Renal cell carcinoma (RCC) is a group of neoplastic
diseases that affect the renal parenchyma and represent 2–3% of all
cancer diagnoses (1). In 2022, the
GLOBOCAN database estimated the global incidence of RCC as 434,419
cases, which were associated with 155,702 deaths (2). RCC is more commonly diagnosed in men
(ratio men to women, 2:1), and in the sixth decade of life
(3). Up to 70% of patients are
incidentally diagnosed with RCC, 50% of them with metastatic
disease (4); although localized RCC
is curable, in up to 30% of patients this will progress to
metastatic disease, which has a median survival time of 6–10 months
(5). Among the different types of
RCCs, 80% of all diagnoses consist of the clear cell histological
variety (ccRCC), which is followed in frequency by the papillary
variant (10–15% of cases), the chromophobe cell variant (4–5% of
cases), and other molecularly defined phenotypes (<1% of cases)
(6).
The high rates of mortality that are observed in RCC
have been associated with factors such as the high prevalence of
advanced disease at diagnosis (7)
and the intrinsic chemoresistance of RCC tumors, which is mostly
related to apoptotic resistance, the upregulation of xenobiotic
excretion systems (8–10) and the metabolic dependence of such
tumors on the Warburg effect (11).
In total, <10% of patients with RCC will respond to cytotoxic
chemotherapy (12), which makes it
necessary to use novel approaches for which little clinical
information is available. Current RCC treatments can be grouped
into the following categories: cytotoxic chemotherapy, surgery,
local therapy, recombinant-cytokine therapy, targeted
anti-angiogenic therapy, immunotherapy [immune checkpoint blockade
(ICB)], and other therapies that include the use of inhibitors of
the mechanistic target of rapamycin pathway (Table I). Despite the broad diversity and
availability of RCC treatments, the need for a prognostic tool for
use in selecting the treatment of choice and evaluating the
clinical responses of patients remains a matter of controversy
(13). Treatment selection depends
on factors such as the histological variety, cellular grade and
clinical stage of the disease, as well as the failure of previous
treatments (14–16). In this regard, there are currently
several scales that have been validated for risk classification and
clinical decision-making in patients with RCC; among these, the
Heng score (International Metastatic RCC Database Consortium or
IMDC), the MSKCC scale (Memorial Sloan-Kettering Cancer Center),
and the general physical status according to ECOG-PS criteria (the
Eastern Cooperative Oncology Group performance score) (17–19)
stand out (Table II).
In the last decade, the use of monoclonal antibodies
for reversing the ‘exhaustion’ state in tumor infiltrating
lymphocytes (TILs) has proven to be a valuable treatment for
numerous solid tumors and hematologic cancers. In 2015, the
ChekMate-025 study demonstrated the efficacy of nivolumab (which
was initially approved for treating melanoma and non-small cell
lung cancer) in treating patients with RCC (20). In addition to nivolumab [which
blocks the programmed cell death protein 1 (PD-1) receptor], four
other monoclonal antibodies are currently approved for use in RCC,
all of which reverse the inhibition of lymphocyte immune effectors:
Pembrolizumab, which is also a PD-1 blocker; ipilimumab, which
blocks the cytotoxic T-lymphocyte associated protein 4; and
atezolizumab/avelumab, which blocks PD-1 ligand in myeloid and
tumor cells and prevents PD-1 inhibitory signals in lymphocytes
(Table III) (20–30).
In the following section, the current knowledge of
the cellular mechanisms that lead to lymphocyte exhaustion within
the highly dynamic tumor-immune microenvironment (TIME) was
reviewed. It was concluded with a short description of experimental
biomarkers that have been used to predict the patients' responses
to ICB therapy.
The TIME comprises a network of interacting elements
within the tumor tissue that can be categorized as follows: Cells
(tumor, stroma and infiltrating immune cells), small soluble
elements (proteins, cytokines, growth factors, metabolites and
chemokines), and the extracellular matrix (31). The growth of tumors requires two
conditions: Cell transformation, whether genetic or acquired
(32) and immune dysfunction
(33). This second requirement
explains the association between immunodeficiency status and cancer
development (34). The clearance of
transformed cells from tissues requires the activation of cytotoxic
immunity that is achieved through the infiltration of
CD8+ cytotoxic lymphocytes and natural killer cells into
a tumor (35). Antitumor immunity
is so efficient that even though transformation and cell damage
occur over the course of every life, cancer develops only in a
small proportion of patients; the occurrence of cancer is promoted
by immune dysfunction.
Tumor cells proliferate at higher rates compared
with normal cells. This accelerated proliferation, which is linked
to metabolic changes that take place within the tumor bed, results
in dysfunctional local immunity and further selection of the
best-adapted tumor cells (36–38).
Cytotoxic chemotherapy exploits this increased proliferative rate,
and cytotoxic treatments are widely used in most neoplastic
diseases other than RCC. The peculiarities of RCC include its
intrinsic aggressive nature, its increased infiltration with
lipids, its high rate of metastasis, and its resistance to
cytotoxic chemotherapy (39). This
chemo-resistance has numerous causal factors, both intrinsic (or
genetic) and acquired. It is important to note that the epithelial
renal cells normally have secretion systems for xenobiotics and
intrinsic antioxidant mechanisms protecting the cells from toxic
damage (40). Given that most RCC
subtypes arise from renal epithelia, it is not surprising that such
mechanisms are enhanced in renal carcinoma. In addition, renal
tumor cells have increased expression of anti-apoptotic proteins
Bcl-2, Bcl-XL, ARC (apoptosis repressor with a caspase
recruitment domain) and XIAP (X-linked inhibitor of apoptosis)
while pro-apoptotic proteins such as Bim are decreased.
Anti-apoptotic and pro-apoptotic proteins are regulated by NF-κB
and von Hippel-Lindau (VHL) pathways, respectively (8,10,41).
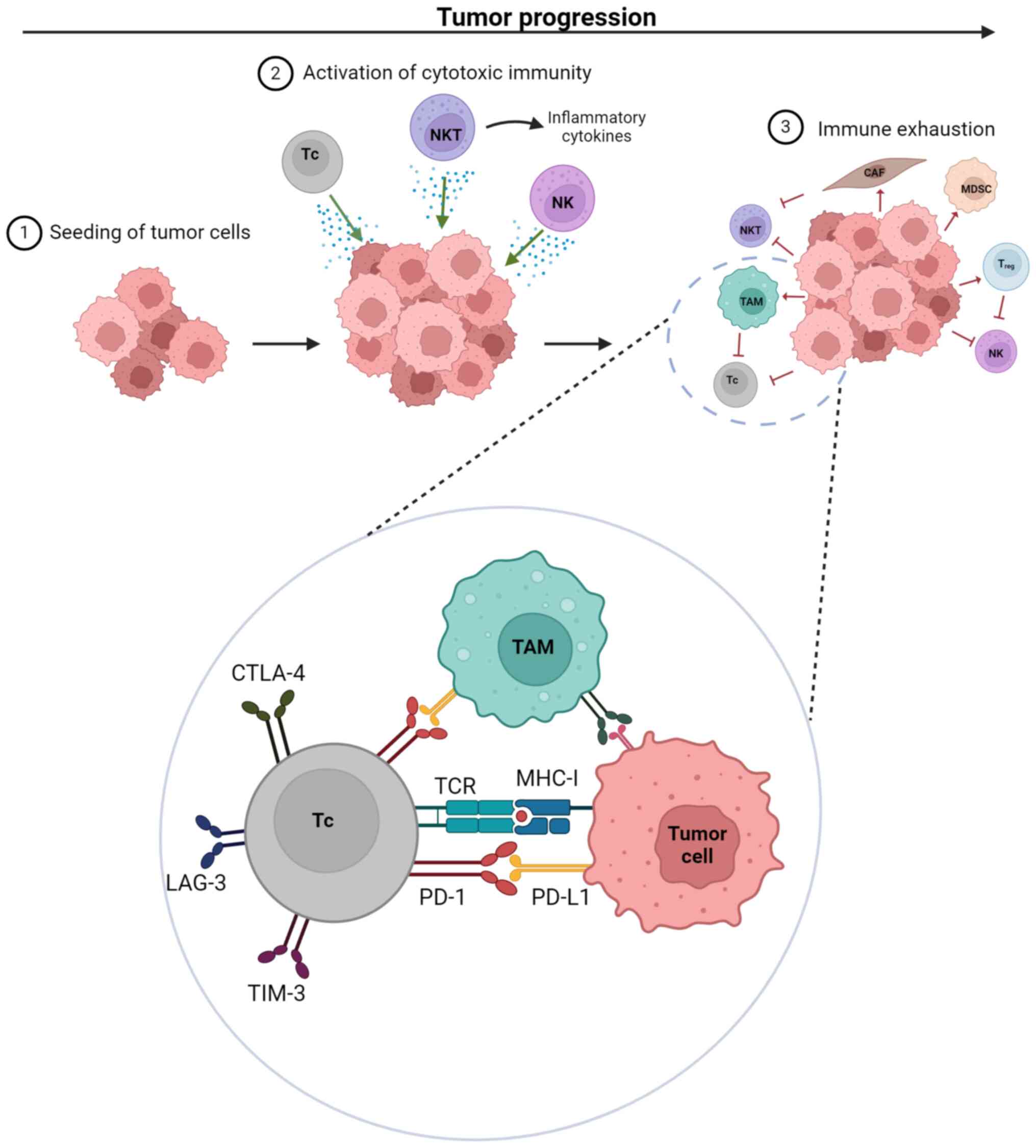
In addition, although the presence of TILs is a
favorable prognostic factor for most cancers, such infiltrative
cells are associated with poor outcomes in RCC (44,45).
Consequently, a hostile TIME develops that leads to the selection
of tumors that are resistant to hypoxia, acidosis and the low
availability of nutrients. In addition, a hostile TIME negatively
affects lymphocyte-dependent antitumor immunity (Fig. 1 and Table IV) (31,46–51).
Previous studies suggested that dysbiosis is an important element
to be considered when evaluating either the responses of tumors to
ICB or the general prognosis of patients with neoplastic diseases
(52,53). The molecular basis for lymphocyte
dysfunction involves several mechanisms that are associated with
peripheral tolerance, namely anergy, suppression and exhaustion
(54). Immune cell exhaustion is
reversed by ICB, which blocks interactions between the inhibitory
receptors of lymphocytes and their ligands (55). The PD-1/programmed death-ligand 1
(PD-L1) axis is the most studied of these interactions within TILs
(56,57).
Even though numerous patients achieve complete
clinical response to ICB therapy, a significant proportion of
patients show only a partial response or no response at all, which
is occasionally accompanied by signs of systemic toxicity (58). This situation explains why the
search for prognostic markers for ICB in patients with RCC is a
highly active area of research. Most prognostic markers for RCC can
be divided into those addressing disease progression and those
addressing responses to treatments, in particular immunotherapy and
directed therapies. Thus, predicting the ICB responsiveness will
impact in selecting the patients who will benefit the most and have
the lowest probability of developing adverse events after receiving
immunotherapy. There are currently no standardized and validated
methods for properly assessing the risk/benefit ratio of using ICB
therapy. Therefore, the goal of the present review was to
synthesize the reported findings of the studies that have focused
on this important topic.
ICB therapy is associated with an average mortality
rate of 1%, with death mostly caused by immune-related adverse
events (58). After the reporting
of successful results from the CheckMate-025 study, and given the
low predictability and consistency of ICB responsiveness (59), interest has been increasing in the
search for reliable markers of ICB responsiveness. Among the
biomarkers that have been studied, three stand out for their
consistency: the level of C-reactive protein (CRP), the number of
TILs and the basal expression levels of exhaustion markers in tumor
tissue. CRP is an acute phase reactant that is frequently used for
evaluating systemic inflammation, making it a logical putative
marker for ICB responsiveness given the close association between
inflammation and cancer. Previous studies have indicated that low
basal CRP levels or low normalized levels of CRP measured after
patients received their first ICB doses were predictive of their
ICB responsiveness (60–63). Studies investigating TILs found that
a patient's prognosis is associated with the nature of his or her
infiltrating cells; infiltration with inflammatory leukocytes was
associated with the best responses to ICB therapy (64–66).
In peripheral blood, a recent study observed that increased numbers
of circulating eosinophils were associated with an improved
response to ICB, which is an intriguing result given the regulatory
role that eosinophils play in systemic inflammation (67). Interestingly, the basal expression
level of PD-L1 has not been consistently predictive of ICB
responsiveness (68–70), whereas the basal expression level of
T cell immunoglobulin and mucin-domain containing-3 (TIM-3) appears
to be (71).
Several additional markers have been studied for
predicting the response to ICB in patients with RCC. For example,
longer responses to ICB have been associated with decreased levels
of circulating tumor DNA, increased levels of chemokine CXCL14,
increased levels of circulating miR-22 and miR-24, and the presence
of immunogenic transcriptional signatures (72–75).
In this context, several markers have been associated with poor
responses after ICB therapy (increased levels of interleukin-8) or
predictive of the development of immune-related adverse events
(decreased levels of miR-146a) (76,77).
All the studies described in this section are summarized in
Table V.
From a technical perspective, the evaluation of
leukocytes' exhaustion markers require the performance of
histopathology after the direct sampling of tissue, which is not
feasible for all diagnoses (78).
In this context, the studies assessing the suitability of
peripheral biomarkers for predicting ICB responsiveness are
encouraging (79,80).
Several limitations currently prevent the formal use
of markers to predict ICB responsiveness. First, the inconsistency
of reported outcomes is mostly related to heterogeneity in the
target population and the low number of patients that have been
studied. In addition, a lack of standardization in the use of
methods and reagents is complicating the replication of pioneering
studies. Furthermore, the relationship between the expression of
such potential biomarkers and the underlying mechanisms of
resistance to ICB is not fully understood, making it difficult not
only to predict ICB responsiveness but also to know the clinical
safety of using ICB. Finally, the intrinsic resistance of RCCs to
chemotherapy and the complex combinations required for treatment
make it difficult to determine a logical approach for studying the
expression of putative cancer biomarkers. In the future, by
integrating multi-omics data and machine-learning approaches, it
should be possible to create more accurate prediction models that
will help in the identification of novel biomarkers for ICB
responsiveness.
The authors are grateful to Dr. Susana Chávez
(Department for Research Assistance, University Hospital ‘José
Eleuterio González’, Autonomous University of Nuevo León) for
reviewing the English language in this manuscript.
The present study was supported by CONAHCYT México (grant no.
783446).
Not applicable.
RGG and MMT performed the literature review, wrote
the manuscript, made the illustrations and constructed the tables.
AGG, MCSC and MMT revised the manuscript. RGG and MMT were involved
in the conception of the study. All authors read and approved the
final version of the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Padala SA and Barsouk A, Thandra KC,
Saginala K, Mohammed A, Vakiti A, Rawla P and Barsouk A:
Epidemiology of renal cell carcinoma. World J Oncol. 11:79–87.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bukavina L, Bensalah K, Bray F, Carlo M,
Challacombe B, Karam JA, Kassouf W, Mitchell T, Montironi R and
O'Brien T: Epidemiology of renal cell carcinoma: 2022 update. Eur
Urol. 82:529–542. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Musaddaq B, Musaddaq T, Gupta A, Ilyas S
and von Stempel C: Renal cell carcinoma: The evolving role of
imaging in the 21st century. Semin Ultrasound CT MR. 41:344–350.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scosyrev E, Messing EM, Sylvester R and
Van Poppel H: Exploratory subgroup analyses of renal function and
overall survival in european organization for research and
treatment of cancer randomized trial of Nephron-sparing surgery
versus radical nephrectomy. Eur Urol Focus. 3:599–605. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goswami PR, Singh G, Patel T and Dave R:
The WHO 2022 classification of renal neoplasms (5th Edition):
Salient updates. Cureus. 16:e584702024.PubMed/NCBI
|
|
7
|
Didwaniya N, Edmonds RJ, Fang X,
Silberstein PT and Subbiah S: Survival outcomes in metastatic renal
carcinoma based on histological subtypes: SEER database analysis. J
Clin Oncol. 29:381. 2011. View Article : Google Scholar
|
|
8
|
Morais C, Gobe G, Johnson DW and Healy H:
Inhibition of nuclear factor kappa B transcription activity drives
a synergistic effect of pyrrolidine dithiocarbamate and cisplatin
for treatment of renal cell carcinoma. Apoptosis. 15:412–425. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toth C, Funke S, Nitsche V, Liverts A,
Zlachevska V, Gasis M, Wiek C, Hanenberg H, Mahotka C, Schirmacher
P and Heikaus S: The role of apoptosis repressor with a CARD domain
(ARC) in the therapeutic resistance of renal cell carcinoma (RCC):
The crucial role of ARC in the inhibition of extrinsic and
intrinsic apoptotic signalling. Cell Commun Signal. 15:162017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang WZ, Zhou H and Yan Y: XIAP underlies
apoptosis resistance of renal cell carcinoma cells. Mol Med Rep.
17:125–130. 2018.PubMed/NCBI
|
|
11
|
Wang L, Fu B, Hou DY, Lv YL, Yang G, Li C,
Shen JC, Kong B, Zheng LB, Qiu Y, et al: PKM2 allosteric converter:
A self-assembly peptide for suppressing renal cell carcinoma and
sensitizing chemotherapy. Biomaterials. 296:1220602023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diamond E, Molina AM, Carbonaro M, Akhtar
NH, Giannakakou P, Tagawa ST and Nanus DM: Cytotoxic chemotherapy
in the treatment of advanced renal cell carcinoma in the era of
targeted therapy. Crit Rev Oncol Hematol. 96:518–526. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saliby RM, Saad E, Kashima S, Schoenfeld
DA and Braun DA: Update on biomarkers in renal cell carcinoma. Am
Soc Clin Oncol Educ Book. 44:e4307342024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lam JS, Pantuck AJ, Belldegrun AS and
Figlin RA: Protein expression profiles in renal cell carcinoma:
Staging, prognosis, and patient selection for clinical trials. Clin
Cancer Res. 13((2 Pt 2)): 703s–708s. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JN, Chun SY, Ha YS, Choi KH, Yoon GS,
Kim HT, Kim TH, Yoo ES, Kim BW and Kwon TG: Target molecule
expression profiles in metastatic renal cell carcinoma: Development
of individual targeted therapy. Tissue Eng Regen Med. 13:416–427.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patard JJ, Leray E, Rioux-Leclercq N,
Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani
W, Abbou CC, et al: Prognostic value of histologic subtypes in
renal cell carcinoma: A multicenter experience. J Clin Oncol.
23:2763–2771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heng DY, Xie W, Regan MM, Harshman LC,
Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan
MH, et al: External validation and comparison with other models of
the International Metastatic Renal-Cell Carcinoma Database
Consortium prognostic model: A population-based study. Lancet
Oncol. 14:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kattan MW, Reuter V, Motzer RJ, Katz J and
Russo P: A Postoperative prognostic nomogram for renal cell
carcinoma. J Urol. 166:63–67. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
Renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Motzer RJ, Tannir NM, McDermott DF, Arén
Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: Nivolumab plus ipilimumab versus
sunitinib in advanced renal-cell carcinoma. N Engl J Med.
378:1277–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Motzer R, Alekseev B, Rha SY, Porta C, Eto
M, Powles T, Grünwald V, Hutson TE, Kopyltsov E, Méndez-Vidal MJ,
et al: Lenvatinib plus pembrolizumab or everolimus for advanced
renal cell carcinoma. N Engl J Med. 384:1289–1300. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Motzer RJ, Penkov K, Haanen J, Rini B,
Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S,
Uemura M, et al: Avelumab plus axitinib versus sunitinib for
advanced renal-cell carcinoma. N Engl J Med. 380:1103–1115. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rini BI, Plimack ER, Stus V, Gafanov R,
Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B,
et al: Pembrolizumab plus axitinib versus sunitinib for advanced
Renal-cell carcinoma. N Engl J Med. 380:1116–1127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rini BI, Powles T, Atkins MB, Escudier B,
McDermott DF, Suarez C, Bracarda S, Stadler WM, Donskov F, Lee JL,
et al: Atezolizumab plus bevacizumab versus sunitinib in patients
with previously untreated metastatic renal cell carcinoma
(IMmotion151): A multicentre, open-label, phase 3, randomised
controlled trial. The Lancet. 393:2404–2415. 2019. View Article : Google Scholar
|
|
26
|
Vogelzang NJ, Olsen MR, McFarlane JJ,
Arrowsmith E, Bauer TM, Jain RK, Somer B, Lam ET, Kochenderfer MD,
Molina A, et al: Safety and efficacy of nivolumab in patients with
advanced Non-clear cell renal cell carcinoma: Results from the
Phase IIIb/IV CheckMate 374 study. Clin Genitourin Cancer.
18:461–468.e3. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tykodi SS, Gordan LN, Alter RS, Arrowsmith
E, Harrison MR, Percent I, Singal R, Van Veldhuizen P, George DJ,
Hutson T, et al: Safety and efficacy of nivolumab plus ipilimumab
in patients with advanced non-clear cell renal cell carcinoma:
Results from the phase 3b/4 CheckMate 920 trial. J Immunother
Cancer. 10:e0038442022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pal SK, Albiges L, Tomczak P, Suárez C,
Voss MH, de Velasco G, Chahoud J, Mochalova A, Procopio G,
Mahammedi H, et al: Atezolizumab plus cabozantinib versus
cabozantinib monotherapy for patients with renal cell carcinoma
after progression with previous immune checkpoint inhibitor
treatment (CONTACT-03): A multicentre, randomised, Open-label,
phase 3 trial. The Lancet. 402:185–195. 2023. View Article : Google Scholar
|
|
29
|
Choueiri TK, Powles T, Burotto M, Escudier
B, Bourlon MT, Zurawski B, Oyervides Juárez VM, Hsieh JJ, Basso U,
Shah AY, et al: Nivolumab plus cabozantinib versus sunitinib for
advanced renal-cell carcinoma. N Engl J Med. 384:829–841. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choueiri TK, Tomczak P, Park SH, Venugopal
B, Ferguson T, Chang YH, Hajek J, Symeonides SN, Lee JL, Sarwar N,
et al: Adjuvant pembrolizumab after nephrectomy in renal-cell
carcinoma. N Engl J Med. 385:683–694. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Monjaras-Avila CU, Lorenzo-Leal AC,
Luque-Badillo AC, D'Costa N, Chavez-Muñoz C and Bach H: The tumor
immune microenvironment in clear cell renal cell carcinoma. Int J
Mol Sci. 24:79462023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karras P, Black JRM, McGranahan N and
Marine JC: Decoding the interplay between genetic and Non-genetic
drivers of metastasis. Nature. 629:543–554. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ucche S and Hayakawa Y: Immunological
aspects of cancer cell metabolism. Int J Mol Sci. 25:52882024.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bucciol G, Delafontaine S, Meyts I and
Poli C: Inborn errors of immunity: A field without frontiers.
Immunol Rev. 322:15–27. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kramer G, Blair T, Bambina S, Kaur AP,
Alice A, Baird J, Friedman D, Dowdell AK, Tomura M, Grassberger C,
et al: Fluorescence tracking demonstrates T cell recirculation is
transiently impaired by radiation therapy to the tumor. Sci Rep.
14:119092024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tan D, Miao D, Zhao C, Shi J, Lv Q, Xiong
Z, Yang H and Zhang X: Comprehensive analyses of A
12-metabolism-associated gene signature and its connection with
tumor metastases in clear cell renal cell carcinoma. BMC Cancer.
23:2642023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu Y and Li X: Senescence gene expression
in clear cell renal cell carcinoma: Role of tumor immune
microenvironment and senescence-associated survival prediction.
Medicine (Baltimore). 102:e352222023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Q, Lin B, Chen H, Ye Y, Huang Y,
Chen Z and Li J: Lipid Metabolism-related gene expression in the
immune microenvironment predicts prognostic outcomes in renal cell
carcinoma. Front Immunol. 14:13242052023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bahadoram S, Davoodi M, Hassanzadeh S,
Bahadoram M, Barahman M and Mafakher L: Renal cell carcinoma: An
overview of the epidemiology, diagnosis, and treatment. G Ital
Nefrol. 39:2022–vol3. 2022.PubMed/NCBI
|
|
40
|
Mickisch G, Bier H, Bergler W, Bak M,
Tschada R and Alken P: P-170 glycoprotein, glutathione and
associated enzymes in relation to chemoresistance of primary human
renal cell carcinomas. Urol Int. 45:170–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo Y, Schoell MC and Freeman RS: The von
Hippel-lindau protein sensitizes renal carcinoma cells to apoptotic
stimuli through stabilization of BIMEL. Oncogene. 28:18642009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Büscheck F, Fraune C, Simon R, Kluth M,
Hube-Magg C, Möller-Koop C, Sarper I, Ketterer K, Henke T,
Eichelberg C, et al: Prevalence and clinical significance of VHL
mutations and 3p25 deletions in renal tumor subtypes. Oncotarget.
11:237–249. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ascierto ML, McMiller TL, Berger AE,
Danilova L, Anders RA, Netto GJ, Xu H, Pritchard TS, Fan J, Cheadle
C, et al: The intratumoral balance between metabolic and
immunologic gene expression is associated with Anti-PD-1 response
in patients with renal cell carcinoma. Cancer Immunol Res.
4:726–733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rooney MS, Shukla SA, Wu CJ, Getz G and
Hacohen N: Molecular and genetic properties of tumors associated
with local immune cytolytic activity. Cell. 160:48–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Möller K, Fraune C, Blessin NC, Lennartz
M, Kluth M, Hube-Magg C, Lindhorst L, Dahlem R, Fisch M, Eichenauer
T, et al: Tumor cell PD-L1 expression is a strong predictor of
unfavorable prognosis in immune checkpoint Therapy-naive clear cell
renal cell cancer. Int Urol Nephrol. 53:2493–2503. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kawashima A, Kanazawa T, Kidani Y, Yoshida
T, Hirata M, Nishida K, Nojima S, Yamamoto Y, Kato T, Hatano K, et
al: Tumour grade significantly correlates with total dysfunction of
tumour Tissue-infiltrating lymphocytes in renal cell carcinoma. Sci
Rep. 10:62202020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Yin C, Geng L and Cai W: Immune
infiltration landscape in clear cell renal cell carcinoma
implications. Front Oncol. 10:4916212021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sabrina S, Takeda Y, Kato T, Naito S, Ito
H, Takai Y, Ushijima M, Narisawa T, Kanno H, Sakurai T, et al:
Initial myeloid cell status is associated with clinical outcomes of
renal cell carcinoma. Biomedicines. 11:12962023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu B, Chen X, Zhan Y, Wu B and Pan S:
Identification of a gene signature for renal cell
Carcinoma-associated fibroblasts mediating cancer progression and
affecting prognosis. Front Cell Dev Biol. 8:6046272021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Y, Chen X, Fu Q, Wang F, Zhou X,
Xiang J, He N, Hu Z and Jin X: Comprehensive analysis of pyroptosis
regulators and tumor immune microenvironment in clear cell renal
cell carcinoma. Cancer Cell Int. 21:6672021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ballesteros PÁ, Chamorro J, Román-Gil MS,
Pozas J, Gómez Dos Santos V, Granados ÁR, Grande E, Alonso-Gordoa T
and Molina-Cerrillo J: Molecular mechanisms of resistance to
immunotherapy and antiangiogenic treatments in clear cell renal
cell carcinoma. Cancers. 13:59812021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Derosa L, Hellmann MD, Spaziano M,
Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC,
Chaft JE, et al: Negative association of antibiotics on clinical
activity of immune checkpoint inhibitors in patients with advanced
renal cell and Non-small-cell lung cancer. Ann Oncol. 29:1437–1444.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xing Y and Hogquist KA: T-cell tolerance:
Central and peripheral. Cold Spring Harb Perspect Biol.
4:a0069572012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Braun DA, Street K, Burke KP, Cookmeyer
DL, Denize T, Pedersen CB, Gohil SH, Schindler N, Pomerance L,
Hirsch L, et al: Progressive immune dysfunction with advancing
disease stage in renal cell carcinoma. Cancer Cell. 39:632–648.e8.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
McKay RR, Bossé D, Xie W, Wankowicz SAM,
Flaifel A, Brandao R, Lalani AA, Martini DJ, Wei XX, Braun DA, et
al: The clinical activity of PD-1/PD-L1 inhibitors in metastatic
non-clear cell renal cell carcinoma. Cancer Immunol Res. 6:758–765.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pichler R, Siska PJ, Tymoszuk P, Martowicz
A, Untergasser G, Mayr R, Weber F, Seeber A, Kocher F, Barth DA, et
al: A chemokine network of T cell exhaustion and metabolic
reprogramming in renal cell carcinoma. Front Immunol.
14:10951952023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors. JAMA
Oncol. 4:1721–1728. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lin E, Liu X, Liu Y, Zhang Z, Xie L, Tian
K, Liu J and Yu Y: Roles of the dynamic tumor immune
microenvironment in the individualized treatment of advanced clear
cell renal cell carcinoma. Front Immunol. 12:6533582021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ishihara H, Takagi T, Kondo T, Fukuda H,
Tachibana H, Yoshida K, Iizuka J, Okumi M, Ishida H and Tanabe K:
Predictive impact of an early change in serum C-reactive protein
levels in nivolumab therapy for metastatic renal cell carcinoma.
Urol Oncol Semin Orig Investig. 38:526–532. 2020.
|
|
61
|
Noguchi G, Nakaigawa N, Umemoto S,
Kobayashi K, Shibata Y, Tsutsumi S, Yasui M, Ohtake S, Suzuki T,
Osaka K, et al: C-reactive protein at 1 month after treatment of
nivolumab as a predictive marker of efficacy in advanced renal cell
carcinoma. Cancer Chemother Pharmacol. 86:75–85. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yano Y, Ohno T, Komura K, Fukuokaya W,
Uchimoto T, Adachi T, Hirasawa Y, Hashimoto T, Yoshizawa A,
Yamazaki S, et al: Serum C-reactive protein level predicts overall
survival for clear cell and Non-clear cell renal cell carcinoma
treated with ipilimumab plus nivolumab. Cancers. 14:56592022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tomita Y, Larkin J, Venugopal B, Haanen J,
Kanayama H, Eto M, Grimm MO, Fujii Y, Umeyama Y, Huang B, et al:
Association of C-reactive protein with efficacy of avelumab plus
axitinib in advanced renal cell carcinoma: Long-term follow-up
results from JAVELIN renal 101. ESMO Open. 7:1005642022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim JH, Kim GH, Ryu YM, Kim SY, Kim HD,
Yoon SK, Cho YM and Lee JL: Clinical implications of the tumor
microenvironment using multiplexed immunohistochemistry in patients
with advanced or metastatic renal cell carcinoma treated with
nivolumab plus ipilimumab. Front Oncol. 12:9695692022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sammarco E, Rossetti M, Salfi A, Bonato A,
Viacava P, Masi G, Galli L and Faviana P: Tumor microenvironment
and clinical efficacy of first line Immunotherapy-based
combinations in metastatic renal cell carcinoma. Med Oncol.
41:1502024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kazama A, Bilim V, Tasaki M, Anraku T,
Kuroki H, Shirono Y, Murata M, Hiruma K and Tomita Y:
Tumor-infiltrating immune cell status predicts successful response
to immune checkpoint inhibitors in renal cell carcinoma. Sci Rep.
12:203862022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Herrmann T, Ginzac A, Molnar I, Bailly S,
Durando X and Mahammedi H: Eosinophil counts as a relevant
prognostic marker for response to nivolumab in the management of
renal cell carcinoma: A retrospective study. Cancer Med.
10:6705–6713. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Atkins MB, Jegede OA, Haas NB, McDermott
DF, Bilen MA, Stein M, Sosman JA, Alter R, Plimack ER, Ornstein M,
et al: Phase II study of nivolumab and salvage Nivolumab/Ipilimumab
in Treatment-naive patients with advanced clear cell renal cell
carcinoma (HCRN GU16-260-Cohort A). J Clin Oncol. 40:2913–2923.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Motzer RJ, Choueiri TK, McDermott DF,
Powles T, Vano YA, Gupta S, Yao J, Han C, Ammar R,
Papillon-Cavanagh S, et al: Biomarker analysis from CheckMate 214:
Nivolumab plus ipilimumab versus sunitinib in renal cell carcinoma.
J Immunother Cancer. 10:e0043162022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Brown LC, Zhu J, Desai K, Kinsey E, Kao C,
Lee YH, Pabla S, Labriola MK, Tran J, Dragnev KH, et al: Evaluation
of tumor microenvironment and biomarkers of immune checkpoint
inhibitor response in metastatic renal cell carcinoma. J Immunother
Cancer. 10:e0052492022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kato R, Jinnouchi N, Tuyukubo T, Ikarashi
D, Matsuura T, Maekawa S, Kato Y, Kanehira M, Takata R, Ishida K
and Obara W: TIM3 expression on tumor cells predicts response to
anti-PD-1 therapy for renal cancer. Transl Oncol. 14:1009182020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Koh Y, Nakano K, Katayama K, Yamamichi G,
Yumiba S, Tomiyama E, Matsushita M, Hayashi Y, Yamamoto Y, Kato T,
et al: Early dynamics of circulating tumor DNA predict clinical
response to immune checkpoint inhibitors in metastatic renal cell
carcinoma. Int J Urol. 29:462–469. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Incorvaia L, Fanale D, Badalamenti G,
Brando C, Bono M, De Luca I, Algeri L, Bonasera A, Corsini LR,
Scurria S, et al: A ‘Lymphocyte MicroRNA Signature’ as predictive
biomarker of immunotherapy response and plasma PD-1/PD-L1
expression levels in patients with metastatic renal cell carcinoma:
Pointing towards epigenetic reprogramming. Cancers. 12:33962020.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pan Q, Liu R, Zhang X, Cai L, Li Y, Dong
P, Gao J, Liu Y and He L: CXCL14 as a potential marker for
immunotherapy response prediction in renal cell carcinoma. Ther Adv
Med Oncol. 15:175883592312179662023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pabla S, Seager RJ, Van Roey E, Gao S,
Hoefer C, Nesline MK, DePietro P, Burgher B, Andreas J, Giamo V, et
al: Integration of tumor inflammation, cell proliferation, and
traditional biomarkers improves prediction of immunotherapy
resistance and response. Biomark Res. 9:562021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Schalper KA, Carleton M, Zhou M, Chen T,
Feng Y, Huang SP, Walsh AM, Baxi V, Pandya D, Baradet T, et al:
Elevated serum interleukin-8 is associated with enhanced intratumor
neutrophils and reduced clinical benefit of immune-checkpoint
inhibitors. Nat Med. 26:688–692. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ivanova E, Asadullina D, Rakhimov R,
Izmailov A, Izmailov A, Gilyazova G, Galimov S, Pavlov V,
Khusnutdinova E and Gilyazova I: Exosomal miRNA-146a is
downregulated in clear cell renal cell carcinoma patients with
severe immune-related adverse events. Noncoding RNA Res. 7:159–163.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Petitprez F, Ayadi M, de Reyniès A,
Fridman WH, Sautès-Fridman C and Job S: Review of prognostic
expression markers for clear cell renal cell carcinoma. Front
Oncol. 11:6430652021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lee A, Lee HJ, Huang HH, Tay KJ, Lee LS,
Sim SPA, Ho SSH, Yuen SPJ and Chen K: Prognostic significance of
inflammation-associated blood cell markers in nonmetastatic clear
cell renal cell carcinoma. Clin Genitourin Cancer. 18:304–313.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Teishima J, Inoue S, Hayashi T and
Matsubara A: Current status of prognostic factors in patients with
metastatic renal cell carcinoma. Int J Urol. 26:608–617. 2019.
View Article : Google Scholar : PubMed/NCBI
|