Introduction
Multiple sclerosis (MS) is a chronic autoimmune
disease of the central nervous system (CNS), characterized by
recurrent episodes of inflammatory demyelination resulting in
damage of axons present in the brain, optic nerve, and spinal cord
(1,2). There are four types of MS: Clinically
isolated syndrome, relapsing-remitting, secondary progressive, and
primary progressive (1). A recent
disease burden study in Egypt published in 2019, estimated an
average of 59,671 patients nationwide (3).
Disease pathogenesis is known to be initiated
through the activation of peripheral B and T-cells towards
self-antigens resulting in damage to the myelin sheath and nerve
block (4). One of the main key
players in disease activity is cytotoxic T-cells as they are found
to be abundant in MS lesions compared to other subsets of immune
cells (5). CD8+ T-cells
are known for their killing ability as they produce serine protease
granzyme B, responsible for apoptosis in target cells due to loss
of cellular integrity (6). This is
complemented by the presence of perforin pores facilitating the
exit of granzyme B from CD8+ T-cells and its attack on
target cells (7). Moreover,
CD8+ T-cells express surface receptors such as
intracellular adhesion molecule-1 (ICAM1) and integrin subunit β2
(ITGB2/CD18). ICAM1 and ITGB2 provide the secondary signal needed
for cellular activation following antigen presentation along with
their role in migration through the blood-brain barrier (BBB)
(8).
Unfortunately, current immunomodulatory approaches
have severe side effects and complications for the patients, since
they become more prone to infections due to immune response
suppression (9). To overcome the
limitations, modern approaches need to target specifically
excessive immune responses against self-antigens in autoimmune
diseases such as MS by the administration of self-antigens in high
doses (10).
A promising therapeutic approach is personalized
therapy, that could be achieved through the use of RNA
interference, which involves gene silencing at the messenger RNA
(mRNA) level mediated by small complementary non-coding RNA species
such as small interfering RNAs (siRNAs) or microRNAs (miRNAs or
miRs) (11). Upon investigating
promising epigenetics in MS pathogenesis, miR-155 was identified to
be a favorable therapeutic target as it was reported to be
upregulated in peripheral blood mononuclear cells (PBMCs), the
spinal cord, and white matter lesions of patients with MS compared
to healthy controls (12-14).
Moreover, miR-155 was reported to regulate immune cell activity of
innate and adaptive immunity (15).
However, miR-155 was revealed to be downregulated in the serum
samples of patients with MS in remission compared to patients with
post-acute attack MS and upregulated in the PBMCs of patients with
MS in remission compared to patients with relapsed MS and healthy
controls (16,17). This raises the question as to the
role of miR-155 in regulating CD8+ T-cell activity in MS
pathogenesis of patients with RRMS. Previous research indicated
that a deficiency of miR-155 caused decreased CD8+
T-cell responses, whereas miR-155 overexpression increased
CD8+ T-cell responses during inflammation (18). Moreover, CD8+ T-cells
lacking miR-155 exhibited reduced frequency of interferon (IFN)-γ
production, reduced ability to lyse targets, reduced
antigen-specific CD8+ T-cells in cases of viral
infection and impaired primary response, hence, the decreased viral
clearance (15,19). The aim of the present study was to
investigate the role of miR-155 on CD8+ T-cell activity
through the monitoring of ICAM1 and ITGB2 levels reflecting
migration and activation, along with perforin and granzyme B levels
reflecting cytolytic activity on oligodendrocytes.
Materials and methods
Sample collection
Blood samples were collected from 25 patients with
RRMS and 10 healthy controls, according to the inclusion and
exclusion criteria. Patients diagnosed with RRMS, without treatment
with steroids in the past 3 months, were included in the present
study. Patients were recruited from May 2019 to May 2020. The mean
age of patients was 39.12 years with an age range of 28-55 years,
while the mean age of controls was 30.3 years with an age range of
24-50 years. All subjects involved provided their written informed
consent, and the Ethics Review Committee of the German University
in Cairo (Cairo, Egypt) approved the study (approval no.
PTX-2018-11-HET). The study followed the ethical guidelines of the
1975 Declaration of Helsinki. PBMCs were isolated from whole blood
using Ficoll density gradient technique. All samples were stored at
-80˚C until further use. The clinical characteristics of patients
and controls are presented in Tables
I, SI and SII.
 | Table ICharacteristics of patients and
healthy controls. |
Table I
Characteristics of patients and
healthy controls.
| A, Patients
(n=25) | Percentage (%) |
|---|
| Sex | |
|
Female
(17/25) | 68 |
|
Male
(8/25) | 32 |
| Age | |
|
<50 years
old (22/25) | 88 |
|
≥50 years
old (3/25) | 12 |
| Family history | |
|
Positive
family history (1/25) | 4 |
|
Negative
family history (24/25) | 96 |
| Type | |
|
RRMS
(15/25) | 100 |
|
PRMS
(0/25) | 0 |
|
PPMS
(0/25) | 0 |
|
SPMS
(0/25) | 0 |
| CSF findings | |
|
+ve
Oligoclonal antibodies (25/25) | 100 |
|
Protein
(0/25) | 0 |
| Treatment | |
|
Untreated
(naïve) (3/25) | 12 |
|
DMT-treated
(22/25) | 88 |
|
IFNβ-1a
(6/25) | 24 |
|
IFNβ-1b
(6/25) | 24 |
|
Fingolimod
(10/25) | 40 |
| B, Controls
(n=10) | Percentage (%) |
| Sex | |
|
Female
(7/10) | 70 |
|
Male
(3/10) | 30 |
| Age | |
|
<50 years
old (9/10) | 90 |
|
≥50 years
old (1/10) | 10 |
Ficoll density gradient technique
PBMCs were isolated using Ficoll (Greiner Bio-One
International GmbH), as per the manufacturer's instructions.
Harvested cells were washed twice in Roswell Park Memorial
Institute Medium-1640 (RPMI-1640; cat. no. SR263-10L; Serox GmbH)
supplemented with L-glutamine, phenol red, 10% fetal bovine serum
(FBS; cat. no. 10270098) and 1% penicillin/streptomycin (cat. no.
15140122; both from Applied Biosystems; Thermo Fisher Scientific,
Inc.), and viable cells were counted using a hemocytometer. Cells
were frozen at -80˚C at a density of 107 cells/ml in 50%
v/v supplemented media, 40% v/v FBS and 10% v/v dimethyl sulfoxide
(DMSO; cat. no. D12345; Applied Biosystems; Thermo Fisher
Scientific, Inc.) for later use. Samples were stored at -80˚C for a
maximum of 6 months and after thawing, viability was verified using
0.4% Trypan blue (cat. no. 15250061; Thermo Fisher Scientific,
Inc.) with an acceptable viability of >80%.
Isolation of CD8+ T-cells
by negative depletion using magnetic nanobeads
Frozen PBMCs were thawed at 37˚C and transferred to
10 ml of supplemented media and centrifuged at 300 x g for 5 min at
room temperature. Cells were isolated to obtain CD8+
T-cells by negative depletion using MojoSort™ Human
CD8+ T-cell Isolation Kit (cat. no 480012), MojoSort
Buffer (cat. no 480017) and MojoSort Magnet (cat. no 480019; all
Biolegend, Inc.) as per manufacturer's instructions. Collected pure
CD8+ T-cells were centrifuged (at 300 x g for 5 min at
room temperature) and re-suspended in culture media.
Flow cytometry
Confirmation of CD8+ T-cell isolation was
performed using flow cytometry on the isolated population, and
CD8-PE antibody (product no. IM0452U; Beckman Coulter, Inc.) for 30
min at room temperature, followed by a washing step and
acquisition. Samples were analyzed by flow cytometry (CytoFLEX
benchtop flow cytometer; Beckman Coulter Inc.) gating for the
CD8-PE-positive population. Fluorescence data were acquired and
analyzed using the CytExpert software (version 2.3.3.84; Beckman
Coulter Inc.) to determine the purity of the sample, as shown in
Fig. S1 and previously described
(20). With regard to the isolation
process and the size scatter of the resultant populations, of note,
a small percentage of cells (30%), were remaining monocytes and
other T-cells that were not completely depleted.
Cell culture
Isolated CD8+ T-cells were incubated in
supplemented media at 37˚C with an atmosphere of 5% CO2
and 95% humidity. The cultured cells were then screened for
miR-155, ICAM1, ITGB2, perforin, and granzyme B expression.
Transfection
Before transfection, seeding of 4-7x104
isolated CD8+ T-cells per well of a 96-well plate was
performed. The cells were incubated under normal growth conditions
(37˚C and 5% CO2). Isolated CD8+ T-cells were
transfected for 5-10 min at room temperature, with mimics of
miR-155 (syn-hsa-miR-155-5p miScript miRNA mimic; cat. no.
MSY0000646) and antagomirs of miR-155 (anti-hsa-miR-155-5p miScript
miRNA inhibitor; cat. no. MIN0000646), along with both siRNAs of
ICAM1 (Hs_ICAM1_3 FlexiTube siRNA; cat. no. SI00004347) and ITGB2
(Hs_ITGB2_3 FlexiTube siRNA; cat. no. SI00004571; all from Qiagen
GmbH), in addition to a negative control. The mass of miR-155
mimics and antagomirs, as well as all siRNAs including all negative
controls was 250 ng. The negative controls for miRNA mimics and
antagomirs were purchased from Invitrogen; Thermo Fisher
Scientific, Inc. (cat. nos. AM17110 and AM17010, respectively) and
transfected similar to miR-155 mimics and antagomirs. The negative
control for siRNA was purchased from Qiagen GmbH (cat. no. 1022076)
and was transfected similarly to ICAM1 and ITGB2 siRNA. All
transfection experiments were performed in triplicate using
HiPerfect Transfection Reagent (cat. no. 301704; Qiagen GmbH)
according to the manufacturer's instructions, and experiments were
repeated three times. Cells exposed to transfection reagent only
were designated as mock cells, cells transfected with miR-155
mimics and antagomirs were designated as mimics and antagomirs,
respectively, and cells transfected with ICAM1 and ITGB2 siRNA were
designated as siICAM1 and siITGB2 cells. Negative controls
transfected with pre-miR negative control, anti-miR negative
control and negative control siRNA were designated as pre-miR NC,
anti-miR NC and siRNA NC, respectively. siRNA NC was not utilized
in silencing experiments as it is widely interchanged with
pre-miRNA negative controls (as they have the same makeup), hence
the data obtained from the pre-miRNA were proof enough. This was
followed by RNA extraction, screening for miR-155, ICAM1, ITGB2,
perforin, and granzyme B expression, and finally, comparison to
CD8+ T-cell mock cells, 48 h after transfection.
RNA isolation
RNA was isolated from cultured CD8+
T-cells using RNeasy Minikit (cat. no. 74104; Qiagen GmBH) as per
the extraction protocol. RNA was stored at -80˚C until further use.
RNA concentration was calculated using Nanodrop and RNA purity was
evaluated using A260/280 with an acceptable range of 1.9-2.2. Total
RNA used per sample was 30-50 ng.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA extracted was reverse-transcribed into
single-stranded cDNA using the high-capacity cDNA reverse
transcription kit (cat. no. 4368814; Applied Biosystems; Thermo
Fisher Scientific, Inc.). The relative expression of ICAM1, ITGB2,
perforin and granzyme B, with β-actin (as a housekeeping gene for
normalization), along with miR-155 and RNU6 (as a housekeeping gene
for normalization) was quantified and amplified using TaqMan
RT-quantitative polymerase chain reaction (qPCR; Assay IDs:
Hs00164932_m1, Hs00164957_m1, Hs00169473_m1, Hs00188051_m, and
Hs99999903_m1 respectively for genes of interest along with 002623
and 001093 for miR-155 and RNU6, respectively; Applied Biosystems;
Thermo Fisher Scientific, Inc.) on a StepOne™ Real-Time PCR
instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.).
For every sample, a reaction mix was prepared according to the
manufacturer's instructions, and 4 µl of the respective cDNA was
added. The RT-qPCR run was performed in the standard mode,
consisting of two stages: A first 10-min stage at 95˚C where the
Taq-polymerase enzyme was activated, followed by a second stage of
40 amplification cycles (15 sec at 95˚C and 60 sec at 60˚C). qPCR
runs with negative controls as undetermined were taken into
account, relative expression was calculated using the
2-ΔΔCq method (21). All
PCR reactions including controls were run in triplicate.
Statistical analysis
All data were expressed in relative quantitation
(RQ). One Way ANOVA was employed, followed by Dunnett's multiple
comparison test to compare the basal expression of two different
studied groups. Unpaired t-test was used to compare the effect of
manipulations within each group (compared to mock). Data were
expressed as the mean ± standard error of the mean (SEM).
Correlation analyses were performed using Spearman's correlation
coefficient, denoted by a rho value, indicating that when the
strength of the correlation approaches 1, the degree of correlation
increases. Analysis was performed using GraphPad Prism 6.0 software
(GraphPad Software, Inc.). All experiments were performed in
triplicate. P<0.05 was considered to indicate a statistically
significant difference.
Bioinformatics analysis
Target prediction was performed using Tools for miRs
(https://tools4mirs.org/software/target_prediction/),
which included the algorithm Probability of Interaction by Target
Accessibility (PITA), and TargetSpy (http://webclu.bio.wzw.tum.de/targetspy/index.php?search=true).
Hits found between miR-155 and genes of interest are reported in
Table SIII.
Results
Effect of miR-155 overexpression and
knockdown on the mRNA expression of ICAM1, ITGB2, perforin and
granzyme B in cytotoxic T-cells of patients with RRMS
First, to understand the relationship between
miR-155 and the genes of interest, bioinformatics studies were
performed and interactions between miR-155 and genes of interest
were found and reported in Table
SIII. The expression profile of miR-155, ICAM1, ITGB2, perforin
and granzyme B in cytotoxic T-cells isolated from different
treatment groups of patients with RRMS is presented in a previous
study (20). Subsequently, the
effect of miR-155 on the expression of ICAM1, ITGB2, perforin and
granzyme B was studied through the overexpression and knockdown of
miR-155 ex vivo. Efficient overexpression of miR-155 was
confirmed in cultured cells as shown in Fig. S2 (P=0.0008). As a result of miR-155
overexpression using mimics, a significant downregulation of ICAM1
mRNA was observed in healthy controls, and all patients with RRMS,
treated with fingolimod, IFNβ-1a, and IFNβ-1b (P=0.0048; P=0.0161;
P=0.0097; and P=0.0248; respectively) compared to mock. However,
cells from naïve RRMS patients exhibited a significant increase in
ICAM1 mRNA following miR-155 overexpression (P=0.0073) compared to
the mock group. Of note, anti-miR-155 produced no significant
changes except in IFNβ-1a-treated patients, with anti-miR-155
exhibiting similar effects to mimics (Fig. 1A). Moreover, miR-155
mimic-transfection resulted in significant consistent
downregulation of ITGB2 mRNA in healthy controls and all patients
with RRMS, including naïve-, fingolimod-, IFNβ-1a-, and
IFNβ-1b-treated patients (P=0.0133; P=0.04011; P=0,0250; P=0.0224;
and P=0.0214; respectively), compared to the mock group.
Conversely, anti-miR-155 caused no significant changes except for
healthy controls and RRMS-naïve patients, where anti-miR-155
exhibited similar effects to mimics (Fig. 1B). In addition, miR-155
mimic-transfection induced a downregulation in perforin mRNA levels
in healthy controls and RRMS naïve-, fingolimod-, and
IFNβ-1a-treated patients (P=0.0137; P=0.0153; P=0.0206; and
P=0.0001) with an unexpected upregulation the the mRNA levels of
perforin in IFNβ-1b-treated patients (P=0.0340) compared to the
mock group. Anti-miR-155 caused no significant changes, except for
fingolimod-treated patients where a significant opposite effect to
mimics was observed and in IFNβ-1b patients, where anti-miR-155
exhibited a similar effect to mimics (Fig. 1C). Finally, overexpression of miR-155
caused a significant decrease in granzyme B mRNA in healthy
controls and all patients with RRMS, treated with fingolimod,
IFNβ-1a and IFNβ-1b (P=0.0345; P=0.0118; P=0.0334; and P=0.0397;
respectively) except for naïve patients with RRMS, where a
significant increase in granzyme B expression was observed
following miR-155-mimic transfection (P=0.0405) (Fig. 1D).
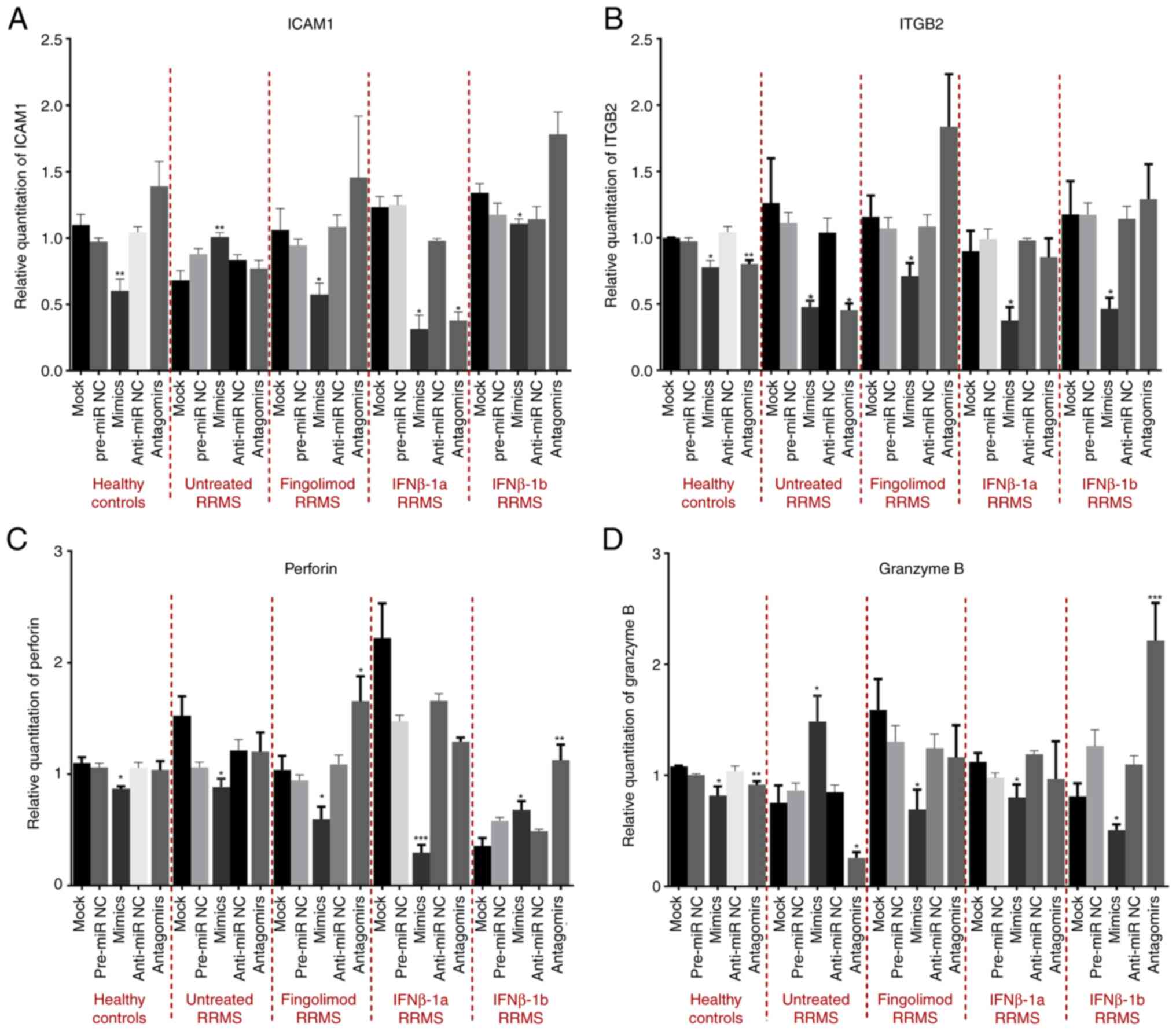 | Figure 1Effect of miR-155 overexpression and
knockdown on the mRNA levels of ICAM1, ITGB2, perforin, and
granzyme B in CD8+ T-cells isolated from different
treatment groups of patients with relapsing-remitting multiple
sclerosis and healthy controls. (A-D) Effect of miR-155
overexpression and knockdown on the mRNA levels of (A) ICAM1, (B)
ITGB2, (C) perforin and (D) granzyme B compared to the mock group.
*P<0.05, **P<0.01 and
***P<0.001. Data are presented as the mean ± standard
error of the mean. miR-155, microRNA-155; ICAM1, intracellular
adhesion molecule-1; ITGB2, integrin subunit β2; RRMS,
relapsing-remitting multiple sclerosis; NC, negative control. |
Effect of ICAM1 and ITGB2 knockdown on
the expression levels of miR-155, perforin and granzyme B
Secondly, to understand the relationship between
ICAM1, ITGB2 with miR-155, perforin, and granzyme B, the effect of
ICAM1 and ITGB2 knockdown on the expression of miR-155, perforin
and granzyme B was investigated. Efficient knockdown of ICAM1 and
ITGB2 was confirmed as shown in Fig.
S3 (P=0.0367 and P=0.0105, respectively). Silencing of ICAM1
resulted in a significant downregulation of miR-155 expression in
healthy controls and all patients with RRMS, including naïve,
fingolimod, IFNβ-1a and IFNβ-1b (P=0.0013; P=0.0142; P=0.0243;
P=0.0143; and P=0.0405; respectively) treatment groups compared to
the mock group. Moreover, knockdown of ITGB2 caused a significant
downregulation in miR-155 expression in cells isolated from healthy
controls and all patients with RRMS, including naïve, fingolimod,
IFNβ-1a and IFNβ-1b treatment groups (P=0.0053; P=0.0001; P=0.0458;
P=0.0074; and P=0.0425; respectively) compared to the mock group
(Fig. 2A). Investigation of the
effect of ICAM1 silencing on ITGB2 revealed a significant
downregulation of ITGB2 in healthy controls, naïve and IFNβ-1b
patients with RRMS (P=0.0015; P=0.0201; and P=0.0494; respectively)
compared to the mock group. By contrast, a significant increase was
observed in the fingolimod-treated RRMS patients (P=0.0019) with a
non-significant decrease in IFNβ-1a-treated RRMS patients compared
to the mock group (Fig. 2B). In
addition, the effect of ICAM1 and ITGB2 knockdown on perforin mRNA
was investigated. ICAM1 knockdown caused no significant change in
the mRNA levels of perforin in healthy controls, however, it did
induce a significant increase in IFNβ-1b-treated RRMS patients
(P=0.0006), and a significant decrease in the naïve, fingolimod and
IFNβ-1a treatment groups (P=0.0033; P=0.0422; and P=0.0067;
respectively) compared to the mock group. Furthermore, ITGB2
knockdown exerted a significant decrease in perforin mRNA levels in
healthy controls and naïve patients with RRMS, as well as groups
treated with fingolimod and IFNβ-1a (P=0.0311; P=0.0022; P=0.0125;
and P=0.0019; respectively), and a significant increase in
IFNβ-1b-treated patients with RRMS (P=0.0352) compared to the mock
group (Fig. 2C). With regard to the
effect of ICAM1 silencing on granzyme B mRNA levels, no significant
change in healthy controls, naïve and IFNβ-1b-treated RRMS patients
was observed, while a significant decrease was observed in the
groups treated with fingolimod and IFNβ-1a (P=0.0057 and P=0.0075,
respectively) compared to the mock group. Furthermore, silencing of
ITGB2 resulted in a decrease in granzyme B mRNA levels in healthy
controls, a non-significant change in naïve and fingolimod-treated
RRMS patients and a significant increase in RRMS patients treated
with IFNβ-1a and IFNβ-1b (P=0.0213 and P<0.0001, respectively)
compared to the mock group (Fig.
2D).
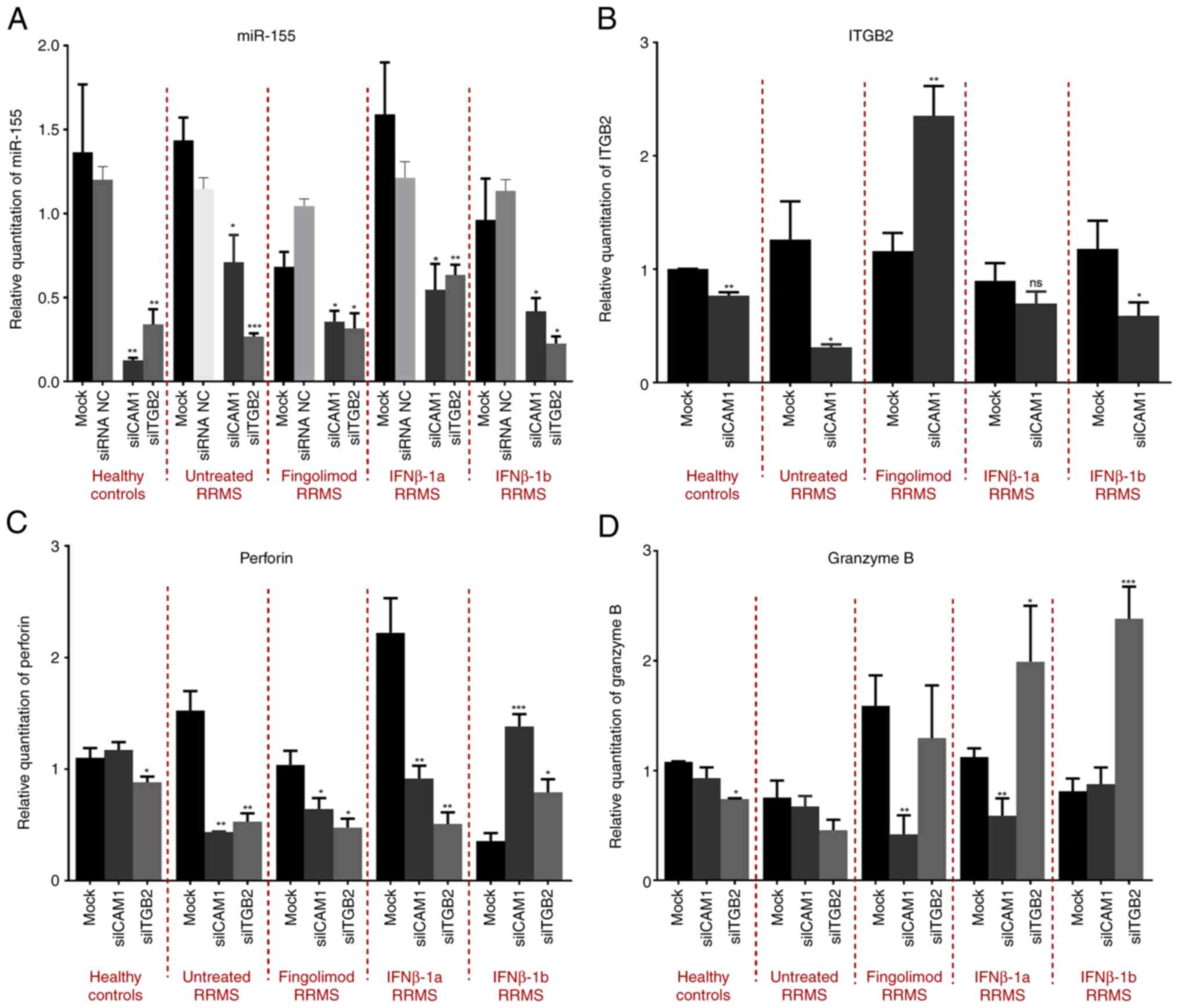 | Figure 2Effect of ICAM1 and ITGB2 knockdown
on the mRNA levels of miR-155, ITGB2, perforin, and granzyme in
CD8+ T-cells isolated from different treatment groups of
patients with relapsing-remitting multiple sclerosis and healthy
controls. (A-D) Effect of ICAM1 and ITGB2 knockdown on the mRNA
levels of (A) miR-155, (B) ITGB2, (C) perforin and (D) granzyme B
compared to the mock groups. *P<0.05,
**P<0.01 and ***P<0.001. Data are
presented as the mean ± standard error of the mean. ICAM1,
intracellular adhesion molecule-1; ITGB2, integrin subunit β2;
miR-155, microRNA-155; siRNA or si, small interfering RNA; NC,
negative control; RRMS, relapsing-remitting multiple sclerosis; ns,
not significant. |
Correlation analysis between the
effect of miR-155 overexpression on target genes and the expanded
disability status scale (EDSS) score of patients
In a previous study, correlation analyses revealed a
positive correlation between miR-155 and ITGB2 with the EDSS of
patients and a negative correlation between ICAM1, perforin, and
granzyme B with the EDSS (20).
Moreover, the correlation between miR-155 and genes of interest
ex vivo showed a consistent negative correlation between
miR-155 and genes of interest in patients with RRMS (20). Finally, to investigate whether the
clinical score of a patient could affect the manipulation outcome,
correlation analysis was performed between the fold change (RQ) in
the genes of interest following miR-155 overexpression and the EDSS
of patients, using Spearman's correlation coefficient. The analysis
revealed a consistent positive correlation between the effect of
miR-155 on ICAM1, ITGB2, perforin and granzyme B with the EDSS of
patients (P=0.0082; P=0.0188; P=0.0003; and P=0.0045; respectively
and rho=0.5738; rho=0.4489; rho=0.6586; and r=0.4697,
respectively), raising the question as to whether patients with
high clinical scores may be responding differently to treatments
than patients with low EDSS scores (Fig.
3).
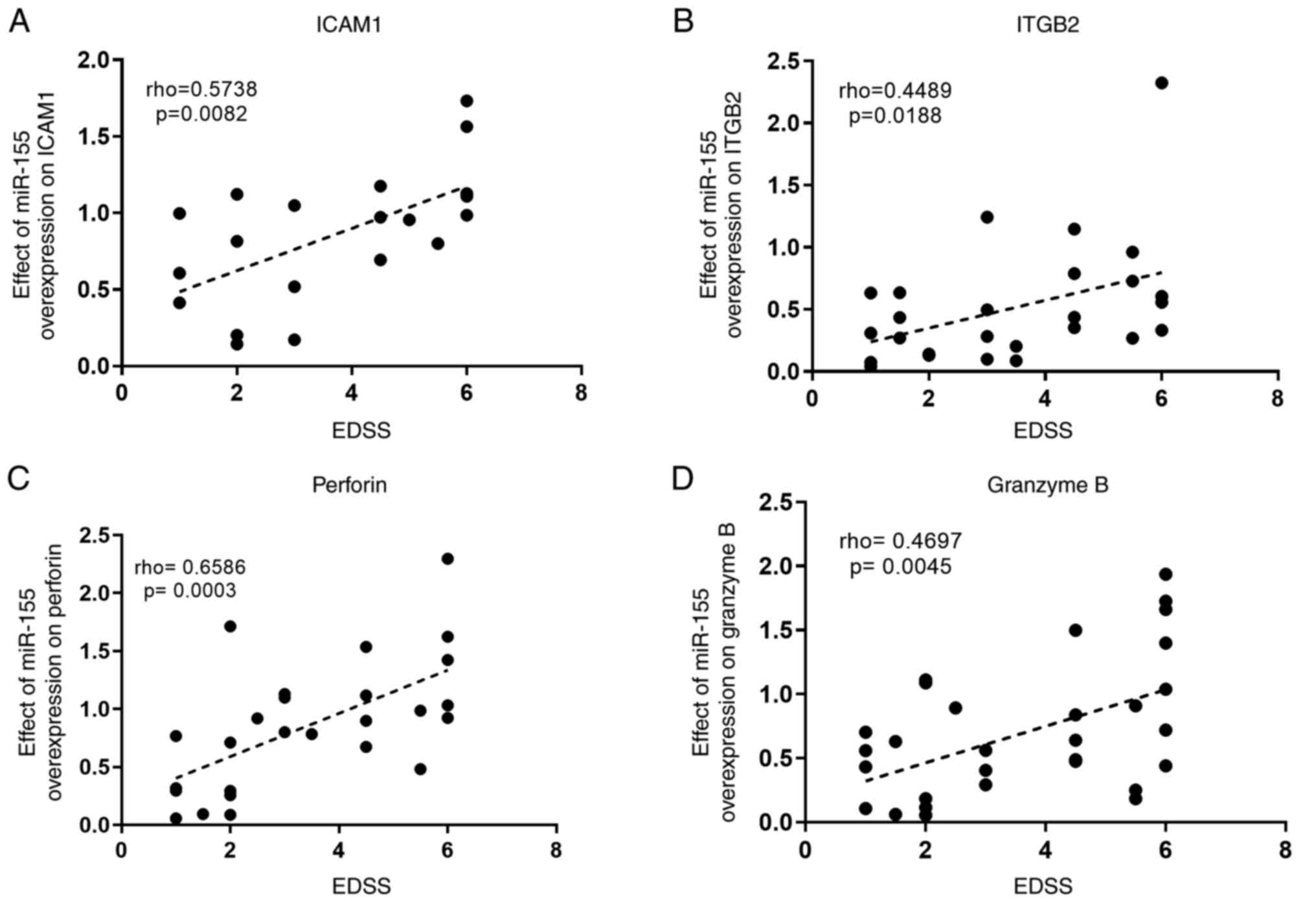 | Figure 3Correlation analysis between the
relative expression of target genes following miR-155
overexpression, normalized to the mock groups, and the EDSS score
of patients, determined using Spearman's correlation coefficient.
(A-D) Correlation between the effect of miR-155 overexpression on
(A) ICAM1, (B) ITGB2, (C) perforin and (D) granzyme B expression,
as the RQ values of each gene normalized to the mock groups in
CD8+ T-cells isolated from patients with
relapsing-remitting multiple sclerosis, and the EDSS score of
patients. Correlation analysis was performed using Spearman's
correlation coefficient, and the rho values and P-values are
presented in each graph. miR-155, microRNA-155; ICAM1,
intracellular adhesion molecule-1; ITGB2, integrin subunit β2;
EDSS, Expanded Disability Status Scale. |
Discussion
MS is a chronic neuroinflammatory disease and
considered one of the leading causes of disability worldwide. Due
to the heterogeneity of the disease, an optimized targeted
therapeutic approach is required to achieve efficient treatments
for the diverse subpopulations of the disease. Molecular proteins
of interest to regulate are CD8+ T-cell surface
receptors, ICAM1 and ITGB2, along with cytotoxic proteins produced
by the cells, perforin and granzyme B (7,22).
Accumulating evidence has demonstrated non-coding RNAs as pivotal
tools in targeting the molecular make-up of MS pathogenesis and
miR-155 has multiple roles in innate and adaptive immunity
(15,23,24). Its
role in carcinogenesis has been studied previously in various
cancers such as hepatocellular carcinoma (HCC), and its
immunomodulatory role in regulating the programmed cell death
protein 1 (PD-1), programmed death ligand 1 (PDL-1) pathway has
been highlighted (25,26). Specific upregulation of miRNA-155 is
witnessed in various immunopathologic conditions including MS
(27), rheumatoid arthritis
(28), and systemic lupus
erythematosus (29,30) where it affects both T lymphocyte and
blood-brain barrier functions (31).
Prior to investigating the role of miR-155 in the
regulation of crucial proteins for CD8+ T-cells, a
screening step for the basal expression levels of miR-155, ICAM1,
ITGB2, perforin, and granzyme B in CD8+ T-cells was
performed to identify the endogenous levels of these genes.
Significant downregulation of miR-155 was observed in
CD8+ T-cells of patients with RRMS (20). The downregulation in miR-155 in
isolated CD8+ T-cells coincides with previous studies
reporting variation in miRNA expression in CD8+ T-cells
during the differentiation process and an inverse correlation with
activation status (32-34).
Moreover, upregulation of ICAM1, ITGB2, perforin, and granzyme B
was observed in all patients with RRMS compared to healthy controls
(20). This upregulation of ICAM1
and ITGB2 [β sub-unit of lymphocyte function-associated antigen 1
(LFA-1)] is consistent with previous results showing overexpression
of ICAM1 and LFA-1 on mononuclear cells from the blood of patients
with RRMS compared to controls (35). Another interesting study by Fujii
et al studied the levels of cytotoxic proteins perforin and
granzyme B in patients administered fingolimod and found a
significant increase in perforin and granzyme B expression in both
relapse-free and relapsing patients with higher overexpression in
the latter compared to the healthy controls (36). To the best of our knowledge, the
present study is the first to investigate the interplay of the
aforementioned genes of interest on CD8+ T-cells of MS
patients.
The role of miR-155 on CD8+ T-cell
auto-activity and cytotoxicity was studied by ex vivo
overexpression and knockdown of miR-155 in isolated CD8+
T-cells of patients with RRMS of different treatments representing
the effect of the epigenetic manipulation on the four target genes
in each subtype of patients with RRMS. miR-155 mimic transfection
induced a downregulation in the mRNA of ICAM1 in all subtypes
except naïve RRMS patients (Fig. 1A)
and a downregulation in the mRNA levels of ITGB2 in all subtypes of
RRMS (Fig. 1B). The inconsistency in
the effect of miR-155 on ICAM1 could be an indirect mechanism of
the effect of miR-155 on leukocyte adhesion other than regulating
gene expression of adhesion molecules as stated previously by
Cerutti et al (37).
Moreover, miR-155 mimic transfection caused a significant
downregulation in the mRNA expression of perforin in all groups
except for IFNβ-1b-treated RRMS patients (Fig. 1C) and a downregulation in the mRNA
expression of granzyme B in all groups except for untreated
patients with RRMS (Fig. 1D). An
inconsistent pattern in the effect of miR-155 on pro-inflammatory
mediators was observed in previous studies on CD8+
T-cells in viral infection settings, where miR-155 knockdown
decreased IFN-γ production and had no effect on granzyme B and
TNF-α levels (19). Nevertheless,
another study revealed a significant decrease in IFN-γ and granzyme
B levels in CD8+ T-cells in miR-155-knockdown mice
following viral infection induction (38). Building on the complex effects of
miR-155, miR-155 knockdown in an RA mouse model had no effect on
the levels of IFN-γ following induction of disease in those mice
(39). Another study investigating
the role of miR-155 in PBMCs isolated from juvenile systemic lupus
erythematosus (SLE) patients reported an anti-inflammatory response
as the upregulation of miR-155 relieved the immune modulator IL-2
from the inhibitory effect of PP2A (40). These conflicting results give rise to
the hypothesis that miR-155 has a diverse, non-specific role in
regulating CD8+ T-cell immune response depending on the
differentiation stage of the cell.
Furthermore, the role of ICAM1 and ITGB2 in the
regulation of cytolytic proteins perforin and granzyme B as well as
miR-155, was investigated. The silencing of ICAM1 and ITGB2 induced
significant downregulation of miR-155 compared to the mock group in
all patients with RRMS and healthy controls (Fig. 2A). Additionally, ICAM1 silencing
caused an inconsistent downregulation of ITGB2, perforin and
granzyme B (Fig. 2B-D). Moreover,
ITGB2 silencing induced an inconsistent downregulation of ICAM1 and
a consistent downregulation of perforin (Fig. 2C). The inconsistent increase in
perforin mRNA levels in IFNβ-1b-treated patients following all
manipulations could be due to the increase in the number of
perforin-dependent CD8+ T-cells in this subtype. A
previous study investigating the effects of β integrins on other
adhesion molecules revealed that stimulation of β1 integrin by
cross-linking or ligation with matrix proteins reduced ICAM1
expression in lung cancer cell lines (41). If the same relationship applies
herein, then the increase in the expression of ICAM1 or ITGB2
following the silencing of either, is expected. However, immune
cells rather than cancer cells are in question hence, this could
explain the different results.
As aforementioned, ICAM1 expression on
antigen-presenting cells or T lymphocytes is crucial for
antigen-specific interactions leading to CD8+ T-cell
activation, proliferation, and differentiation into effector
T-cells (42). The results of ICAM1
silencing are consistent with previous studies indicating that
ICAM-1 expression is critical on T-cells and other cell types for
the development of demyelinating disease (43). Additionally, the previous deletion of
Mac-1 (CD11b/CD18) resulted in profound protection in both active
and adoptive-transferred EAE, indicating that Mac-1 (partially
CD18) expression is critical not only to phagocytic cells but also
to T-cells for the development of demyelinating disease, concluding
that Mac-1 is an important integrin target for MS immunotherapy
(44). Collectively, the results
confirm the hypothesis that silencing of ICAM1 and ITGB2 could be
of therapeutic value in modulating cytotoxic T-cells of patients
with MS.
Interestingly, ICAM1 silencing caused similar
changes to miR-155 overexpression and miR-155 overexpression caused
a decrease in ICAM1 expression in all treated subtypes which
suggests that the modulations observed with miR-155 overexpression
could be due to ICAM1 downregulation rather than miR-155
manipulation. This indicates that ICAM1 may have a dominant effect
in modulating the aforementioned target genes in CD8+
T-cells of treated patients with MS. For further insight, it was
also examined whether the disease state affects the manipulation
outcomes, hence the same manipulations on CD8+ T-cells
isolated from healthy controls were performed. The genetic and
epigenetic manipulations performed caused similar outcomes in all
diseased cells and healthy controls cells with two exceptions.
First, the upregulation of ICAM1 in untreated naïve patients
following miR-155-mimic transfection (Fig. 1A) could be due to the increased
expression of the endogenous levels as observed in the previous
screening of ICAM1(20). Second, the
upregulation of perforin following miR-155 overexpression as well
as ICAM1 and ITGB2 knockdown in the IFNβ-1b-treated subtype
(Figs. 1C and 2C) could be due to the increased
upregulation of perforin in those samples before manipulation as
observed in the previous screening of perforin (20).
Relating the experimental data obtained to the
clinical data of the patients was intriguing, hence, correlation
studies between mRNA expression of miR-155, target genes, and the
EDSS of the patients were carried out. The positive correlation
between miR-155, ITGB2, and EDSS, and the negative one with ICAM1,
perforin, and granzyme B, determined in a previous study by the
authors, could be further exploited to enhance the use of these
molecules as biomarkers for diagnostic and prognostic purposes
(20). Moreover, in this previous
study, the negative correlation between miR-155 and the target
genes reflects the results observed during the ex vivo
experiments of the present study (20). Considering the probability of
personalized, optimized therapy, correlating the effect of miR-155
overexpression on the expression of target genes with the EDSS of
patients revealed a significant positive correlation between the
effect of miR-155 overexpression on all genes and the EDSS of
patients (Fig. 3) leading to the
theory that the manipulation of miR-155 could be more effective in
patients with high EDSS. It is also worth mentioning that this is
the first reported correlation study discussing miR-155 and the
target genes. If miR-155 could really downregulate the expression
of surface receptors responsible for migration and target attack,
or cytolytic proteins responsible for destruction, then it could be
one of the targets to be used to downregulate those key players in
CD8+ T-cells. This would decrease their migration
through the BBB following activation and their attack on
oligodendrocytes following migration. Regarding the vulnerability
of patients to infections following CD8+ T-cell
manipulation, this is unfortunately the case with most
immunomodulatory drugs. A method to tone down the activated immune
system against the oligodendrocytes of patients may be a first
approach until research discovers selective activation markers or
auto-receptors present on immune cells activated against
self-antigens only.
Considering the multi-target influence afforded by a
single miRNA, it is reasonable to hypothesize that studies directed
at establishing the effect of drugs on miRNA gene expression could
disclose possible unrevealed, to date, modes of action of drugs
(45). This explains the aim of
screening for the expression of miR-155 throughout different
treatments. The differences in results between treatment groups
reveal the potential role of epigenetic modulations in treatment
outcome and efficacy. Hence, a biomarker for treatment responses in
MS would be of considerable clinical value. Thus, prospective
studies using cohorts of patients with MS at different stages of
disease would validate whether miR-155 could fulfill this
additional role.
In conclusion, the ex vivo overexpression of
miR-155 in CD8+ T-cells caused significant
downregulation of pro-inflammatory ICAM1, ITGB2, perforin, and
granzyme B expression, indicating a probable anti-inflammatory role
of the recognized to be pro-inflammatory miRNA (Fig. 4). Interestingly, the knockdown of
ICAM1 and ITGB2 caused downregulation of miR-155 and a similar
anti-inflammatory profile to that observed with miR-155
overexpression, suggesting that the changes observed during
overexpression could be a result of ICAM1 downregulation rather
than the direct effect of miR-155 modulation. Future
recommendations involve a larger cohort in a longitudinal study
setting, where patients are followed prior to and further into
treatment, to identify cellular and molecular changes occuring due
to treatments. The present study revealed the interplay between
miR-155, ICAM1, and ITGB2, paving the road for their beneficial use
as probable therapeutic regulators and diagnostic biomarkers of
disease.
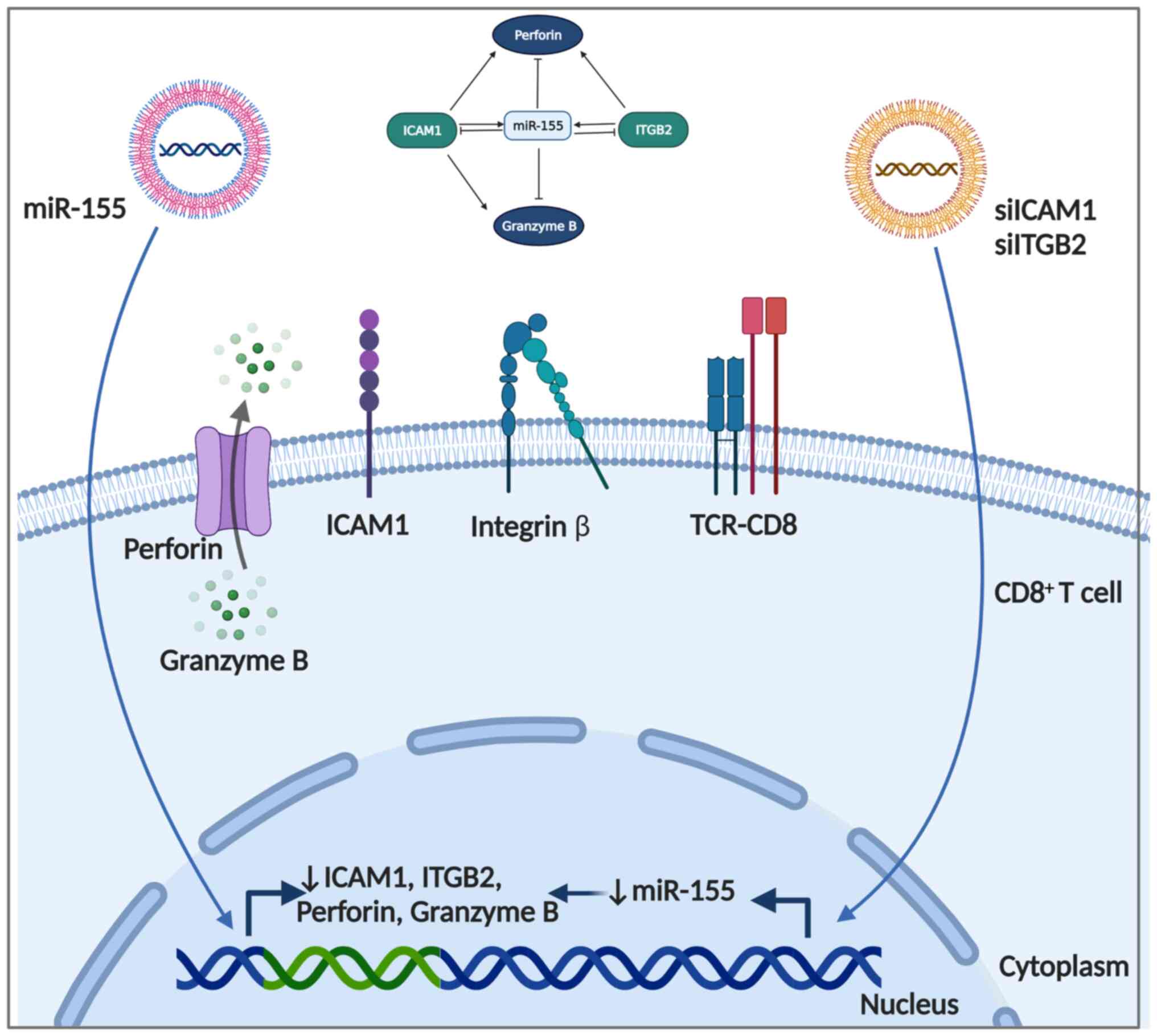 | Figure 4Summary of the regulatory roles of
miR-155 mimics, siICAM1 and siITGB2 on ICAM1, ITGB2, perforin and
granzyme B. The inhibitory effect of miR-155 on ICAM1, ITGB2,
perforin and granzyme B, as well as that of siICAM1 on miR-155,
ITGB2, perforin and granzyme B, and that of siITGB2 on miR-155 and
perforin is illustrated. Created by Biorender. miR-155,
microRNA-155; si, small interfering RNA; ICAM1, intracellular
adhesion molecule-1; ITGB2, integrin subunit β2. |
Supplementary Material
Flow cytometricanalysis of an isolated
sample of cells confirming CD8+ T cells.
Transfection efficiency of miR-155
upregulation and knockdown using mimics and antagomirs in
CD8+ T cells from all groups. Confirmed efficient
upregulation of miR-155 compared to mock (P=0.0008). The same
pattern was observed in all groups. ***P<0.001.
miR-155, microRNA-155.
Transfection efficiency of ICAM1 and
ITGB2 knockdown using siRNA in CD8+ T cells from all
groups. (A and B) Confirmed efficient knockdown of (A) ICAM1 and
(B) ITGB2 compared to the mock group (P=0.0367 and P=0.0105
respectively). *P<0.05. siRNA, small interfering RNA;
ICAM1, intracellular adhesion molecule-1; ITGB2, integrin subunit
β2.
Characteristics of patients with
RRMS.
Characteristics of healthy
controls.
Results of target prediction analysis
between miR-155 and genes of interest using bioinformatics
analysis.
Acknowledgements
The authors acknowledge the German University in
Cairo (Cairo, Egypt) for providing the required facilities to
conduct the research work.
Funding
Funding: Partial financial support for the present study was
received from the DAAD/BMBF Funded M.Sc. Scholarship.
Availability of data and materials
Data is contained within the article or
supplementary material. The data presented in this study are
available in Table SI, Table SII and Table SIII and Fig. S1, Fig.
S2 and Fig. S3.
Authors' contributions
AAE carried out all the experiments, analyzed the
data and contributed to the writing of the manuscript. DAZ is the
clinical neurologist who provided all samples and clinical data,
and contributed to the data acquisition and revision of manuscript
drafts. HMET is the principal investigator and the main supervisor
of this research work, and contributed to the conception and design
of the work, revising and approving the drafts and final version of
the manuscript. AAE and HMET confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki, and approved (approval
no. PTX-2018-11-HET) by Ethics Committee of the German University
in Cairo (Cairo, Egypt). All subjects involved provided their
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bishop M and Rumrill PD: Multiple
sclerosis: Etiology, symptoms, incidence and prevalence, and
implications for community living and employment. Work. 52:725–734.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kamm CP, Uitdehaag BM and Polman CH:
Multiple sclerosis: Current knowledge and future outlook. Eur
Neurol. 72:132–141. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zakaria M, Sharawy M and Anan I: Economic
burden of multiple sclerosis in Egypt: A societal perspective. Mult
Scler Relat Disord. 37(101563)2020.
|
|
4
|
Ghasemi N, Razavi S and Nikzad E: Multiple
sclerosis: Pathogenesis, symptoms, diagnoses and cell-based
therapy. Cell J. 19:1–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hauser SL, Bhan AK, Gilles F, Kemp M, Kerr
C and Weiner HL: Immunohistochemical analysis of the cellular
infiltrate in multiple sclerosis lesions. Ann Neurol. 19:578–587.
1986.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shi L, Yang X, Froelich CJ and Greenberg
AH: Purification and use of granzyme B. Methods Enzymol.
322:125–143. 2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Osińska I, Popko K and Demkow U: Perforin:
An important player in immune response. Cent Eur J Immunol.
39:109–115. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alexander JS, Zivadinov R, Maghzi AH,
Ganta VC, Harris MK and Minagar A: Multiple sclerosis and cerebral
endothelial dysfunction: Mechanisms. Pathophysiology. 18:3–12.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bascones-Martinez A, Mattila R, Gomez-Font
R and Meurman JH: Immunomodulatory drugs: Oral and systemic adverse
effects. Med Oral Patol Oral Cir Bucal. 19:e24–e31. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Critchfield JM, Racke MK, Zúñiga-Pflücker
JC, Cannella B, Raine CS, Goverman J and Lenardo MJ: T cell
deletion in high antigen dose therapy of autoimmune
encephalomyelitis. Science. 263:1139–1143. 1994.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ding SW, Li H, Lu R, Li F and Li WX: RNA
silencing: A conserved antiviral immunity of plants and animals.
Virus Res. 102:109–115. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Paraboschi EM, Soldà G, Gemmati D, Orioli
E, Zeri G, Benedetti MD, Salviati A, Barizzone N, Leone M, Duga S
and Asselta R: Genetic association and altered gene expression of
mir-155 in multiple sclerosis patients. Int J Mol Sci.
12:8695–8712. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ma X, Zhou J, Zhong Y, Jiang L, Mu P, Li
Y, Singh N, Nagarkatti M and Nagarkatti P: Expression, regulation
and function of microRNAs in multiple sclerosis. Int J Med Sci.
11:810–818. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Junker A, Krumbholz M, Eisele S, Mohan H,
Augstein F, Bittner R, Lassmann H, Wekerle H, Hohlfeld R and Meinl
E: MicroRNA profiling of multiple sclerosis lesions identifies
modulators of the regulatory protein CD47. Brain. 132:3342–3352.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Seddiki N, Brezar V, Ruffin N, Lévy Y and
Swaminathan S: Role of miR-155 in the regulation of lymphocyte
immune function and disease. Immunology. 142:32–38. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Niwald M, Migdalska-Sęk M,
Brzeziańska-Lasota E and Miller E: Evaluation of selected MicroRNAs
expression in remission phase of multiple sclerosis and their
potential link to cognition, depression, and disability. J Mol
Neurosci. 63:275–282. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Baulina N, Kulakova O, Kiselev I, Osmak G,
Popova E, Boyko A and Favorova O: Immune-related miRNA expression
patterns in peripheral blood mononuclear cells differ in multiple
sclerosis relapse and remission. J Neuroimmunol. 317:67–76.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Song J and Lee JE: miR-155 is involved in
Alzheimer's disease by regulating T lymphocyte function. Front
Aging Neurosci. 7(61)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tsai CY, Allie SR, Zhang W and Usherwood
EJ: MicroRNA miR-155 affects antiviral effector and effector Memory
CD8 T cell differentiation. J Virol. 87:2348–2351. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Elkhodiry AA, Zamzam DA and El Tayebi HM:
miR-155 and functional proteins of CD8+ T cells as potential
prognostic biomarkers for relapsing-remitting multiple sclerosis.
Mult Scler Relat Disord. 53(103078)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yusuf-Makagiansar H, Anderson ME,
Yakovleva TV, Murray JS and Siahaan TJ: Inhibition of LFA-1/ICAM-1
and VLA-4/VCAM-1 as a therapeutic approach to inflammation and
autoimmune diseases. Med Res Rev. 22:146–167. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang X, Wu Y, Zhang B and Ni B: Noncoding
RNAs in multiple sclerosis. Clin Epigenetics.
10(149)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lukiw WJ, Surjyadipta B, Dua P and
Alexandrov PN: Common micro RNAs (miRNAs) target complement factor
H (CFH) regulation in Alzheimer's disease (AD) and in age-related
macular degeneration (AMD). Int J Biochem Mol Biol. 3:105–116.
2012.PubMed/NCBI
|
|
25
|
El Tayebi HM, Waly AA, Assal RA, Hosny KA,
Esmat G and Abdelaziz AI: Transcriptional activation of the
IGF-II/IGF-1R axis and inhibition of IGFBP-3 by miR-155 in
hepatocellular carcinoma. Oncol Lett. 10:3206–3212. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Atwa S, Hosny K, Handoussa H and El Tayebi
H: Paradoxically functioning onco-miR-155 and the tumor suppressor
miR-194 consensus on PD-L1 immune checkpoint upregulation via
MALAT-1 and XIST in hepatocellular carcinoma. J Hepatol. 68 (Suppl
1):S610–S611. 2018.
|
|
27
|
Junker A: Pathophysiology of translational
regulation by microRNAs in multiple sclerosis. FEBS Lett.
585:3738–3746. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Reyes-Long S, Cortes-Altamirano JL,
Clavijio-Cornejo D, Gutiérrez M, Bertolazzi C, Bandala C, Pineda C
and Alfaro-Rodríguez A: Nociceptive related microRNAs and their
role in rheumatoid arthritis. Mol Biol Rep. 47:7265–7272.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Leng RX, Pan HF, Qin WZ, Chen GM and Ye
DQ: Role of microRNA-155 in autoimmunity. Cytokine Growth Factor
Rev. 22:141–147. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Azzaoui K, Blommers M, Götte M, Zimmermann
K, Liu H and Fretz H: Discovery of small molecule drugs targeting
the biogenesis of microRNA-155 for the treatment of systemic lupus
erythematosus. Chimia (Aarau). 74:798–802. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kamphuis WW, Troletti C, Reijerkerk A,
Romero IA and de Vries HE: The blood-brain barrier in multiple
sclerosis: microRNAs as key regulators. CNS Neurol Disord Drug
Targets. 14:157–167. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu H, Neilson JR, Kumar P, Manocha M,
Shankar P, Sharp PA and Manjunath N: miRNA profiling of naïve,
effector and memory CD8 T cells. PLoS One. 2(e1020)2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Neilson JR, Zheng GXY, Burge CB and Sharp
PA: Dynamic regulation of miRNA expression in ordered stages of
cellular development. Genes Dev. 21:578–589. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Elovaara I, Ukkonen M, Leppäkynnäs M,
Lehtimäki T, Luomala M, Peltola J and Dastidar P: Adhesion
molecules in multiple sclerosis: Relation to subtypes of disease
and methylprednisolone therapy. Arch Neurol. 57:546–551.
2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fujii C, Kondo T, Ochi H, Okada Y, Hashi
Y, Adachi T, Shin-Ya M, Matsumoto S, Takahashi R, Nakagawa M and
Mizuno T: Altered T cell phenotypes associated with clinical
relapse of multiple sclerosis patients receiving fingolimod
therapy. Sci Rep. 6(35314)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cerutti C, Soblechero-Martin P, Wu D,
Lopez-Ramirez MA, de Vries H, Sharrack B, Male DK and Romero IA:
MicroRNA-155 contributes to shear-resistant leukocyte adhesion to
human brain endothelium in vitro. Fluids Barriers CNS.
13(8)2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lind EF, Elford AR and Ohashi PS:
Micro-RNA 155 is required for optimal CD8+ T cell responses to
acute viral and intracellular bacterial challenges. J Immunol.
190:1210–1216. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Blüml S, Bonelli M, Niederreiter B,
Puchner A, Mayr G, Hayer S, Koenders MI, van den Berg WB, Smolen J
and Redlich K: Essential role of microRNA-155 in the pathogenesis
of autoimmune arthritis in mice. Arthritis Rheum. 63:1281–1288.
2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lashine YA, Salah S, Aboelenein HR and
Abdelaziz AI: Correcting the expression of miRNA-155 represses
PP2Ac and enhances the release of IL-2 in PBMCs of juvenile SLE
patients. Lupus. 24:240–247. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yasuda M, Tanaka Y, Tamura M, Fujii K,
Sugaya M, So T, Takenoyama M and Yasumoto K: Stimulation of beta1
integrin down-regulates ICAM-1 expression and ICAM-1-dependent
adhesion of lung cancer cells through focal adhesion kinase. Cancer
Res. 61:2022–2030. 2001.PubMed/NCBI
|
|
42
|
Scholer A, Hugues S, Boissonnas A, Fetler
L and Amigorena S: Intercellular adhesion molecule-1-dependent
stable interactions between T cells and dendritic cells determine
CD8+ T cell memory. Immunity. 28:258–270. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bullard DC, Hu X, Schoeb TR, Collins RG,
Beaudet AL and Barnum SR: Intercellular adhesion molecule-1
expression is required on multiple cell types for the development
of experimental autoimmune encephalomyelitis. J Immunol.
178:851–857. 2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bullard DC, Hu X, Schoeb TR, Axtell RC,
Raman C and Barnum SR: Critical requirement of CD11b (Mac-1) on T
cells and accessory cells for development of experimental
autoimmune encephalomyelitis. J Immunol. 175:6327–6333.
2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Faraoni I, Antonetti FR, Cardone J and
Bonmassar E: miR-155 gene: A typical multifunctional microRNA.
Biochim Biophys Acta. 1792:497–505. 2009.PubMed/NCBI View Article : Google Scholar
|


















