Introduction
Ionizing radiation exerts biological effects, such
as cell death and chromosomal aberration due to the direct
radiation of the cells. However, evidence suggests that this
radiation affects not only the cells irradiated directly, but also
the surrounding non-irradiated cells (1-3).
This response, known as the non-targeted effect, includes genomic
instability and other radiation-induced bystander effects. Genomic
instability refers to biological effects, such as delayed gene
mutations and chromosomal aberrations that occur in the progeny of
the irradiated cells (3), whereas
radiation-induced bystander effects are caused by the transmission
of signals from the irradiated cells to the non-irradiated cells
via gap junctions or soluble factors (1,2).
Various factors, such as transformation growth factor-β (TGF-β),
tumor-necrosis factor-α, and reactive oxygen species have been
reported to be possible candidate bystander factors (4). In general, to examine the bystander
effects mediated by soluble factors in vitro, non-irradiated
cells are co-cultured with irradiated cells or cultured in the
presence of irradiated cell conditioned medium (ICCM).
Non-irradiated cells co-cultured with irradiated cells or treated
with ICCM have been reported to undergo various biological effects,
such as DNA double-strand breaks and apoptosis, generally observed
in irradiated cells (1,3).
Radiation therapy is widely used in the the
treatment of various types of cancer. Although radiation therapy is
considered to control the tumor cells locally, there is evidence to
indicate additional systemic antitumor effects of this therapy
(5,6); these effects have been referred to
as the abscopal effect. In the abscopal effect, the reduction or
disappearance of tumors occurs not only in the irradiated lesions,
but also in the non-irradiated lesions, suggesting that signals
from irradiated tissues can affect the unirradiated tissues outside
of the irradiated volume. There is recent evidence to suggest the
involvement of the immune system in the abscopal effect (7). Briefly, irradiated tumors release
immunostimulatory molecules, such as inflammatory cytokines and
damage-associated molecular patterns, which are endogenous
molecules released due to cellular damage. The released signals
activate the innate immune system, leading to T-cell-mediated
cytotoxicity against tumors in non-irradiated lesions (8).
The factors released from irradiated cells exert
biological effects, such as the induction of apoptosis and activate
antitumor immunity, which may prove beneficial for the treatment of
cancers (5,9,10).
However, little is known about the effects of factors released from
irradiated cells on the response of cancer cells to anticancer
treatment. Therefore, the present study investigated the effects of
ICCM on the response of human lung cancer cells to anticancer
treatment in terms of the induction of apoptosis and cellular
migration.
Materials and methods
Reagents
Dimethyl sulfoxide (DMSO) and propidium iodide (PI)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Gefitinib
was purchased from Selleckchem (Houston, TX, USA).
Cells and cell culture
Human lung cancer cell lines A549 and H1299 cells
were purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The cells were maintained at 37˚C in a
humidified atmosphere containing 5% CO2 and cultured in
RPMI-1640 medium (Gibco®; Invitrogen/Thermo Fisher
Scientific, Waltham, MA, USA) supplemented with 1%
penicillin/streptomycin (Gibco®) and 10%
heat-inactivated fetal bovine serum (Japan Bioserum Co., Ltd.,
Nagoya, Japan).
In vitro irradiation
The cells were irradiated (150 kVp; 20 mA; 0.5 mm Al
and 0.3 mm Cu filters) using an X-ray generator (MBR 1520R 3;
Hitachi, Ltd., Tokyo, Japan) at a distance of 45 cm from the focus
and a dose rate of 1.01-1.07 Gy/min.
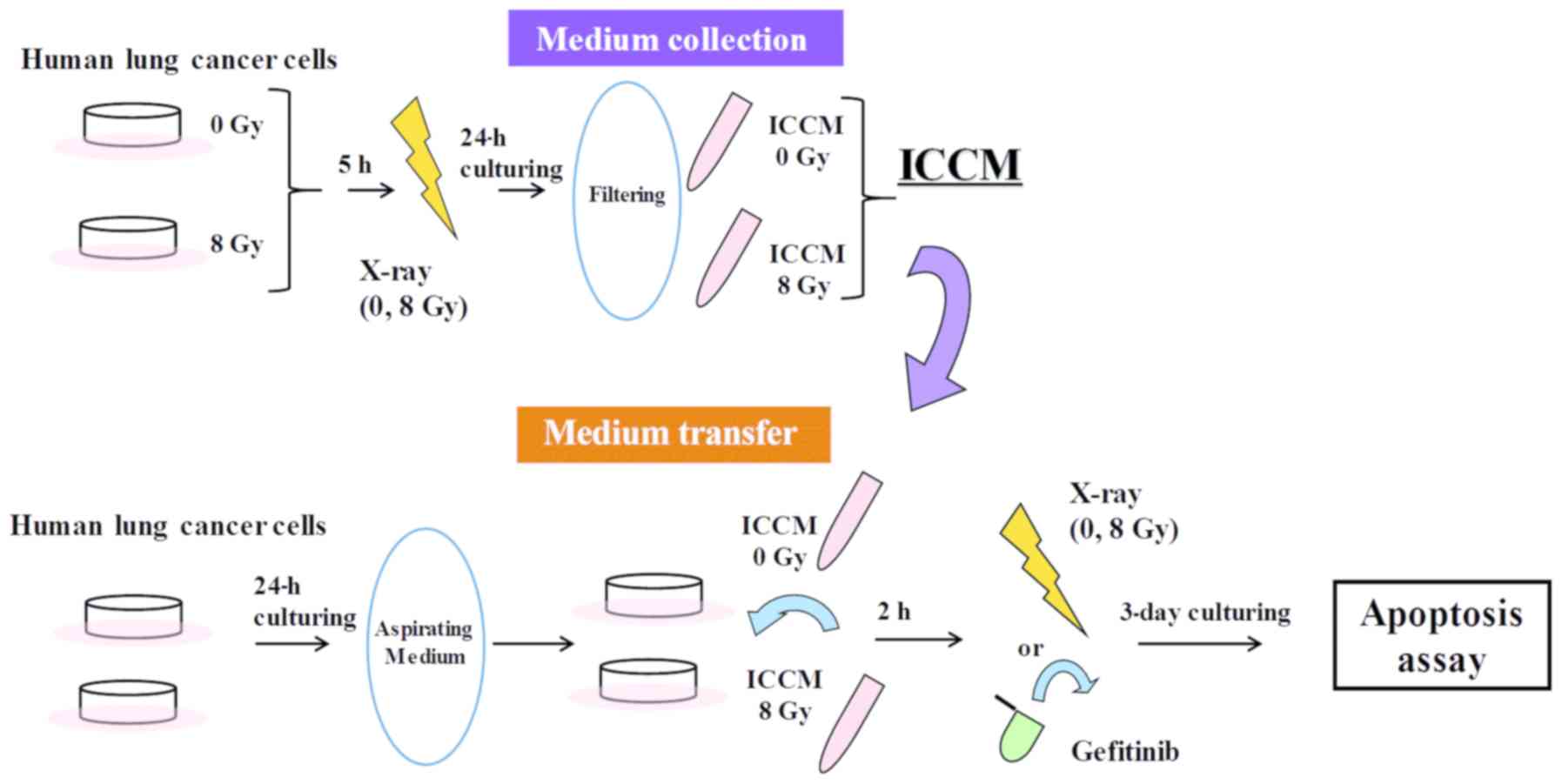
Medium transfer experiments
Medium transfer experiments were performed, as
previously reported (11). A
schematic illustration of the medium transfer experiments is
presented in Fig. 1.
Approximately 2.4x105 cells were seeded onto 35-mm
culture dishes (Iwaki, Chiba, Japan) and cultured for 5 h at 37˚C
to promote their adherence to the dish. The cells were then exposed
to 8 Gy X-ray and cultured for 24 h at 37˚C. The conditioned medium
was then collected by centrifugation (180 x g for 5 min at room
temperature) wherein, the supernatant was collected and filtered
using a 0.45-µm syringe filter (2053-025; Iwaki) to remove cells
and debris. The filtrated cell conditioned medium (hereafter
referred to as ICCM) was used in the subsequent experiments.
One day prior to the collection of the ICCM,
approximately 6.0x104 cells were seeded onto 35-mm
culture dishes and cultured overnight at 37˚C to allow for their
adherence to the dish. On the following day, the medium was
aspirated and ICCM was added to the 35-mm culture dishes. After 2 h
of culturing at 37˚C, the cells were exposed to 8 Gy X-ray or 20 µM
of gefitinib, which was added to the culture medium. DMSO (0.1%)
was used as a vehicle control for gefitinib. Following 3 days of
culture, the cells were harvested using 0.1% trypsin-ethylene
diamine tetraacetic acid (Gibco®; Thermo Fisher
Scientific) to perform the apoptosis assay.
Apoptosis assay
Apoptosis was analyzed using Annexin V-FITC, PI and
Annexin V binding buffer (all BioLegend Inc., San Diego, CA, USA),
as reported previously (12). The
stained cells were analyzed by performing flow cytometry (Cytomics
FC500 with CXP software version 2; Beckman Coulter, Inc., Brea, CA,
USA).
Scratch assay
The A549 cells were cultured in a 24-well plate (BD
Falcon; BD Biosciences, Franklin Lakes, NJ, USA) until they reached
approximately 90% confluence. The cell monolayer was scratched
using a yellow tip. After the culture medium containing floating
cells was aspirated, ICCM (500 µl) was added to the plate. The
cell-free scratched area was measured using an Olympus LX71
microscope and DP2-BSW software version 2.1 (both Olympus, Tokyo,
Japan) immediately, and at 24 and 48 h after scratching, and the
percentage of wound closure area was calculated. In some
experiments, the cells were exposed to 8 Gy X-ray at 2 h following
the addition of ICCM.
Statistical analysis
Data are presented as the means ± standard deviation
(SD). Comparisons between the control and experimental groups were
performed using a two-sided Student's t-test or a two-sided
Mann-Whitney U test depending on the data distribution. Multiple
data were analyzed using one-factor analysis of variance followed
by the Tukey-Kramer test. Differences were considered statistically
significant at P<0.05. All statistical analyses were performed
using Excel 2016 software version 1903 (Microsoft, USA), with an
add-on software Statcel 4 (OMS Publishing, Inc., Tokyo, Japan).
Results
Effects of ICCM on the induction of
apoptosis by X-ray irradiation
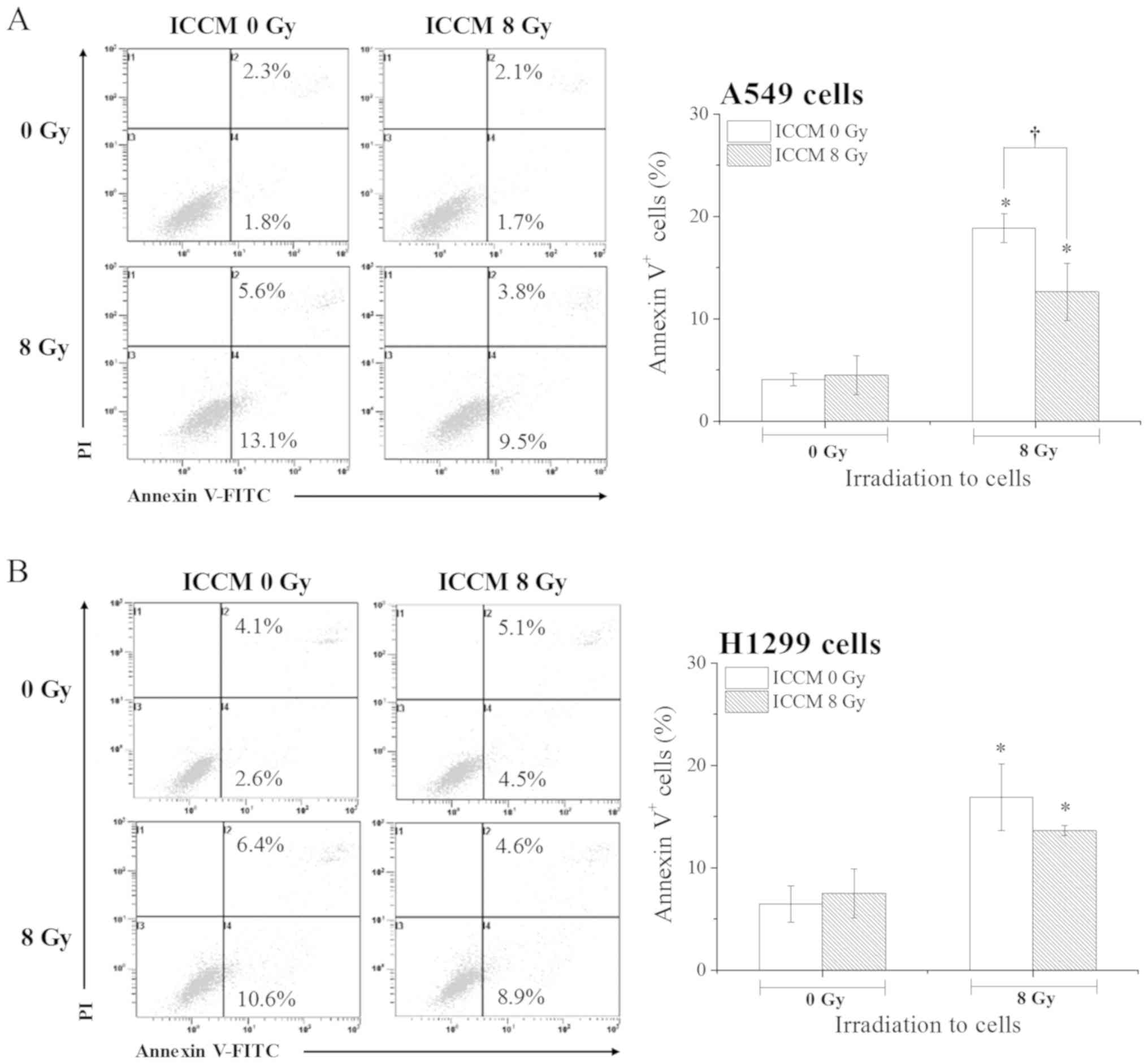
We examined the effects of ICCM on the induction of
apoptosis of the A549 and H1299 cells following ionizing radiation.
Yang et al previously reported that the cell conditioned
medium from irradiated A549 cells exerted cyototoxic effects
against non-irradiated A549 cells (9). However, under the present
experimental conditions, at 0 Gy irradiation no significant
differences in the proportion of Annexin V+ apoptotic
cells were observed between the cells treated with ICCM from the
non-irradiated cells and those treated with ICCM from 8
Gy-irradiated cells (Fig. 2A), as
we have previously reported (11). In the A549 cells, the proportion
of Annexin V+ apoptotic cells was significantly
increased following exposure to 8 Gy X-ray when compared to the
cells exposed to 0 Gy X-ray after being cultured with ICCM from
irradiated and non-irradiated cells (P<0.01). Notably, the
proportion of Annexin V+ apoptotic cells was
significantly lower in the 8 Gy-irradiated cells treated with ICCM
from 8 Gy-irradiated cells when compared with those treated with
ICCM from non-irradiated cells (P<0.05; Fig. 2A). However, this effect was not
observed in the experiments using the H1299 cells (Fig. 2B) and ICCM from 2 Gy-irradiated
cells (data not shown). These results suggest that the effects of
ICCM vary depending on the cell type, as well as the radiation dose
(13).
Effects of ICCM on the induction of
apoptosis by gefitinib treatment
Inhibitors of the epidermal growth factor receptor
(EGFR) tyrosine kinase, such as gefitinib have been used in the
treatment of lung cancer (14).
Therefore, in this study, we examined the effect of ICCM on the
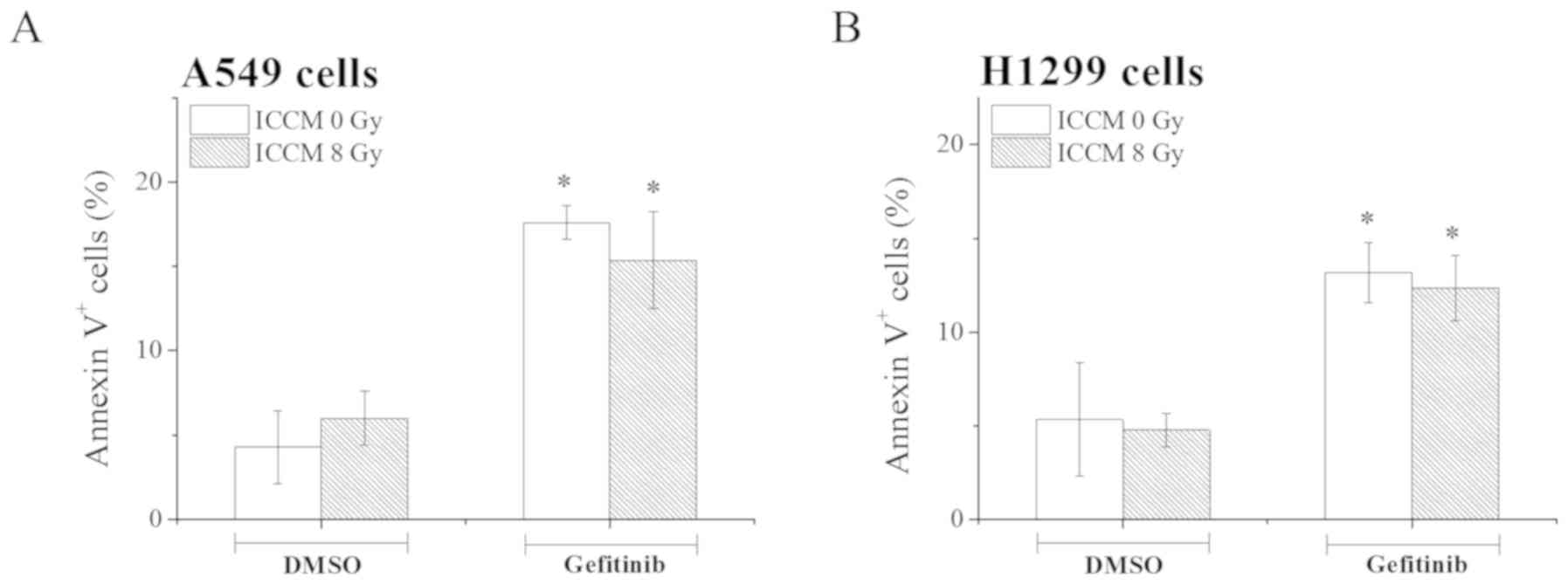
induction of apoptosis by gefitinib in the A549 and H1299 cells. As
shown in Fig. 3, gefitinib
treatment significantly increased the proportion of Annexin
V+ apoptotic A549 and H1299 (P<0.05) when compared
with the cells treated with DMSO. However, in contrast to the
results obtained with X-ray irradiation (Fig. 2), no significant differences in
the proportion of Annexin V+ apoptotic cells were noted
between ICCM obtained from non-irradiated cells and that from
irradiated cells (Fig. 3).
Effect of ICCM on cellular
migration
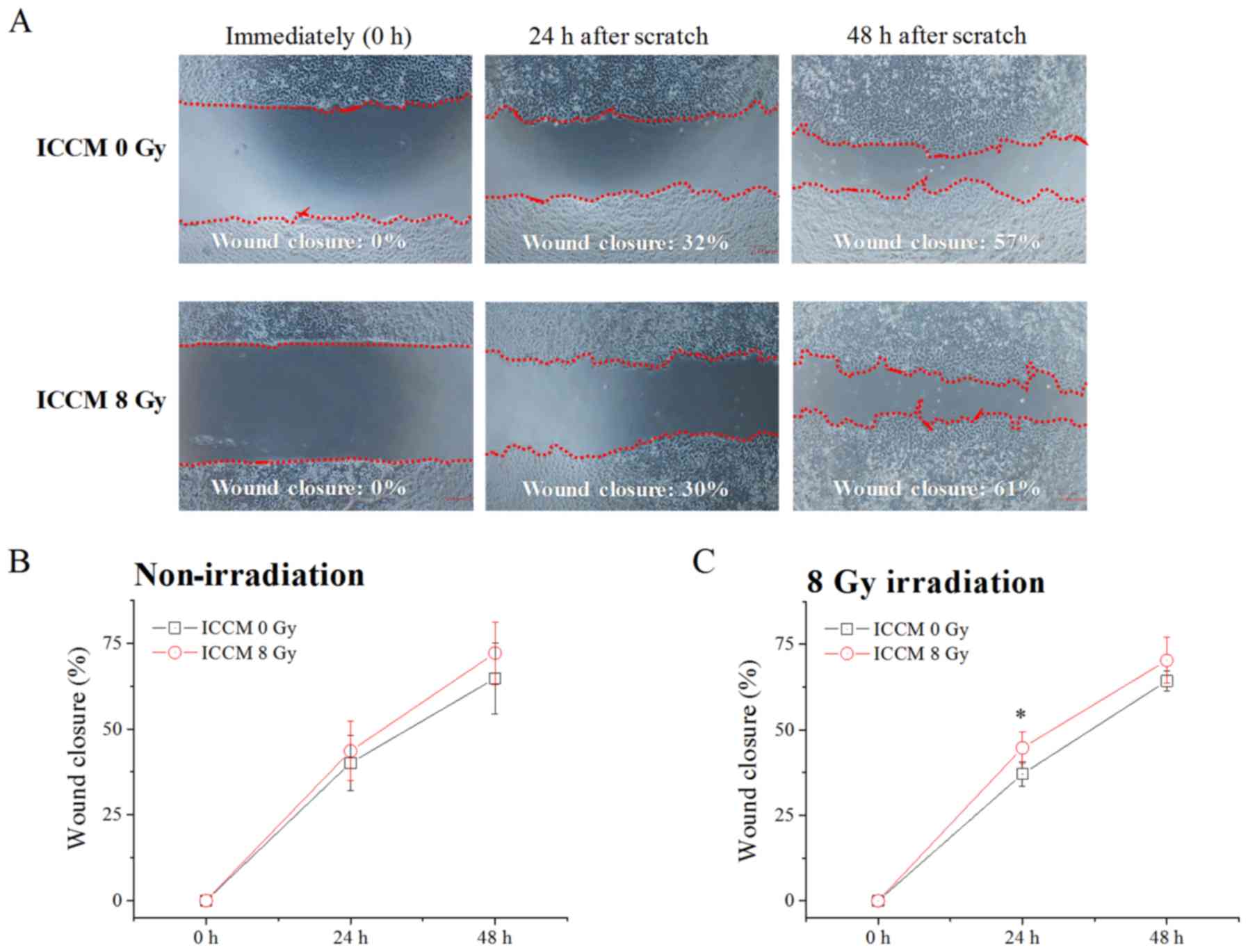
As shown in Fig.
4, both ICCM from non-irradiated cells and that from 8
Gy-irradiated cells exerted minimal effects on the migration of the
non-irradiated A549 cells. Additionally, no significant difference
in the wound closure area was observed between the non-irradiated
cells and 8 Gy-irradiated cells (ICCM 0 Gy in Fig. 4B vs. ICCM 0 Gy in Fig. 4C). However, as shown in Fig. 4C, the wound closure area in the 8
Gy-irradiated A549 cells at 24 h after the scratch was made was
significantly greater in the cells treated with ICCM from
irradiated cells when compared with those from non-irradiated cells
(P<0.01).
Discussion
The factors released from irradiated cells induce
various biological effects, such as cell death and inflammatory
responses, and these effects may be preferential for cancer
treatment. In this study, we investigated the effects of ICCM from
irradiated human lung cancer cells on the response to anticancer
treatment (ionizing radiation or gefitinib). Although ICCM did not
induce the apoptosis of non-irradiated cells in the current study,
it attenuated the induction of apoptosis by ionizing radiation, but
not by gefitinib, depending on the cell type. We also demonstrated
that ICCM enhanced the migration of 8 Gy-irradiated cells, but not
that of non-irradiated cells. Taken together, these results suggest
that cancer cells treated with ICCM exhibit resistance to ionizing
radiation in terms of apoptosis and cellular migration. In line
with our results, Iyer and Lehnert reported that clonogenic
survival after γ-irradiation (2 Gy or 4 Gy) in normal human lung
fibroblasts (HFL-1) was increased when the cells were treated with
ICCM (15). In this study, we did
not determine the underlying mechanisms through which ICCM
treatment increased the resistance to ionizing radiation.
Nonetheless, some factors released as bystander signals, such as
interleukin-6 are known to induce radioresistance (16,17). Therefore, it is possible that
these cytokines were involved in the ICCM-induced resistance to
ionizing radiation in this study.
It is known that the cells exposed to a low
radiation priming dose exhibit resistance to a subsequent high dose
of radiation, namely radiation-induced adaptive response. This
effect is observed at both the cellular and individual level, and
it seems to occur in a p53-dependent manner (18,19). p53 is a tumor suppressor
gene that plays critical roles in cellular responses, such as the
induction of apoptosis following DNA damage, including ionizing
radiation (20). In this study,
we found that ICCM treatment attenuated the induction of apoptosis
of p53-wild type A549 cells, but not that of p53-null
H1299 cells, following ionizing radiation. Therefore, the cell type
specific-effect of ICCM, which is similarly to a priming low-dose
irradiation, may be dependent on the p53 status.
Tumor cells sometimes acquire radioresistance, a
major cause of treatment failure during radiation therapy (21). In the current study, ICCM
treatment resulted in resistance to ionizing radiation, whereas no
such effect was noted with gefitinib. This may be attributed to the
differences in the mechanisms of action between gefitinib and
ionizing radiation. Gefitinib induces tumor growth arrest and
apoptosis by inhibiting EGFR signaling (22), while ionizing radiation exerts
cell-killing effects by inducing DNA damage (23). Kuwahara et al reported that
radioresistant cancer cells established by daily repeated
irradiation in vitro showed cross-resistance to X-rays and
the anti-microtubule agent, docetaxel (24). Further studies are warranted to
investigate the effects of ICCM on the anticancer effects of
various types of anticancer agents.
For a favorable prognosis of cancer patients, it is
important to control tumor migration and invasion. Akino et
al reported that carbon-ion beam and X-ray irradiation
suppressed the migration and invasion of human lung cancer cells
in vitro (25). In the
present study, 8 Gy X-ray irradiation had very minimal effects on
cell migration. However, we observed that ICCM enhanced the
migration of 8 Gy-irradiated cells, but not that of the
non-irradiated cells. Zhou et al reported that 2 Gy
γ-irradiation promoted the migration and invasion of cancer cells,
including A549 cells, via TGF-β-mediated epithelial-mesenchymal
transition (26). TGF-β is known
as a bystander signal (27) and
may possibly facilitate the ICCM-mediated cellular migration of
irradiated cells. The involvement of TGF-β in the increase in
cellular migration of irradiated cells by ICCM treatment warrants
further investigation in the future.
In conclusion, although the present study is limited
in terms of the in vitro nature of the analysis, our results
suggest that cancer cells treated with ICCM exhibit resistance to
ionizing radiation, which may be unfavorable for cancer treatment.
Therefore, further studies clarifying the underlying mechanisms
involved are required to achieve an effective treatment strategy
for cancer.
Acknowledgements
The authors would like to thank Enago (https://www.enago.jp) for the English language review.
Funding
The present study was supported by JSPS KAKENHI
(grant no. JP15K09985).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY and IK initiated the research. HY, MN and KM
performed the experiments, and collected and analyzed the data. HY
and IK wrote, reviewed, and revised the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tomita M and Maeda M: Mechanisms and
biological importance of photon-induced bystander responses: Do
they have an impact on low-dose radiation responses. J Radiat Res
(Tokyo). 56:205–219. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hamada N, Maeda M, Otsuka K and Tomita M:
Signaling pathways underpinning the manifestations of ionizing
radiation-induced bystander effects. Curr Mol Pharmacol. 4:79–95.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Matsumoto H, Tomita M, Otsuka K, Hatashita
M and Hamada N: Nitric oxide is a key molecule serving as a bridge
between radiation-induced bystander and adaptive responses. Curr
Mol Pharmacol. 4:126–134. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kadhim MA and Hill MA: Non-targeted
effects of radiation exposure: Recent advances and implications.
Radiat Prot Dosimetry. 166:118–124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Grass GD, Krishna N and Kim S: The immune
mechanisms of abscopal effect in radiation therapy. Curr Probl
Cancer. 40:10–24. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Siva S, MacManus MP, Martin RF and Martin
OA: Abscopal effects of radiation therapy: A clinical review for
the radiobiologist. Cancer Lett. 356:82–90. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Levy A, Chargari C, Marabelle A,
Perfettini JL, Magné N and Deutsch E: Can immunostimulatory agents
enhance the abscopal effect of radiotherapy? Eur J Cancer.
62:36–45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Demaria S, Ng B, Devitt ML, Babb JS,
Kawashima N, Liebes L and Formenti SC: Ionizing radiation
inhibition of distant untreated tumors (abscopal effect) is immune
mediated. Int J Radiat Oncol Biol Phys. 58:862–870. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang S, Xu J, Shao W, Geng C, Li J, Guo F,
Miao H, Shen W, Ye T, Liu Y, et al: Radiation-induced bystander
effects in A549 cells exposed to 6 MV X-rays. Cell Biochem Biophys.
72:877–882. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jiang Y, Chen X, Tian W, Yin X, Wang J and
Yang H: The role of TGF-β1-miR-21-ROS pathway in bystander
responses induced by irradiated non-small-cell lung cancer cells.
Br J Cancer. 111:772–780. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yoshino H, Murakami K, Nawamaki M and
Kashiwakura I: Effects of Nrf2 knockdown on the properties of
irradiated cell conditioned medium from A549 human lung cancer
cells. Biomed Rep. 8:461–465. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yoshino H, Konno H, Ogura K, Sato Y and
Kashiwakura I: Relationship between the regulation of
caspase-8-mediated apoptosis and radioresistance in human
THP-1-derived macrophages. Int J Mol Sci. 19(E3154)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Morgan WF and Sowa MB: Non-targeted
effects induced by ionizing radiation: Mechanisms and potential
impact on radiation induced health effects. Cancer Lett. 356:17–21.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kucharczuk CR, Ganetsky A and Vozniak JM:
Drug-drug interactions, safety, and pharmacokinetics of EGFR
tyrosine kinase inhibitors for the treatment of non-small cell lung
cancer. J Adv Pract Oncol. 9:189–200. 2018.PubMed/NCBI
|
|
15
|
Iyer R and Lehnert BE: Low dose, low-LET
ionizing radiation-induced radioadaptation and associated early
responses in unirradiated cells. Mutat Res. 503:1–9.
2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pasi F, Facoetti A and Nano R: IL-8 and
IL-6 bystander signalling in human glioblastoma cells exposed to
gamma radiation. Anticancer Res. 30:2769–2772. 2010.PubMed/NCBI
|
|
17
|
Tamari Y, Kashino G and Mori H:
Acquisition of radioresistance by IL-6 treatment is caused by
suppression of oxidative stress derived from mitochondria after
γ-irradiation. J Radiat Res (Tokyo). 58:412–420. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Takahashi A, Matsumoto H and Ohnishi T:
Hdm2 and nitric oxide radicals contribute to the
p53-dependent radioadaptive response. Int J Radiat Oncol
Biol Phys. 71:550–558. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yonezawa M: Induction of radio-resistance
by low dose X-irradiation. Yakugaku Zasshi. 126:833–840. 2006.(In
Japanese). PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lakin ND and Jackson SP: Regulation of p53
in response to DNA damage. Oncogene. 18:7644–7655. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim JJ and Tannock IF: Repopulation of
cancer cells during therapy: An important cause of treatment
failure. Nat Rev Cancer. 5:516–525. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Rho JK, Choi YJ, Ryoo BY, Na II, Yang SH,
Kim CH and Lee JC: p53 enhances gefitinib-induced growth inhibition
and apoptosis by regulation of Fas in non-small cell lung cancer.
Cancer Res. 67:1163–1169. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Klammer H, Mladenov E, Li F and Iliakis G:
Bystander effects as manifestation of intercellular communication
of DNA damage and of the cellular oxidative status. Cancer Lett.
356:58–71. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kuwahara Y, Roudkenar MH, Suzuki M,
Urushihara Y and Fukumoto M, Saito Y and Fukumoto M: The
involvement of mitochondrial membrane potential in cross-resistance
between radiation and docetaxel. Int J Radiat Oncol Biol Phys.
96:556–565. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Akino Y, Teshima T, Kihara A,
Kodera-Suzumoto Y, Inaoka M, Higashiyama S, Furusawa Y and Matsuura
N: Carbon-ion beam irradiation effectively suppresses migration and
invasion of human non-small-cell lung cancer cells. Int J Radiat
Oncol Biol Phys. 75:475–481. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou YC, Liu JY, Li J, Zhang J, Xu YQ,
Zhang HW, Qiu LB, Ding GR, Su XM, Mei Shi and Guo GZ: Ionizing
radiation promotes migration and invasion of cancer cells through
transforming growth factor-beta-mediated epithelial-mesenchymal
transition. Int J Radiat Oncol Biol Phys. 81:1530–1537.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gow MD, Seymour CB, Ryan LA and Mothersill
CE: Induction of bystander response in human glioma cells using
high-energy electrons: A role for TGF-beta1. Radiat Res.
173:769–778. 2010.PubMed/NCBI View
Article : Google Scholar
|