Introduction
Multiple sclerosis (MS) is a chronic
auto-inflammatory disease of the central nervous system (CNS)
(1). As a chronic autoimmune
disease, MS has a higher incidence in women than men, and it often
occurs in young adults. Abnormal inflammatory cells infiltrate into
the CNS to initiate lesion formation and finally lead to
demyelination of the neuronal axon (2). While, the exact etiological
mechanisms remain unclear. Accumulated evidence has revealed that
chronic demyelinating disorders of the CNS are primarily induced by
self-reactive CD4+ helper-T (TH) cells. While
mice lacking TH1 cells can develop this pathology
(3), and it has been considered
that interferon (IFN)-γ produced by TH1 cells had a key
role in the pathogenesis of MS (4). Mice deficient in TH1
cells-associated molecules, such as IFN-γ or IL-12p35 were more
susceptible to EAE, while IL-12p40 deficient mice were resistant to
disease. With the discoveries that p40 is also a subunit of IL-23
and IL-23 plays a pivotal role in mediating the development of
disease. It was revealed that IL23-p19-deficient mice could
abrogate the pathogenesis of experimental autoimmune
encephalomyelitis (EAE) and downregulate the expression of
TH17 cell-associated proteins (5–7).
These results highlighted the importance of IL-17-producing
TH17 cells in the pathogenesis of EAE.
Chalcones are aromatic ketones that form the central
core of a variety of biological compounds. Derivatives of these
agents exhibit a broad range of biological activities, in great
part due to the presence of their αβ-unsaturated carbonyl system.
As such, chalcones could be broadly used in drug research through
the use of structural modifications. In previous studies, it was
reported that N-heterocyclic-substituted chalcone compounds
display a variety of biological activities in tumor cell lines and
macrophages (such as A549, HeLa and RAW264.7 cell lines) (8–10).
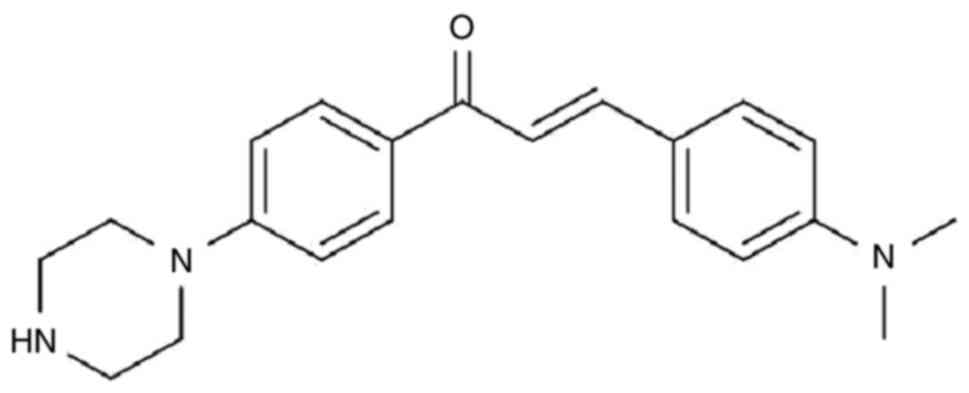
Among these, MW-9 (Fig. 1)
appeared to impart a significant inhibitory effect on the
generation of nitric oxide and markedly suppressed the production
of tumor necrosis factor α in the RAW264.7 murine
monocyte/macrophage cell line.
Nonetheless, the therapeutic potential of MW-9 in
treating immune-based pathologies remains unknown. In order to
explore the biological effects of MW-9 on autoimmune-related
pathologies, the present study presented examined the effect of
this agent on murine EAE. In the context of EAE, it remains unclear
whether MW-9 may affect the levels of IL-17 and TH17
cells. It was expected that information obtained in these studies
about potential immunomodulating mechanisms of MW-9 could then be
used to assess if the agent could be used for other
autoimmune-based pathologies, including MS.
Materials and methods
Experimental animals
A total of 12 C57BL/6 mice (weight 18±2 g; female;
8–10 weeks-old) were obtained from the Shanghai Laboratory Animal
Center of the Chinese Academy of Sciences (Shanghai, China). Upon
arrival, all mice were housed under specific pathogen-free
conditions (12-h light/dark cycles, room temperature of 22±1°C, and
a 55±5% relative humidity). All mice had ad libitum access
to standard rodent chow and filtered tap water. All mice were
allowed to acclimatize for 4 weeks before initiation of any
experiment. All experiments were carried out according to the
institutional ethical guidelines on animal care, and were approved
(approval no. S2019-001) by the Institute Animal Care and Usage
Committee of The First Hospital Affiliated Yunnan University of
Traditional Chinese Medicine (Kunming, China).
Reagents
MOG35-55 used to induce EAE in the mice
was purchased from ProSpec-Tany TechnoGene, Ltd. Freund's complete
adjuvant (CFA) containing Mycobacterium tuberculosis (M.
tuberculosis) strain H37Rv was obtained from BD Biosciences.
Pertussis toxin (PTX) was obtained from List Biological Labs. Mouse
allophycocyanin (APC)-conjugated IL-17antibody (cat. no. 506916;
cloneTC11-18H10), mouse fluorescein isothiocyanate
(FITC)-conjugated interferon (IFN)-γ antibody (cat. no. 505805;
clone XMG1.2), mouse PE-Cy7-conjugated CD4 antibody (cat. no.
100528; clone RM4.5), mouse Alexa Fluor@ 488-conjugated
Foxp3 (cat. no. 320012; clone MF23) antibody, mouse FITC-conjugated
anti-mGr.1 (cat. no 108405; clone RB6-8C5, BD), phycoerythrin
(PE)-anti-mCD11b (cat. no. 101255; clone M1/7), mouse
FITC-anti-mCD8a (cat. no. 100705; clone 53-6.7), PE-anti-mB220
(cat. no. 103207; clone RA3-682), PE-Cy7-anti-mCD4 (clone GK.1.5),
mouse APC-conjugated CD3 (cat. no 100312; clone 145-2C11) were
purchased from BioLegend, Inc. ELISA kits for IL-17A (cat. no.
88-7371), IgG (cat. no. 88-50400), IgG1 (cat. no.
88-50410), IgG2a (cat. no. 88-50420) and IgG3
(cat. no. 88-50440) were purchased eBioscience. Reverse
transcription kit and SYBR Premix Ex Taq II were purchased from
Takara Biotechnology Co., Ltd. RNA extraction kit was purchased
from Qiagen, Inc. All primers used were synthesized by Shanghai
Bioengineering Co., Ltd.
General procedure for the preparation
of MW-9
MW-9 were synthesized in house according to the
method previously described (10). Briefly, 20% KOH (10 ml) was added
to a solution of 4-dimethyl amino benzaldehyde (0.75 g, 5 mmol) and
4-fluoroacetophenone (0.69 g, 5 mmol) in EtOH (10 ml), and left to
react overnight at room temperature. After filtration, the
precipitate was washed twice with 50% EtOH-H2O and dried
without further purification. The solid was dissolved in dried DMF
(15 ml), then Cs2CO3 (3.26 g, 10 mmol) and
piperazine (258 mg, 3 mmol) was added and the mixture was stirred
for 12 h at 110°C. After completion of the reaction as indicated by
thin layer chromatography, the reaction was quenched by the
addition of DCM (30 ml) and was washed with water (3×20 ml). The
organic layer was dried using anhydrous sodium sulfate,
concentrated in vacuo and purified by column chromatography
to afford product as yellow solid.
Induction of EAE
MOG35-55-induced EAE in C57BL/6 mice was
established as previously described (11–13). In brief, the mice were immunized
on day 0 with an intracutaneous injection of 150 mg of
MOG35-55 emulsified in CFA-bearing M.
tuberculosis strain H37Rv. Injection volume was set at 150 µl.
Thereafter, each mouse received an additional 300 ng of PTX by
intraperitoneal injection in 100 ml of phosphate-buffered saline
(PBS) on day 0 and again on day 2 post-immunization. Clinical
assessment of EAE was performed daily and mice were scored
according to the following criteria: 0, no overt signs of disease;
1, limp tail or hind limb weakness but not both; 2, limp tail and
hind limb weakness; 3, partial hind limb paralysis; 4, complete
hind limb paralysis; 5, moribund state or dead.
Treatment protocols
MW-9 was dissolved in PBS containing 0.5% sodium
CM-cellulose (Qiangshun Inc.). The MW-9 solution was prepared in a
manner that the mice could receive a per os dose of 40 mg/kg
in each treatment (volume=200 µl). Control mice received an equal
volume of PBS containing 0.5% CM-cellulose. MW-9 and vehicle were
respectively administered from day 1 post-immunization and then
daily for 25 days. This particular dose of MW-9 was chosen based
upon pilot study results assessing antiarthritic potential (data
not shown). On the 25th day of the experiment, all mice were
euthanized using 30% carbon dioxide of the cage volume per min,
then used the following methods to confirm the death of animals,
including lack of a heartbeat, lack of respiration, lack of corneal
reflex and presence of rigor mortis. Samples (spinal columns,
spleen, blood for serum) were collected for analyses as described
below.
Hematoxylin-Eosin (H&E)
staining
At necropsy, the spinal cord of each mouse was
collected. A portion of this tissue was then fixed with 4% formalin
at room temperature for 3 days. Thereafter, each underwent
dehydration in alcohols and embedment in paraffin, then these were
sectioned (to 5 µm) and stained with H&E at room temperature
for 1 h. Each slide was evaluated using a light microscope to
detect any inflammatory cell aggregation in the myelin and the
extent of any demyelination.
Production of cytokines and specific
antibodies
At necropsy, lymph nodes of all the
MOG35-55-induced mice were collected and lymphocytes
from each group were then isolated using standard protocols. After
counting, the isolated cells were placed in 24-well plates
(2×106 cells/well) and then stimulated by addition of
MOG35-55 (to yield a final dose of 10 µg
MOG35-55/ml in each well); and parallel wells received
only vehicle as ‘stimulant’. Plates were then cultured at 37°C. The
supernatants from all wells were harvested after 48 h in order to
assess IL-17 produced by the cells. Each sample was evaluated using
specific murine ELISA IL-17 assays (Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. The sensitivity level of the
kits was 4 pg/ml. The concentration of IL-17A and anti-mouse IgG,
IgG1, IgG2a and IgG3 in serum were
isolated from the mice at the peak of the disease process.
Reverse transcription-quantitative
(RT-q) PCR assay
Total RNA from remaining spinal cord tissue isolated
from each mouse was isolated using RNA simple Total RNA kit
(Tiangen Biotech Co., Ltd.). After confirming the purity
(wavelength 260/280 nm) and concentration (wavelength 260 nm) of
each isolate using a NANODrop2000 (Thermo Fisher Scientific, Inc.),
1 µg of total RNA was used to synthesize cDNA using a PrimeScript
RT Master Mix Perfect Real-Time kit (Takara Biotechnology Co.,
Ltd.). The transcriptional conditions were 37°C for 15 min and 85°C
for 5 sec, and then followed by maintenance at 4°C (14,15). Synthesized cDNA was used in
RT-qPCR experiments using SYBR Premix Ex Taq II kits (Takara
Biotechnology Co., Ltd.). Each 20 µl of reaction contained 10 µl of
SYBR Green Mix, 8.4 µl of nuclease-free H2O, 1 µl of
cDNA, 0.3 µl of forward primer and 0.3 µl of reverse primer. The
thermocycling conditions for qPCR were as follows: Initial
denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for
5 sec and 62°C for 20 sec. From this, the relative mRNA expression
of several TH17 cell-related genes (Il17a, Il17f,
Il6 and Ccr6) were measured. The primer sequences were
synthesized by Tsingke Biotechnology Co., Ltd. All samples were
assayed on a Stratagene MX3000P Real-Time PCR system (Agilent
Technologies, Inc.). Relative quantitation of mRNA expression was
calculated as the fold-increase in expression by using the
2−ΔΔCq method and the β-actin housekeeping gene
(16). The primer sequences of
specific genes were as follows: Il17a forward,
5′-TTTAACTCCCTTGGCGCAAAA-3′ and reverse,
5′-CTTTCCCTCCGCATTGACAC-3′; Il17f forward,
5′-CTGTTGATGTTGGGACTTGCC-3′ and reverse,
5′-TCACAGTGTTATCCTCCAGG-3′; Ccr6 forward,
5′-CCTGGGCAACATTATGGTGGT-3′ and reverse,
5′-CAGAACGGTAGGGTGAGGACA-3′; Il6 forward,
5′-CGGAGAGGAGACTTCACAGAG-3′ and reverse,
5′-CATTTCCACGATTTCCCAGA-3′; and β-actin forward,
5′-GGCTGTATTCCCCTCCATCG-3′ and reverse,
5′-CCAGTTGGTAACAATGCCATGT-3′.
Flow cytometry
To assess expression of various lymphocyte types in
the hosts with EAE, lymphocytes were harvested (centrifugation at
300 g × at 4°C for 5 min) at necropsy from lymph nodes of the
MOG35-55-treated mice using standard protocols (15). Aliquots of the cells
(106 total) were then blocked by incubation in 100 ml of
PBS containing 1 µg/ml of rat-anti-mouse CD16/CD32 (clone 2.4G2;
BioLegend, Inc.) for 10 min at 4°C. Thereafter, dedicated sets of
cells from each mouse were labeled by incubation for 15 min (at
4°C) in a solution of staining buffer containing one of the
following rat anti-mouse monoclonal antibodies (1:100 dilution; all
from BioLegend, Inc.): FITC-conjugated anti-mGr.1 (clone RB6-8C5),
phycoerythrin (PE)-anti-mCD11b (clone M1/7), FITC-anti-mCD8a (clone
53–6.7), PE-anti-mB220 (clone RA3-682), or PE-Cy7-anti-mCD4
(cloneGK.1.5), APC-conjugated CD3 (clone 145-2C11).
Intracellular cytokine staining for IFN-γ and IL-17A
was performed using Foxp3 fixation-permeabilization reagent,
following a protocol from eBioscience; Thermo Fisher Scientific,
Inc. In brief, aliquots of the isolated lymphocytes
(2×106 total) were plated into 24-well dishes and then
stimulated for 4 h with 50 ng/ml phorbol myristate acetate and 750
ng ionomycin/ml (both from Sigma-Aldrich; Merck KGaA) in the
presence of Brefeldin A (BioLegend, Inc.). The cells were then
collected, blocked with rat-anti-mouse CD16/CD32 (as
aforementioned), stained with PE-Cy7-conjugated rat anti-mCD4 (as
aforementioned), then fixed by fixation/permeabilization buffer
(eBioscience; cat. no. 005123-43) for 30 min at 4°C permeabilized,
and again stained for 45 min at 4°C with APC-conjugated rat
anti-mIL-17A (clone TC11-18H10.1) or FITC-rat anti-mIFN-γ (clone
XMG1.2) (1:100 dilution; both from BioLegend, Inc.). The
aforementioned stimulation was not required for the staining of
intracellular Foxp3 that was also performed using Alexa Fluor@
488-conjugated rat anti-mFoxp3 (clone 150 D; BioLegend, Inc.).
In all of the above cases, samples were evaluated on
a FACs Canto II flow cytometer (BD Biosciences) and analyzed using
FlowJo software v10.6.2 (FlowJo LLC) (17,18). A minimum of 10,000 events/sample
was acquired.
TH17 differentiation of
CD4+ cells in vitro
The methods used to evaluate TH17 cell
polarization here are those previously described in Li et al
(11) and Hou et al
(19). In brief, murine
splenocytes from untreated ‘naïve’ mice (no EAE) were prepared and
incubated (1:4 dilution) with Biotin-Antibody Cocktail (Miltenyi
Biotec GmbH) for 10 min at 4°C to deplete CD11b+,
CD8α+ and CD19+ cells. Then, these cells were
exposed to Anti-biotin Microbeads (Miltenyi Biotec GmbH) for 10 min
at 4°C, and the enriched CD4+ T cells were isolated by
MACS separator. Purity of the isolated cells was evaluated by
staining of representative aliquots with PE-Cy7-rat anti-mCD4 as
aforementioned.
For the assay, the ‘naïve’ CD4 T-cells from each
mouse were plated into 24-well plates (4×105/well) whose
wells had been pre-coated with rat anti-mCD3 (clone 154-2C11, 5
µg/ml: BioLegend, Inc.), anti-mCD28 (clone 37.51, 2.5 µg/ml:
BioLegend, Inc.), and a set of four polarizing cytokines. The
latter included recombinant mouse IL-6 (10 ng/ml), recombinant
human TGF-β1 (2 ng/ml), anti-mouse IL-4 (10 µg/ml), and
anti-mouse IFN-γ (10 µg/ml) (all from BioLegend, Inc.). After 72 h
of incubation at 37°C, the supernatants from each well were
collected to permit quantification of IL-17A levels by ELISA.
To detect IL-17A expression on cells that were
induced to differentiate into TH17 cells, other wells
containing isolated ‘naïve’ CD4 T-cells from each mouse plated into
24-well plates (106 total) were stimulated with phorbol
myristate acetate and ionomycin in the presence of Brefeldin A for
4 h, and then fixed (as aforementioned) for 30 min at 4°C,
permeabilized and stained for 45 min at 4°C with
fluorochrome-conjugated anti-mIL-17A (1:100 dilution). These cells
then underwent flow analyses as aforementioned.
Statistical analysis
All data are expressed as the mean ± SEM. Two
independent sample t-test was used to determine whether differences
between two given groups were significant. One-way analysis of
variance (one-way ANOVA) and Bonferroni test were used for
comparison among multiple groups and pairwise comparison from
multiple groups, respectively. P<0.05 was considered to indicate
a statistically significant difference. All data were analyzed
using SPSS 20.0 software (IBM Corp.).
Results
MW-9 alleviates development of EAE in
MOG35-55-immunized C57BL/6 mice
To assess therapeutic potential of MW-9, mice were
immunized with MOG35-55 peptide emulsified with CFA on
day 0 and then gavaged with MW-9 (40 mg/kg) or vehicle on the day
of immunization to the end of experiment. Severity of EAE due to
the presence or absence of MW-9 treatment was then evaluated. As
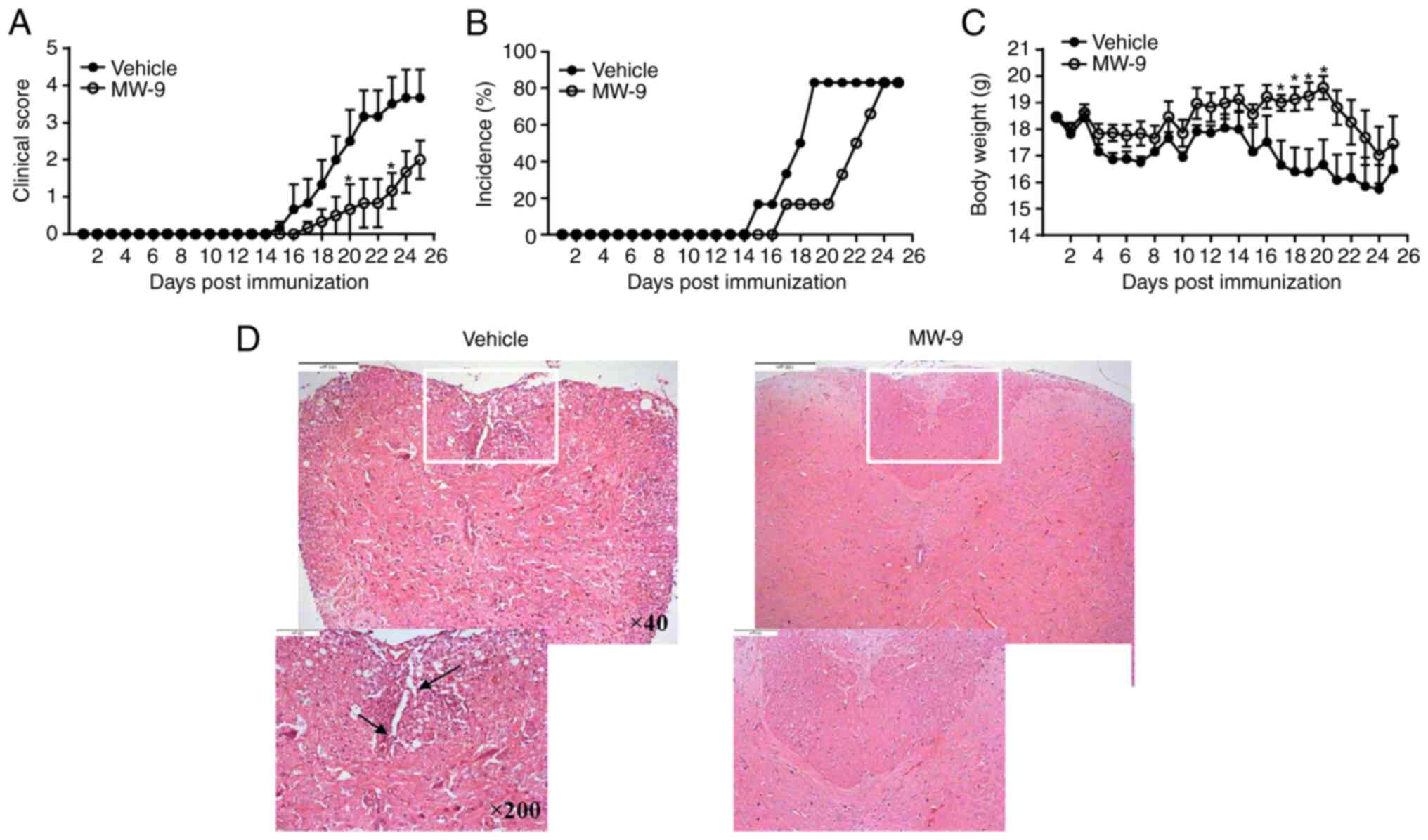
revealed in Fig. 2A, clinical
scores gradually increased as EAE progressed. Scores in the MW-9
treatment group were significantly reduced as compared with those
in the vehicle group. In addition, by day 19, 80% of the control
mice developed severe EAE. In comparison, <20% of mice showed
mild signs of disease in the MW-9-treated group (Fig. 2B). MW-9 treatment also markedly
prevented a loss of body weight due to EAE (Fig. 2C). Although MW-9 exhibited delayed
and ameliorated disease, the incidence in MW-9 treated group was
increased after day 24 post immunization, thus the difference
between these two groups decreased after day 24.
To assess effects of MW-9 on infiltration of immune
cells and demyelination of the spinal cord, histologic analysis was
performed after the treatments ended. The data indicated that by
day 25, mice in the vehicle group had typical pathological changes,
including severe infiltration of inflammatory cells and
demyelination of their spinal cords. By contrast, in mice treated
with MW-9, the extent of both infiltration and demyelination was
markedly reduced (Fig. 2D).
MW-9 blocks anti-MOG35-55
IgG antibody production
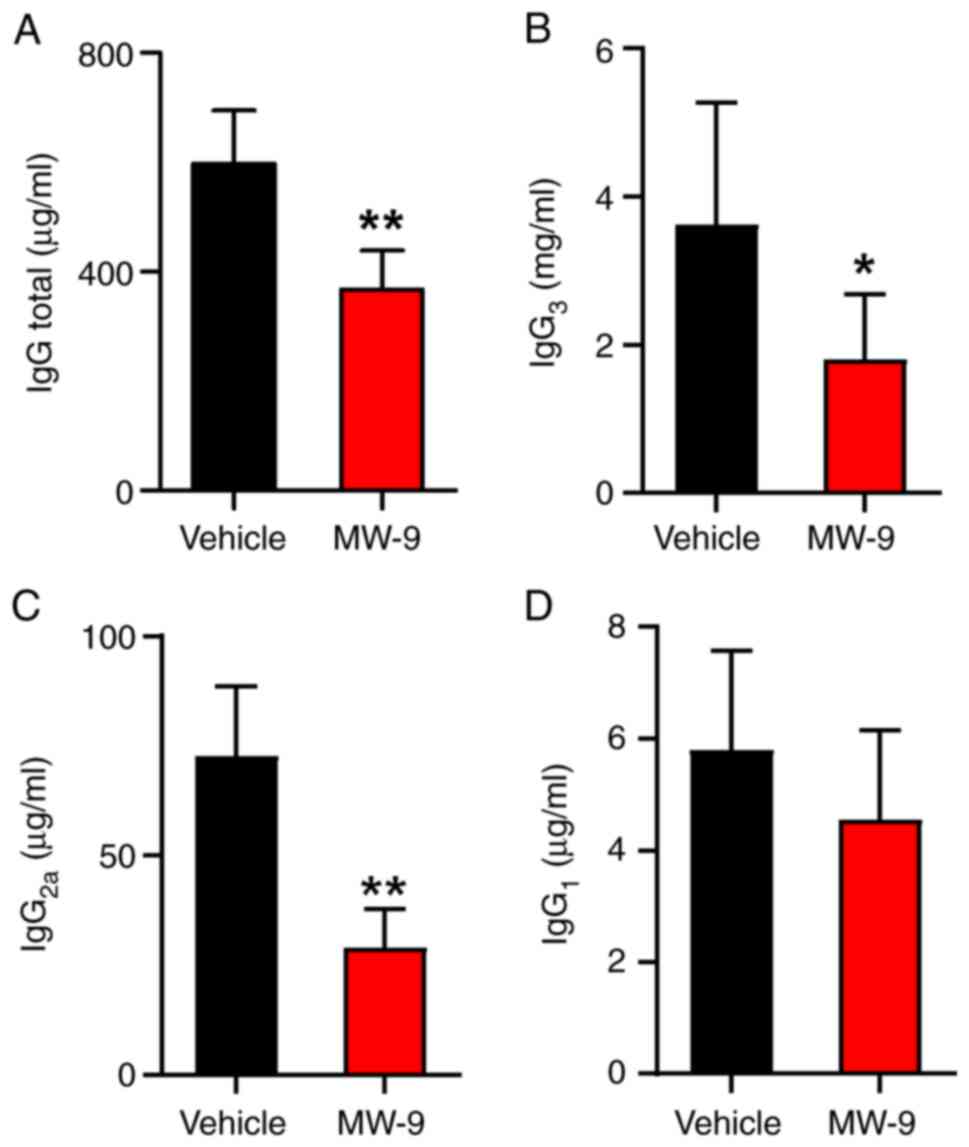
To monitor effects of MW-9 on antibody responses to
MOG35-55 peptide, serum levels of
anti-MOG35-55 peptide IgG, IgG1,
IgG2a and IgG3 were determined by ELISA. As
revealed in Fig. 3, serum levels
of total IgG in the MW-9 treatment group were significantly
decreased on day 25 compared with the control EAE mice (Fig. 3A). Furthermore, compared with the
control group, MW-9 treatment also reduced the production of
anti-MOG35-55 IgG3 (Fig. 3B) and IgG2a (Fig. 3C). However, MW-9 appeared to
impart no effect on anti-MOG35-55 IgG1 levels
(Fig. 3D).
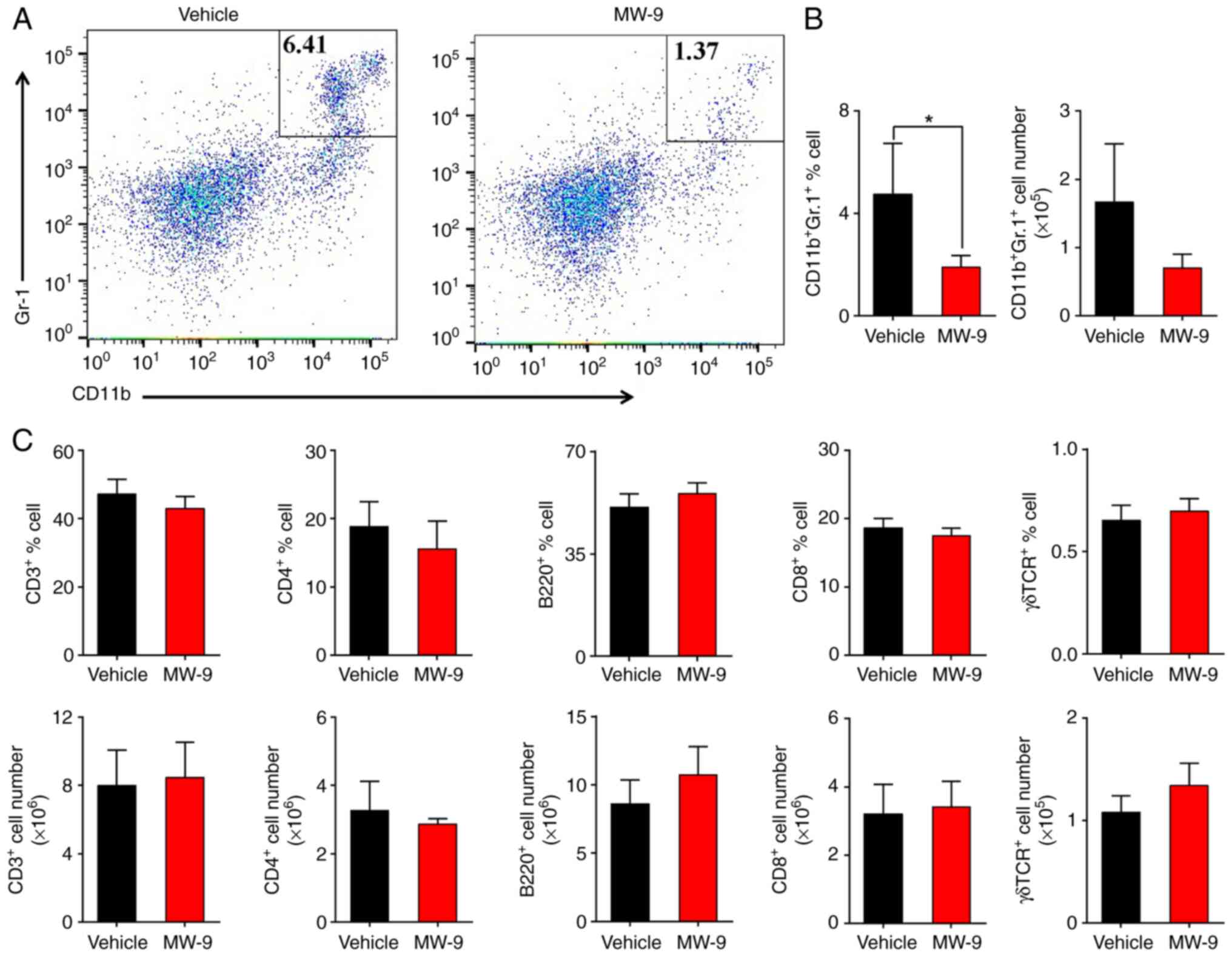
MW-9 inhibits accumulation of
neutrophils
To examine the effects of MW-9 on the types of
leukocytes in the spleens of mice with EAE, flow cytometric
analysis was performed. On day 25, the proportion and cell number
of CD11b+Gr-1+ neutrophils in the MW-9
treated mice was decreased compared with the vehicle group
(Fig. 4A and B). The data
indicated that MW-9 did not influence the expression of
CD3+, CD8+, or CD4+ T cells, and
no effects on γδ TCR+ T or B220+ B cells were
observed (Fig. 4C).
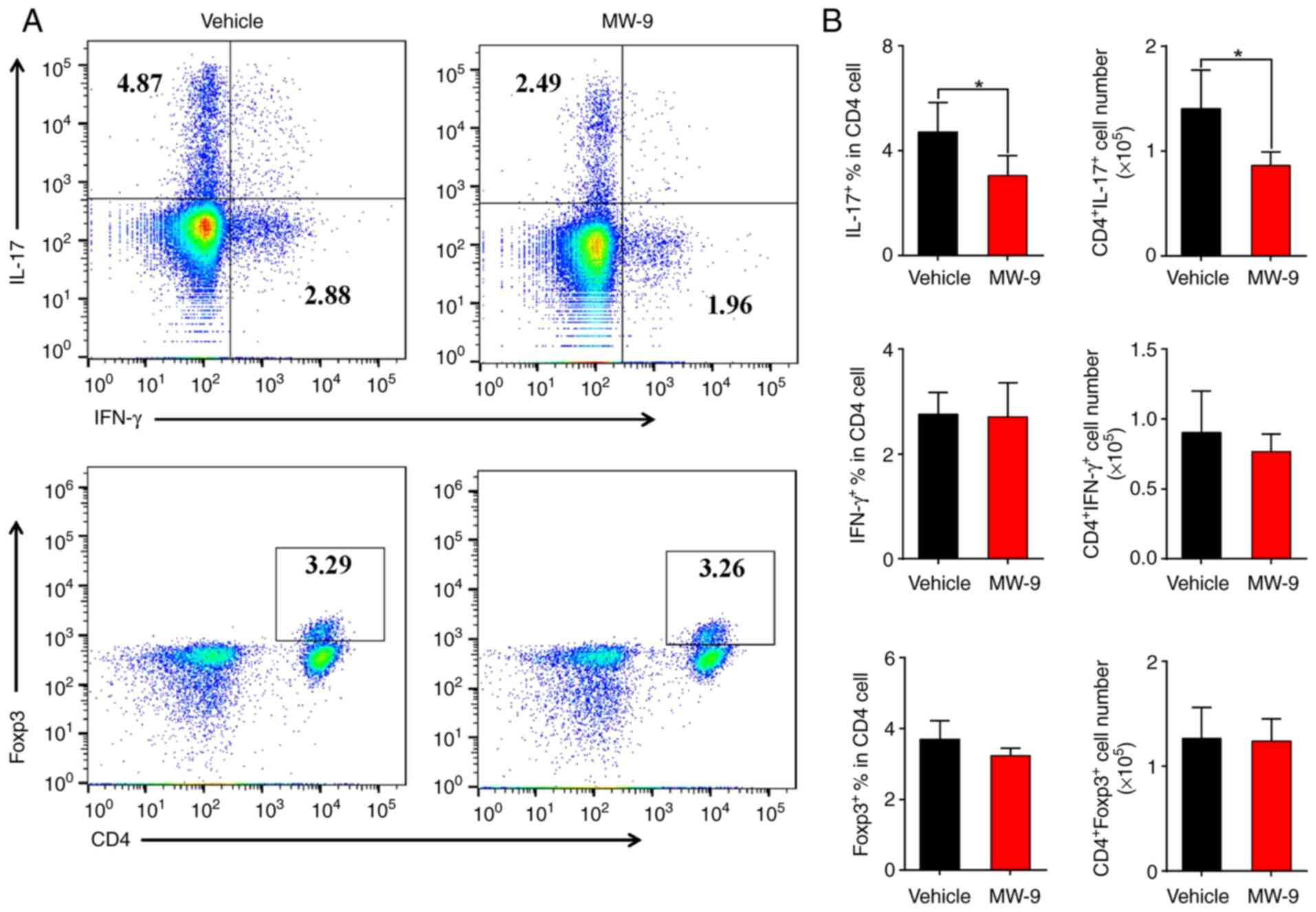
MW-9 markedly suppresses
TH17 cell responses in EAE
Since TH17 cells and IL-17 contribute to
the pathogenesis of autoimmune disorders such as EAE (20), it was further determined whether
MW-9 could influence these parameters during EAE through evaluating
the expression of intracellular cytokines in CD4+ T
cells via flow cytometry. As revealed in Fig. 5, compared with the vehicle control
mice, MW-9 treatment significantly suppressed IL-17A expression in
CD4+ T cells (Fig. 5A and
B). However, MW-9 treatment did not affect the expression of
IFN-γ+ and Foxp3+ CD4+ T cells in
the hosts (Fig. 5A and B).
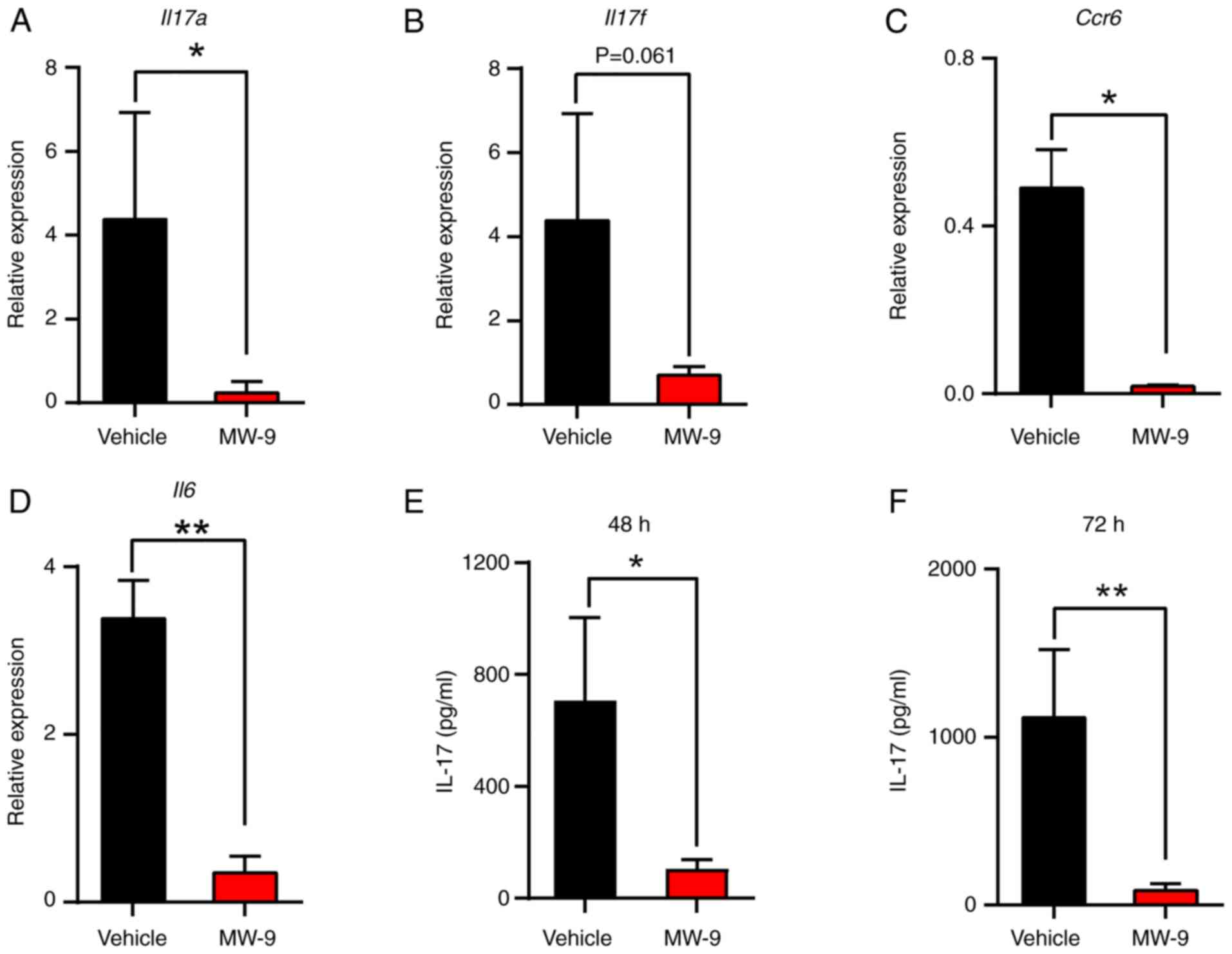
MW-9 suppresses production of IL-17
and expression of TH17 cell-related genes
There is evidence that TH17 cells play a
critical pathogenic role in EAE (21) and the present data indicated that
MW-9 markedly inhibited TH17 cell responses during EAE.
To assess the expression of TH17 cell-related
cytokines/proteins, spinal cords were isolated on day 25 and
analyzed by RT-qPCR. The results demonstrated that MW-9 treatment
significantly downregulated Il17a, Il17f, Ccr6 and
Il6 mRNA expression (Fig.
6A-D). The data also indicated that IL-17A produced by cells
from the MW-9-treated mice was lower than that from the vehicle
control. These findings were consistent with the gene expression
data (Fig. 6E and F).
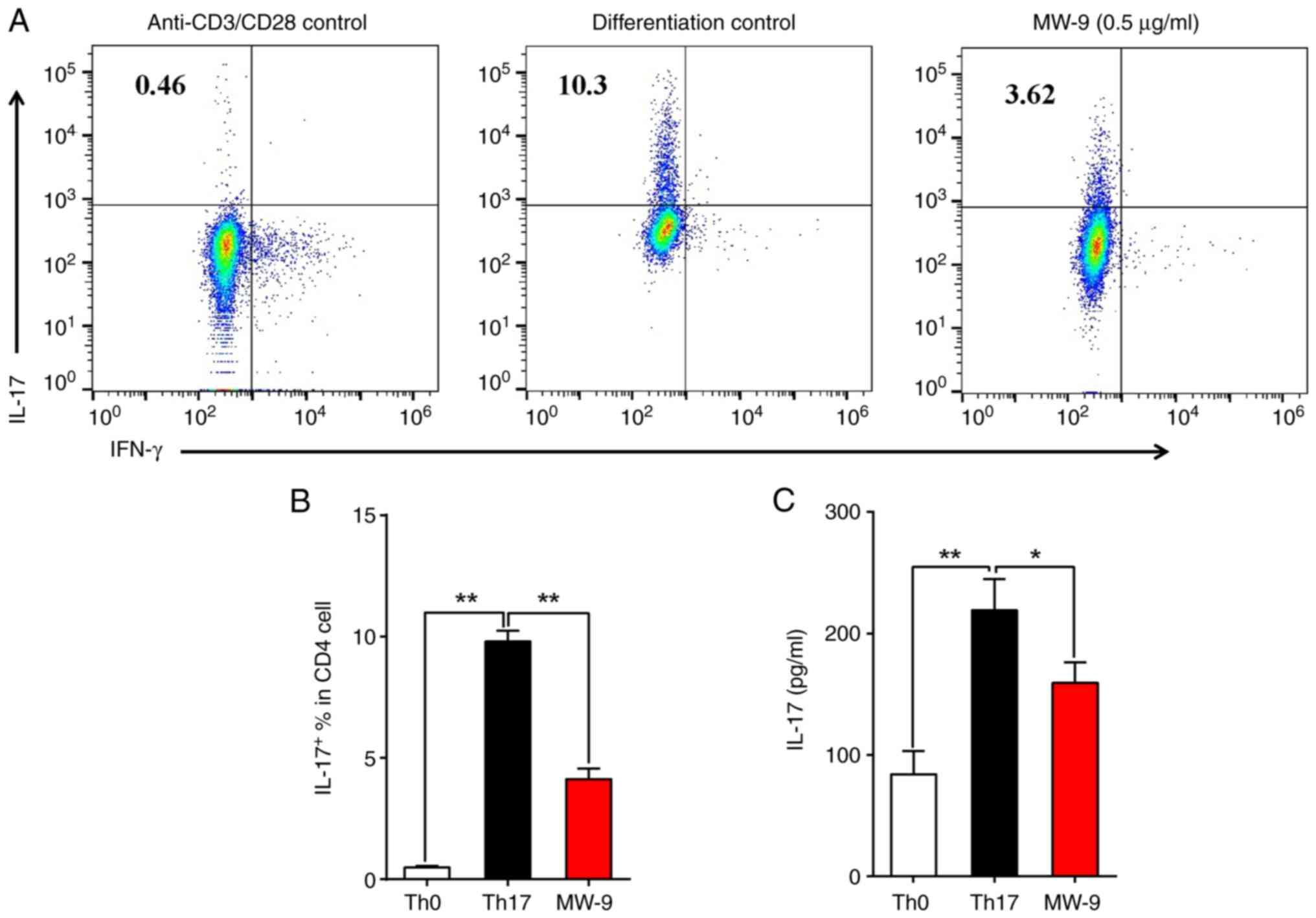
MW-9 suppresses TH17 cell
differentiation in vitro
To ascertain whether MW-9 could affect
TH17 cell differentiation, CD4+ T cells were
isolated from the spleens of naive mice and then induced to
differentiate into TH17 cells under polarizing
conditions for 3 days in the presence/absence of MW-9. The results
indicated that MW-9 treatment significantly suppressed
TH17 cell differentiation (Fig. 7A and B). In addition, IL-17A
production was also reduced in MW-9-treated cells (Fig. 7C).
Discussion
MS is an autoimmune disease affecting both adults
and children. It is driven by TH cells and white blood
cells that result in inflammatory attacks on the brain and spinal
cord, and ultimately the degradation of the protective myelin
sheath on nerve fibers. There are numerous different kinds of
TH cells, but which ones are the main pathogenic forms
in MS remain unknown. EAE, a classical animal model for MS, is
characterized by abnormal inflammatory cells that infiltrate into
the CNS, initiating lesion formation and demyelination of neuronal
axons (2). In the present study,
the therapeutic potential of the chalcone MW-9 on autoimmune
disorders such as MS was evaluated in vivo in murine hosts
with EAE. Notably, oral administration of MW-9 for a period of 19
days ameliorated the severity of the induced EAE. This effect was
accompanied by significant reduction in leukocyte infiltration and
demyelination of the spinal columns. It was reported that T-cells
played a pivotal role in the pathogenesis of numerous autoimmune
diseases and chronic inflammatory disorders, including EAE
(22). CD4+
TH cells are important mediators in immune responses, as
they regulate other cellular components of the immune system. Upon
activation by antigens or cellular signals, naïve CD4 cells can
differentiate into various subtypes, including TH1,
TH2, TH17 and Treg, which in turn
produce their own arrays of cytokines and perform specific immune
regulatory functions (22).
Inflammatory TH17 cells have been identified as a
distinct TH cell lineage that can mediate tissue
specificity during autoimmunity (23,24), including pathologies such as EAE
(25).
The therapeutic potentials of MW-9 on treating
immune-based pathologies remains unknown. In order to explore the
biological effects of MW-9 on autoimmune-related pathologies, the
effect of this agent on murine EAE was examined in the present
study. In the context of EAE, it remains unclear what effects MW-9
may have on IL-17 levels, TH17 cells, and on key
cytokines in hosts treated with this chalcone. The results showed
that MW-9 treatment (under the specified paradigm) significantly
reduced the production of TH17-related cytokine IL-17A
in the MOG35-55-induced immune responses in the mice.
Intracellular cytokine staining revealed that MW-9 suppressed
IL-17A expression in CD4+ T-cells without affecting the
proportions of IFN-γ+ and Foxp3+
CD4+ T-cells. This suggested that MW-9 could selectively
inhibit TH17-mediated tissue inflammatory responses, but
may exert no influence on TH1 and Treg cell
levels/activities, at least in the context of an ongoing EAE
response.
It has been reported that TH17
differentiation is initiated by TGF-b and IL-6, and is further
reinforced by IL-23 (26).
TH17 cells produce IL-17A, IL-17F and IL-22, that
regulate inflammatory responses (21,27), but the present study did not
directly examine the effect of MW-9 on these cytokines. Thus, the
changes in TH17 levels/activities observed could be a
result of either changes in T-cell responsivity to one or more of
the aforenoted activating cytokines or in the host ability to form
these agents. The results from the differentiation assay using
naïve T-cells revealed that MW-9 treatment markedly inhibited
TH17 differentiation. Thus, it holds true that TH17
responses are altered in the EAE mice. Ongoing studies to
re-analyze the mice sera for levels of TGF-b, IL-6, IL-23 and IL-22
will help to clarify the underlying mechanisms in an improved
way.
Analogous to STAT4 and STAT1 in Th1 and STAT6 in Th2
cell, STAT3 was found to selectively mediate TH17
differentiation (28).
Overexpression of a hyperactive STAT3 enhanced Th17
differentiation, whereas STAT3 deficiency impaired Th17
differentiation in vitro. Th17-specific transcription factor
RORγt was recently shown to be selectively expressed in Th17 cells
and regulated by STAT3 (29).
Overexpression of RORγt promotes Th17 differentiation when Th1 and
Th2 development is inhibited. Conversely, RORγτ deficiency results
in profound Th17 deficiency. In vivo, oral administration of
MW-9 significantly reduced the expression of IL-17 producing T
cells compared with the vehicle group. Consistent with in
vivo results, MW-9 also impaired Th17 cell differentiation and
IL-17-production in vitro, confirming that the inhibitory
effect of MW-9 on Th17 cell differentiation is responsible for the
effect of MW-9 on EAE. In the present study, oral administration of
MW-9 significantly decreased the IL-17 production in
MOG35-55 induced-specific immune response and in serum
of mice. Importantly, MW-9 treatment significantly inhibited IL-17
expression in CD4+ T-cells in EAE model, accompanied by
the reduced production of MOG35-55 peptide-specific
antibodies (IgG, IgG2a and IgG3), which may contribute to the
protective effect of MW-9 on EAE.
Overall, the data demonstrated that oral
administration of MW-9 could attenuate EAE in mice by inhibiting
pathogenic TH17 cells. Importantly, MW-9 treatment
significantly inhibited IL-17 expression in CD4+ T-cells
and TH17 cell differentiation in vitro. Both of these
changes induced by MW-9 may help to explain why under the current
experimental paradigm, MW-9 may provide a protective effect against
EAE.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of China (grant nos. 81960745 and 81960868), the Yunnan
Provincial Science and Technology Department-Applied Basic Research
Joint Special Fund of Yunnan University of Traditional Chinese
Medicine [grant no. 2018FF001(−009)] and the Yunnan Applicative and
Basic Research Program (grant no. 2018FY001-001).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
CW and ZM designed the present study; BL, SY and NY
performed biological research, analyzed data and wrote the
manuscript; and QG, YQ, XL, HY, ZW and NZ participated in data
analysis. CW reviewed and edited the manuscript. CW, BL and SY
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
All experiments were carried out according to the
institutional ethical guidelines on animal care and were approved
(approval no. S2019-001) by the Institute Animal Care and Usage
Committee of the No. 1 Hospital Affiliated Yunnan University of
Traditional Chinese Medicine (Kunming, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Q, Gao Q, Zhang Y, Li Z and Mei X:
MicroRNA-590 promotes pathogenic Th17 cell differentiation through
targeting Tob1 and is associated with multiple sclerosis. Biochem
Biophys Res Commun. 493:901–908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Handel AE, Lincoln MR and Ramagopalan SV:
Of mice and men: Experimental autoimmune encephalitis and multiple
sclerosis. Eur J Clin Invest. 41:1254–1258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gran B, Zhang GX, Yu S, Li J, Chen XH,
Ventura ES, Kamoun M and Rostami A: IL-12p35-deficient mice are
susceptible to experimental autoimmune encephalomyelitis: Evidence
for redundancy in the IL-12 system in the induction of central
nervous system autoimmune demyelination. J Immunol. 169:7104–7110.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-behi M, Rostami A and Ciric B: Current
views on the roles of Th1 and Th17 cells in experimental autoimmune
encephalomyelitis. J Neuroimmune Pharmacol. 5:189–197. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cua DJ, Sherlock J, Chen Y, Murphy CA,
Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al:
Interleukin-23 rather than interleukin-12 is the critical cytokine
for autoimmune inflammation of the brain. Nature. 421:744–748.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Langrish CL, McKenzie BS, Wilson NJ, de
Waal Malefyt R, Kastelein RA and Cua DJ: IL-12 and IL-23: Master
regulators of innate and adaptive immunity. Immunol Rev.
202:96–105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Komiyama Y, Nakae S, Matsuki T, Nambu A,
Ishigame H, Kakuta S, Sudo K and Iwakura Y: IL-17 plays an
important role in the development of experimental autoimmune
encephalomyelitis. J Immunol. 177:566–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin Y, Hu C, Zheng X, Wang X and Mao Z:
Synthesis and anti-tumor activities of novel
4′-(N-substitued-1-piperazinyl) chalcone derivatives. Chin J Org
Chem. 37:237–241. 2017. View Article : Google Scholar
|
|
9
|
Hui G, Xi Z, Ping Z, Si W, Chunping W,
Gaoxionga R and Zewei M: Synthesis and biological evaluation of
novel substituted chalcone-piperazine derivatives. Chin J Org Chem.
38:684–691. 2018. View Article : Google Scholar
|
|
10
|
Mao Z, Zheng X, Lin Y, Qi Y, Hu C, Wan C
and Rao G: Concise synthesis and biological evaluation of chalcone
derivatives bearing N-heterocyclic moieties. Heterocycles.
92:1102–1110. 2016. View Article : Google Scholar
|
|
11
|
Li X, Li TT, Zhang XH, Hou LF, Yang XQ,
Zhu FH, Tang W and Zuo JP: Artemisinin analogue SM934 ameliorates
murine experimental autoimmune encephalomyelitis through enhancing
the expansion and functions of regulatory T cell. PLoS One.
8:e741082013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Contarini G, Giusti P and Skaper SD:
Active induction of experimental autoimmune encephalomyelitis in
C57BL/6 mice. Neurotrophic Factors. Springer; pp. 353–360. 2018,
View Article : Google Scholar
|
|
13
|
Hou L, Rao DA, Yuki K, Cooley J, Henderson
LA, Jonsson AH, Kaiserman D, Gorman MP, Nigrovic PA, Bird PI, et
al: SerpinB1 controls encephalitogenic T helper cells in
neuroinflammation. Proc Natl Acad Sci USA. 116:20635–20643. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rho TW, Lee SY, Han SY, Kim JH, Lee KH,
Kim DS, Kwak HB and Kim YK: Glycyrrhizae radix inhibits osteoclast
differentiation by inhibiting c-Fos-dependent NFATc1 expression. Am
J Chin Med. 45:283–298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu S, Zuo A, Guo Z and Wan C: Ethyl
caffeate ameliorates collagen-induced arthritis by suppressing TH1
immune response. J Immunol Res. 2017. 74167922017.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao YJ, Xu Y, Liu B, Zheng X, Wu J, Zhang
Y, Li XS, Qi Y, Sun YM, Wen WB, et al: Dioscin, a steroidal saponin
isolated from dioscorea nipponica, attenuates collagen-induced
arthritis by inhibiting Th17 cell response. Am J Chin Med.
47:423–437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou LF, He SJ, Li X, Yang Y, He PL, Zhou
Y, Zhu FH, Yang YF, Li Y, Tang W and Zuo JP: Oral administration of
artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr
mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum.
63:2445–2455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou L, Cooley J, Swanson R, Ong PC, Pike
RN, Bogyo M, Olson ST and Remold-O'Donnell E: The protease
cathepsin L regulates Th17 cell differentiation. J Autoimmun.
65:56–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Langrish CL, Chen Y, Blumenschein WM,
Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA and
Cua DJ: IL-23 drives a pathogenic T cell population that induces
autoimmune inflammation. J Exp Med. 201:233–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chung Y, Yang X, Chang SH, Ma L, Tian Q
and Dong C: Expression and regulation of IL-22 in the
IL-17-producing CD4+ T lymphocytes. Cell Res. 16:902–907. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brzustewicz E and Bryl E: The role of
cytokines in the pathogenesis of rheumatoid arthritis-practical and
potential application of cytokines as biomarkers and targets of
personalized therapy. Cytokine. 76:527–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weaver CT, Harrington LE, Mangan PR,
Gavrieli M and Murphy KM: Th17: An effector CD4 T cell lineage with
regulatory T cell ties. Immunity. 24:677–688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bettelli E, Oukka M and Kuchroo VK:
T(H)-17 cells in the circle of immunity and autoimmunity. Nat
Immunol. 8:345–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Louten J, Boniface K and de Waal Malefyt
R: Development and function of TH17 cells in health and disease. J
Allergy Clin Immunol. 123:1004–1011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou L, Ivanov II, Spolski R, Min R,
Shenderov K, Egawa T, Levy DE, Leonard WJ and Littman DR: IL-6
programs T(H)-17 cell differentiation by promoting sequential
engagement of the IL-21 and IL-23 pathways. Nat Immunol. 8:967–974.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng Y, Danilenko DM, Valdez P, Kasman I,
Eastham-Anderson J, Wu J and Ouyang W: Interleukin-22, a T(H)17
cytokine, mediates IL-23-induced dermal inflammation and
acanthosis. Nature. 445:648–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang XO, Panopoulos AD, Nurieva R, Chang
SH, Wang D, Watowich SS and Dong C: STAT3 regulates
cytokine-mediated generation of inflammatory helper T cells. J Biol
Chem. 282:9358–9363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ivanov II, McKenzie BS, Zhou L, Tadokoro
CE, Lepelley A, Lafaille JJ, Cua DJ and Littman DR: The orphan
nuclear receptor RORgammat directs the differentiation program of
proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. 2006.
View Article : Google Scholar : PubMed/NCBI
|