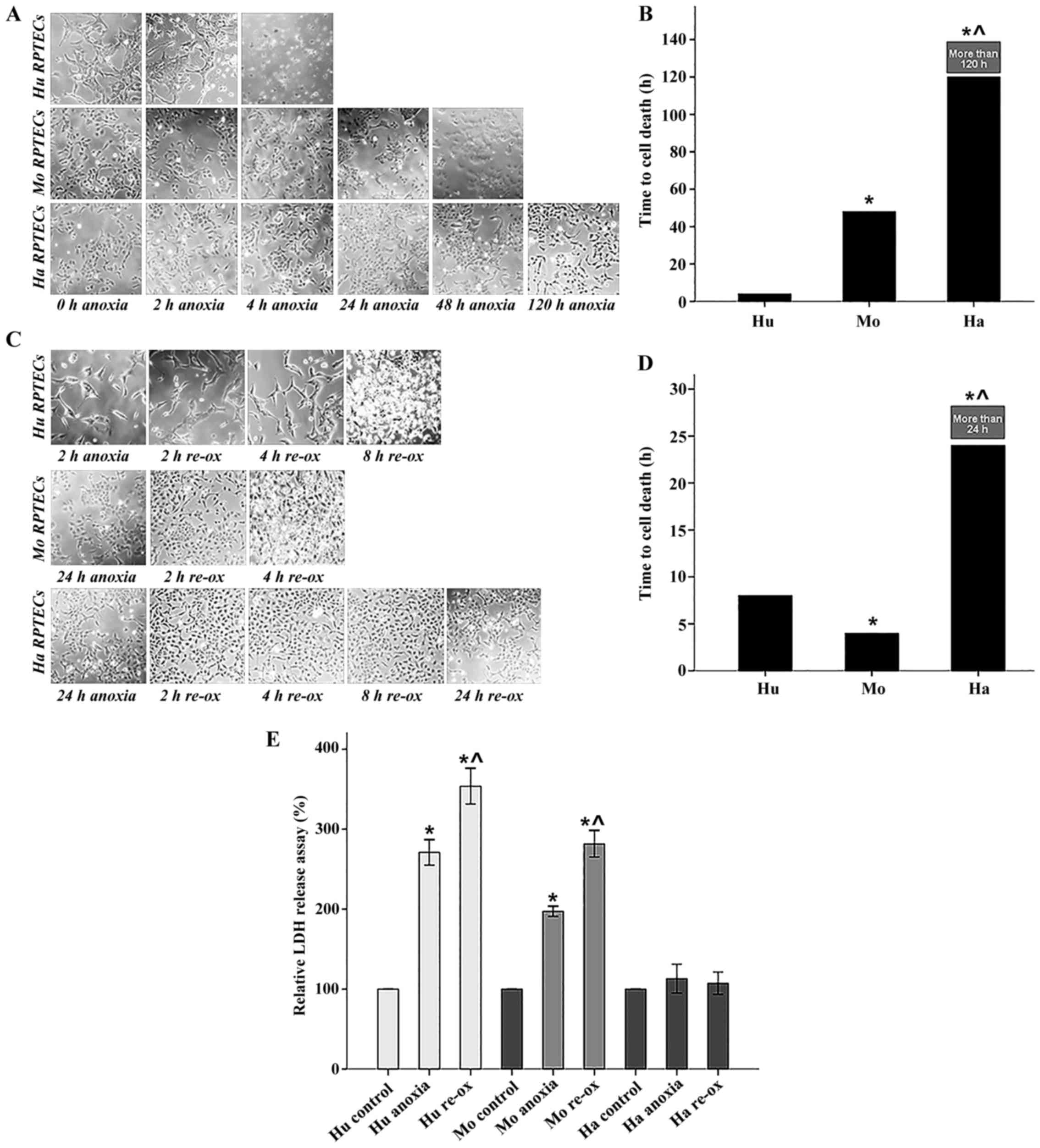
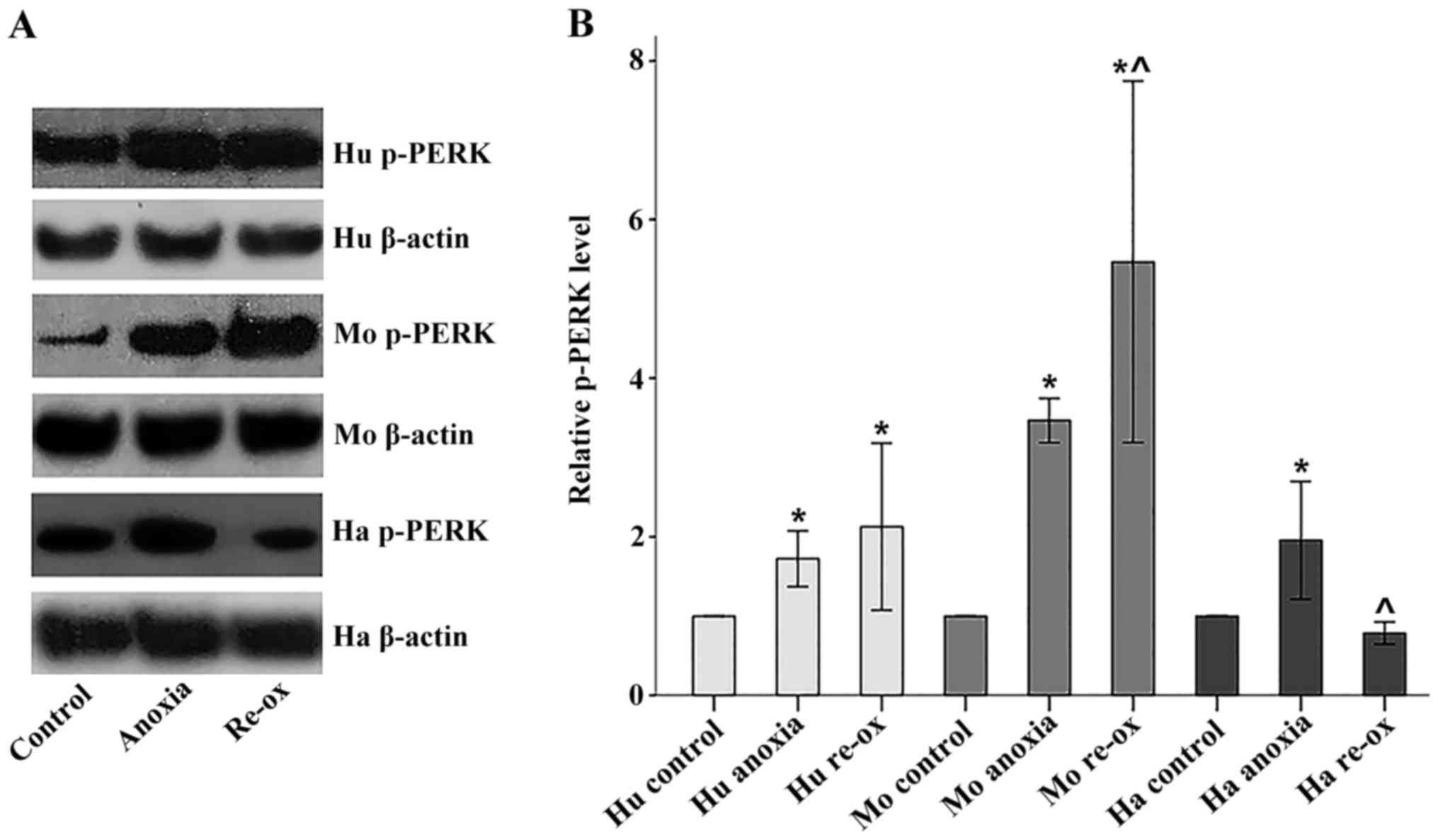
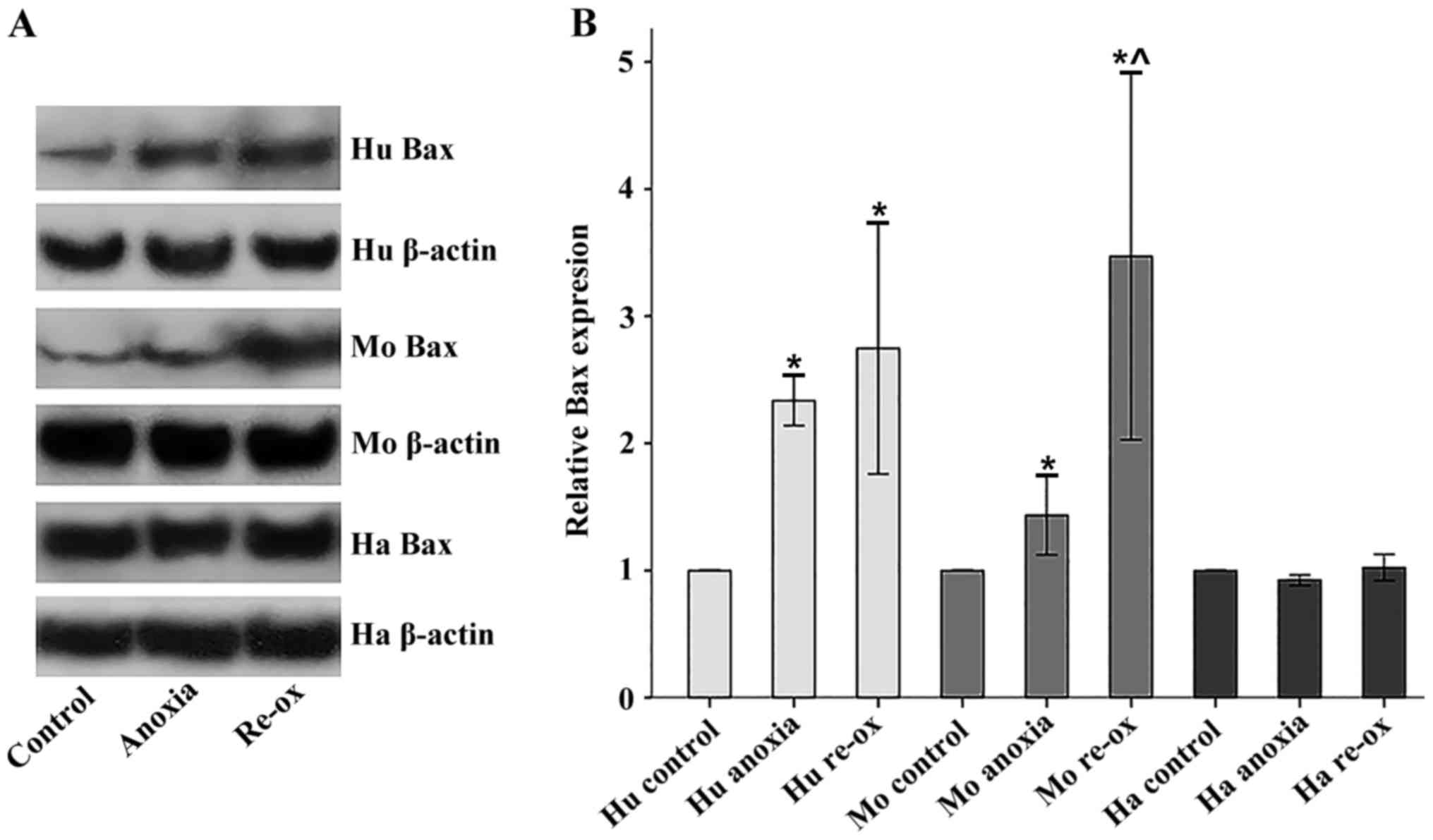
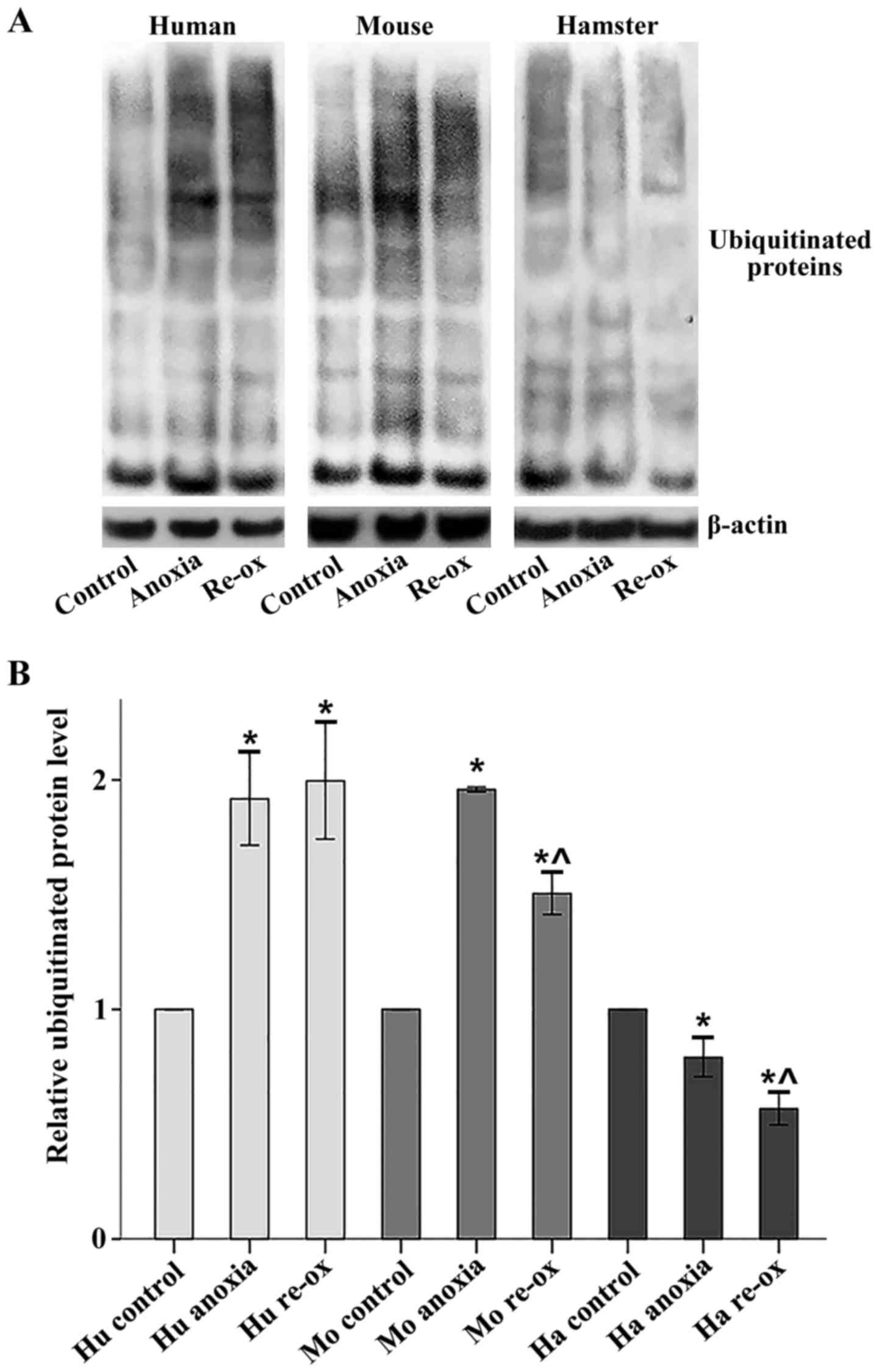
|
1
|
Neri M, Riezzo I, Pascale N, Pomara C and
Turillazzi E: Ischemia/reperfusion injury following acute
myocardial infarction: A critical issue for clinicians and forensic
pathologists. Mediators Inflamm. 2017(7018393)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bakthavachalam P and Shanmugam PST:
Mitochondrial dysfunction - Silent killer in cerebral ischemia. J
Neurol Sci. 375:417–423. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tsukamoto T, Chanthaphavong RS and Pape
HC: Current theories on the pathophysiology of multiple organ
failure after trauma. Injury. 41:21–26. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bonventre JV and Yang L: Cellular
pathophysiology of ischemic acute kidney injury. J Clin Invest.
121:4210–4221. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Lieberthal W and Nigam SK: Acute renal
failure. I. Relative importance of proximal vs. distal tubular
injury. Am J Physiol. 275:F623–F631. 1998.PubMed/NCBI
|
|
6
|
Carey HV, Andrews MT and Martin SL:
Mammalian hibernation: Cellular and molecular responses to
depressed metabolism and low temperature. Physiol Rev.
83:1153–1181. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Storey KB and Storey JM: Metabolic rate
depression The biochemistry of mammalian hibernation. Adv Clin
Chem. 52:77–108. 2010.PubMed/NCBI
|
|
8
|
Dave KR, Prado R, Raval AP, Drew KL and
Perez-Pinzon MA: The arctic ground squirrel brain is resistant to
injury from cardiac arrest during euthermia. Stroke. 37:1261–1265.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Quinones QJ, Zhang Z, Ma Q, Smith MP,
Soderblom E, Moseley MA, Bain J, Newgard CB, Muehlbauer MJ,
Hirschey M, et al: Proteomic profiling reveals adaptive responses
to surgical myocardial ischemia-reperfusion in hibernating arctic
ground squirrels compared to rats. Anesthesiology. 124:1296–1310.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dausmann KH, Glos J, Ganzhorn JU and
Heldmaier G: Physiology: Hibernation in a tropical primate. Nature.
429:825–826. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Hetz C and Papa FR: The unfolded protein
response and cell fate control. Mol Cell. 69:169–181.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu X and Rapoport TA: Mechanistic insights
into ER-associated protein degradation. Curr Opin Cell Biol.
53:22–28. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li Y, Guo Y, Tang J, Jiang J and Chen Z:
New insights into the roles of CHOP-induced apoptosis in ER stress.
Acta Biochim Biophys Sin (Shanghai). 46:629–640. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Maekawa H and Inagi R: Stress signal
network between hypoxia and ER stress in chronic kidney disease.
Front Physiol. 8(74)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu Y, Guo M, Jiang W, Dong H, Han Y, An XF
and Zhang J: Endoplasmic reticulum stress and its effects on renal
tubular cells apoptosis in ischemic acute kidney injury. Ren Fail.
38:831–837. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vekich JA, Belmont PJ, Thuerauf DJ and
Glembotski CC: Protein disulfide isomerase-associated 6 is an
ATF6-inducible ER stress response protein that protects cardiac
myocytes from ischemia/reperfusion-mediated cell death. J Mol Cell
Cardiol. 53:259–267. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tang JY, Jin P, He Q, Lu LH, Ma JP, Gao
WL, Bai HP and Yang J: Naringenin ameliorates
hypoxia/reoxygenation-induced endoplasmic reticulum stress-mediated
apoptosis in H9c2 myocardial cells: Involvement in ATF6, IRE1α and
PERK signaling activation. Mol Cell Biochem. 424:111–122.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao Y, Fang Y, Zhao H, Li J, Duan Y, Shi
W, Huang Y, Gao L and Luo Y: Chrysophanol inhibits endoplasmic
reticulum stress in cerebral ischemia and reperfusion mice. Eur J
Pharmacol. 818:1–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fougeray S, Bouvier N, Beaune P, Legendre
C, Anglicheau D, Thervet E and Pallet N: Metabolic stress promotes
renal tubular inflammation by triggering the unfolded protein
response. Cell Death Dis. 2(e143)2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu W, Sheng M, Xu R, Yu J, Cui K, Tong J,
Shi L, Ren H and Du H: Berberine protects human renal proximal
tubular cells from hypoxia/reoxygenation injury via inhibiting
endoplasmic reticulum and mitochondrial stress pathways. J Transl
Med. 11(24)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Whelan RS, Konstantinidis K, Wei AC, Chen
Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, et
al: Bax regulates primary necrosis through mitochondrial dynamics.
Proc Natl Acad Sci USA. 109:6566–6571. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Morin P Jr, Dubuc A and Storey KB:
Differential expression of microRNA species in organs of
hibernating ground squirrels: A role in translational suppression
during torpor. Biochim Biophys Acta. 1779:628–633. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Biggar KK and Storey KB: Identification
and expression of microRNA in the brain of hibernating bats, Myotis
lucifugus. Gene. 544:67–74. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu Z and Lu Y, Xiao Y and Lu Y:
Upregulation of miR-21 expression is a valuable predicator of
advanced clinicopathological features and poor prognosis in
patients with renal cell carcinoma through the p53/p21-cyclin
E2-Bax/caspase-3 signaling pathway. Oncol Rep. 37:1437–1444.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gui F, Hong Z, You Z, Wu H and Zhang Y:
MiR-21 inhibitor suppressed the progression of retinoblastoma via
the modulation of PTEN/PI3K/AKT pathway. Cell Biol Int.
40:1294–1302. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Logan SM and Storey KB: Avoiding apoptosis
during mammalian hibernation. Temp Austin. 4:15–17. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Takaoka M, Itoh M, Hayashi S, Kuro T and
Matsumura Y: Proteasome participates in the pathogenesis of
ischemic acute renal failure in rats. Eur J Pharmacol. 384:43–46.
1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Huber JM, Tagwerker A, Heininger D, Mayer
G, Rosenkranz AR and Eller K: The proteasome inhibitor bortezomib
aggravates renal ischemia-reperfusion injury. Am J Physiol Renal
Physiol. 297(F451-F460)2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Keller JN, Huang FF, Zhu H, Yu J, Ho YS
and Kindy TS: Oxidative stress-associated impairment of proteasome
activity during ischemia-reperfusion injury. J Cereb Blood Flow
Metab. 20:1467–1473. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tian Z, Zheng H, Li J, Li Y, Su H and Wang
X: Genetically induced moderate inhibition of the proteasome in
cardiomyocytes exacerbates myocardial ischemia-reperfusion injury
in mice. Circ Res. 111:532–542. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Epperson LE, Dahl TA and Martin SL:
Quantitative analysis of liver protein expression during
hibernation in the golden-mantled ground squirrel. Mol Cell
Proteomics. 3:920–933. 2004.PubMed/NCBI View Article : Google Scholar
|