days (73).
Bendamustine related side-effects in
clinical trials
Patients with cancer frequently experience problems
which can be caused by their disease, antineoplastic treatment, or
other, unrelated causes. This should be considered when
interpreting published data. Detailed data on frequency of most
important side-effects are presented in Table II.
 | Table II.Bendamustine-related side-effects in
clinical trials. |
Table II.
Bendamustine-related side-effects in
clinical trials.
| Clinical trial | Disease
(status) | Objective | Phase | N | Bendamustine
dose | Side-effects (% of
patients) | (Refs.) |
|---|
|
| iNHL (R-r) | B | II | 76 | 120
mg/m2 IV on days 1 and 2 of six 3-week cycles | Grade 3–4
neutropenia (54%), thrombocytopenia (25%), and anemia (12%). All
grade nausea (72%), vomiting (41%), fatigue (49%), constipation
(26%), anorexia (34%), fever (25%), cough (29%) and diarrhea
(30%). | Friedberg et
al (74) |
|
| CLL (TN) | CLB vs. B | III | 319 | 100
mg/m2 on days 1 to 2 of six 4-week cycles | Grade 3–4
neutropenia (10.6 vs. 23%), thrombocytopenia (7.9 vs. 11.8%), and
anemia (0 vs. 2.5%). All grade nausea (19.3 vs. 13.9%), vomiting
(15.5 vs. 6.6%), and diarrhea (9.9 vs. 4%). | Knauf et al
(75) |
|
|
|
|
|
|
| Grade 3–4
infections (3 vs. 8%) |
|
| NCT00769522
CLL10 | CLL (TN) | FCR vs. BR | III | 561 | 90 mg/m2
IV on days 1 and 2 of six 4-week cycles | Grade 3–4
hematologic AEs (90 vs. 67%)-neutropenia (85 vs. 59%), leukopenia
(81 vs. 48%), thrombocytopenia (21 vs. 14%), anemia (14 vs.
11%). | Eichhorst et
al (30) |
|
|
|
|
|
|
| Grade 3–4
infections (39 vs. 25%). |
|
| NCT02005471
MURANO | CLL (r/r) | VenR vs. BR | III | 389 | 70 mg/m2
IV on days 1 and 2 of six 4-week cycles | Grade 3–4 AEs (82
vs. 70.2%). Grade 3–4 neutropenia (57.7 vs. 38.8%), anemia (10.8
vs. 13.8%), thrombocytopenia (5.7 vs. 10.1%), and febrile
neutropenia (3.6 vs. 9.6%). Grade 3–4 infections and infestations
(17.5 vs. 21.8%) | Seymour et
al (34) |
| NCT01332968
GALLIUM | FL (TN) | G + CHOP/CVP/B vs.
R + CHOP/CVP/B | III | 1202 | 90 mg/m2
IV on days 1 and 2 of six 4-week cycles | G-group
(CHOP/CVP/B)-grade 3–5 AEs (89%/69%/69%), grade 3–5 neutropenia
(71%/46%/30%), grade 3–5 infections (12%/13%/20%), fatal AEs
(2%/2%/6%) | Hiddemann et
al (36) |
|
|
|
|
|
|
| R-group
(CHOP/CVP/B)-grade 3–5 |
|
|
|
|
|
|
|
| AEs (74%/54%/67%),
grade 3–5 |
|
|
|
|
|
|
|
| neutropenia
(55%/23%/30%), grade 3–5 |
|
|
|
|
|
|
|
| infections
(12%/13%/26%), fatal AEs (2%/2%/5%) |
|
| NCT00991211 StiL
NHL 1–2003 | iNHL, MCL (TN) | BR vs. R-CHOP | III | 514 | 90 mg/m2
IV over 30–60 min on days 1 and 2 of six 4-week cycles | Hematological AEs
(30 vs. 68%). Grade 3–4 leukopenia (37 vs. 72%) and neutropenia (29
vs. 69%). All grade alopecia (0 vs. 100%), infections (37 vs. 50%),
peripheral neuropathy (7 vs. 29%), stomatitis (6 vs. 19%),
erythematous skin reactions (16 vs. 9%). | Rummel et al
(37) |
| NCT00877006
BRIGHT | iNHL, MCL (TN) | BR vs.
R-CHOP/R-CVP | III | 447 | 90 mg/m2
IV on days 1 and 2 of six 4-week cycles | Grade 3–4
neutropenia (39–49% vs. 87%/56%) and lymphopenia (61–63% vs.
33%/28%). All grade vomiting (25–29% vs. 13%/13%),
drug-hypersensitivity (13–17% vs. 6%/3%), peripheral
neuropathy/paresthesia (9–14% vs. 44%/47%), and alopecia (3–4% vs.
51%/21%). Grade 3–4 infections (7–12% vs. 5%/7%) | Flinn et al
(38) |
| NCT01456351 StiL
NHL 2–2003 | iNHL, MCL
(re-lapsed) | BR vs. FR | III | 230 | 90 mg/m2
IV on days 1 and 2 of six 4-week cycles | Grade 3–4
leukopenia (13 vs. 12%), neutropenia. (9 vs. 9%), anemia (1 vs.
1%), and thrombocytopenia (2 vs. 2%). All grade infections (30 vs.
27%) | Rummel et al
(41) |
| NCT01412879
S1106 | MCL (TN) | RH vs. BR | II | 52 | 90 mg/m2
IV on days 1 and 2 of six 4-week cycles | Grade 3–4 anemia
(59 vs. 8.6%), neutropenia (65 vs. 34%), febrile neutropenia (29
vs. 14%), and thrombocytopenia (71 vs. 17%). | Chen et al
(61) |
In patients treated with bendamustine monotherapy,
most frequently reported treatment-emergent adverse events (AE)
were fever, skin reactions, nausea, vomiting and hematological AEs:
granulocytopenia and thrombocytopenia (74–76)
Fatigue, mucositis and infections were very common, but were rarely
higher than grade 2, at least during and immediately after
treatment (74–76).
Toxicity reports on studies comparing
bendamustine-MoAb combinations with CHOP-MoAb combinations are not
completely consistent. Universally, bendamustine was associated
with a very low rate of alopecia and peripheral sensory neuropathy
and higher incidence of skin reactions and gastrointestinal
problems (6,8,37,57).
Granulocytopenia and thrombocytopenia as well as infections during
treatment and neutropenic fever were usually more frequent and
severe with CHOP (37).
Lymphopenia, a frequently disregarded side-effect, universally
occurs more frequently with bendamustine (57).
Toxicities of BR and fludarabine + rituximab were
similar, most commonly myelosuppression and infections (41). As already mentioned, FCR was more
toxic than BR. There were more cases of severe neutropenia and
infections (84 vs. 59% and 39 vs. 25%, respectively) (30). The increased frequency of
infectious complications with FCR was, as aforementioned, more
pronounced in patients older than 65 years (30).
The combination of venetoclax plus rituximab was
associated with more AEs of grade 3 or 4 in comparison with BR in
the MURANO study (82.0 vs. 70.2%) (34). Particularly grade 3–4 neutropenia
was more frequent in the former arm (57.7 vs. 38.8%) (34). On the other hand, febrile
neutropenia and grade 3 or 4 infections or infestations were more
common in the BR group (34).
In the GALLIUM study, treatment with bendamustine
was associated with marked and prolonged reductions in T-cell
counts and higher rates of grade 3 to 5 infection and second
neoplasm during the maintenance and follow-up phases, whereas CHOP
was associated with higher rates of grade 3 to 5 neutropenia during
the induction phase (35,36). Non-relapse-related fatal AEs,
although with small absolute numbers, were more common among
patients who received bendamustine (5.6% of patients in the
obinutuzumab group and 4.4% of those in the rituximab group) than
among those treated with CHOP (1.6 and 2.0%, respectively) or CVP
(1.6 and 1.8%), and pose a concern in this population of patients
(35). The frequency of deaths was
higher in patients treated with bendamustine (10%) than in patients
treated with CHOP (5%) or CVP (8%) (36). Furthermore, patients who had not
previously started new anticancer treatment had higher proportion
of fatal AEs when treated with bendamustine (4%) than with CHOP
(2%) or CVP (2%) (36).
These data suggested that, while the acute
toxicities of bendamustine are less prominent than that of CHOP,
the drug seems to have a prolonged effect, probably immunological,
leading to an increased risk of late infections, particularly in
patients receiving prolonged anti-CD20 MoAb maintenance.
Immunological effects of bendamustine
A recent detailed review by Stokes et al
(77) reported bendamustine
effects in both murine models and clinical setting as
pre-transplant conditioning drug and its immunomodulatory
properties in graft-versus-host disease (GVHD). In the present
review, general immunological effects of bendamustine not
necessarily restricted to GVHD are discussed.
Hematological malignancies are known to have a
direct effect on the immunological system; in patients with CLL
there is an impaired production of polyclonal immunoglobulins due
to anomalous CD40-CD40 ligand relations and reduction of CD40
ligand, suppression of CD95+ plasma cells in the bone
marrow, and impaired inhibition by T cells (78). Furthermore, in those patients the
numbers of T helper cells are reduced with augmented number of T
suppressor cells; a CD200 marker expressed on CLL cells stimulates
differentiation of CD4+ T cells into regulatory T cells
(Tregs), which express CTLA-4, CD270 and PD-L1. NK-cell activity,
phagocytosis and complement amounts have been also reported to be
impaired and all these facts greatly contribute to insufficient
immunological response to infectious stimuli in patients with CLL
(78). Although no differences on
the rate of infections between bendamustine and other alkylating
agents or fludarabine were reported in a meta-analysis of
randomized controlled trials by Gafter-Gvili et al (79), bendamustine is associated with an
increased risk of bacterial infections and opportunistic
infections, including cytomegalovirus, varicella zoster virus,
histoplasmosis and Pneumocystis jirovecii-caused pneumonia
(59). Furthermore, a recent
retrospective multicenter cohort study by Lamure et al
(80) on the determinants of
outcome in COVID-19-hospitalized patients with lymphoma, reported
on bendamustine-associated death in patients with
relapsed/refractory lymphoma. A similar effect has been observed in
our patients (Aurer I et al: Blood 138: 3553, 2021).
Bendamustine-induced lymphopenia, whether as
monotherapy or in combination, has been widely reported in both
hematological and non-hematological malignancies (37,45,57,70,75,81–90).
Lymphopenia ranged from 5% in rituximab-refractory patients with
iNHL (85) to 75% of patients with
grade 3–4 hematological toxicity receiving BR (37,90)
or even to 91% in patients treated for triple negative breast
cancer (89). The latter group was
characterized with pronounced decline in CD4+ cells,
with 86% having grade 4 depressed CD4+ counts
(<50/µl) (89). In FL patients
treated with bendamustine, marked reductions in CD3+ and
CD3+CD4+ T cells were seen during induction
with prolonged recovery during and after maintenance (36). Prolonged lymphopenia and low
CD4+ T-cell counts, for at least 7–9 months were also
observed in relapsed or refractory patients with iNHL and MCL
(83). Recent population-based
analysis by Martínez-Calle et al (91) following BR treatment in patients
with low grade lymphoproliferative disease revealed that median
times to lymphocyte count recovery (≥1×109/l) and
CD4+ recovery (≥0.2×109/l) were 26 and 24
months, respectively, and late recovery was associated with risk of
serious infection.
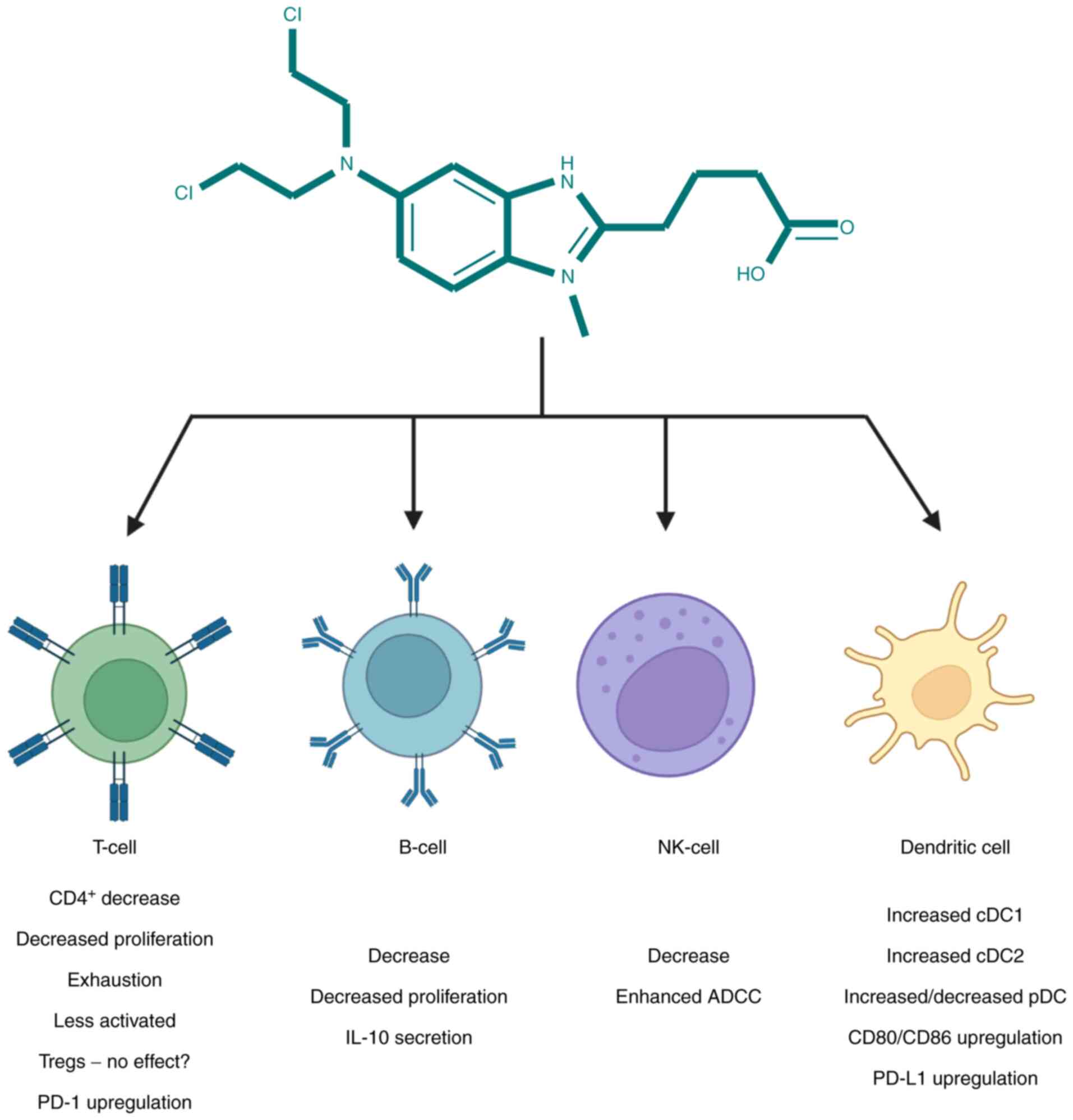
T-cells showed less proliferative properties when
incubated with bendamustine (92).
In a case report of systemic CMV infection following BR treatment,
low γ/δ T-cell frequency and hyperactivated/exhausted
CD4+ and CD8+ T-cell phenotypes unable to
face CMV challenge was reported (93). In a major histocompatibility
complex mismatched murine transplant model, combination of
bendamustine with total body irradiation (BEN-TBI) showed no
difference in donor Tregs, defined as
CD4+CD25+FoxP3+ and measured in peripheral
blood, when compared with cyclophosphamide plus TBI, and
proliferative properties of splenic Tregs did not differ between
groups either (94). In the same
study, in vitro generation of Tregs was not affected by the
increasing concentrations of bendamustine, but mice treated with
bendamustine had less activated donor T-cells measured by CD25
expression (94). In a study of
FOXP3+ Tregs in patients with gastric malt lymphoma,
immunohistochemistry revealed depletion of Tregs at the end of
treatment, that was slightly deeper in cases treated with
bendamustine or fludarabine than in those treated with antibiotics,
with a continuous decline in FOXP3+ cells up to one year
(95). Stokes et al
(94) observed that in peripheral
blood of BEN-TBI conditioned mice, treatment induced an increase in
absolute numbers of Th2 cells on day +7 and decrease in numbers of
Th17 on day +14 but the effects were not detected at any other time
points (94). The same group
exposed murine bone marrow-derived dendritic cells (BMDC) to
bendamustine and incubated them with allogenic T-cells. Upon
incubation, T-cells exhibited an increase in markers of T-cell
exhaustion, as well as markers of activated T-cells, ICOS and CD69,
and an increase in PD-1, negative regulator of immune response,
followed by allogenic CD4+ cell death (96). Since PD-1 is so far considered to
be crucial in T-cell exhaustion, combining bendamustine with
anti-PD-1 antibody could be beneficial in both anticancer and
anti-microbe setting (97).
Notably, CD69 expression on CLL cells is considered to be a
predictor of response to bendamustine since tumor cells derived
from lymphoid tumor niches harbored higher CD69 expression and were
less sensitive to bendamustine than their peripheral blood
counterparts (98). The majority
of the studies support findings that it is mostly CD4+ T
cell count that is decreased with a concomitant decrease in CD4/CD8
ratio and an insufficient T cell recovery in patients treated with
bendamustine, independent of the type of malignancy involved
(99), a fact most recently
corroborated in a study by Yamasaki et al (100).
Bendamustine-mediated lymphopenia is also extended
to B cells, with a previous study reporting predominant B-cell
cytotoxicity (86), resulting in
secondary hypogammaglobulinemia and susceptibility to infections.
Nonetheless, incidence of hypogammaglobulinemia after BR is not
very different from that reported in association with rituximab,
suggesting rituximab- or disease-mediated causes of reduced
immunoglobulin levels (75,79,86,87,91,99).
Suggestive of not only diminished numbers but also altered function
of B-cells are reports whereupon incubation of murine B cells with
bendamustine showed less proliferation in response to LPS (92). In addition, IL-10 production by B
cells among peripheral blood mononuclear cell was significantly
enhanced by addition of bendamustine (101).
NK-cells are known to be impaired in both numbers
and activity in hematological malignancies (78). In a study by Bremer et al
(87), NK-cells dropped by ~60%
within the first 3 weeks after bendamustine therapy. However, data
of a recent study presented on ASH in 2020 showed that in a model
with obinutuzumab-induced antibody-dependent cell cytotoxicity
(ADCC) resistant clones, pretreatment of effector NK cells with
bendamustine enhanced ADCC induction of obinutuzumab, which was
followed by the increased expression of CD107, a NK-cell
degranulation marker (Yamashita-Kashima Y et al: Blood 136:
11–12, 2020).
Due to the crucial role of dendritic cells (DC) in
both pathogenesis of GVHD and graft-vs. leukemia (102), their function and phenotype have
been studied in response to bendamustine. In a murine model,
bendamustine + TBI increased the proportion of plasmacytoid DC,
type 1 conventional DC (cDC1s), and type 2 conventional DCs
(cDC2s), whereas in human monocyte-derived DCs, bendamustine
treatment decreased the number of plasmacytoid DCs and increased
those of cDC1 and cDC2s (96). The
same study demonstrated that bendamustine-treated murine BMDC
showed concentration-dependent increase in CD80, CD86, and PD-L1
expression and dampened response to lipopolysaccharide (LPS).
Bendamustine immunomodulatory properties were additionally
confirmed by a decrease in secretion of pro-inflammatory cytokines
IL-6, TNFα, CCL5, and CCL2 by BMDC in response to LPS (96). Bendamustine effects on T, B, NK and
DC are shown in Fig. 3.
Various and often insufficiently explained
immunological effects induced by bendamustine can be found in a
study where two cases demonstrated hypersensitivity to bendamustine
but with different mechanisms: one with type I hypersensitivity
reaction and another with type IVb or type IVc hypersensitivity
reaction. Additionally, one patient exhibited bendamustine-induced
drug fever in whom neither a type I nor a type IV hypersensitivity
mechanism to this drug was demonstrated (103). Furthermore, in a recent study by
Chan et al a case of severe bendamustine-induced autoimmune
hemolytic anemia in a patient with splenic marginal zone lymphoma
was reported (104).
Conclusion
The approval by FDA of bendamustine in 2008 was
referred to as the revival of an old unjustly ignored drug, an
alkylator that, by virtue of its structure, also has antimetabolite
properties and therefore improved antitumor efficacy than classical
alkylators without increased toxicity. At one time, it seemed to be
the best cytotoxic agent for treatment of indolent
lymphoproliferative disorders. Bendamustine is acutely relatively
well tolerated, easier to handle than CHOP or similar regimens. It
does not cause alopecia, a side-effect which physicians frequently
ignore, but patients, particularly female, not infrequently find
difficult to bear, is not cardiotoxic and not excreted by kidneys
in a meaningful amount, thus increasing its target patient
population. However, bendamustine causes deep and prolonged
lymphopenia, affecting both T and B-cell lineages. The latter in
the current COVID-19 pandemics, together with the appearance of
more effective and less toxic targeted agents for treatment of CLL
resulted in a sharp decline in its use, which remains more or less
unaffected only in mantle-cell lymphoma, at least for the time
being.
Experimental data suggested that bendamustine
causes cytotoxicity through multiple mechanisms, some of which seem
independent of p53. This, coupled with its profound effect on
various lymphocyte subsets and lack of major hematotoxicity
presents an opportunity to develop it further, as in the Pola-BR
combination or CAR-T setting. Physicians using bendamustine must be
aware of its prolonged effects and to continue to carefully and
regularly monitor treated patients, in order to be able to
recognize and treat late complications and thus derive the greatest
benefit from this versatile and interesting drug.
Acknowledgements
Not applicable.
Funding
The present study was supported by the University of Zagreb
support project (grant no. 1120005).
Availability of data and materials
Not applicable.
Authors' contributions
HL and IA conceived the manuscript and wrote the
article. HL, TS and AB performed the literature research and
prepared the figures. DV and DB revised and edited the manuscript.
All authors read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
IA received financial support from Roche, Takeda,
Janssen, Novartis/Sandoz, AbbVie, Oktal Pharma/Celltrion,
SanofiGenzyme, Alvogen, Teva/Pliva, and BMS. The remaining authors
declare that they have no competing interests.
References
|
1
|
WHO collaborating centre for drug
statistics methodology, . ATC classification index with DDDs. Oslo,
Norway: 2021
|
|
2
|
Cheson BD and Rummel MJ: Bendamustine:
Rebirth of an Old Drug. J Clin Oncol. 27:1492–1501. 2009.
View Article : Google Scholar
|
|
3
|
Cheson BD and Leoni L: Bendamustine:
Mechanism of action and clinical data. Clin Adv Hematol Oncol. 9 (8
Suppl 19):S1–S11. 2011.
|
|
4
|
World Health Organization model list of
essential medicines, 21st list 2019, . Geneva: World Health
Organization; 2019
|
|
5
|
Gandhi V and Burger JA: Bendamustine in
B-cell malignancies: The new 46-year-old kid on the block. Clin
Cancer Res. 15:7456–7461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garnock-Jones KP: Bendamustine: A review
of its use in the management of indolent non-Hodgkin's lymphoma and
mantle cell lymphoma. Drugs. 70:1703–1718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tageja N: Bendamustine: Safety and
efficacy in the management of indolent non-hodgkins lymphoma. Clin
Med Insights Oncol. 5:145–156. 2011. View Article : Google Scholar
|
|
8
|
Hoy SM: Bendamustine: A review of its use
in the management of chronic lymphocytic leukaemia,
rituximab-refractory indolent non-Hodgkin's lymphoma and multiple
myeloma. Drugs. 72:1929–1950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu D, Calvo JA and Samson LD: Balancing
repair and tolerance of DNA damage caused by alkylating agents. Nat
Rev Cancer. 12:104–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leoni LM, Bailey B, Reifert J, Bendall HH,
Zeller RW, Corbeil J, Elliott G and Niemeyer CC: Bendamustine
(Treanda) displays a distinct pattern of cytotoxicity and unique
mechanistic features compared with other alkylating agents. Clin
Cancer Res. 14:309–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leoni LM and Hartley JA: Mechanism of
action: The unique pattern of bendamustine-induced cytotoxicity.
Semin Hematol. 48 (Suppl 1):S12–S23. 2011. View Article : Google Scholar
|
|
12
|
Leoni LM: Bendamustine: Rescue of an
effective antineoplastic agent from the mid-twentieth century.
Semin Hematol. 48 (Suppl 1):S4–S11. 2011. View Article : Google Scholar
|
|
13
|
Hiraoka N, Kikuchi J, Yamauchi T, Koyama
D, Wada T, Uesawa M, Akutsu M, Mori S, Nakamura Y, Ueda T, et al:
Purine analog-like properties of bendamustine underlie rapid
activation of DNA damage response and synergistic effects with
pyrimidine analogues in lymphoid malignancies. PLoS One.
9:e906752014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arimany-Nardi C, Montraveta A, Lee-Vergés
E, Puente XS, Koepsell H, Campo E, Colomer D and Pastor-Anglada M:
Human organic cation transporter 1 (hOCT1) as a mediator of
bendamustine uptake and cytotoxicity in chronic lymphocytic
leukemia (CLL) cells. Pharmacogenomics J. 15:363–371. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hagos Y, Hundertmark P, Shnitsar V, Marada
VV, Wulf G and Burckhardt G: Renal human organic anion transporter
3 increases the susceptibility of lymphoma cells to bendamustine
uptake. Am J Physiol Physiol. 308:F330–F338. 2015. View Article : Google Scholar
|
|
16
|
Schwänen C, Hecker T, Hübinger G, Wölfle
M, Rittgen W, Bergmann L and Karakas T: In vitro evaluation of
bendamustine induced apoptosis in B-chronic lymphocytic leukemia.
Leukemia. 16:2096–2105. 2002. View Article : Google Scholar
|
|
17
|
Matthews HK, Bertoli C and de Bruin RAM:
Cell cycle control in cancer. Nat Rev Mol Cell Biol. 23:74–88.
2022. View Article : Google Scholar
|
|
18
|
Vitale I, Galluzzi L, Castedo M and
Kroemer G: Mitotic catastrophe: A mechanism for avoiding genomic
instability. Nat Rev Mol Cell Biol. 12:385–392. 2011. View Article : Google Scholar
|
|
19
|
Cai B, Lyu H, Huang J, Wang S, Lee CK, Gao
C and Liu B: Combination of bendamustine and entinostat
synergistically inhibits proliferation of multiple myeloma cells
via induction of apoptosis and DNA damage response. Cancer Lett.
335:343–350. 2013. View Article : Google Scholar
|
|
20
|
Cai B, Wang S, Huang J, Lee CK, Gao C and
Liu B: Cladribine and bendamustine exhibit inhibitory activity in
dexamethasone-sensitive and -resistant multiple myeloma cells. Am J
Transl Res. 5:36–46. 2013.PubMed/NCBI
|
|
21
|
Visco C, Castegnaro S, Chieregato K,
Bernardi M, Albiero E, Zanon C, Madeo D and Rodeghiero F: The
cytotoxic effects of bendamustine in combination with cytarabine in
mantle cell lymphoma cell lines. Blood Cells Mol Dis. 48:68–75.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gaul L, Mandl-Weber S, Baumann P, Emmerich
B and Schmidmaier R: Bendamustine induces G2 cell cycle arrest and
apoptosis in myeloma cells: The role of ATM-Chk2-Cdc25A and
ATM-p53-p21-pathways. J Cancer Res Clin Oncol. 134:245–253. 2008.
View Article : Google Scholar
|
|
23
|
Kaneko N, Mitsuoka K, Amino N, Yamanaka K,
Kita A, Mori M, Miyoshi S and Kuromitsu S: Combination of YM155, a
survivin suppressant, with bendamustine and rituximab: A new
combination therapy to treat relapsed/refractory diffuse large
B-cell lymphoma. Clin Cancer Res. 20:1814–1822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beeharry N, Rattner JB, Bellacosa A, Smith
MR and Yen TJ: Dose dependent effects on cell cycle checkpoints and
DNA repair by bendamustine. PLoS One. 7:e403422012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Darwish M, Bond M, Hellriegel E, Robertson
P Jr and Chovan JP: Pharmacokinetic and pharmacodynamic profile of
bendamustine and its metabolites. Cancer Chemother Pharmacol.
75:1143–1154. 2015. View Article : Google Scholar
|
|
26
|
Dubbelman AC, Rosing H, Darwish M,
D'Andrea D, Bond M, Hellriegel E, Robertson P Jr, Beijnen JH and
Schellens JH: Pharmacokinetics and excretion of 14C-bendamustine in
patients with relapsed or refractory malignancy. Drugs R D.
13:17–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim T, Choi HY, Lee HS, Jung SH, Ahn JS,
Kim HJ, Lee JJ, Yoo HD and Yang DH: Clinical response and
pharmacokinetics of bendamustine as a component of salvage R-B(O)AD
therapy for the treatment of primary central nervous system
lymphoma (PCNSL). BMC Cancer. 18:7292018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cephalon Inc., . Treanda (bendamustine
hydrochloride for injection) for intravenous infusion. US
prescribing information. 2021.
|
|
29
|
Knauf WU, Lissitchkov T, Aldaoud A,
Liberati AM, Loscertales J, Herbrecht R, Juliusson G, Postner G,
Gercheva L, Goranov S, et al: Bendamustine compared with
chlorambucil in previously untreated patients with chronic
lymphocytic leukaemia: Updated results of a randomized phase III
trial. Br J Haematol. 159:67–77. 2012. View Article : Google Scholar
|
|
30
|
Eichhorst B, Fink AM, Bahlo J, Busch R,
Kovacs G, Maurer C, Lange E, Köppler H, Kiehl M, Sökler M, et al:
First-line chemoimmunotherapy with bendamustine and rituximab
versus fludarabine, cyclophosphamide, and rituximab in patients
with advanced chronic lymphocytic leukaemia (CLL10): An
international, open-label, randomised, phase 3, non-inferiority
trial. Lancet Oncol. 17:928–942. 2016. View Article : Google Scholar
|
|
31
|
Woyach JA, Ruppert AS, Heerema NA, Zhao W,
Booth AM, Ding W, Bartlett NL, Brander DM, Barr PM, Rogers KA, et
al: Ibrutinib regimens versus chemoimmunotherapy in older patients
with untreated CLL. N Engl J Med. 379:2517–2528. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghia P, Pluta A, Wach M, Lysak D, Kozak T,
Simkovic M, Kaplan P, Kraychok I, Illes A, de la Serna J, et al:
ASCEND: Phase III, randomized trial of acalabrutinib versus
idelalisib plus rituximab or bendamustine plus rituximab in
relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol.
38:2849–2861. 2020. View Article : Google Scholar
|
|
33
|
Fraser GAM, Chanan-Khan A, Demirkan F,
Santucci Silva R, Grosicki S, Janssens A, Mayer J, Bartlett NL,
Dilhuydy MS, Loscertales J, et al: Final 5-year findings from the
phase 3 HELIOS study of ibrutinib plus bendamustine and rituximab
in patients with relapsed/refractory chronic lymphocytic
leukemia/small lymphocytic lymphoma. Leuk Lymphoma. 61:3188–3197.
2020. View Article : Google Scholar
|
|
34
|
Seymour JF, Kipps TJ, Eichhorst B, Hillmen
P, D'Rozario J, Assouline S, Owen C, Gerecitano J, Robak T, De la
Serna J, et al: Venetoclax-rituximab in relapsed or refractory
chronic lymphocytic leukemia. N Engl J Med. 378:1107–1120. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marcus R, Davies A, Ando K, Klapper W,
Opat S, Owen C, Phillips E, Sangha R, Schlag R, Seymour JF, et al:
Obinutuzumab for the first-line treatment of follicular lymphoma. N
Engl J Med. 377:1331–1344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hiddemann W, Barbui AM, Canales MA,
Cannell PK, Collins GP, Dürig J, Forstpointner R, Herold M,
Hertzberg M, Klanova M, et al: Immunochemotherapy with obinutuzumab
or rituximab for previously untreated follicular lymphoma in the
GALLIUM study: Influence of chemotherapy on efficacy and safety. J
Clin Oncol. 36:2395–2404. 2018. View Article : Google Scholar
|
|
37
|
Rummel MJ, Niederle N, Maschmeyer G, Banat
GA, von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M,
Balser C, et al: Bendamustine plus rituximab versus CHOP plus
rituximab as first-line treatment for patients with indolent and
mantle-cell lymphomas: An open-label, multicentre, randomised,
phase 3 non-inferiority trial. Lancet. 381:1203–1210. 2013.
View Article : Google Scholar
|
|
38
|
Flinn IW, van der Jagt R, Kahl B, Wood P,
Hawkins T, MacDonald D, Simpson D, Kolibaba K, Issa S, Chang J, et
al: First-line treatment of patients with indolent non-Hodgkin
lymphoma or mantle-cell lymphoma with bendamustine plus rituximab
versus R-CHOP or R-CVP: Results of the BRIGHT 5-year follow-up
study. J Clin Oncol. 37:984–991. 2019. View Article : Google Scholar
|
|
39
|
Sehn LH, Chua N, Mayer J, Dueck G, Trněný
M, Bouabdallah K, Fowler N, Delwail V, Press O, Salles G, et al:
Obinutuzumab plus bendamustine versus bendamustine monotherapy in
patients with rituximab-refractory indolent non-Hodgkin lymphoma
(GADOLIN): A randomised, controlled, open-label, multicentre, phase
3 trial. Lancet Oncol. 17:1081–1093. 2016. View Article : Google Scholar
|
|
40
|
Cheson BD, Chua N, Mayer J, Dueck G,
Trněný M, Bouabdallah K, Fowler N, Delwail V, Press O, Salles G, et
al: Overall survival benefit in patients with rituximab-refractory
indolent non-Hodgkin lymphoma who received obinutuzumab plus
bendamustine induction and obinutuzumab maintenance in the GADOLIN
study. J Clin Oncol. 36:2259–2266. 2018. View Article : Google Scholar
|
|
41
|
Rummel M, Kaiser U, Balser C, Stauch M,
Brugger W, Welslau M, Niederle N, Losem C, Boeck HP, Weidmann E, et
al: Bendamustine plus rituximab versus fludarabine plus rituximab
for patients with relapsed indolent and mantle-cell lymphomas: A
multicentre, randomised, open-label, non-inferiority phase 3 trial.
Lancet Oncol. 17:57–66. 2016. View Article : Google Scholar
|
|
42
|
Visco C, Chiappella A, Nassi L, Patti C,
Ferrero S, Barbero D, Evangelista A, Spina M, Molinari A, Rigacci
L, et al: Rituximab, bendamustine, and low-dose cytarabine as
induction therapy in elderly patients with mantle cell lymphoma: A
multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet
Haematol. 4:e15–e23. 2017. View Article : Google Scholar
|
|
43
|
McCulloch R, Visco C, Eyre TA, Frewin R,
Phillips N, Tucker DL, Quaglia FM, McMillan A, Lambert J, Crosbie N
and Rule S: Efficacy of R-BAC in relapsed, refractory mantle cell
lymphoma post BTK inhibitor therapy. Br J Haematol. 189:684–688.
2020. View Article : Google Scholar
|
|
44
|
Kamdar M, Li H, Chen RW, Rimsza LM,
Leblanc ML, Fenske TS, Shea TC, Barr PM, Phillips TJ, Leonard JP,
et al: Five-year outcomes of the S1106 study of R-hyper-CVAD vs
R-bendamustine in transplant-eligible patients with mantle cell
lymphoma. Blood Adv. 3:3132–3135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park SI, Grover NS, Olajide O, Asch AS,
Wall JG, Richards KL, Sobol AL, Deal AM, Ivanova A, Foster MC, et
al: A phase II trial of bendamustine in combination with rituximab
in older patients with previously untreated diffuse large B-cell
lymphoma. Br J Haematol. 175:281–289. 2016. View Article : Google Scholar
|
|
46
|
Flinn IW, Erter J, Daniel DB, Mace JR and
Berdeja JG: Phase II study of bendamustine and ofatumumab in
elderly patients with newly diagnosed diffuse large B-cell lymphoma
who are poor candidates for R-CHOP chemotherapy. Oncologist.
24:1035–e623. 2019. View Article : Google Scholar
|
|
47
|
Sehn LH, Hertzberg M, Opat S, Herrera AF,
Assouline S, Flowers CR, Kim TM, McMillan A, Ozcan M, Safar V, et
al: Polatuzumab vedotin plus bendamustine and rituximab in
relapsed/refractory DLBCL: Survival update and new extension cohort
data. Blood Adv. 6:533–543. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
O'Connor OA, Lue JK, Sawas A, Amengual JE,
Deng C, Kalac M, Falchi L, Marchi E, Turenne I, Lichtenstein R, et
al: Brentuximab vedotin plus bendamustine in relapsed or refractory
Hodgkin's lymphoma: An international, multicentre, single-arm,
phase 1–2 trial. Lancet Oncol. 19:257–266. 2018. View Article : Google Scholar
|
|
49
|
Broccoli A, Argnani L, Botto B, Corradini
P, Pinto A, Re A, Vitolo U, Fanti S, Stefoni V and Zinzani PL;
Fondazione Italiana Linfomi ONLUS, : First salvage treatment with
bendamustine and brentuximab vedotin in Hodgkin lymphoma: A phase 2
study of the Fondazione Italiana Linfomi. Blood Cancer J.
9:1002019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
LaCasce AS, Bociek RG, Sawas A, Caimi P,
Agura E, Matous J, Ansell SM, Crosswell HE, Islas-Ohlmayer M,
Behler C, et al: Three-year outcomes with brentuximab vedotin plus
bendamustine as first salvage therapy in relapsed or refractory
Hodgkin lymphoma. Br J Haematol. 189:e86–e90. 2020. View Article : Google Scholar
|
|
51
|
Hueso T, Gastinne T, Garciaz S, Tchernonog
E, Delette C, Casasnovas RO, Durot E, Houot R, Tessoulin B,
Tournilhac O, et al: Bendamustine-EAM versus BEAM regimen in
patients with mantle cell lymphoma undergoing autologous stem cell
transplantation in the frontline setting: A multicenter
retrospective study from lymphoma study association (LYSA) centers.
Bone Marrow Transplant. 55:1076–1084. 2020. View Article : Google Scholar
|
|
52
|
Gomez-Arteaga A, Mark TM, Guarneri D,
Christos PJ, Gergis U, Greenberg JD, Hsu J, Mayer SA, Niesvizky R,
Pearse RN, et al: High-dose bendamustine and melphalan conditioning
for autologous stem cell transplantation for patients with multiple
myeloma. Bone Marrow Transplant. 54:2027–2038. 2019. View Article : Google Scholar
|
|
53
|
Lee HC, Feng L, Oriabure O, Graham V, Chen
W, Badillo M, Lu R, Lee HJ, Jain P, Manasanch EE, et al: A phase
one trial of carfilzomib, bendamustine, and dexamethasone in
relapsed and/or refractory multiple myeloma. Am J Hematol.
96:E243–E246. 2021. View Article : Google Scholar
|
|
54
|
Chanan-Khan A, Cramer P, Demirkan F,
Fraser G, Silva RS, Grosicki S, Pristupa A, Janssens A, Mayer J,
Bartlett NL, et al: Ibrutinib combined with bendamustine and
rituximab compared with placebo, bendamustine, and rituximab for
previously treated chronic lymphocytic leukaemia or small
lymphocytic lymphoma (HELIOS): A randomised, double-blind, phase 3
study. Lancet Oncol. 17:200–211. 2016. View Article : Google Scholar
|
|
55
|
Fraser G, Cramer P, Demirkan F, Silva RS,
Grosicki S, Pristupa A, Janssens A, Mayer J, Bartlett NL, Dilhuydy
MS, et al: Updated results from the phase 3 HELIOS study of
ibrutinib, bendamustine, and rituximab in relapsed chronic
lymphocytic leukemia/small lymphocytic lymphoma. Leukemia.
33:969–980. 2019. View Article : Google Scholar
|
|
56
|
Cheson BD, Brugger W, Damaj G, Dreyling M,
Kahl B, Kimby E, Ogura M, Weidmann E, Wendtner CM and Zinzani PL:
Optimal use of bendamustine in hematologic disorders: Treatment
recommendations from an international consensus panel-an update.
Leuk Lymphoma. 57:766–782. 2016. View Article : Google Scholar
|
|
57
|
Flinn IW, van der Jagt R, Kahl BS, Wood P,
Hawkins TE, Macdonald D, Hertzberg M, Kwan YL, Simpson D, Craig M,
et al: Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP
in first-line treatment of indolent NHL or MCL: The BRIGHT study.
Blood. 123:2944–2952. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Martin P, Chen Z, Cheson BD, Robinson KS,
Williams M, Rajguru SA, Friedberg JW, van der Jagt RH, LaCasce AS,
Joyce R, et al: Long-term outcomes, secondary malignancies and stem
cell collection following bendamustine in patients with previously
treated non-Hodgkin lymphoma. Br J Haematol. 178:250–256. 2017.
View Article : Google Scholar
|
|
59
|
Fung M, Jacobsen E, Freedman A, Prestes D,
Farmakiotis D, Gu X, Nguyen PL and Koo S: Increased risk of
infectious complications in older patients with indolent
non-Hodgkin lymphoma exposed to bendamustine. Clin Infect Dis.
68:247–255. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pezzullo L, Giudice V, Serio B, Fontana R,
Guariglia R, Martorelli MC, Ferrara I, Mettivier L, Bruno A, Bianco
R, et al: Real-world evidence of cytomegalovirus reactivation in
non-Hodgkin lymphomas treated with bendamustine-containing
regimens. Open Med (Wars). 16:672–682. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen RW, Li H, Bernstein SH, Kahwash S,
Rimsza LM, Forman SJ, Constine L, Shea TC, Cashen AF, Blum KA, et
al: RB but not R-HCVAD is a feasible induction regimen prior to
auto-HCT in frontline MCL: Results of SWOG study S1106. Br J
Haematol. 176:759–769. 2017. View Article : Google Scholar
|
|
62
|
Smith A, Roman E, Appleton S, Howell D,
Johnson R, Burton C and Patmore R: Impact of novel therapies for
mantle cell lymphoma in the real world setting: A report from the
UK's haematological malignancy research network (HMRN). Br J
Haematol. 181:215–228. 2018. View Article : Google Scholar
|
|
63
|
Bašić-Kinda S, Mišura Jakobac K,
Sinčić-Petričević J, Deak D, Vodanović M, Jakić-Bubalo M, Mitrović
Z, Grubešić A, Dreta B, Županić Krmek D, et al: Improvement in the
outcomes of mantle cell lymphoma in the last decade: A real-life
non interventional study of the croatian cooperative group for
hematologic diseases. Croat Med J. 62:455–463. 2021. View Article : Google Scholar
|
|
64
|
Roué G, López-Guerra M, Milpied P,
Pérez-Galán P, Villamor N, Montserrat E, Campo E and Colomer D:
Bendamustine is effective in p53-deficient B-cell neoplasms and
requires oxidative stress and caspase-independent signaling. Clin
Cancer Res. 14:6907–6915. 2008. View Article : Google Scholar
|
|
65
|
Aurer I: Mantle cell lymphoma in patients
not eligible for autologous stem cell transplantation. Curr Opin
Oncol. 31:374–379. 2019. View Article : Google Scholar
|
|
66
|
Aukema SM, Hoster E, Rosenwald A, Canoni
D, Delfau-Larue MH, Rymkiewicz G, Thorns C, Hartmann S,
Kluin-Nelemans H, Hermine O, et al: Expression of TP53 is
associated with the outcome of MCL independent of MIPI and Ki-67 in
trials of the European MCL network. Blood. 131:417–420. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
FDA approves polatuzumab vedotin-piiq for
diffuse large B-cell lymphoma. 2019.
|
|
68
|
Nikitorowicz-Buniak J: CHMP recommends EMA
approval of polatuzumab vedotin for the treatment of adult patients
with R/R DLBCL. 2019.
|
|
69
|
Moskowitz AJ, Hamlin PA Jr, Perales MA,
Gerecitano J, Horwitz SM, Matasar MJ, Noy A, Palomba ML, Portlock
CS, Straus DJ, et al: Phase II study of bendamustine in relapsed
and refractory Hodgkin lymphoma. J Clin Oncol. 31:456–460. 2013.
View Article : Google Scholar
|
|
70
|
Rinnerthaler G, Gampenrieder SP, Petzer A,
Hubalek M, Petru E, Sandholzer M, Andel J, Balic M, Melchardt T,
Hauser-Kronberger C, et al: Capecitabine in combination with
bendamustine in pretreated women with HER2-negative metastatic
breast cancer: Results of a phase II trial (AGMT MBC-6). Ther Adv
Med Oncol. 13:175883592110423012021. View Article : Google Scholar
|
|
71
|
Lammers PE, Shyr Y, Li CI, Hutchison AS,
Sandler A, Carbone DP, Johnson DH, Keedy VL and Horn L: Phase II
study of bendamustine in relapsed chemotherapy sensitive or
resistant small-cell lung cancer. J Thorac Oncol. 9:559–562. 2014.
View Article : Google Scholar
|
|
72
|
Hartmann JT, Mayer F, Schleicher J, Horger
M, Huober J, Meisinger I, Pintoffl J, Käfer G, Kanz L and Grünwald
V; German sarcoma group, : Bendamustine hydrochloride in patients
with refractory soft tissue sarcoma: A noncomparative multicenter
phase 2 study of the German sarcoma group (AIO-001). Cancer.
110:861–866. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chambers SK: Open trial of bendamustine
hydrochloride in women with advanced ovarian cancer. NCT00867503.
2012.
|
|
74
|
Friedberg JW, Cohen P, Chen L, Robinson
KS, Forero-Torres A, La Casce AS, Fayad LE, Bessudo A, Camacho ES,
Williams ME, et al: Bendamustine in patients with
rituximab-refractory indolent and transformed non-Hodgkin's
lymphoma: Results from a phase II multicenter, single-agent study.
J Clin Oncol. 26:204–210. 2008. View Article : Google Scholar
|
|
75
|
Knauf WU, Lissichkov T, Aldaoud A,
Liberati A, Loscertales J, Herbrecht R, Juliusson G, Postner G,
Gercheva L, Goranov S, et al: Phase III randomized study of
bendamustine compared with chlorambucil in previously untreated
patients with chronic lymphocytic leukemia. J Clin Oncol.
27:4378–4384. 2009. View Article : Google Scholar
|
|
76
|
Cheson BD, Friedberg JW, Kahl BS, Van der
Jagt RH and Tremmel L: Bendamustine produces durable responses with
an acceptable safety profile in patients with rituximab-refractory
indolent non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk.
10:452–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Stokes J, Molina MS, Hoffman EA, Simpson
RJ and Katsanis E: Immunomodulatory effects of bendamustine in
hematopoietic cell transplantation. Cancers (Basel). 13:17022021.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Allegra A, Tonacci A, Musolino C, Pioggia
G and Gangemi S: Secondary immunodeficiency in hematological
malignancies: Focus on multiple myeloma and chronic lymphocytic
leukemia. Front Immunol. 12:7389152021. View Article : Google Scholar
|
|
79
|
Gafter-Gvili A, Gurion R, Raanani P,
Shpilberg O and Vidal L: Bendamustine-associated
infections-systematic review and meta-analysis of randomized
controlled trials. Hematol Oncol. 35:424–431. 2017. View Article : Google Scholar
|
|
80
|
Lamure S, Duléry R, Di Blasi R, Chauchet
A, Laureana C, Deau-Fischer B, Drenou B, Soussain C, Rossi C, Noël
N, et al: Determinants of outcome in Covid-19 hospitalized patients
with lymphoma: A retrospective multicentric cohort study.
EClinicalMedicine. 27:1005492020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fraser C, Brown P, Megason G, Ahn HS, Cho
B, Kirov I, Frankel L, Aplenc R, Bensen-Kennedy D, Munteanu M, et
al: Open-label bendamustine monotherapy for pediatric patients with
relapsed or refractory acute leukemia: Efficacy and tolerability. J
Pediatr Hematol Oncol. 36:e212–e218. 2014. View Article : Google Scholar
|
|
82
|
García Muñoz R, Izquierdo-Gil A, Muñoz A,
Roldan-Galiacho V, Rabasa P and Panizo C: Lymphocyte recovery is
impaired in patients with chronic lymphocytic leukemia and indolent
non-Hodgkin lymphomas treated with bendamustine plus rituximab. Ann
Hematol. 93:1879–1887. 2014. View Article : Google Scholar
|
|
83
|
Saito H, Maruyama D, Maeshima AM, Makita
S, Kitahara H, Miyamoto K, Fukuhara S, Munakata W, Suzuki T,
Kobayashi Y, et al: Prolonged lymphocytopenia after bendamustine
therapy in patients with relapsed or refractory indolent B-cell and
mantle cell lymphoma. Blood Cancer J. 5:e3622015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Coutre SE, Flinn IW, de Vos S, Barrientos
JC, Schreeder MT, Wagner-Johnson ND, Sharman JP, Boyd TE, Fowler N,
Dreiling L, et al: Idelalisib in combination with rituximab or
bendamustine or both in patients with relapsed/refractory chronic
lymphocytic leukemia. Hemasphere. 2:e392018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rummel MJ, Janssens A, MacDonald D,
Keating MM, Zaucha JM, Davis J, Lasher J, Babanrao Pisal C,
Izquierdo M and Friedberg JW: A phase 3, randomized study of
ofatumumab combined with bendamustine in rituximab-refractory iNHL
(COMPLEMENT A + B study). Br J Haematol. 193:1123–1133. 2021.
View Article : Google Scholar
|
|
86
|
Schöffski P, Seeland G, Engel H, Grünwald
V, Paul H, Merkle K, Kowalski R and Ganser A: Weekly administration
of bendamustine: A phase I study in patients with advanced
progressive solid tumours. Ann Oncol. 11:729–734. 2000. View Article : Google Scholar
|
|
87
|
Bremer K: High rates of long-lasting
remissions after 5-day bendamustine chemotherapy cycles in
pre-treated low-grade non-Hodgkin's-lymphomas. J Cancer Res Clin
Oncol. 128:603–609. 2002. View Article : Google Scholar
|
|
88
|
Klippstein A, Schneider CP, Sayer HG and
Höffken K: Pneumocystis carinii pneumonia as a complication of
bendamustine monotherapy in a patient with advanced progressive
breast cancer. J Cancer Res Clin Oncol. 129:316–319. 2003.
View Article : Google Scholar
|
|
89
|
Layman RM, Ruppert AS, Lynn M, Mrozek E,
Ramaswamy B, Lustberg MB, Wesolowski R, Ottman S, Carothers S,
Bingman A, et al: Severe and prolonged lymphopenia observed in
patients treated with bendamustine and erlotinib for metastatic
triple negative breast cancer. Cancer Chemother Pharmacol.
71:1183–1190. 2013. View Article : Google Scholar
|
|
90
|
Burotto M, Stetler-Stevenson M, Arons E,
Zhou H, Wilson W and Kreitman RJ: Bendamustine and rituximab in
relapsed and refractory hairy cell leukemia. Clin Cancer Res.
19:6313–6321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Martínez-Calle N, Hartley S, Ahearne M,
Kasenda B, Beech A, Knight H, Balotis C, Kennedy B, Wagner S, Dyer
MJS, et al: Kinetics of T-cell subset reconstitution following
treatment with bendamustine and rituximab for low-grade
lymphoproliferative disease: A population-based analysis. Br J
Haematol. 184:957–968. 2019.
|
|
92
|
Stokes J, Hoffman EA, Zeng Y, Larmonier N
and Katsanis E: Post-transplant bendamustine reduces GvHD while
preserving GvL in experimental haploidentical bone marrow
transplantation. Br J Haematol. 174:102–116. 2016. View Article : Google Scholar
|
|
93
|
Cona A, Tesoro D, Chiamenti M, Merlini E,
Ferrari D, Marti A, Codecà C, Ancona G, Tincati C, d'Arminio
Monforte A and Marchetti G: Disseminated cytomegalovirus disease
after bendamustine: A case report and analysis of circulating B-
and T-cell subsets. BMC Infect Dis. 19:8812019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Stokes J, Hoffman EA, Molina MS, Kummet N,
Simpson RJ, Zeng Y and Katsanis E: Bendamustine with total body
irradiation conditioning yields tolerant T-cells while preserving
T-cell-dependent graft-versus-leukemia. Oncoimmunology.
9:17580112020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
García M, Bellosillo B, Sánchez-González
B, García-Payarols F, Seoane A, Ferrer AM, Gimeno E, Barranco LE,
Torner A, Solé F, et al: Study of regulatory T-cells in patients
with gastric malt lymphoma: Influence on treatment response and
outcome. PLoS One. 7:e516812012. View Article : Google Scholar
|
|
96
|
Molina MS, Hoffman EA, Stokes J, Kummet N,
Smith KA, Baker F, Zúñiga TM, Simpson RJ and Katsanis E: Regulatory
dendritic cells induced by bendamustine are associated with
enhanced Flt3 expression and alloreactive T-cell death. Front
Immunol. 12:6991282021. View Article : Google Scholar
|
|
97
|
Lepik KV, Mikhailova NB, Kondakova EV,
Zalyalov YR, Fedorova LV, Tsvetkova LA, Kotselyabina PV, Borzenkova
ES, Babenko EV, Popova MO, et al: A study of safety and efficacy of
nivolumab and bendamustine (NB) in patients with
relapsed/refractory Hodgkin lymphoma after nivolumab monotherapy
failure. Hemasphere. 4:e4012020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Montraveta A, Lee-Vergés E, Roldán J,
Jiménez L, Cabezas S, Clot G, Pinyol M, Xargay-Torrent S, Rosich L,
Arimany-Nardí C, et al: CD69 expression potentially predicts
response to bendamustine and its modulation by ibrutinib or
idelalisib enhances cytotoxic effect in chronic lymphocytic
leukemia. Oncotarget. 7:5507–5520. 2016. View Article : Google Scholar
|
|
99
|
Gafter-Gvili A and Polliack A:
Bendamustine associated immune suppression and infections during
therapy of hematological malignancies. Leuk Lymphoma. 57:512–519.
2016. View Article : Google Scholar
|
|
100
|
Yamasaki S, Matsushima T, Minami M,
Kadowaki M, Takase K and Iwasaki H: Clinical impact of bendamustine
exposure on lymphopenia risk after bendamustine and rituximab
combination therapy for follicular lymphoma: A single-institute
retrospective study. Ann Hematol. 101:209–211. 2022. View Article : Google Scholar
|
|
101
|
Lu L, Yoshimoto K, Morita A, Kameda H and
Takeuchi T: Bendamustine increases interleukin-10 secretion from B
cells via p38 MAP kinase activation. Int Immunopharmacol.
39:273–279. 2016. View Article : Google Scholar
|
|
102
|
Stenger EO, Turnquist HR, Mapara MY and
Thomson AW: Dendritic cells and regulation of graft-versus-host
disease and graft-versus-leukemia activity. Blood. 119:5088–5103.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Barbarroja-Escudero J, Sanchez-Gonzalez
MJ, Antolin-Amerigo D, Rodriguez-Rodriguez M and Alvarez-Mon M:
Hypersensitivity reactions and drug fever by bendamustine: A case
report of three patients. Allergol Int. 64:109–111. 2015.
View Article : Google Scholar
|
|
104
|
Chan M, Silverstein WK, Nikonova A,
Pavenski K and Hicks LK: Bendamustine-induced immune hemolytic
anemia: A case report and systematic review of the literature.
Blood Adv. 4:1756–1759. 2020. View Article : Google Scholar : PubMed/NCBI
|