Introduction
Lung cancer is one of the main health burdens
worldwide; non-small cell lung cancer (NSCLC) accounts for ~85% of
lung cancer cases, with most patients diagnosed at a metastatic or
advanced stage (1,2). For patients with locally advanced
NSCLC, concurrent chemoradiotherapy is the backbone of treatment;
however, the efficacy of chemotherapy is hampered by the emergence
of NSCLC recurrence and distant metastasis (3,4).
Radiotherapy (RT), particularly stereotactic body RT (SBRT), exerts
beneficial effects on the tumor microenvironment (TME) (5-7). For
example, lethal dose irradiation can induce immunogenic cell death;
non-lethal dose irradiation can alter the immunological phenotype
of tumor cells (such as by upregulating the expression of tumor
MHC-I molecules) and can enhance the ability of T cells to kill
tumor cells. However, RT can also recruit immunosuppressive cells
to aggregate in tumor tissues, induce immune checkpoint generation
[e.g., programmed cell death 1 ligand 1 (PD-L1)] and promote tumor
immune tolerance (8-10). Our previous study reported that
increasing the dose of SBRT cannot improve its curative effect
(11). Moreover, the 4-year
failure rate of SBRT in the treatment of early NSCLC was 38%
(12). Treatment with SBRT alone
is not enough to change the immunosuppressive TME. It is still
difficult to trigger a systemic antitumor immune effect even though
RT can result in the release of tumor antigens locally in the
lesion (7).
In recent years, the use of immune checkpoint
inhibitors, including programmed cell death protein 1 (PD-1) and
PD-L1, has transformed tumor therapy, promoting significant
clinical outcomes in numerous tumor types (13-15).
In addition, it has been reported that the TME contains inhibitory
factors, including CTLA-4, TIM-3, LAG-3, CD160 and indoleamine
2,3-dioxygenase 1 (IDO1) (16).
IDO1 is a rate-limiting enzyme that catalyzes the conversion of
tryptophan (TRP) to kynurenine (KYN) for immune regulation. IDO1 is
upregulated in some types of cancer (such as colorectal, esophageal
and breast cancer) and can lead to immune escape and metastasis,
resulting in poor patient prognosis (17-19).
The high expression levels of IDO1 leads to TRP depletion and the
accumulation of metabolites (such as KYN, kynurenic acid and
3-hydroxykynurenine), to activate general control nonderepressible
2 (GCN2). The activation of GCN2 can block the cell cycle of T
cells or induce apoptosis, thereby mediating the immune escape of
tumor cells (20). Emerging
studies, including our previous study, have reported that a high
expression of IDO1 is positively associated with poor cancer
prognosis (21,22). Furthermore, IDO1 may regulate the
sensitivity of tumor cells to RT and chemotherapy drugs (23,24),
and could be associated with immunotherapeutic drug resistance
(25,26). Although the major findings of IDO1
have been described, the association of IDO1 with RT in NSCLC
remains ambiguous.
STAT5 has two subtypes, STAT5A and STAT5B, that are
encoded by two genes in series on the human chromosome 17q21.2
(27). STAT5A is strongly
associated with the occurrence and progression of various types of
cancer, such as prostate cancer, breast cancer, malignant melanoma
and lung cancer (28-30); it can inhibit cell apoptosis,
promote tumor cell proliferation and invasion, and has been
reported to be related to DNA repair in patients with chronic
myeloid leukemia (31). Maranto
et al (31) demonstrated
that inhibiting STAT5 activation could increase the
radiosensitivity of prostate cancer and reduce the ability of DNA
repair. However, to the best of our knowledge, targeting STAT5A and
IDO1 in lung cancer treatment has not been reported, and the effect
of STAT5A on T-cell response through IDO1 in the lung cancer TME is
not well characterized.
Therefore, the present study aimed to investigate
the role of the STAT5A/IDO1 axis in RT-mediated immunosuppression
in the NSCLC and to provide insights into the development of more
efficient immunotherapy regimens for NSCLC treatment.
Materials and methods
Datasets and databases
The TIMER database (TIMER2.0,
cistrome.shinyapps.io/timer; Immune Association dataset) is a web
resource used for comprehensive analysis of tumor-infiltrating
immune cells, which was established by the Department of
Statistics, Harvard University and Dana-Farber Cancer Institute.
The UCSC database (http://genome.ucsc.edu/cgi-bin/hgNear), the PROMO
database (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3)
and GeneCards (https://www.genecards.org) are searchable, integrative
database that provides comprehensive, user-friendly information on
all annotated and can predict the transcription factor of
genes.
Cell culture
The normal human bronchial epithelial cell line
BEAS-2B, the human lung adenocarcinoma cell lines (A549, NCI-H1975,
NCI-H1650, NCI-H460 and PC-9), the Jurkat T cells and the mouse
Lewis lung carcinoma (LLC) cell line were purchased from The Cell
Bank of Type Culture Collection of Chinese Academy of Sciences
(https://cellbank.org.cn) and were stored at the
Cancer Institute of Henan Cancer Hospital (Zhengzhou, China). The
human lung adenocarcinoma cells, Jurkat T cells and BEAS-2B cells
were cultured in conventional RPMI-1640 medium containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 mg/ml streptomycin, whereas the LLC cells were cultured in DMEM
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 100 mg/ml streptomycin.
Irradiation
Tumor cells (A549 and H1975) were seeded in 6-cm
dishes and exposed to 0, 2, 4, 6, 8 or 10 Gy irradiation using an
X-ray irradiator (Varian Medical Systems, Inc.). The irradiation
field, source skin distance (that is, the distance from the center
of radiation source to the center of body surface radiation field)
and dose rate were 13×13 cm, 100 cm and 400 cGy/min, respectively.
In addition, BALB/c nu/nu and C57BL/6 mice were exposed to 8 Gy
irradiation after the tumor volume reached 100 mm3 using
a small animal radiation platform.
Cell transfection
The short hairpin (sh)IDO1-1 (5′-CCA TCT GCA AAT CGT
GAC TAA-3′), shIDO1-2 (5′-CGT AAG GTC TTG CCA AGA AAT-3′),
shSTAT5A-1 (5′-GCG CTT TAG TGA CTC AGA AAT-3′), shSTAT5A-2 (5′-GAG
AAG TTC ACA GTC CTG TTT-3′) and scrambled control (Scr; 5′-TTC TCC
GAA CGT GTC ACG T-3′) sequences inserted into the GV248 lentiviral
vector were purchased from Shanghai GeneChem Co., Ltd. (cat. no.
CON077). A549 and H1975 cells were infected with the viral
particles in 4% polybrene (cat. no. REVG005; Shanghai GeneChem Co.,
Ltd.) at a multiplicity of infection of 20. After 72 h of
infection, the cells were cultured in complete medium containing 2
µl/ml puromycin to screen stable cell lines for 48 h. The
GFP tag was carried in the shRNA lentiviral vector and used to
observe the infection efficiency of lentivirus under fluorescent
microscope.
RNA extraction and reverse
transcription-quantitative PCR (qPCR)
Total RNA was extracted from cells in the different
treatment groups using TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc.) and then reverse-transcribed to cDNA using
a Reverse Transcription Kit (cat. no. RR047A; Takara Bio, Inc.).
The cDNA was then used as the template for qPCR using a SYBR Premix
Ex Taq kit (Takara Bio, Inc.) on an Applied Biosystems Real-time
PCR System (Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: 95°C for 30 sec; followed by 40 cycles
of denaturation at 95°C for 5 sec and annealing at 60°C for 30 sec.
GAPDH was used as an internal control. Relative RNA expression
levels were calculated by the 2−ΔΔCq method (33). The primer sequences were as
follows: IDO1 forward, 5′-GCC TGA TCT CAT AGA GTC TG GC-3′ and
reverse, 5′-TGC ATC CCA GAA CTA GAC GTG C-3′; GAPDH forward, 5′-GTC
TCCT CTG ACT TCA ACA GCG-3′ and reverse, 5′-ACC ACC CTG TTG CTG TAG
CCA A-3′.
Western blot analysis
Cells were lysed with RIPA lysis buffer (Beyotime
Institute of Biotechnology) on ice for 30 min, centrifuged at
20,000 × g for 30 min at 4°C and the supernatant was collected to
obtain total proteins. The protein concentration was determined by
the BCA assay (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Equal amounts (40 µg) of cell
lysates were separated by 8-12% SDS-PAGE and transferred to a
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.).
The membrane was subsequently blocked for 1-2 h using 5% non-fat
milk at room temperature, then incubated with the primary
antibodies (1:1,000 dilution) at 4°C overnight. After washing with
TBS + 0.05% Tween 20, the membrane was incubated with the secondary
antibody for 2 h at room temperature. Proteins were visualized
using the SuperSignal™ West Pico PLUS Chemiluminescent Substrate
(cat. no. 34580; Thermo Fisher Scientific, Inc.), and a gel
electrophoresis image analyzer (LI-COR Biosciences) was used for
color development. The primary and secondary antibody details are
shown in Table SI.
Cell immunofluorescence assay
Sterile glass coverslips were placed in 24-well
plates, and 2×104 cells were seeded into the wells. The
cells were exposed to 8 Gy RT for 6, 12, 24 or 48 h. After washing
with PBS, the cells were fixed with 4% paraformaldehyde for 15 min
at room temperature, incubated with 5% bovine serum albumin (cat.
no. ST023-1000g; Beyotime Institute of Biotechnology) at room
temperature for 30 min. Subsequently, a primary antibody
(anti-IDO1; 1:100; cat. no. 13268-1-AP; Proteintech Group, Inc.)
was added to the cells at 4°C for 15 h and the cells were then
incubated with Alexa Fluor 488 goat anti-rabbit IgG (1:400; cat.
no. A11008; Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 2 h. Finally, the coverslips were mounted onto
glass slides with anti-fluorescence quenching sealing agent
containing DAPI (ProLong™ Gold Antifade Mountant; cat. no. P36930;
Invitrogen; Thermo Fisher Scientific, Inc.) and imaged using a
Leica SP5II confocal microscope (Leica Microsystems GmbH).
Cell co-culture system
Jurkat T cells were used as a model for the analysis
of the anti-T cell proliferation effect of the cancer cell culture
supernatant, as previously described (34). H1975 cells (1×105) in
800 µl RPMI-1640 with 10% FBS were added to the bottom of
the Transwell chamber. The Jurkat T cells (2×105) in 300
µl RPMI-1640 with 10% FBS were added to the upper chamber.
The cells were co-cultured at 37°C for 24 h. Finally, western blot
analysis and flow cytometry were used to detect apoptosis of Jurkat
T cells. The following four groups were established: i) Negative
control group; ii) EPA group; iii) RT group; iv) EPA + RT group.
H1975 cells were pretreated for 1 h at 37 with 10 µmol/l EPA
(Selleck Chemicals; dissolved in DMSO), a selective IDO1 inhibitor,
and then treated with RT as aforementioned. The following six
groups were also established: i) Scr group; ii) shIDO1#1 group;
iii) shIDO1#2 group; iv) RT-only group; v) shIDO1#1 + RT group; vi)
shIDO1#2 + RT group. An additional set of experiments included the
following groups: i) Scr group; ii) shSTAT5A#1 group; iii)
shSTAT5A#2 group; iv) RT-only group; v) shSTAT5A#1 + RT group; vi)
shSTAT5A#2 + RT group.
Colony formation assays
Transduced A549 and H1975 cells (Scr, shIDO1#1,
shIDO1#2; shSTAT5A#1, shSTAT5A#2) were seeded (1,000 cells/well) in
6-well plates in 2 ml medium and incubated for 24 h. Subsequently,
cells were exposed to 0, 2, 4, 6 or 8 Gy irradiation using an X-ray
irradiator and were further incubated for an additional 14 days.
The medium was then removed and cells were fixed with 0.1%
formaldehyde at room temperature for 20 min; 0.5% crystal violet
was used to stain the colonies for 10 min at room temperature. The
dye solution was slowly rinsed away with distilled water and the
cells were air-dried. A colony was considered as having >50
cells, and visualized under an IX73 inverted optical microscope
(Olympus Corporation) and the number of colonies was counted using
ImageJ software (version 1.51K; National Institutes of Health). The
classic multitarget single-hit model was applied to plot the dose
survival curves. Survival fraction (SF)=1-(1-e−D/D0) N,
where the mean lethal dose (D0) represents the dose needed to
decrease the fraction of surviving cells to 37% of its previous
value, N is the extrapolation number, and the quasi-threshold dose
(Dq) indicates repair capacity of tumor cells after radiation
therapy. Radiation sensitivity parameters included survival
fraction at 2 Gy (SF2), D0, Dq and the sensitivity enhancement
ratio was calculated. The sensitivity enhancement ratio was
calculated by dividing D0 in the control group by D0 in the
treatment group. The test was repeated three times.
Cell cycle analysis by flow
cytometry
Cells were seeded in 6-well plates at a density of
1×106 cells/well, then divided into the six experimental
groups (Scr, shSTAT5A#1, shSTAT5A#2, RT, shSTAT5A#1 + RT and
shSTAT5A#2 + RT). After 24 h of treatment, the cells were
harvested, washed with phosphate-buffered saline (PBS) and fixed
with 70% ice-cold ethanol at −20°C overnight. The cells were then
washed further with PBS and stained using the CycletestPlus DNA
Reagent kit (BD Biosciences, San Jose, CA, USA), according to the
manufacturer's protocol. The cell cycle distribution was analyzed
by a flow cytometer (BD Biosciences).
Apoptosis analysis by flow cytometry
For apoptosis, transfected A549 and H1975 cells were
seeded into 6-well plates. Once they reached confluence (60-70%),
the cells were collected and incubated with Annexin V-FITC (5
µl; Biogot Technology Co., Ltd.) and propidium iodide
solution (5 µl; Biogot Technology Co., Ltd.) at room
temperature for 15 min according to the manufacturer's
instructions. Cells were subsequently suspended in 400 µl
binding buffer (Biogot Technology Co., Ltd.). Early + late stage
apoptosis was analyzed using a FACSAria flow cytometer (BD
Biosciences). All data were analyzed with ModFit version 4.0
(Verity Software House, Inc.).
ELISA
The A549 and H1975 cell culture supernatants were
directly collected, and then the concentration of KYN was
determined with a Human KYN ELISA kit (CUSABIO; catalog number
CSB-E13659h) according to the manufacturer's instructions.
Animal studies
Mice were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. and were housed under
pathogen-free conditions. All animal experiments were performed in
accordance with the institutional guidelines and were approved by
the Committee on the Ethics of Animal Experiments of Zhengzhou
University (NO.CUHCI2021-003). The animals were housed under
controlled environmental conditions: 12-h dark/light cycle;
20-22°C; humidity, 55±5%, and were allowed free access to normal
food and water. Two murine models were used in the present study
and were established as follows: i) BALB/c nu/nu mice (age, 4-6
weeks; female; weight, 15-17 g) were subcutaneously injected with
5×106 H1975 cells on their dorsal flanks; ii) C57BL/6
mice (age, 4-6 weeks; female; weight, 15-17 g) were subcutaneously
injected with 5×106 LLC cells, which are syngeneic to
C57BL/6, on their dorsal flanks. The mice were randomly divided
into four groups (n=6 for BALB/c nu/nu mice and n=5 for C57BL/6
mice): i) Scr group, ii) shSTAT5A group, iii) RT group and iv)
shSTAT5A + RT group. Mice were subcutaneously inoculated in the
right flank with 5×106 NCI-H1975 and LLC cells with or
without stable shSTAT5A transduction. The tumor sizes were recorded
every 3 days for BALB/c nu/nu mice and twice per week for C57BL/6
mice. Tumor volume was measured with a digital caliper [(length x
width2)/2] and body weight was recorded periodically.
The mice were sacrificed by intraperitoneal injection of sodium
pentobarbital 20 days later (200 mg/kg) and death was verified by
respiratory and cardiac arrest, and pupil dilation. Tumor
ulceration, tissue swelling and cachexia were the humane endpoints
evaluated, and none of the mice used in the study met these
endpoints.
Flow cytometric profiling of
lymphocytes
Mouse spleens were excised and ground through a
70-µm nylon mesh. Single spleen cells were then resuspended
in PBS and lysed by Red Blood Cell Lysis Buffer (cat. no. C3702;
Beyotime Institute of Biotechnology). Cells were Fc-blocked with
TruStain FcX™ (anti-mouse CD16/3; Clone 93; BioLegend, Inc.) for 30
min at 4°C. In addition, mouse tumor tissues were minced, underwent
enzymatic digestion with 0.1% collagenase (cat. no. C5138;
MilliporeSigma), hyaluronidase (0.1 mg/ml; cat. no. H6254;
MilliporeSigma) and DNase IV (20 U/ml; cat. no. D5025;
MilliporeSigma) in Hank's balanced salt solution (Gibco; Thermo
Fisher Scientific, Inc.) and ground through a 70-µm nylon
mesh. The filtrate was collected and lysed with the Red Blood Cell
Lysis Buffer. A single cell suspension of 1-2×106
tumor-infiltrating lymphocytes and spleen cells was used for cell
surface and intracellular marker staining. The single cell
suspension was incubated with a conjugated monoclonal antibody
(BioLegend, Inc., unless otherwise stated) at 4°C for 30 min. All
antibodies used for flow cytometric analysis of mouse tumor samples
and spleen are shown in Table
SII. All samples were assessed using a Cytek Aurora flow
cytometer (Cytek Biosciences) and analyzed using FlowJo software
(FlowJo V10.5.3; Becton Dickinson).
Immunohistochemistry (IHC)
IHC was performed on 10% formalin-fixed (at room
temperature for 4 h) paraffin-embedded tumor tissue sections (4
µm). The paraffin-embedded sections were deparaffinized,
underwent antigen retrieval using microwave heating in 10 mM
citrate buffer (pH 7.0) at 80°C for 20 min; endogenous peroxidases
were blocked with 3% H2O2 (OriGene
Technologies, Inc.) at room temperature for 30 min and the sections
were blocked with 5% goat serum (Beyotime Institute of
Biotechnology) for 40 min at room temperature before being
incubated with the primary anti-bodies at 4°C overnight. The tissue
sections were incubated with primary antibodies at 4°C overnight.
The following primary antibodies were used for IHC: Rabbit
anti-mouse IDO1 (1:1,000; cat. no. 122402; BioLegend, Inc.); rabbit
anti-mouse CD8 (1:200; cat. no. bs-0648R; Bioss antibodies, Inc.),
rabbit anti-STAT5A (1:200; cat. no. ab32043; Abcam, Inc.), rabbit
anti-Ki67 (1:400; cat. no. ab15580; Abcam); and rabbit anti-human
FOXP3 (1:100; cat. no. ab215206; Abcam, Inc.). Following incubation
with the primary antibodies, the samples were incubated with
HRP-conjugated goat anti-rabbit IgG secondary antibody (1:2,000
dilution; cat. no. 7074S; Cell Signaling Technology, Inc.) at room
temperature for 1 h. The samples were stained using DAB (OriGene
Technologies, Inc.) at room temperature for 5 min and
counterstained with hematoxylin at room temperature for 1 min. The
images were obtained using an IX73 inverted light microscope
(Olympus Corporation). Integrated optical density of the IHC image
data were quantitatively analyzed using Image-Pro Plus v.6.0 (Media
Cybernetics, Inc.) image analysis software.
Statistical analysis
All quantitative data are presented as the mean ±
standard deviation, and were analyzed using GraphPad Prism version
8.0 (GraphPad Software, Inc.) and SPSS 22.0 software (IBM Corp.).
The unpaired Student's t-test and one-way ANOVA followed by Tukey's
post hoc test were used for single-group comparisons and multiple
comparisons, respectively. P<0.05 was considered to indicate a
statistically significant difference. All analyses represented at
least three independent in vitro experiments.
Results
Inhibition of IDO1 enhances the efficacy
of radiation in vitro
To elucidate the functional role of IDO1 in NSCLC,
the protein expression levels of IDO1 were detected in immortalized
normal lung epithelial cells (BEAS-2B) and lung cancer cells. IDO1
expression was high in PC-9, A549 and H1975 cells, but not in
H1650, H460 and BEAS-2B cells (Fig.
1A). Moreover, whether radiation affected the expression of
IDO1 was explored. Lung adenocarcinoma cell lines (A549 and H1975)
were irradiated with a gradient dose of radiation, and the
expression of IDO1 was evaluated 48 h later. The results revealed
that with the increase in irradiation dose, the protein expression
levels of IDO1 were increased; with the highest expression detected
in the 8 Gy (A549) and 6 Gy (H1975) (Fig. 1B). It has been reported that
changes in KYN concentration evaluated using ELISA can reflect the
effect of RT on IDO1 enzyme activity (35). The present study results
demonstrated that KYN levels in the culture supernatant were
initially increased in response to irradiation, peaking at 8 Gy
(A549) and 6 Gy (H1975), and then decreased (Fig. 1C). To further examine the effect of
radiation on IDO1 expression, immunofluorescence was used to detect
the localization of IDO1 in irradiated lung adenocarcinoma cells
(Fig. S1). IDO1 expression was
detected 12 h after irradiation and peaked at 48 h. IDO1 was mainly
expressed in the nucleus, with a low expression in the cytoplasm.
These findings indicated that IDO1 was upregulated in human lung
cancer cells after exposure to radiation.
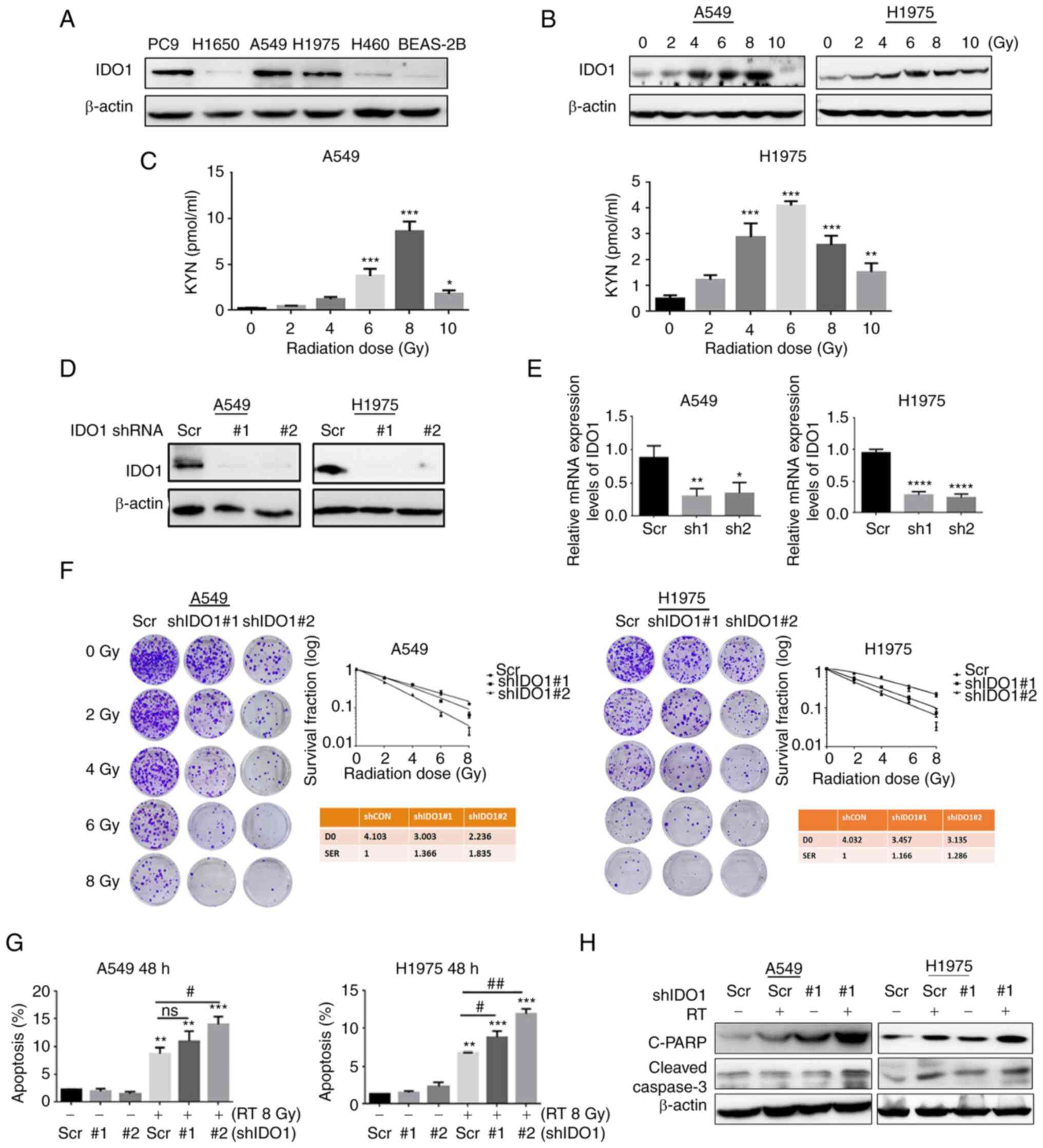 | Figure 1Radiation increases IDO1 expression
in NSCLC cells and knockdown of IDO1 enhances radiosensitivity
in vitro. (A) IDO1 protein expression levels in the human
normal bronchial epithelial cell line BEAS-2B and NSCLC cell lines.
(B) Protein expression levels of IDO1 in lung adenocarcinoma cells
48 h after gradient radiotherapy (lower panel). (C) ELISA was
performed to detect KYN levels in A549 and H1975 cell supernatants
48 h after irradiation. IDO1 knockdown in A549 and H1975 cells, as
determined by (D) western blotting and (E) reverse
transcription-quantitative PCR. (F) Tumor cells were transduced
with lentiviruses carrying Scr, shIDO1#1 or shIDO1#2) and were
selected with 2 µg/ml puromycin. The surviving cells were
then used to examine radiosensitivity following γ-irradiation (0,
2, 4, 6 or 8 Gy) by clonogenic assay. (G) Flow cytometry was used
to detect the effect of shIDO1 + RT for 48 h on the apoptosis of
lung adenocarcinoma cells. (H) Western blotting was used to detect
the expression levels of apoptosis-related proteins. Data are
presented as the mean ± SD. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001 vs. Scr; #P<0.05 and
##P<0.01. C-PARP, cleaved-poly [ADP-ribose]
polymerase 1; D0, Day 0; IDO1, indoleamine-2,3-dioxygenase 1; KYN,
kynurenine; ns, not significant; NSCLC, non-small cell lung cancer;
RT, radiotherapy; Scr, scrambled shRNA negative control; sh/shRNA,
short hairpin RNA. |
To determine the effects of IDO1 on the sensitivity
of lung cancer cells to radiation, A549 and H1975 cells were
collected and their radiosensitivity was investigated after IDO1
knock-down (Fig. 1D and E).
Compared with the Scr cells, the number of IDO1 knockdown A549 and
H1975 cell colonies was notably reduced as radiation dose increased
(Fig. 1F). The sensitivity
enhancement ratios for an estimated SF of IDO1 knockdown cells were
1.366 or 1.835 for shIDO1#1 and shIDO1#2 transduced A549 cells,
respectively, and 1.166 or 1.286 for the two H1975 cell
transductions. These results indicated that IDO1 knockdown
sensitized lung cancer cells to radiation treatment.
Flow cytometry was used to determine the efficacy of
shIDO1 transduction combined with RT on the apoptotic rate of lung
adenocarcinoma cells. The use of the combination was found to
increase the radiation-induced lung adenocarcinoma cell apoptotic
rate (Figs. 1G and S2). Western blot analysis showed that
compared with the Scr group, the expression levels of
apoptosis-related proteins [cleaved-caspase-3 and cleaved-Poly
(ADP-ribose) polymerase 1 (PARP)] were significantly increased in
RT + shIDO1 group (Fig. 1H).
Flow cytometry was also used to detect the apoptotic
state of T cells in a co-culture system. H1975 cells were
co-cultured with Jurkat T cells for 48 h after RT; the apoptotic
rate was significantly higher compared with that in the non-RT
group (Fig. 2A). When shIDO1 or
EPA were used to suppress IDO1 in H1975 cells, the apoptotic rate
of T cells in the RT combined with shIDO1 or EPA group was
significantly lower compared with that in the RT alone group
(Figs. 2A, B, S3A and B). Furthermore, under RT, when
IDO1 expression was blocked with EPA, the expression levels of
GCN2, cleaved-caspase-3 and cleaved-PARP were notably lower
compared with the RT-only group, suggesting that RT-induced T-cell
apoptosis may be IDO1-dependent (Fig.
2C).
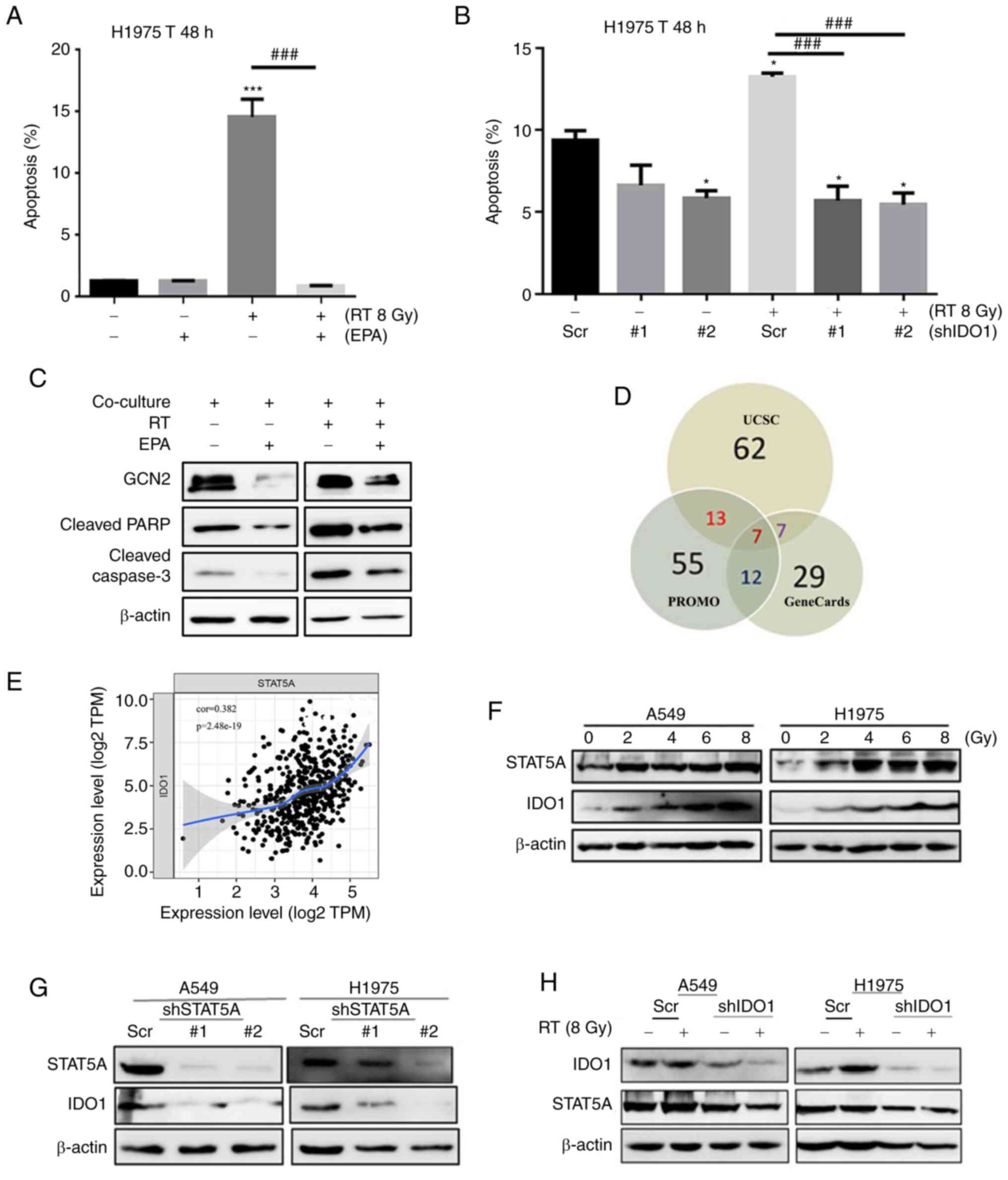 | Figure 2IDO1 inhibition reverses
irradiation-induced apoptosis of Jurkat T cells, and STAT5A serves
an important role in regulating the expression of IDO1. (A) H1975
cells were co-cultured with Jurkat T cells (1:4) and then exposed
to 8 Gy radiation. Subsequently, the apoptotic rate of extracted
Jurkat T cells was detected by flow cytometry. (B) Flow cytometry
was used to detect the effect of shIDO1 combined with RT for 48 h
on the apoptosis of Jurkat T cells. (C) Western blotting was used
to detect the expression levels of apoptosis-related proteins and
GCN2 downstream of IDO1. (D) Several databases (UCSC, PROMO and
GeneCards) were used to predict the transcription factors that may
regulate IDO1. (E) Positive correlation between the expression of
STAT5A and IDO1 in patients with lung cancer in TIMER database. (F)
Western blot analysis showed the effects of RT on the STAT5A
expression in A549 and H1975 cells. (G) Western blot analysis
showed that interfering with the expression of STAT5A decreased the
expression levels of IDO1. (H) Western blot analysis showed that
knocking down the expression of IDO1 did not affect STAT5A protein
expression after irradiation. Data are presented as the mean ± SD.
*P<0.05, ***P<0.001 vs. Scr;
###P<0.01. EPA, epacadostat; IDO1,
indoleamine-2,3-dioxygenase 1; RT, radiotherapy; Scr, scrambled
shRNA negative control; sh, short hairpin RNA. |
STAT5A knockdown contributes to decreased
IDO1 expression in NSCLC, and STAT5A knockdown enhances the
survival of Jurkat T cells by suppressing IDO1 function
Databases (including UCSC, PROMO and GeneCards) were
used to predict the transcription factors that may regulate IDO1
expression (Fig. 2D); seven
transcription factors were identified that may bind to the promoter
region of IDO1, including CEBPB, YY1, GATA1, SP1, STAT5A, STAT1 and
STAT3. The expression of transcription factor STAT5A is
significantly correlated with IDO1 in patients with lung cancer in
the TIMER database (Fig. 2E).
Western blot analysis demonstrated that the expression levels of
STAT5A and IDO1 in lung adenocarcinoma cells following irradiation
were comparable; that is, both expression levels were increased
with higher radiation exposure (Fig.
2F). Interfering with STAT5A expression notably inhibited IDO1
expression (Fig. 2G); however,
after IDO1 knockdown, STAT5A expression was not altered in tumor
cell lines after irradiation (Fig.
2H). These findings indicated that STAT5A may be a
transcription factor that regulates IDO1 expression.
The function of STAT5A in RT of NSCLC in
vitro was also explored. Results from the colony formation
assay revealed that knockdown of STAT5A resulted in decreased
proliferation and increased sensitivity to radiation therapy
compared with the Scr group (Fig.
3A). As determined by flow cytometry, STAT5A knockdown resulted
in cell cycle arrest at G0/G1 phase, whereas
the number of cells at G0/G1 was significantly decreased in cells
treated with RT alone compared with Scr group. Compared with RT
alone, shSTAT5A + RT also resulted in cell cycle arrest at
G0/G1 phase. The percentage of cells in the
G2/M phase in the shSTAT5A groups were significantly decreased,
whereas RT alone resulted in cell cycle arrest at G2/M phase
compared with the Scr group. Compared with RT alone, shSTAT5A + RT
also decreased the percentage of cells in the G2/M phase (Figs. 3B and S4). Flow cytometric apoptosis analysis
showed that shSTAT5A combined with RT significantly increased the
total apoptotic rate of the two cell lines compared with RT alone
(Figs. 3C and S5). Western blot analysis also revealed
that the expression levels of apoptosis-related proteins
(cleaved-caspase-3 and cleaved-PARP) in shSTAT5A + RT were markedly
increased compared with RT alone (Fig.
3D).
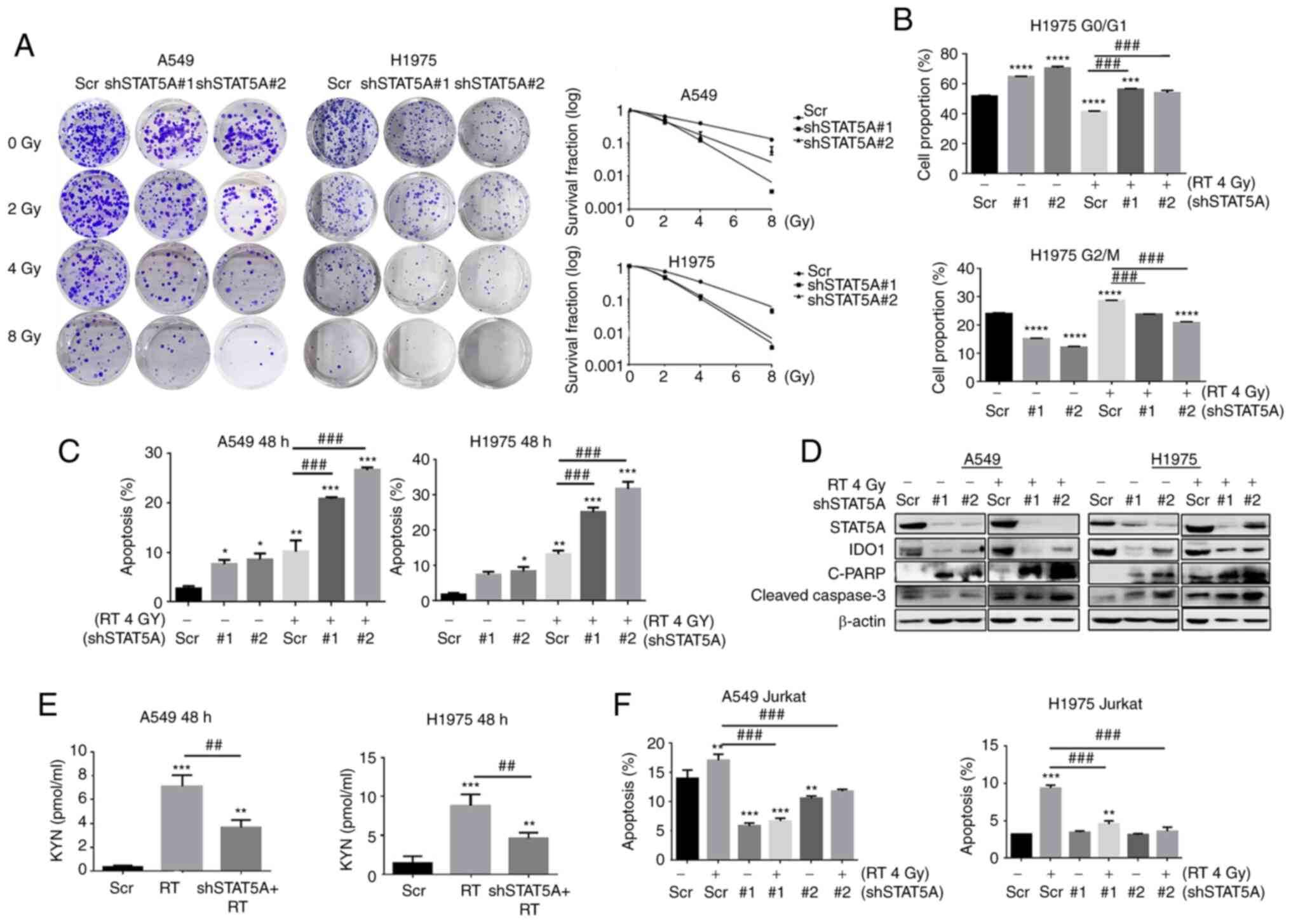 | Figure 3Effects of STAT5A knockdown in
combination with RT on proliferation, cell cycle progression and
apoptosis of lung cancer cells. (A) Cell survival was assessed via
clonogenic assay to examine the radiosensitivity of transduced
cells following irradiation (0, 2, 4 and 8 Gy). The diameter of
each well is 35 mm. (B) Cell cycle arrest at the
G0/G1 phase after shSTAT5A transduction. (C)
Apoptosis was assessed by flow cytometry. (D) Western blotting was
used to detect the expression levels of apoptosis-related proteins.
(E) KYN was analyzed in the culture medium from A549 and H1975
transduced with shSTAT5A with or without RT exposure; the
concentration of KYN was determined by ELISA. (F) Flow cytometry
was used to detect the effect of shSTAT5A + RT for 48 h on the
apoptosis of Jurkat cells. Data are presented as the mean ± SD.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 vs. Scr;
##P<0.01 and ###P<0.001. C-PARP,
cleaved-poly [ADP-ribose] polymerase 1; CON, negative control;
IDO1, indoleamine-2,3-dioxygenase 1; KYN, kynurenine; ns, not
significant; RT, radiotherapy; Scr, scrambled shRNA negative
control; sh, short hairpin RNA. |
The influence and potential underlying molecular
mechanism of shSTAT5A + RT on IDO1 enzyme activity and T-lymphocyte
apoptotic rate in the co-culture system was further investigated
using ELISA and flow cytometry. The ELISA results of the culture
medium of A549 and H1975 cells showed that RT enhanced KYN compared
with the Scr group, whereas shSTAT5A + RT suppressed KYN compared
with RT alone (Fig. 3E).
Furthermore, flow cytometry suggested that the group treated with
Scr + RT exhibited an enhanced percentage of apoptotic Jurkat T
cells compared with the Scr group (Figs. 3F and S6). Moreover, the shSTAT5A + RT group
demonstrated a reverse in the apoptosis of Jurkat T cells compared
with the Scr + RT group. Taken together, these results indicated
that shSTAT5A may suppress the apoptosis of Jurkat T cells by
blocking IDO1 expression.
Knockdown of STAT5A combined with RT
significantly inhibits the growth of NSCLC tumors in vivo
NCI-H1975 and LLC cells were transduced with
shSTAT5A and were then injected subcutaneously into
immune-compromised mice (BALB/c nude) and mice with a functioning
immune system (C57BL/6). Results showed that shSTAT5A#1 and
shSTAT5A#2 notably decreased the protein levels of STAT5A in LLC
cell (Fig. S7A). Mice were
divided into the following four groups: Scr, RT, shSTAT5A and
shSTAT5A + RT; the mice were sacrificed and tumors were harvested
(Figs. 4A and 5A). For BALB/c nude mice, compared with
Scr group, tumor growth was significantly inhibited in the other
three groups. Additionally, compared with RT group, the shSTAT5A +
RT group exhibited more impeded tumor growth (Fig. 4B). For C57BL/6 mice, compared with
Scr group, the other three groups significantly inhibited the tumor
growth; however, there was no difference between the treatment
groups (Fig. 5B). In addition, the
average tumor weight in the shSTAT5A + RT groups were significantly
lower compared with those in the Scr group (Figs. 4C and 5C). Notably, there were no detectable
effects of each treatment regimen on body weights of the treated
mice (Fig. S7B).
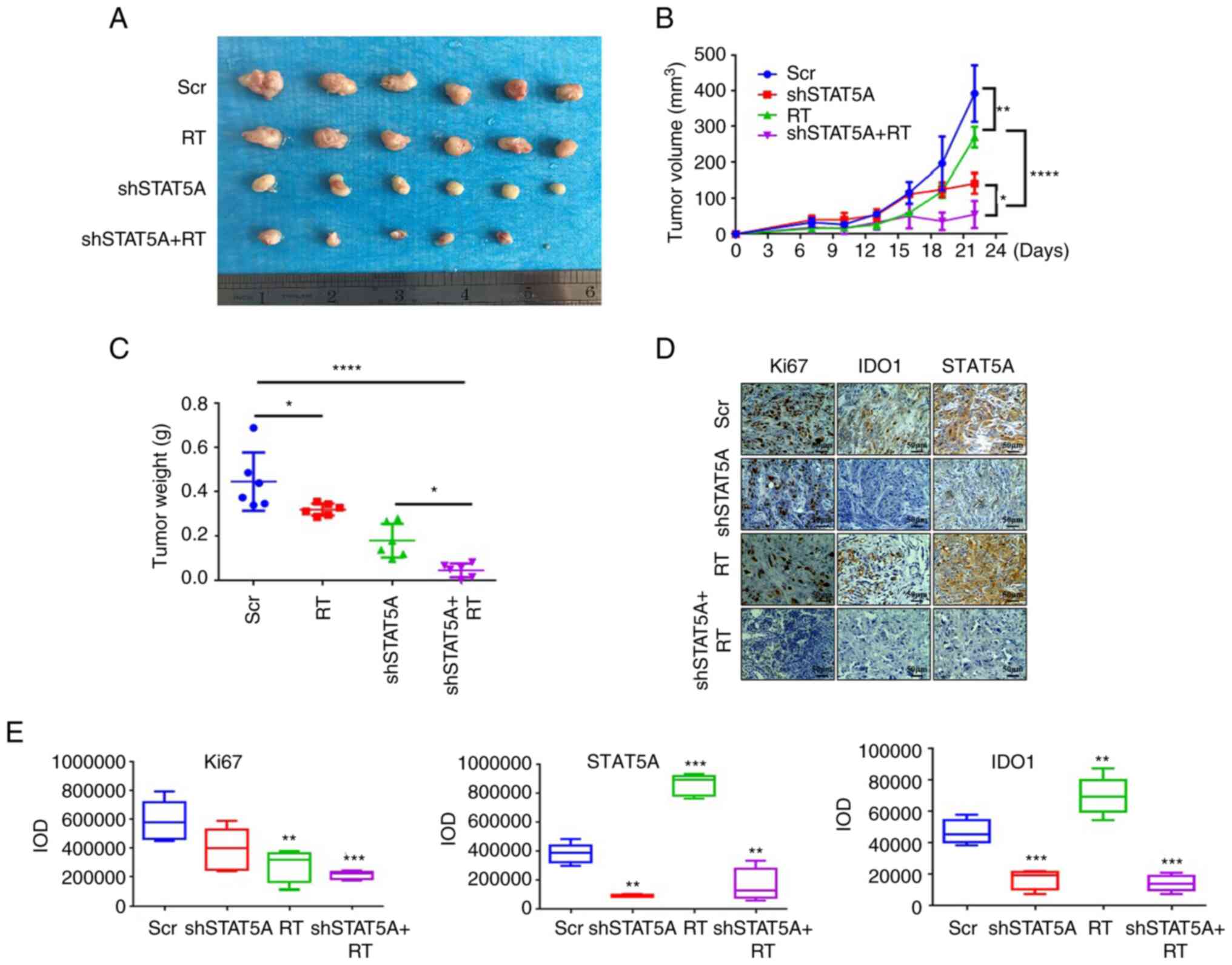 | Figure 4Effects of shSTAT5A combined with RT
on BALB/c nude mice tumor formation and proliferation in
vivo. (A) Images of excised subcutaneous xenografts from BALB/c
nude mice injected with shSTAT5A-transduced H1975 cells. (B) Tumor
volume was measured in all mice every 3 days. (C) Tumor weight was
measured in all mice following excision. (D) Immunohistochemistry
of Ki-67, IDO1 and STAT5A and were performed on the tumor samples
(magnification, ×200). (E) Semi-quantification of Ki67, STAT5A and
IDO1 staining intensity in the images shown in (D). The experiments
were repeated three times. Data are presented as the mean ± SD.
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 vs. Scr. IDO1,
indoleamine-2,3-dioxygenase 1; IOD, integrated optical density; RT,
radiotherapy; Scr, scrambled shRNA negative control; sh, short
harpin RNA. |
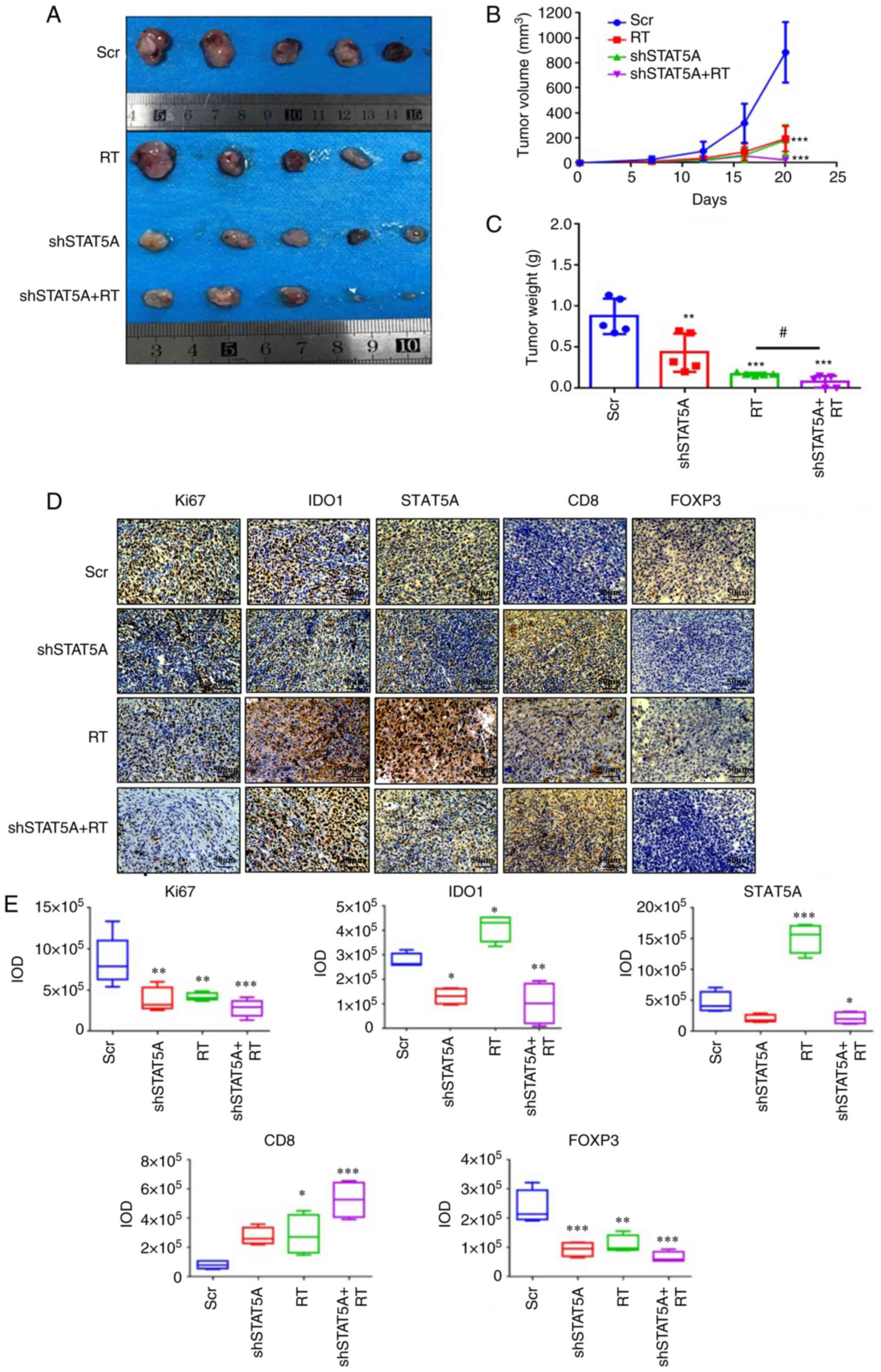 | Figure 5Effects of shSTAT5A combined with RT
on C57BL/6 mice xenograft tumor formation and proliferation in
vivo. (A) Images of excised subcutaneous xenografts of C57BL/6
mice injected with shSTAT5A-transduced LLC cells. (B) Tumor volume
was measured in all mice twice a week. (C) Tumor weight was
measured in all mice. (D) Immunohistochemistry of Ki-67, IDO1,
STAT5A, CD8 and FOXP3 were performed on the tumor samples
(magnification, ×200). (E) Semi-quantification of Ki67, IDO1,
STAT5A, CD8 and FOXP3, staining intensity in the images shown in
(D). The experiments were repeated three times. Data are presented
as the mean ± SD. *P<0.05, **P<0.01,
***P<0.001 vs. Scr, #P<0.05. FOXP3;
forkhead box P3; IDO1, indoleamine-2,3-dioxygenase 1; IOD,
integrated optical density; LLC, Lewis lung carcinoma; RT,
radiotherapy; Scr, scrambled shRNA negative control; sh, short
harpin RNA. |
IHC staining in the sections of LLC tumors revealed
that shSTAT5A + RT significantly inhibited STAT5A and Ki67 protein
levels in BALB/c nude (Fig. 4D and
E) and C57BL/6 models compared with the Scr group (Fig. 5D and E). IHC staining also
demonstrated that the combined shSTAT5A + RT treatment resulted in
a reduction in IDO1 expression, an increase in the number of
CD8+ T cells and a decrease in the number
FOXP3+ Tregs compared with the Scr treatment (Fig. 5D and E). These data indicated that
shSTAT5A + RT may significantly attenuate tumor growth in
vivo, probably through a mechanism involving an increased
recruitment or activation of cytotoxic T cells and a reduced
recruitment or activation of inhibitory Tregs.
Knockdown of STAT5A combined with RT
promotes activation of the antitumor T-cell response in vivo
The abundance of total CD3+,
CD4+ and CD8+ T-cell subsets in the spleen
and dissociated LLC tumor tissue from C57BL/6 mice was assessed
using flow cytometry. Compared with RT-treated mice, shSTAT5A + RT
resulted in a significant increase in the percentage of
CD3+ T cells in the spleen (Fig. 6A and B); there was no significant
difference in CD4+ T cells among the four treatment
groups, whereas the number of CD8+ T cells was
significantly higher in the shSTAT5A + RT group compared with in
the other three groups. In the tumors, shSTAT5A + RT had no
significant effect on CD3+ T cells, and there was no
significant difference among the four treatment groups (Fig. S8A and B). Notably, the number of
CD4+ T cells was decreased in the shSTAT5A + RT group
compared with the Scr group, whereas the number of CD8+
T cells was increased (Fig. S8A and
B).
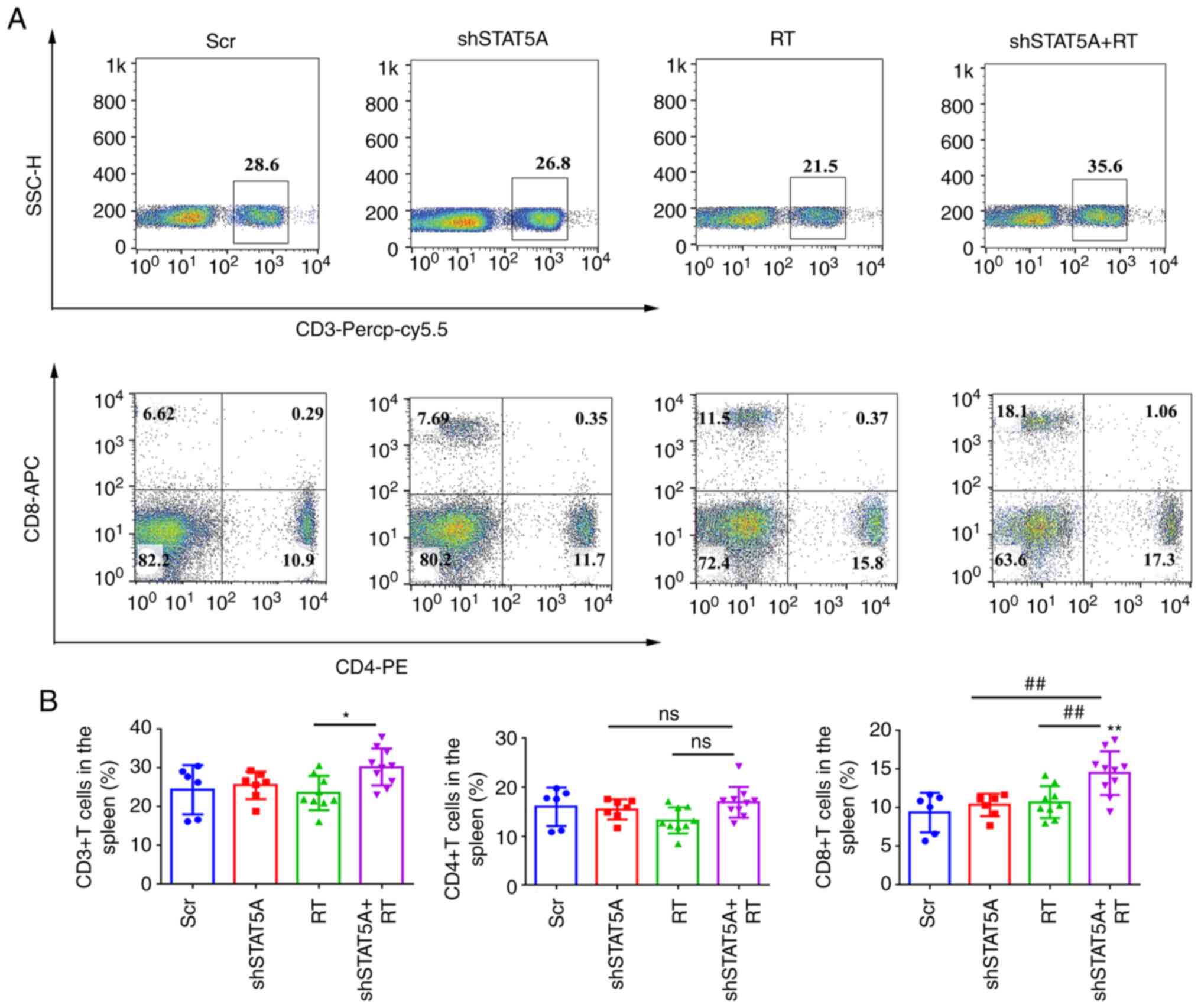 | Figure 6Knockdown of STAT5A combined with RT
promoted the activation of CD8+T cell response in
spleen. (A) Representative flow cytometry plots of CD3+,
CD4+ and CD8+ T cells and (B) quantitative
analysis of the results of CD3+, CD4+ and
CD8+ T cells in the spleen. Data are presented as the
mean ± SD. *P<0.05, **P<0.01 vs. Scr;
##P<0.01. APC, allophycocyanin; Cy, cyanine; ns, not
significant; PE, phycoerythrin; PerCP, peridinin chlorophyll
protein; RT, radiotherapy; Scr, scrambled shRNA negative control;
sh, short hairpin RNA; SSC, side scatter. |
To determine other potential mechanisms underlying
the induction of immunosuppression, the present study analyzed the
changes in the abundance of Tregs in mouse spleen and tumor tissues
using flow cytometry. The results revealed that, compared with the
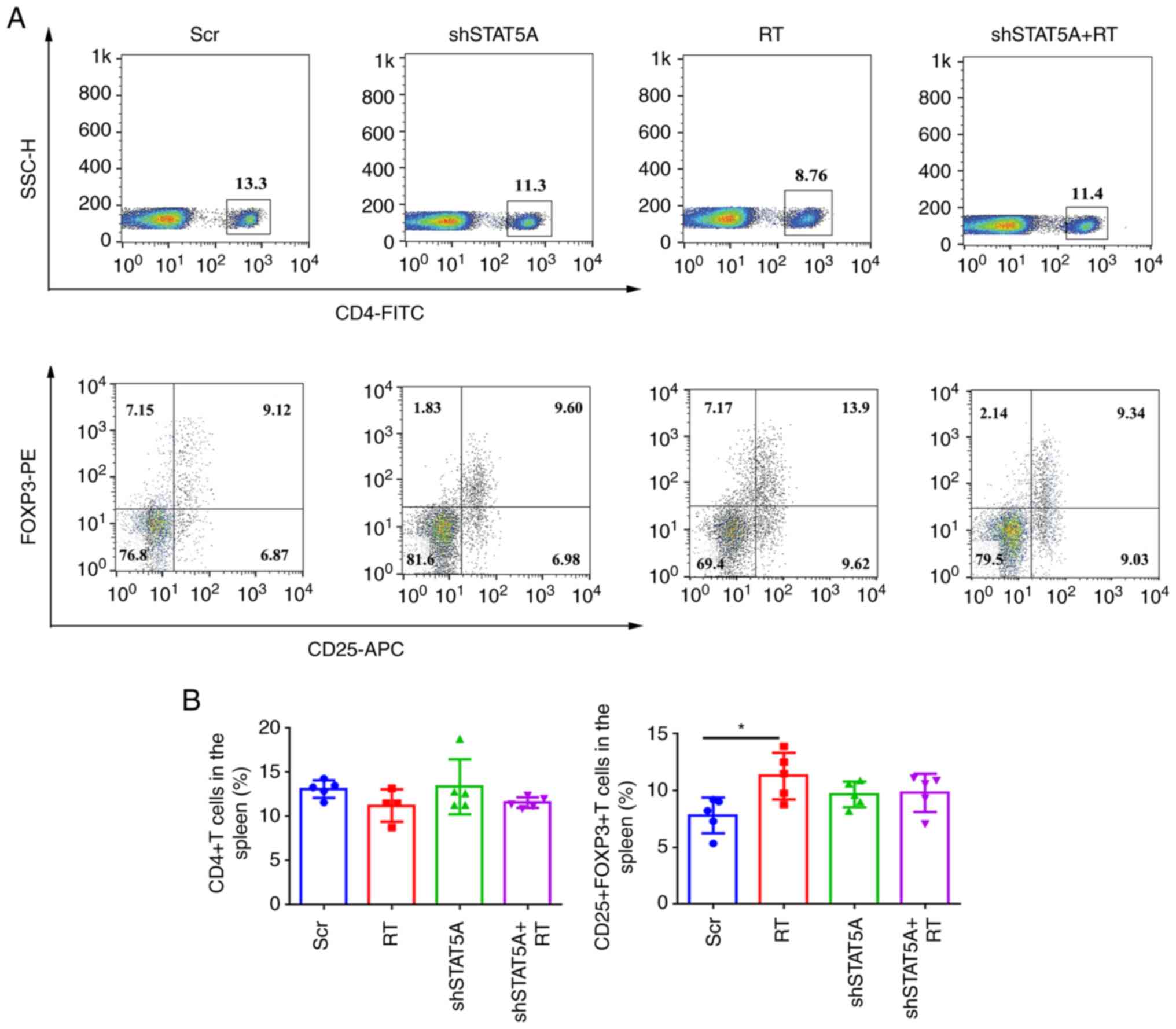
Scr group, the number of
CD25+FOXP3+CD4+ Tregs in the
spleens of mice in the RT group were significantly increased,
whereas no significant difference was identified in the shSTAT5A
and shSTAT5A + RT groups compared with Scr (Fig. 7A and B). Compared with the Scr
group, CD25+FOXP3+CD4+ Treg
infiltration in mouse tumors was significantly reduced in the
shSTAT5A + RT group (Fig. S9A and
B). Collectively, these data suggested that knockdown of STAT5A
combined with RT may promote activation of the antitumor T-cell
response in vivo.
Discussion
RT serves an important role in the treatment of
NSCLC (10,36). In addition to RT and chemotherapy,
immunotherapy has attracted much attention. Immune checkpoint
inhibitors have shown significant clinical efficacy in the
treatment of NSCLC; nevertheless, drug resistance may occur, and
the long-term survival of patients with NSCLC remains
unsatisfactory (37). Therefore,
an in-depth understanding of the molecular mechanism underlying RT
resistance is vital, and potential reversal strategies urgently
need to be resolved for current lung cancer treatment.
A previous study has reported that IDO1 contributes
to tumor progression in vivo by suppressing
tumor-infiltrating T lymphocytes and natural killer cells, and by
activating Tregs (38). In
addition to suppressing the antitumor immune response, IDO1 may
promote tumor development in a non-immunomodulating manner. The
inhibition of IDO1 expression has been shown to sensitize human
lung cancer cells to chemotherapeutic agents by regulating the cell
cycle (39). In colorectal cancer
cells, it was found that TRP metabolites activate the β-catenin
pathway and promote cell proliferation (40). The present study revealed that IDO1
expression was increased after RT, and interfering with the
expression of IDO1 reduced proliferation, promoted apoptosis and
promoted the inherent antitumor effect of radiation on lung cancer
cells. These results were similar to those of a prior study wherein
IDO1 inhibition was found to sensitize colorectal cancer cells to
radiation-induced cell death (41). When the activity or concentration
of local IDO1 increases, TRP in the cell is metabolized to KYN,
resulting in a significant reduction in TRP concentration (24). In the state of low TRP levels,
tryptophanyl tRNA concentration increases, which activates GCN2
(26). In the present study, tumor
cells and Jurkat T cells were co-cultured, and it was revealed that
RT could upregulate IDO1 of lung cells, increase the content of
KYN, and ultimately induce T-cell apoptosis. IDO1 knockdown or the
use of IDO1 inhibitors was able to hinder T-cell apoptosis. These
data indicated that an RT-induced increase in IDO1 expression in
lung cells may be a factor leading to T-cell apoptosis.
A previous report showed that an IL-6-induced
proto-oncogene protein, intestinal-specific homeobox, can induce
the expression of IDO1 and TDO, thereby increasing KYN and aryl
hydrocarbon receptor levels, and promoting the expression of CD86
and PD-L1 in liver cancer cells (42). The present study predicted that
STAT5A is an upstream transcription factor affecting IDO1
expression. Moreover, it was confirmed that RT significantly
promoted the expression levels of STAT5A and IDO1. Interfering with
IDO1 did not affect the expression of STAT5A, whereas interfering
with STAT5A prevented a radiation-induced increase in IDO1
expression, thereby significantly improving the radiosensitivity of
lung cancer cells.
To further evaluate the effects of the STAT5A/IDO1
axis on the efficacy of RT in lung cancer treatment, two cell-line
derived xenograft mouse models (BALB/c nude and C57BL/6) were
selected for in vivo analysis in the present study. The
results demonstrated that shSTAT5A combined with RT decreased tumor
volume and weight, as well as significantly suppressed tumor cell
proliferation in these mouse models, as determined by Ki67 IHC.
Moreover, in the C57BL/6 model, STAT5A knockdown sensitized tumors
to RT by decreasing IDO1 expression, reducing FOXP3 and recruiting
infiltrating CD8+ T cells that drive the antitumor
immune response. In the occurrence and development of tumors,
CD25+FOXP3+CD4+ Tregs can hinder
the effect of antitumor immunity by inducing CD8+ T cell
dysfunction and depletion (37).
Tregs in the TME have a stronger inhibitory effect than Tregs in
the spleen and peripheral blood (43). It has previously been reported that
2 Gy RT can increase the proportion of Tregs in the spleen.
Increasing the RT dose to 20 Gy can double the proportion of Tregs
infiltrating the tumors (44).
However, some studies have reported that Tregs show dose-dependent
apoptosis with an increase in irradiation dose, which downregulates
the expression levels of FOXP3, CD25 and CTLA-4, and which may
weaken their ability to inhibit the proliferation of
CD8+ T cells (45,46).
The present study demonstrated that the C57BL/6 mouse model of lung
cancer had some of the same characteristics as other solid tumors,
such as rich Treg infiltration. STAT5A knockdown with 8 Gy RT
reduced the infiltration of
CD25+FOXP3+CD4+ Tregs, thereby
enhancing the proportion of CD8+ T cells. Targeting the
expression of STAT5A/IDO1 may effectively prevent Treg infiltration
and provide a novel idea for inhibiting the RT-induced
immunosuppressive microenvironment.
In conclusion, the present study demonstrated that
IDO1 and STAT5A were highly expressed in cancer tissues. The
expression levels of both proteins were positively related to the
immune microenvironment. RT significantly promoted the expression
levels of STAT5A and IDO1 in NSCLC, and their activity supported
the apoptosis of T cells. In addition, the results confirmed that
STAT5A knockdown may promote activation of the antitumor T-cell
response by regulating IDO1 expression. In this context, a
regulatory mechanism of STAT5A/IDO1 in RT-mediated
immunosuppression was proposed, which may provide insight into the
development of more comprehensive immunotherapy regimens for
patients with NSCLC.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HG and ZD designed the experiments. YY, XZ, PN, DL
and QD collected the specimens and performed the experiments. YY,
XW, YW, YS, and KL analyzed the statistical data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Zhengzhou University (Zhengzhou, China;
NO.CUHCI2021-003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
FOXP3
|
forkhead box P3
|
|
IDO1
|
indoleamine-2,3-dioxygenase 1
|
|
IHC
|
immunohistochemistry
|
|
KYN
|
kynurenine
|
|
NSCLC
|
non-small cell lung cancer
|
|
RT
|
radiotherapy
|
|
SBRT
|
stereotactic radiotherapy
|
|
TME
|
tumor microenvironment
|
|
TRP
|
tryptophan
|
Acknowledgments
Not applicable.
Funding
This work was supported by The National Natural Science
Foundation of China (grant no. 81372436), The Scientific and
Technological Project in Henan Province (grant no. 222102310015),
and The Medical Science and Technology Project in Henan Province
(grant no. SBGJ202102056).
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Global Burden of Disease Cancer
Collaboration; Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T,
Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, et
al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2016:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 4:1553–688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Y, Peng X, Zhou Y, Xia K and Zhuang
W: Comparing the benefits of chemoradiotherapy and chemotherapy for
resectable stage III A/N2 non-small cell lung cancer: A
meta-analysis. World J Surg Oncol. 16:82018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reck M and Rabe KF: Precision diagnosis
and treatment for advanced non-small-cell lung cancer. N Engl J
Med. 377:849–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herrera FG, Bourhis J and Coukos G:
Radiotherapy combination opportunities leveraging immunity for the
next oncology practice. CA Cancer J Clin. 67:65–85. 2017.
View Article : Google Scholar
|
|
7
|
Chen Y, Gao M, Huang Z, Yu J and Meng X:
SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: A
focus on the mechanisms, advances, and future challenges. J Hematol
Oncol. 13:1052020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ngwa W, Irabor OC, Schoenfeld JD, Hesser
J, Demaria S and Formenti SC: Using immunotherapy to boost the
abscopal effect. Nat Rev Cancer. 18:313–322. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horn L, Mansfield AS, Szczęsna A, Havel L,
Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio
M, et al: First-line atezolizumab plus chemotherapy in
extensive-stage small-cell lung cancer. N Engl J Med.
379:2220–2229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brooks ED and Chang JY: Time to abandon
single-site irradiation for inducing abscopal effects. Nat Rev Clin
Oncol. 16:123–135. 2019. View Article : Google Scholar
|
|
11
|
Zheng X, Sun Y, Ye K, Fan C, Wang X, Yang
Y, Jiao R and Ge H: Stereotactic ablative radiotherapy as single
treatment for early stage non-small cell lung cancer: A single
institution analysis. Thoracic Cancer. 12:899–905. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng XL, Liu ML, Wang XH, Sun Y, Song S,
Yang Y, Jiao R, Ye K, Fan C and Ge H: Analysis of clinical outcomes
and prognostic factors in 109 patients with early-stage non-small
cell lung cancer treated with stereotactic ablation radiotherapy.
Chin J Radiat Oncol. 29:1031–1036. 2020.In Chinese.
|
|
13
|
Gettinger SN, Horn L, Gandhi L, Spigel DR,
Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman
DM, et al: Overall survival and long-term safety of nivolumab
(anti-programmed death 1 antibody, BMS-936558, ONO-4538) in
patients with previously treated advanced non-small-cell lung
cancer. J Clin Oncol. 33:2004–2012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Procureur A, Simonaggio A, Bibault JE,
Oudard S and Vano YA: Enhance the immune checkpoint inhibitors
efficacy with radiotherapy induced immunogenic cell death: A
comprehensive review and latest developments. Cancers (Basel).
13:6782021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Theelen WSME, Chen D, Verma V, Hobbs BP,
Peulen HMU, Aerts JGJV, Bahce I, Niemeijer ALN, Chang JY, de Groot
PM, et al: Pembrolizumab with or without radiotherapy for
metastatic non-small-cell lung cancer: A pooled analysis of two
randomised trials. Lancet Respir Med. 9:467–475. 2021. View Article : Google Scholar
|
|
16
|
Zhou QH, Han H, Lu JB, Liu TY, Huang KB,
Deng CZ, Li ZS, Chen JP, Yao K, Qin ZK, et al: Up-regulation of
indoleamine 2,3-dioxygenase 1 (IDO1) expression and catalytic
activity is associated with immunosuppression and poor prognosis in
penile squamous cell carcinoma patients. Cancer Commun (Lond).
40:3–15. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei L, Wu N, Wei F, Li F, Zhang Y, Liu J
and Ren X: Prognosis significance of indoleamine 2, 3-dioxygenase,
programmed death ligand-1 and tumor-infiltrating immune cells in
microenvironment of breast cancer. Int Immunopharmacol.
84:1065062020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brandacher G, Perathoner A, Ladurner R,
Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G,
Weiss HG, Göbel G, et al: Prognostic value of indoleamine
2,3-dioxygenase expression in colorectal cancer: Effect on
tumor-infiltrating T cells. Clin Cancer Res. 12:1144–1151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kiyozumi Y, Baba Y, Okadome K, Yagi T,
Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Komohara
Y and Baba H: IDO1 expression is associated with immune tolerance
and poor prognosis in patients with surgically resected esophageal
cancer. Ann Surg. 269:1101–1108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Halaby MJ, Hezaveh K, Lamorte S, Ciudad
MT, Kloetgen A, MacLeod BL, Guo M, Chakravarthy A, Medina TDS, Ugel
S, et al: GCN2 drives macrophage and MDSC function and
immunosuppression in the tumor microenvironment. Sci Immunol.
4:eaax81892019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu YY, Hu M, Xu QH, Sun X, Ye Y, Liu Y,
Feng J and Xu Y: The correlation between serum indoleamine
2,3-dioxygenase and the prognosis of stereotactic radiotherapy for
early non-small cell lung cancer. Chin J Radiol Med Prot.
40:512–518. 2020.In Chinese.
|
|
22
|
Jiao R, Zheng X, Sun Y, Feng Z, Song S and
Ge H: IDO1 expression increased after neoadjuvant therapy predicts
poor pathologic response and prognosis in esophageal squamous cell
carcinoma. Front Oncol. 10:10992020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Le Naour J, Galluzzi L, Zitvogel L,
Kroemer G and Vacchelli E: Trial watch: IDO inhibitors in cancer
therapy. Oncoimmunology. 9:17776252020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li A, Barsoumian HB, Schoenhals JE,
Caetano MS, Wang X, Menon H, Valdecanas DR, Niknam S, Younes AI,
Cortez MA and Welsh JW: IDO1 inhibition overcomes radiation-induced
'rebound immune suppression' by reducing numbers of IDO1-expressing
myeloid-derived suppressor cells in the tumor microenvironment. Int
J Radiat Oncol Biol Phys. 104:903–912. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grobben Y, de Man J, van Doornmalen AM,
Muller M, Willemsen-Seegers N, Vu-Pham D, Mulder WR, Prinsen MBW,
de Wit J, Sterrenburg JG, et al: Targeting indoleamine
2,3-dioxygenase in cancer models using the novel small molecule
inhibitor NTRC 3883-0. Front Immunol. 11:6094902021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kocher F, Amann A, Zimmer K, Geisler S,
Fuchs D, Pichler R, Wolf D, Kurz K, Seeber A and Pircher A: High
indoleamine-2,3-dioxygenase 1 (IDO) activity is linked to primary
resistance to immunotherapy in non-small cell lung cancer (NSCLC).
Transl Lung Cancer Res. 10:304–313. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maurer B, Kollmann S, Pickem J,
Hoelbl-Kovacic A and Sexl V: STAT5A and STAT5B-twins with different
personalities in hematopoiesis and leukemia. Cancers (Basel).
11:17262019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koptyra M, Gupta S, Talati P and
Nevalainen MT: Signal transducer and activator of transcription
5a/b: Biomarker and therapeutic target in prostate and breast
cancer. Int J Biochem Cell Biol. 43:1417–1421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brachet-Botineau M, Deynoux M, Vallet N,
Polomski M, Juen L, Hérault O, Mazurier F, Viaud-Massuard MC, Prié
G and Gouilleux F: A novel inhibitor of STAT5 signaling overcomes
chemotherapy resistance in myeloid leukemia. Cancers (Basel).
11:20432019. View Article : Google Scholar
|
|
30
|
Sánchez-Ceja SG, Reyes-Maldonado E,
Vázquez-Manríquez ME, López-Luna JJ, Belmont A and
Gutiérrez-Castellanos S: Differential expression of STAT5 and
Bcl-xL, and high expression of Neu and STAT3 in non-small-cell lung
carcinoma. Lung Cancer. 54:163–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maranto C, Udhane V, Hoang DT, Gu L,
Alexeev V, Malas K, Cardenas K, Brody JR, Rodeck U, Bergom C, et
al: STAT5A/B blockade sensitizes prostate cancer to radiation
through inhibition of RAD51 and DNA repair. Clin Cancer Res.
24:1917–1931. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang JY, Senan S, Paul MA, Mehran RJ,
Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L, et al:
Stereotactic ablative radiotherapy versus lobectomy for operable
stage I non-small-cell lung cancer: A pooled analysis of two
randomised trials. Lancet Oncol. 16:630–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
34
|
Low HY, Lee YC, Lee YJ, Wang HL, Chen YI,
Chien PJ, Li ST and Chang WW: Reciprocal regulation between
indoleamine 2,3-dioxigenase 1 and notch1 involved in radiation
response of cervical cancer stem cells. Cancers (Basel).
12:15472020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lou Q, Liu R, Yang X, Li W, Huang L, Wei
L, Tan H, Xiang N, Chan K, Chen J and Liu H: miR-448 targets IDO1
and regulates CD8+ T cell response in human colon
cancer. J Immunother Cancer. 7:2102019. View Article : Google Scholar
|
|
36
|
Palma DA, Olson R, Harrow S, Gaede S,
Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP,
et al: Stereotactic ablative radiotherapy versus standard of care
palliative treatment in patients with oligometastatic cancers
(SABR-COMET): A randomised, phase 2, open-label trial. Lancet.
393:2051–2058. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Crittenden M, Kohrt H, Levy R, Jones J,
Camphausen K, Dicker A, Demaria S and Formenti S: Current clinical
trials testing combinations of immunotherapy and radiation. Semin
Radiat Oncol. 25:54–64. 2015. View Article : Google Scholar :
|
|
38
|
Maleki Vareki S, Rytelewski M, Figueredo
R, Chen D, Ferguson PJ, Vincent M, Min W, Zheng X and Koropatnick
J: Indoleamine 2,3-dioxygenase mediates immune-independent human
tumor cell resistance to olaparib, gamma radiation, and cisplatin.
Oncotarget. 5:2778–2791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thaker AI, Rao MS, Bishnupuri KS, Kerr TA,
Foster L, Marinshaw JM, Newberry RD, Stenson WF and Ciorba MA: IDO1
metabolites activate β-catenin signaling to promote cancer cell
proliferation and colon tumorigenesis in mice. Gastroenterology.
145:416–425. e1–e4. 2013. View Article : Google Scholar
|
|
40
|
Chen B, Alvarado DM, Iticovici M, Kau NS,
Park H, Parikh PJ, Thotala D and Ciorba MA: Interferon-induced IDO1
mediates radiation resistance and is a therapeutic target in
colorectal cancer. Cancer Immunol Res. 8:451–464. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang LT, Chiou SS, Chai CY, His E,
Yokoyama KK, Wang SN, Huang SK and Hsu SH: Intestine-specific
homeobox gene ISX integrates IL6 signaling, tryptophan catabolism,
and immune suppression. Cancer Res. 77:4065–4077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maj T, Wang W, Crespo J, Zhang H, Wang W,
Wei S, Zhao L, Vatan L, Shao I, Szeliga W, et al: Oxidative stress
controls regulatory T cell apoptosis and suppressor activity and
PD-L1-blockade resistance in tumor. Nat Immunol. 18:1332–1341.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maruyama T, Li J, Vaque JP, Konkel JE,
Wang W, Zhang B, Zhang P, Zamarron BF, Yu D, Wu Y, et al: Control
of the differentiation of regulatory T cells and T(H)17 cells by
the DNA-binding inhibitor Id3. Nat Immunol. 12:86–95. 2011.
View Article : Google Scholar
|
|
44
|
Zhang T, Yu H, Ni C, Zhang T, Liu L, Lv Q,
Zhang Z, Wang Z, Wu D, Wu P, et al: Hypofractionated stereotactic
radiation therapy activates the peripheral immune response in
operable stage I non-small-cell lung cancer. Sci Rep. 7:48662017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cao M, Cabrera R, Xu Y, Liu C and Nelson
D: Gamma irradiation alters the phenotype and function of CD4+CD25+
regulatory T cells. Cell Biol Int. 33:565–571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mortezaee K, Parwaie W, Motevaseli E,
Mirtavoos-Mahyari H, Musa AE, Shabeeb D, Esmaely F, Najafi M and
Farhood B: Targets for improving tumor response to radiotherapy.
Int Immunopharmacol. 76:1058472019. View Article : Google Scholar : PubMed/NCBI
|