Introduction
With the continuous deepening and improvement of
research on the tumor microenvironment and metabolic stress
mechanisms, increasing evidence has shown that tumor cells can
change cysteine metabolism through solute carrier family 7 member
11 (SLC7A11) to promote the occurrence and development of the
tumor. The growth and migration of tumor cells are highly dependent
on the tumor microenvironment, which is different from the normal
tissue environment (1,2). Given the rapid and massive
proliferation of tumor cells, a microenvironment of metabolic
stress, such as hypoxia and nutritional stress, is often formed. In
theory, this metabolic stress microenvironment is not conducive to
the survival and growth of tumors, but tumors can overcome the
stress environment that is not conducive to their growth and
continue to maintain the ability to proliferate indefinitely. The
metabolic reprogramming of tumors (including changes in glucose and
amino acid uptake) plays an important role in this. The results of
correlation studies indicated that the tumor microenvironment
exhibits the characteristics of homocysteine/cysteine (3) and is involved in tumor metabolic
reprogramming and resistance to ferroptosis (4). Ferroptosis is due to the abnormal
activation of the mitochondrial oxidative phosphorylation pathway
due to iron overload, which produces high levels of lipid peroxides
[e.g., reactive oxygen species (ROS)] when ATP is produced.
Ferroptosis can be regarded as cell death caused by the
accumulation of lipid peroxides on the cell membrane; it shows
evident iron dependence and does not belong to the category of
apoptosis or necrosis (5). From
the morphological point of view, ferroptosis is characterized by
mitochondrial atrophy, increased membrane density and reduced
mitochondrial cristae, but does not show the typical morphological
characteristics of traditional apoptosis, such as cell swelling,
cell contraction, cell rupture, apoptotic body formation, and
cytoskeleton disintegration and necrosis. In addition, ferroptosis
does not have the typical characteristics of autophagy, such as
autophagy vacuoles. Ferroptosis is closely related to reduced
glutathione (GSH) metabolism, and GSH is predominantly synthesized
by cysteine (6). Cystine in the
microenvironment of tumor cells is predominantly transported into
cells through the cystine/glutamate antiporter system xc- to
participate in the synthesis of GSH, which leads to the resistance
of tumor cells to ferroptosis, and promotes the occurrence and
development of tumors (4). The
cystine/glutamate antiporter system xc- is a member of the amino
acid transporter family and consists of two subunits, i.e., light
(SLC7A11) and heavy (SLC3A2) chain subunits. SLC7A11 plays a major
biological function as a transporter; it can transport glutamate
out of the cell and cystine into the cell at a ratio of 1:1.
SLC3A2, as a chaperone protein, is involved in maintaining the
stability of SLC7A11 only (7,8).
The present review focuses on the unique role of
SLC7A11 in the resistance of tumors to ferroptosis, and in the
occurrence, development and treatment of tumors with regard to the
regulation of cystine/cysteine metabolism.
Structure and biological function of
cysteine
Cysteine (2-amino-3-mercaptopropionic acid), an
aliphatic sulfhydryl-containing polar α-amino acid, is a
conditional amino acid that is essential for the human body and can
be converted into cystine. Cysteine plays an important role in
protein synthesis, catalysis, transport, post-translational
modification and redox maintenance (9,10).
The two main functions of cysteine in the human body are its
involvement in the production of reduced GSH and protein synthesis.
GSH is a tripeptide composed of three amino acids, namely,
cysteine, glutamic acid and glycine, and is one of the most
important cellular antioxidants in the human body. Cysteine is the
rate-limiting precursor of GSH synthesis, and its participation in
the biosynthesis of GSH comprises two steps (11). The first step is the rate-limiting
reaction, which uses cysteine and glutamate to synthesize
γ-glutamyl-cysteine (γ-Glu-Cys) by glutamate cysteine ligase. The
second step is the addition of glycine to the C-terminus of
γ-Glu-Cys by GSH synthetase to produce glutathione. Once
synthesized, GSH becomes a detoxification substance for lipid
peroxides. For example, glutathione peroxidase 4 (GPX4), which uses
GSH as a cofactor, detoxifies lipid peroxides into lipid alcohols
(12,13). At the same time, GSH is oxidized
into oxidized (GSSG), which then consumes H+ in NADPH through GSH
reductase and is reduced back to GSH, enabling GSH recycling. In
addition, cysteine is a precursor or cofactor of other biomolecules
with antioxidant properties, such as taurine, hydrogen sulfide and
aconitic acid (14). Intracellular
cysteine can be transformed from methionine and serine through
sulfur transfer. In addition, cysteine can be obtained by the
degradation and recovery of GSH and other proteins (15). However, due to the infinite
proliferation characteristics of tumor cells, a tumor
microenvironment of metabolic stress and oxidative stress, such as
hypoxia and nutrient deficiency, is often formed. As a result, in
this poor nutritional tumor microenvironment, the cysteine, which
is provided through traditional biosynthesis or protein catabolism,
cannot meet the high demand of the cancer cells for antioxidative
defense and metabolic stress. Therefore, the method of obtaining
cystine from an exogenous pathway through a nutrient transporter
and then reducing it to cysteine by GAPDH has become the main
strategy for most tumor cells. SLC7A11 is highly specific to
cystine and glutamate, and is the main protein transporter for
tumor cells to transport cysteine (16,17).
Structure and function of SLC7A11 and its
regulatory mechanism
Structure and function of SLC7A11
The human SLC7A11 gene is located on chromosome 4,
contains 14 exons and consists of 502 amino acids. SLC7A11 is
composed of 12 highly hydrophobic channel transmembrane proteins,
the N- and C-termini of which are located in the cytoplasm
(Fig. 1). SLC7A11 is widely
expressed in normal tissues, such as those of the brain and liver,
and cells, such as macrophages (18). In the internal environment of the
human body, SLC7A11 can transport glutamate from the cell. At the
same time, SLC7A11 can transport extracellular cystine into the
cell. The cystine that enters the cytoplasm can be quickly reduced
to cysteine by GAPDH. The acid is used for the synthesis of GSH.
GSH, as a cofactor of the GPX family (such as GPX4), detoxifies
lipid peroxide into lipid alcohol to protect tumor cells from
oxidative stress, block the ferroptosis of tumor cells and promote
tumor cell proliferation (Fig. 1).
In addition, SLAC7A11 can regulate ferroptosis and participate in
tumor proliferation and survival by regulating the nutritional
dependence of glutamine and glucose (5).
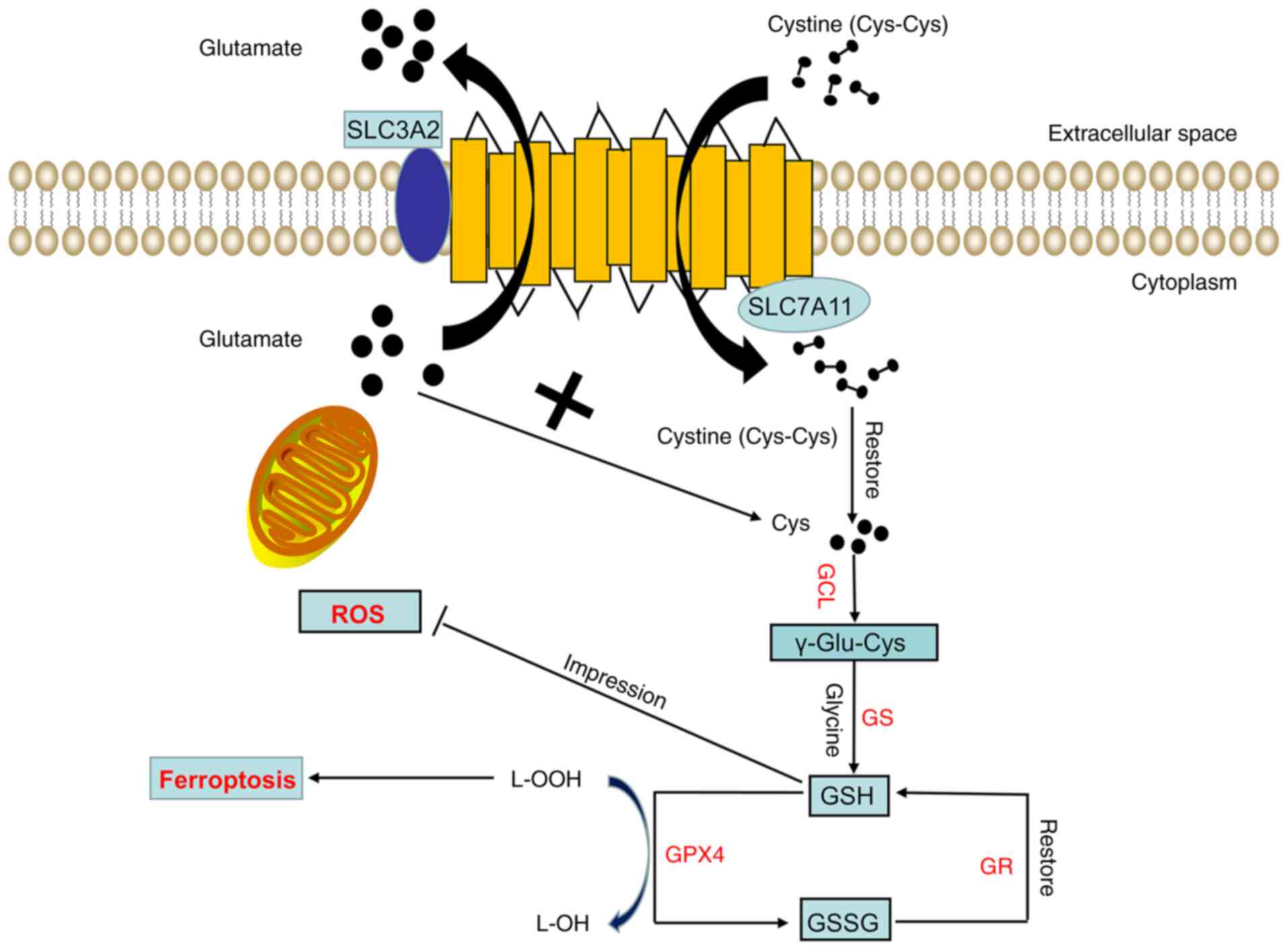 | Figure 1.Structure and function of Xc-. The
Xc- system consists of two subunits, namely, light (SLC7A11) and
heavy (SLC3A2) chain subunits. SLC7A11 exerts the main biological
function and transport activity, whereas SLC3A2 only participates
in maintaining the stability of SLC7A11 as a chaperone protein.
SLC7A11 transfers a molecule of extracellular cystine into the cell
while transferring a molecule of intracellular glutamate out of the
cell. The cystine transported into the cell is quickly reduced to
cysteine. Next, cysteine and glutamic acid are combined under the
action of GCL to form γ-glutamyl cysteine, and glycine is added to
the C-terminus of γ-Glu-Cys through GS to produce reduced GSH. GPX4
then reduces lipid hydroperoxides into lipid alcohols through GSH,
thereby inhibiting ferroptosis. At this time, GSH is oxidized into
GSSG, which can be reduced to GSH again through GR. GCL, glutamate
cysteine ligase; GS, glutathione synthetase; GPX4, glutathione
peroxidase 4; GR, glutathione reductase; GSH, reduced glutathione;
GSSG, oxidized glutathione; L-OOH, lipid hydroperoxide; L-OH, lipid
alcohol; ROS, reactive oxygen species; SLC7A11, solute carrier
family 7 member 11; SLC3A2, solute carrier family 3 member 2. |
Regulation mechanism of SLC7A11
The expression and activity of SLC7A11 are regulated
by a variety of mechanisms, including transcription factors and
epigenetic regulation.
Transcriptional regulation of SLC7A11
by transcription factors
Koppula et al (19) reported that under various stress
conditions, such as oxidative stress and amino acid deficiency, the
expression of SLC7A11 can be significantly upregulated, which is
beneficial for cells to restore redox homeostasis and continue to
survive under stress conditions. Activating transcription factor 4
(ATF4) and nuclear erythroid 2-related factor 2 (NRF2) are the two
main transcription factors involved in the stress-induced
transcription of SLC7A11. ATF4 is a member of the ATF/cAMP
responsive element binding protein (ATF/CREB) transcription factor
family and regulates redox homeostasis, amino acid metabolism and
endoplasmic reticulum stress (20). When the living environment of a
cell lacks amino acids, especially when glutamine and cysteine are
lacking, the cell can transfer ATF4 into the nucleus through the
general control nonderepressible 2 (GCN2)/eukaryotic initiation
factor 2α (eIF2α)/ATF4 signaling axis and bind to the amino acid
response element (AARE) in the gene promoter, thereby promoting the
transcription of genes involved in amino acid metabolism and stress
response, including the transcription of SLC7A11 (21) (Fig.
2). NRF2 is a transcription factor that predominantly mediates
the antioxidant response. Under normal physiological conditions,
NRF2 is extremely unstable, can be ubiquitinated by Kelch-like
ECH-associated protein 1 (KEAP1), and is rapidly degraded by the
proteasome pathway. In the case of oxidative stress, the NRF2
degradation pathway mediated by KEAP1 is blocked, and NRF2 can
remain stable and bind to the AARES region of the antioxidant
response element to induce the transcription of a variety of
antioxidant defense genes, including SLC7A11 (Fig. 2). ATF4 and NRF2 have been found to
have a synergistic effect. Ye et al (22) reported that ATF4 and NRF2 can
interact on the SLC7A11 promoter, and can synergistically regulate
the SLC7A11 transcription under a variety of metabolic stress
conditions.
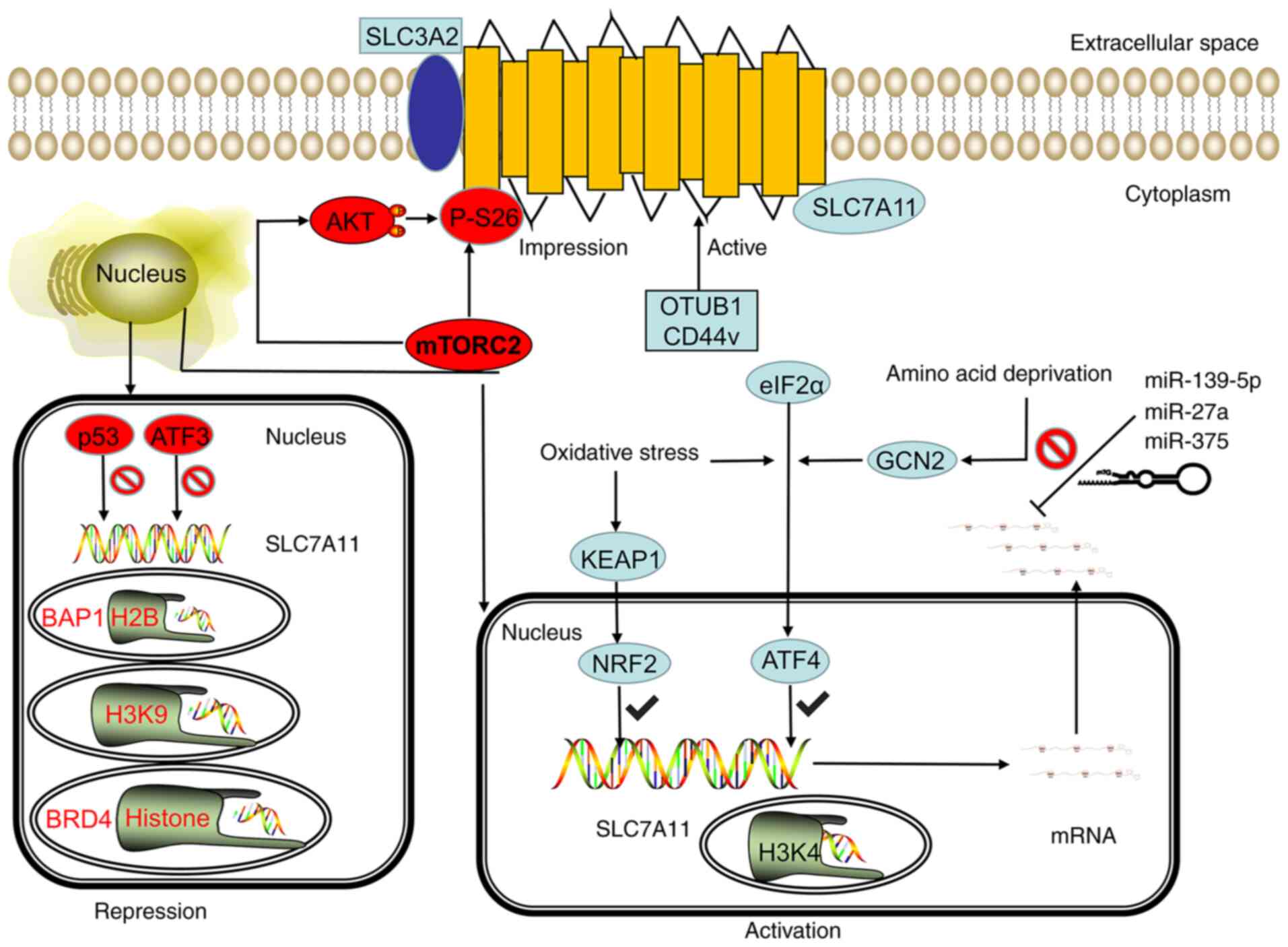 | Figure 2.SLC7A11 is regulated by
transcriptional, post-transcriptional and post-translational
mechanisms. Under stress conditions, such as oxidative stress and
amino acid deficiency, the GCN2-eIF2α and KEAP1-NRF2 signal axes
can induce SLC7A11 transcription, and the SWI/SNF chromatin
remodeling complex can further promote the NRF2-mediated
transcriptional activation of SLC7A11. However, p53 and ATF3
inhibit the expression of SLC7A11 under basal conditions. In
epigenetics, the methylation of H3K4 can activate the transcription
of SLC7A11, whereas the methylation of H3K9me3 and H3K27me3 can
inhibit the transcription activity of SLC7A11. BRD4 can inhibit the
transcription of SLC7A11 by regulating histone acetylation. The
stability of SLC7A11 mRNA can be regulated by microRNA. miR-139-5p,
mir-27a, and mir-375 can destroy the stability of SLC7A11 mRNA and
inhibit its transcription. mTORC2 can regulate the phosphorylation
of SLC7A11 at serine site 26 and inhibit the transport activity of
SLC7A11 directly or through the AKT signaling pathway. CD44v and
OTUB1 can inhibit the degradation of SLC7A11, thereby stabilizing
the SLC7A11 protein and improving its transport activity. GCN2,
general control nonderepressible 2; eIF2α, eukaryotic initiation
factor 2α; KEAP1, kelch-like ECH-associated protein 1; NRF2,
nuclear erythroid 2-related factor 2; mTORC2, mammalian target of
rapamycin complex 2; OTUB1, OTU deubiquitinase ubiquitin aldehyde
binding 1; ATF, activating transcription factor; p53, tumor protein
53; SWI/SNF, switch/sucrose non-fermentable modeling complex; BRD4,
bromodomain-containing protein 4; miR, microRNA; CD44v, CD44
variant; SLC7A11, solute carrier family 7 member 11; SLC3A2, solute
carrier family 3 member 2. |
Transcription factors can negatively regulate
SLC7A11, and the main transcription factors for this are p53 and
ATF3 (Fig. 2). The p53 protein is
a transcription factor, and the gene is a tumor suppressor. A study
has shown that p53 can promote ferroptosis in cells under various
ferroptosis-inducing conditions. Part of its effect is achieved by
inhibiting the expression of SLC7A11. p53 deficiency can evidently
lead to the upregulation of SLC7A11, thereby enhancing the
resistance of tumor cells to ferroptosis and inhibiting ferroptosis
(23). One study has further
revealed that p53 can inhibit the expression of SLC7A11 by
weakening the function of NRF2. ATF3 is another member of the
ATF/CREB transcription factor family. Under basic conditions, ATF3
can bind to the SLC7A11 promoter and inhibit the expression of
SLC7A11. However, under stress conditions that can induce the
expression of SLC7A11, ATF3 does not inhibit the expression and
activity of SLC7A11. Part of the role of Erastin (an inhibitor of
SLC7A11) is achieved by upregulating ATF3 and promoting the
ferroptosis of cells (24).
Overall, various stress conditions can partially
promote the transcription of SLC7A11 through ATF4 and/or NRF2,
whereas p53 and ATF3 predominantly inhibit the expression of
SLC7A11 under basal conditions. These transcription factors affect
the downstream biological effects (including cysteine metabolism)
mediated by SLC7A11 by regulating the expression and
transcriptional activity of SLC7A11, leading to changes in the
sensitivity of cells to ferroptosis. In addition, SLC7A11 mRNA is
directly regulated by miR-139-5p, miR-27a and miR-375 (25–27)
(Fig. 2).
Transcriptional regulation of SLC7A11
by epigenetic modification
The epigenetic regulation of gene transcription is
predominantly achieved through related modifications of DNA or
DNA-related histones, such as DNA methylation, histone acetylation,
methylation and ubiquitination. (28). A study showed that BRCA1-associated
protein-1 (BAP1) is a nuclear protein with deubiquitinase that
removes histone H2A monoubiquitylation (H2Aub) at position 119 of
lysine. In a recent study, whole-genome analysis identified SLC7A11
as a key transcription target of BAP1 (29). At the SLC7A11 promoter, BAP1
deubiquitinates H2Aub and subsequently inhibits the expression of
SLC7A11. The methylation of histone H3 (H3K9me3 and H3K27me3) at
the lysine 9 and 27 positions also leads to the transcriptional
inhibition of SLC7A11. Bromodomain-containing protein 4 (BRD4) is a
member of the bromodomain and extra-terminal domain protein family.
The main function of BRD4 is to recognize acetylated histones and
recruit transcription factors to regulate gene transcription.
Recent research showed that knocking out the BRD4 gene or using
BRD4 inhibitors significantly inhibited the expression of SLC7A11
and promoted ferroptosis (Fig. 2)
(30), indicating that BRD4 is
likely to activate SLC7A11 transcription through epigenetic
mechanisms. This phenomenon inhibits cell ferroptosis.
Chromatin remodeling is another key epigenetic
mechanism that controls genes. Recently, studies showed that
switch/sucrose non-fermentable (SWI/SNF) complex-mediated chromatin
remodeling is involved in regulating SLC7A11 transcription. The
SWI/SNF complex combines with the SLC7A11 promoter to promote the
NRF2-mediated transcriptional activation of SLC7A11 through the
chromatin remodeling of the SWI/SNF complex. SWI/SNF deficiency
leads to the suppression of SLC7A11 transcription, which causes
impaired cystine uptake and GSH biosynthesis, and subsequently
promotes ROS-induced cell ferroptosis (31).
It was recently found that the adhesion molecule
CD44 variant (CD44v) forms a complex by binding to SLC7A11, thereby
maintaining the stability of SLC7A11. The inactivation of CD44v
destroys the stability of SLC7A11 and promotes ferroptosis
(32). In addition, mammalian
target of rapamycin complex 2 can directly phosphorylate SLC7A11 at
serine 26 and serine 25 through the AKT signaling pathway (33,34)
(Fig. 2).
In summary, these studies confirmed that SLC7A11 can
be regulated by a variety of post-translational mechanisms,
including regulation of protein stability, localization and
transporter activity.
SLC7A11 regulates the association between
cysteine metabolism and tumors
Cysteine is involved in the synthesis of GSH, and
GSH can be used as a cofactor of GPX. Lipid peroxides detoxify into
lipid alcohol, thereby protecting tumor cells from oxidative stress
and blocking the ferroptosis of tumor cells. Tumor cells obtain
cysteine from outside the cell through SLC7A11 to maintain the
level of homo-GSH in the cell, thereby meeting the high energy
demand against oxidative stress. This phenomenon is conducive to
the survival and development of tumor cells. SLC7A11 can also
participate in the metabolic reprogramming of tumors by regulating
cysteine metabolism, and promote tumor growth and migration, such
as via increasing ingestion of glutamine (35).
Next, the present review specifically discusses the
association between the regulation of cysteine metabolism and tumor
occurrence, development and prognosis by SLC7A11. The study
attempts to show that SLC7A11 is widely expressed in various tumors
of the human body and can be a potential therapeutic target for a
number of systems.
SLC7A11 regulates cysteine metabolism
and liver cancer
The etiology of liver cancer is related to
environment, diet or lifestyle factors (36). Evidence shows that liver cancer is
closely related to hepatitis B/C virus infection and liver
cirrhosis (37). There are
~700,000 new liver cancer patients worldwide each year, and cases
in China account for more than half of the global total. Given the
low sensitivity of liver cancer to radiotherapy and chemotherapy,
the current main treatments are surgery and liver transplantation.
However, as liver cancer cells are prone to metastasis, these
treatments often fail to achieve the expected results (38). Therefore, finding new and effective
targets for the diagnosis and treatment of liver cancer has become
an urgent problem to be solved. Kinoshita et al (39) showed the association between
SLC7A11 in mRNA transcription and the clinical characteristics of
hepatoceullar carcinoma, and proposed that SLC7A11 has prognostic
value in this disease. However, the study also reported that the
expression of SLC7A11 protein was detected in only one out of eight
liver cancer specimens (39).
Recent studies have found that compared with that in normal tissues
and cells, SLC7A11 expression is high at the protein level in liver
cancer tissues and cells, and is significantly related to an
advanced poor prognosis (40),
further confirming that SLC7A11 can be used as an independent
prognostic factor of liver cancer factor. A further study by Wada
et al (41) found that
SLC7A11 is highly expressed in poorly differentiated liver cancer.
In vitro experiments also confirmed that the SLC7A11Z
inhibitor sulfasalazine (SASP) could inhibit the uptake of
cysteine, reduce the resistance of liver cancer cells to ROS and
lipid peroxide, and enhance the ferroptosis of liver cancer cells
by inhibiting CD44v9-SLC7A11 (41).
The liver is the organ with the highest expression
and activity of cysteine dioxygenase (CDO) protein in the human
body. CDO occupies a key position in the normal synthesis and
degradation of cysteine in the human body and is an important
biological enzyme of cysteine, which is obtained through the diet
(9). The development of liver
cancer often follows the trilogy of hepatitis-cirrhosis-liver
cancer. Abnormalities in liver function are present at each step,
but whether this leads to abnormalities in CDO needs further
investigation. We hypothesize that the occurrence and development
of liver cancer is closely related to the abnormal physiological
pathway of cysteine, and that iver cancer cells can obtain
increased cysteine from extracellular pathways by upregulating the
expression of SLC7A11 in the presence of toxic substances and
oxidative stress. This aspect is a direction worth exploring in the
future. In addition, SLC7A11 is closely related to the ferroptosis
resistance and glutamine deprivation of liver cancer cells.
Therefore, SLC7A11 could be a potential target for the treatment of
liver cancer.
SLC7A11 regulates cysteine metabolism
and lung cancer
The incidence and mortality of lung cancer rank
first in the world, and the rates are increasing every year
(42). With the aging of the
Chinese population, the incidence of lung cancer is predicted to
continue to rise. Traditional treatment options for lung cancer
include surgery, radiation therapy and chemotherapy (43). In recent years, the targeted
therapy of lung cancer has received increasing attention and
recognition. Programmed cell death protein 1 (PD-1), PD ligand
protein-1 and cytotoxicity T lymphocyte-associated antigen-4
inhibitors showed good effects (increased survival time and improve
survival rate) in the treatment of small cell lung cancer (SCLC)
and non-SCLC (NSCLC). However, as treatment progresses, most
patients eventually develop resistance (44). Therefore, finding new targets for
the treatment of lung cancer is important to improve the survival
and quality of life of patients with this disease. Recent studies
showed that SLC7A11 was overexpressed in lung adenocarcinoma,
squamous cell carcinoma and NSCLC. The follow-up analysis of 254
patients with NSCLC found that patients with high expression of
SLC7A11 tended to be at later stages and that the survival times
were significantly shortened (45). These results confirmed that the
expression of SLC7A11 is significantly correlated with cancer stage
and 5-year overall survival rate. SLC7A11 decreases the GSH/GSSG
ratio through overexpression, thereby creating an oxidized
intracellular microenvironment in multiple lung cancer cell lines
and promoting tumor growth. When the inhibitor Erastin is used to
target the expression of SLC7A11, the growth and proliferation of
lung cancer cells are significantly inhibited (45). In normal tracheal epithelial cells,
SLC7A11 has been shown to participate in the metabolic
reprogramming of the cells by regulating cystine metabolism, which
is likely to be closely related to the occurrence of lung cancer
(45). At present, the occurrence
of lung cancer is known to be related to a variety of gene
mutations, such as the translocation of EGFR, TP53, KRAS, ALK, RET
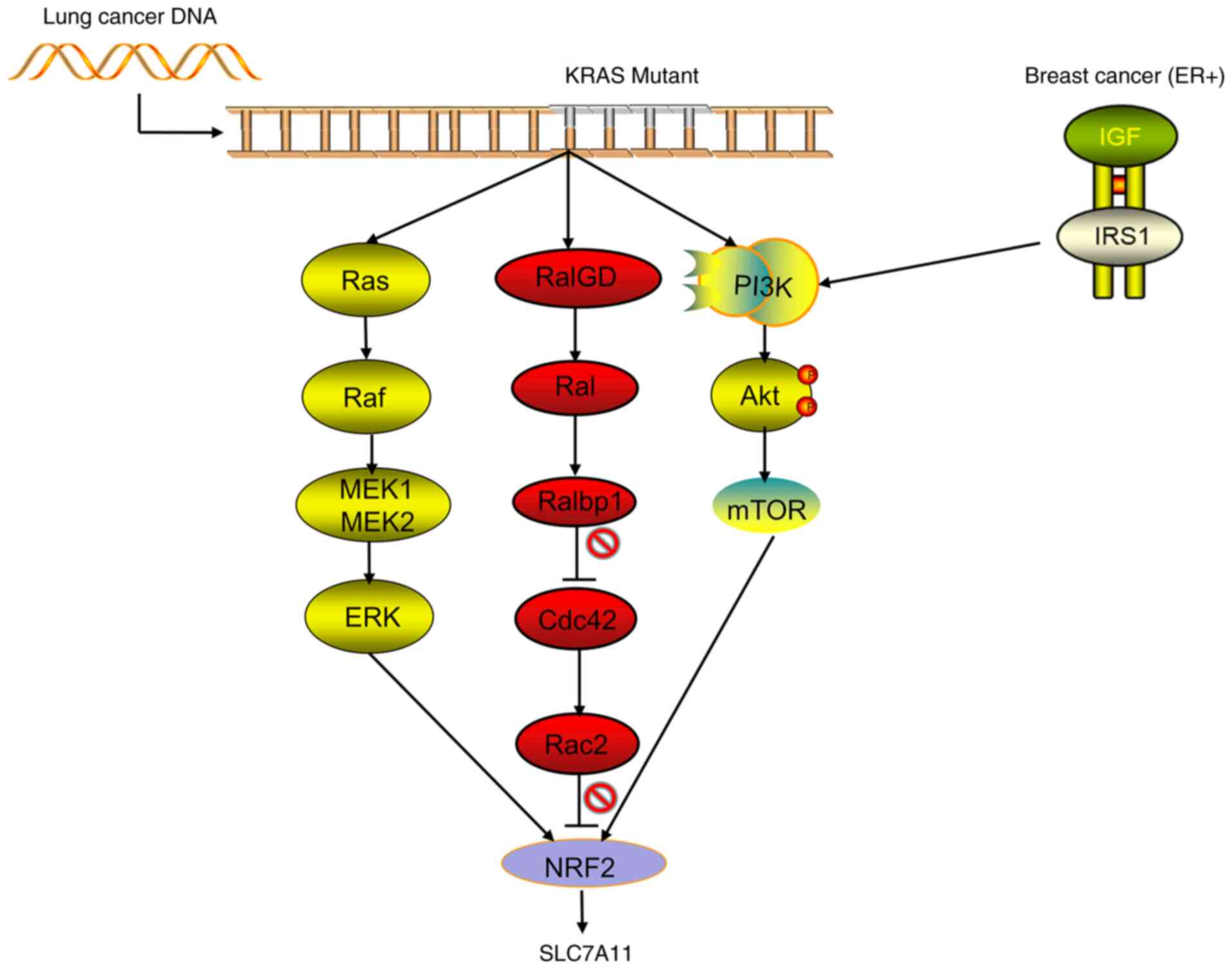
or ROS1. Recent studies by Hu et al (46) confirmed that the KRAS mutant gene
of lung adenocarcinoma acts on NRF2 through its downstream pathways
(including MAPK, PI3K/AKT and RAS-related protein pathways),
regulates the expression of SLC7A11, upregulates the levels of
intracellular cysteine and GSH, and then promotes the progression
of the tumor (Fig. 3). Compared
with patients with lung cancer without KRAS mutation, those with
lung cancer and KRAS mutations have significantly increased
expression levels of NRF2 and SLC7A11, significantly upregulated
intracellular cysteine and a significantly decreased overall
survival rate (46). Wang et
al (47) found that in NSCLC,
the TP53 mutant has lost the functions of inducing cell cycle
arrest and senescence, but still plays a certain role in inhibiting
tumors by blocking the function of SLC7A11 (47). However, Jennis et al
(48) constructed a TP53 mutant
that retained the function of inducing cell cycle arrest, but could
not inhibit the function of SLC7A11, and found that the tumor
suppressor effect of the mutant was markedly decreased (48). Therefore, we hypothesize that the
occurrence of NSCLC is closely related to TP53 and KRAS mutations,
but that the pathogenesis is predominantly a series of functional
abnormalities, such as TP53/KRAS-SLC7A11 cysteine ferroptosis
rather than the abnormalities of cell cycle regulation caused by
TP53 mutation in the traditional sense. Studies showed that CD44v
and SLC7A11 are involved in the resistance of lung cancer to
cisplatin and other drugs. The use of SASP can improve the drug
resistance of lung cancer and promote the death of tumor-related
stem cells (49,50). The relationship among EGFR, ALK and
RET or between ROS1 and SLC7A11 needs to be further explored. SPAP
plays an important role in inhibiting tumor growth and promoting
ferroptosis, but requires a large dose to inhibit SLC7A11, thereby
limiting the clinical application of SPAP. Therefore, Hu et
al (46) also screened out the
compound HG106, which has achieved good effects in lung cancer
modeling mice (46). These studies
showed that SLC7A11 is involved in the occurrence and drug
resistance of lung cancer and is closely related to the survival
rate and prognosis of patients. Therefore, SLC7A11 can be used as a
potential site for the molecular targeted therapy of lung cancer.
At present, relevant research and clear evidence to support SLC7A11
driving the mutations of genes, such as TP53 and KRAS, and the
specific mechanisms driving the aforementioned gene mutations
remain lacking. This gap may be the key research direction of
SLC7A11 in the field of lung cancer in the future.
SLC7A11 and breast cancer
Breast cancer is the most common malignant tumor in
women and the main cause of death in women with cancer (42). The full use of antiestrogen hormone
drugs (e.g., tamoxifen and aromatase inhibitors) and trastuzumab
therapy (Herceptin) has benefited patients with breast cancer, but
metastasis and relapse affect the survival of patients, especially
in those with triple-negative breast cancer (TNBC) (51). Therefore, targeted and minimally
toxic treatments for breast cancer are needed. SLC7A11 is reported
to be highly expressed in a variety of breast cancer cells. Breast
cancer cells with high expression of SLC7A11 have strong resistance
to oxidative stress and ferroptosis, but are sensitive to the
nutritional deprivation of glucose (52). Along with the high expression of
SLC7A11, glucose deprivation can increase the ROS level of breast
cancer cells through the AMPK/ACC pathway, thereby promoting the
death of breast cancer cells (52). In estrogen receptor-positive breast
cancer, the expression of SLC7A11 can be upregulated through the
IGF-1/IRS-1/PI3K signaling axis to improve the ability of the tumor
to withstand oxidative stress, which is beneficial to the survival
of tumor cells and the occurrence of chemotherapy resistance
(53) (Fig. 3). Ruiu et al (54) showed that the use of anti-SLC7A11
antibody vaccines to inhibit the expression of SLC7A11 can increase
the sensitivity of breast cancer to chemotherapy (such as
doxorubicin) and molecular targeted (such as PD-1) drugs. In
addition, the use of this vaccine can induce autoimmune responses
by targeting differentiated cancer stem cells, thereby achieving
good therapeutic effects and having minimal toxicity. The
expression can also be used to diagnose and treat the autoimmune
response of tumor stem cells to achieve good results and have an
excellent response, such as killing tumor cells, limiting tumor
metastasis and exhibiting a less immunotoxic response (54). Conti et al (55) also confirmed that anti-HER2 vaccine
predominantly inhibits tumor growth and that anti-SLC7A11 vaccine
predominantly inhibits tumor metastasis, thereby showing that
SLC7A11 can be used as a breast cancer treatment target. However,
studies on the relationship between SLC7A11 and TNBC are few. Given
the limited available treatments for TNBC, new methods are urgently
needed. Therefore, the relationship between SLC7A11 and TNBC still
needs to be further explored, and SLC7A11 still has marked
prospects as a breast cancer treatment target.
SLC7A11 and glioma
Glioma is the most common malignant primary brain
tumor. The rapid growth of this tumor benefits from the
tumor-mediated release of glutamate, and the release of glutamate
from gliomas is considered to cause the death of peritumoral
neurons and make room for tumor growth (56). A previous study showed that the
expression of SLC7A11 in the tumor tissues of ~50% of patients with
glioma was higher than that in normal tissues adjacent to the
cancer. Compared with those lacking the transporter, tumors with
high expression of SLC7A11 had strong proliferation ability and
could produce increased excitotoxicity of glutamate, thus
shortening the overall survival of the patients (57). This phenomenon indicates that
SLC7A11 can be used as an independent prognostic indicator for
patients with glioma. SLC7A11 is reported to promote the occurrence
and development of gliomas by participating in the promotion of
tumor-associated epilepsy, an important cause of glioma in
patients. Although various brain tumors may induce seizures, the
highest incidence and prevalence of epilepsy is found within
patients with glioma (≤80%) (58,59).
Consistent with the research in lung cancer, Polewski et al
(60) also found in glioma that
SLC7A11 transports cysteine into tumor cells, participates in the
synthesis of GSH and downregulates cellular ROS levels, thereby
generating increased cancer-like stem cells. The functions of
SLC7A11 and tumor stem cells are basically the same and are
involved in the recurrence and metastasis of glioma. SLC7A11 may be
regulated by EGFR/CD44 in glioma cells (61). The study by Long et al
(62) showed that treatment with
VEGF blockers can lead to increased expression of SLC7A11. In
addition, the study pointed out that the glutamate output by
SLC7A11 from tumor cells can promote the proliferation of
regulatory T cells and improve the immunosuppressive function,
thereby helping in improving the adaptability and drug resistance
of glioblastoma against VEGF treatment (62). Robert et al (57) also found that the use of SLC7A11
inhibitors or targeted interference downregulates SLC7A11 levels
due to cysteine uptake disorders, and that the resistance of the
tumor to ROS is weakened, which can significantly increase the
death of glioma cells (57).
Although in vivo experiments need to be further improved,
the aforementioned studies have confirmed that SLC7A11 is closely
related to the prognosis, recurrence and metastasis of glioma, and
that it is expected to become a new therapeutic target for
glioma.
In addition, existing studies have also shown the
relationship between SLC7A11 and pancreatic cancer, colorectal
cancer and lymphoma in terms of prognosis and metastasis, and that
SLC7A11 also plays a role in promoting cancer (25,63,64),
but the conclusions and mechanisms of action need further
experimental verification.
Future prospects
SLC7A11, as an amino acid transport active subunit
on the cell membrane, exerts a wide range of biological effects in
organisms. At the same time, abnormality in the regulatory
mechanism of SLC7A11 plays an important role in the process of
tumor invasion, migration and drug resistance in multiple systems,
such as the digestive, respiratory, reproductive and nervous
systems. SLC7A11 is an important biomarker for tumor diagnosis and
prognosis in various systems. Evidence has indicated that SLC7A11
can be used as a broad target for tumor treatment. At present, the
two strategies with regard to SLC7A11 are the direct inhibition of
SLC7A11-mediated cystine uptake and the targeting of
SLC7A11-induced glucose or glutamine dependence. The first strategy
occurs via the direct use of SLC7A11 inhibitors, such as SASP, to
inhibit its uptake of cystine, antagonize its role in tumor
resistance and tumor stem cell formation, and improve tumor
treatment efficacy. At present, these studies have achieved only
preliminary results. A number of clinical trials on SASP in tumor
resistance and stem cell therapy (e.g., EPOC1205, EPOC1407 and
UMIN00017854) are underway. In addition, a vaccine against SLC7A11
has been created, but the safety of the vaccine needs to be further
explored, as it may require a large dose to achieve the inhibitory
effect, and any toxicity and side effects should be assessed.
Therefore, the development of new high-efficiency small-molecule
drugs targeting SLC7A11 is one of the development directions of
tumor treatment. The second strategy is to select a glucose
transporter or glutaminase inhibitors for the treatment of SLC7A11
overexpression tumors, which can induce tumor cell death by using
the high sensitivity of SLC7A11 overexpression tumors to glucose
and glutamine deficiency. Recent studies showed that the
combination of immunotherapy (or radiotherapy) and SLC7A11
inhibitors (e.g., imidazole ketone erastin sulfadiazine and
sorafenib) has a strong therapeutic effect (65,66).
In addition, SLC7A11 promotes the synthesis of GSH by transporting
cysteine, thereby increasing the drug resistance of various tumors,
such as lung cancer, breast cancer and glioma. The drug resistance
caused by SLC7A11 is also related to the activation of MAPK and
other pathways. The development of drugs that inhibit or block
these pathways can reverse tumor resistance and enhance the
efficacy of tumor radiotherapy and chemotherapy. After overcoming
various limitations and drawbacks, SLC7A11 may become a new
prognostic indicator and a new therapeutic target for tumors in the
future.
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
Not applicable.
Authors' contributions
XT and JW searched the literature and drafted the
manuscript. WC and HL performed revisions to the manuscript. DC
reviewed and edited the manuscript. NL and DT were involved in the
conception of the study. All authors read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zheng J and Gao P: Toward normalization of
the tumor microenvironment for cancer therapy. Integr Cancer Ther.
18:15347354198623522019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bissell MJ and Hines WC: Why don't we get
more cancer? A proposed role of the microenvironment in restraining
cancer progression. Nat Med. 17:320–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meng X, Yang Y, Zhou L, Zhang L, Lv Y, Li
S, Wu Y, Zheng M, Li W, Gao G, et al: Dual-responsive molecular
probe for tumor targeted imaging and photodynamic therapy.
Theranostics. 7:1781–1794. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stockwell BR, Angeli JP, Bayir H, Bush AI,
Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, et al:
Ferroptosis: A regulated cell death nexus linking metabolism, redox
biology, and disease. Cell. 171:273–285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dolma S, Lessnick SL, Hahn WC and
Stockwell BR: Identification of genotype-selective antitumor agents
using synthetic lethal chemical screening in engineered human tumor
cells. Cancer Cell. 3:285–296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sato H, Tamba M, Ishii T and Bannai S:
Cloning and expression of a plasma membrane cystine/glutamate
exchange transporter composed of two distinct proteins. J Biol
Chem. 274:11455–11458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kandasamy P, Gyimesi G, Kanai Y and
Hediger MA: Amino acid transporters revisited: New views in health
and disease. Trends Biochemical Sci. 43:752–789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stipanuk MH, Dominy JE Jr, Lee JI and
Coloso RM: Mammalian cysteine metabolism: New insights into
regulation of cysteine metabolism. J Nutr. 136 (Suppl
6):1652S–1659S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Combs JA and DeNicola GM: The
non-essential amino acid cysteine becomes essential for tumor
proliferation and survival. Cancers (Basel). 11:6782019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu SC: Regulation of glutathione
synthesis. Mol Aspects Med. 30:42–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Friedmann JP, Schneider M, Proneth B,
Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A,
Eggenhofer E, et al: Inactivation of the ferroptosis regulator Gpx4
triggers acute renal failure in mice. Nat Cell Biol. 16:1180–1191.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scalise M, Pochini L, Pingitore P, Hedfalk
K and Indiveri C: Cysteine is not a substrate but a specifific
modulator of human ASCT2 (SLC1A5) transporter. FEBS Lett.
589:3617–3623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanigan MH and Ricketts WA: Extracellular
glutathione is a source of cysteine for cells that express
gamma-glutamyl transpeptidase. Biochemistry. 32:6302–6306. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zerbib E, Arif T, Shteinfer-Kuzmine A,
Chalifa-Caspi V and Shoshan-Barmatz V: VDAC1 silencing in cancer
cells leads to metabolic reprogramming that modulates tumor
microenvironment. Cancers (Basel). 13:28502021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei G, Zhang Y, Koppula P, Liu X, Zhang J,
Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H, et al: The role of
ferroptosis in ionizing radiation-induced cell death and tumor
suppression. Cell Res. 30:146–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fotiadis D, Kanai Y and Palacín M: The
SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med.
34:139–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koppula P, Zhang Y, Shi J, Li W and Gan B:
The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell
dependency on glucose by exporting glutamate. J Biol Chem.
292:14240–14249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pakos-Zebrucka K, Koryga I, Mnich K,
Ljujic M, Samali A and Gorman AM: The integrated stress response.
EMBO Rep. 17:1374–1395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kilberg MS, Shan J and Su N:
ATF4-dependent transcription mediates signaling of amino acid
limitation. Trends Endocrinol Metab. 20:436–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye P, Mimura J, Okada T, Sato H, Liu T,
Maruyama A, Ohyama C and Itoh K: Nrf2-and ATF4-dependent
upregulation of xCT modulates the sensitivity of T24 bladder
carcinoma cells to proteasome inhibition. Mol Cell Biol.
34:3421–3434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clemons NJ, Liu DS, Duong CP and Phillips
WA: Inhibiting system xC (−) and glutathione biosynthesis-a
potential Achilles'hell in mutant-p53. Mol Cell Oncol.
4:e13447572017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu JH, De Mello RA, Yan QL, Wang JW, Chen
Y, Ye QH, Wang ZJ, Tang HJ and Huang T: MiR-139-5p/SLC7A11 inhibits
the proliferation, invasion and metastasis of pancreatic carcinoma
via PI3K/Akt signaling pathway. Biochim Biophys Acta Mol Basis Dis.
1866:1657472020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Drayton RM, Dudziec E, Peter S, Bertz S,
Hartmann A, Bryant HE and Catto JW: Reduced expression of miRNA-27a
modulates cisplatin resistance in bladder cancer by targeting the
cystine/glutamate exchanger SLC7A11. Clin Cancer Res. 20:1990–2000.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Y, Sun X, Song B, Qiu X and Zhao J:
MiR-375/SLC7A11 axis regulates oral squamous cell carcinoma
proliferation and invasion. Cancer Med. 6:1686–1697. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jaenisch R and Bird A: Epigenetic
regulation of gene expression: How the genome integrates intrinsic
and environmental signals. Nat Genet. 33 (Suppl):S245–S254. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Shi J, Liu X, Feng L, Gong Z,
Koppula P, Sirohi K, Li X, Wei Y, Lee H, et al: BAP1 links
metabolic regulation of ferroptosis to tumour suppression. Nat Cell
Biol. 20:1181–1192. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sui S, Zhang J, Xu S, Wang Q, Wang P and
Pang D: Ferritinophagy is required for the induction of ferroptosis
by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells.
Cell Death Dis. 10:3312019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ogiwara H, Takahashi K, Sasaki M, Kuroda
T, Yoshida H, Watanabe R, Maruyama A, Makinoshima H, Chiwaki F,
Sasaki H, et al: Targeting the vulnerability of glutathione
metabolism in ARID1A defificient cancers. Cancer Cell. 35:177–190.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu T, Jiang L, Tavana O and Gu W: The
deubiquitylase OTUB1 mediates ferroptosis via stabilization of
SLC7A11. Cancer Res. 79:1913–1924. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu Y, Albuquerque CP, Braas D, Zhang W,
Villa GR, Bi J, Ikegami S, Masui K, Gini B, Yang H, et al: mTORC2
regulates amino acid metabolism in cancer by phosphorylation of the
cystineglutamate antiporter xCT. Mol Cell. 67:128–138. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim J and Guan KL: mTOR as a central hub
of nutrient signalling and cell growth. Nat Cell Biol. 21:63–71.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goji T, Takahara K, Negishi M and Katoh H:
Cystine uptake through the cystine/glutamate antiporter xCT
triggers glioblastoma cell death under glucose deprivation. J Biol
Chem. 292:19721–19732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schlageter M, Terracciano LM, D'Angelo S
and Sorrentino P: Histopathology of hepatocellular carcinoma. World
J Gastroenterol. 20:15955–15964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cabibbo G, Maida M, Genco C, Antonucci M
and Cammà C: Causes of and prevention strategies for hepatocellular
carcinoma. Semin Oncol. 39:374–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu L, Zhu XD, Wang WQ, Shen Y, Qin Y, Ren
ZG, Sun HC and Tang ZY: Activation of beta-catenin by hypoxia in
hepatocellular carcinoma contributes to enhanced metastatic
potential and poor prognosis. Clin Cancer Res. 16:2740–2750. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kinoshita H, Okabe H, Beppu T, Chikamoto
A, Hayashi H, Imai K, Mima K, Nakagawa S, Ishimoto T, Miyake K, et
al: Cystine/glutamic acid transporter is a novel marker for
predicting poorsurvival in patients with hepatocellular carcinoma.
Oncol Rep. 29:685–689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim DH, Kim WD, Kim SK, Moon DH and Lee
SJ: TGF-β1-mediated repression of SLC7A11 drives vulnerability to
GPX4 inhibition in hepatocellular carcinoma cells. Cell Death Dis.
11:4062020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wada F, Koga H, Akiba J, Niizeki T,
Iwamoto H, Ikezono Y, Nakamura T, Abe M, Masuda A, Sakaue T, et al:
High expression of CD44v9 and xCT in chemoresistant hepatocellular
carcinoma: Potential targets by sulfasalazine. Cancer Sci.
109:2801–2810. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bianco A, Perrotta F, Barra G, Malapelle
U, Rocco D and De Palma R: Prognostic factors and biomarkers of
responses to immune checkpoint inhibitors in lung cancer. Int J Mol
Sci. 20:49312019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ji X, Qian J, Rahman SM, Siska PJ, Zou Y,
Harris BK, Hoeksema MD, Trenary IA, Heidi C, Eisenberg R, et al:
xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small
cell lung cancer progression. Oncogene. 37:5007–5019. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu K, Li K, Lv J, Feng J, Chen J, Wu H,
Cheng F, Jiang W, Wang J, Pei H, et al: Suppression of the
SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant
lung adenocarcinoma. J Clin Invest. 130:1752–1766. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang SJ, Li D, Ou Y, Jiang L, Chen Y, Zhao
Y and Gu W: Acetylation is crucial for p53-mediated ferroptosis and
tumor suppression. Cell Rep. 17:366–373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jennis M, Kung CP, Basu S, Budina-Kolomets
A, Leu JIJ, Khaku S, Scott JP, Cai KQ, Campbell MR, Porter DK, et
al: An African-specific polymorphism in the TP53 gene impairs p53
tumor suppressor function in a mouse model. Genes Dev. 30:918–930.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Okamoto K, Saito Y, Narumi K, Furugen A,
Iseki K and Kobayashi M: Different mechanisms of cisplatin
resistance development in human lung cancer cells. Biochem Biophys
Res Commun. 530:745–750. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Otsubo K, Nosaki K, Imamura CK, Ogata H,
Fujita A, Sakata S, Hirai F, Toyokawa G, Iwama E, Harada T, et al:
Phase I study of salazosulfapyridine in combination with cisplatin
and pemetrexed for advanced non-small-cell lung cancer. Cancer Sci.
108:1843–1849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Winston JS, Ramanaryanan J and Levine E:
HER-2/neu evaluation in breast cancer: Are we there yet? Am J Clin
Pathol. 121 (Suppl):S33–S49. 2004.PubMed/NCBI
|
|
52
|
Chen MC, Hsu LL, Wang SF, Hsu CY, Lee HC
and Tseng LM: ROS Mediate xCT-dependent cell death in human breast
cancer cells under glucose deprivation. Cells. 9:15982020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang Y and Yee D: IGF-I regulates redox
status in breast cancer cells by activating the amino acid
transport molecule xC-. Cancer Res. 74:2295–2305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ruiu R, Rolih V, Bolli E, Barutello G,
Riccardo F, Quaglino E, Merighi IF, Pericle F, Donofrio G, Cavallo
F and Conti L: Fighting breast cancer stem cells through the
immune-targeting of the xCT cysteine-glutamate antiporter. Cancer
Immunol Immunother. 68:131–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Conti L, Bolli E, Di Lorenzo A, Franceschi
V, Macchi F, Riccardo F, Ruiu R, Russo L, Quaglino E, Donofrio G
and Cavallo F: Immunotargeting of the xCT cystine/glutamate
antiporter potentiates the efficacy of HER2-targeted
immunotherapies in breast cancer. Cancer Immunol Res. 8:1039–1053.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada
N, Hagimura N, Okado H, Miwa A, Kurihara H, Nakazato Y, Tamura M,
et al: Blockage of Ca(2+)-permeable AMPA receptors suppresses
migration and induces apoptosis in human glioblastoma cells. Nat
Med. 8:971–978. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
57
|
Robert SM, Buckingham SC, Campbell SL,
Robel S, Holt KT, Ogunrinu-Babarinde T, Warren PP, White DM, Reid
MA, Eschbacher JM, et al: SLC7A11 expression is associated with
seizures and predicts poor survival in patients with malignant
glioma. Sci Transl Med. 7:289ra862015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Breemen MS, Wilms EB and Vecht CJ:
Epilepsy in patients with brain tumours: Epidemiology, mechanisms,
and management. Lancet Neurol. 6:421–430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sørensen MF, Heimisdóttir SB, Sørensen MD,
Mellegaard CS, Wohlleben H, Kristensen BW and Beier CP: High
expression of cysteine-glutamate antiporter xCT (SLC7A11) is an
independent biomarker for epileptic seizures at diagnosis in
glioma. J Neurooncol. 138:49–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Polewski MD, Reveron-Thornton RF,
Cherryholmes GA, Marinov GK and Aboody KS: SLC7A11 overexpression
in glioblastoma is associated with increased cancer stem cell-like
properties. Stem Cells Dev. 26:1236–1246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tsuchihashi K, Okazaki S, Ohmura M,
Ishikawa M, Sampetrean O, Onishi N, Wakimoto H, Yoshikawa M,
Seishima R, Iwasaki Y, et al: The EGF receptor promotes the
malignant potential of glioma by regulating amino acid transport
system xc(−). Cancer Res. 76:2954–2963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Long Y, Tao H, Karachi A, Grippin AJ, Jin
L, Chang YE, Zhang W, Dyson KA, Hou AY, Na M, et al: Dysregulation
of glutamate transport enhances treg function that promotes VEGF
blockade resistance in glioblastoma. Cancer Res. 80:499–509. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xu X, Zhang X, Wei C, Zheng D, Lu X, Yang
Y, Luo A, Zhang K, Duan X and Wang Y: Targeting SLC7A11
specifically suppresses the progression of colorectal cancer stem
cells via inducing ferroptosis. Eur J Pharm Sci. 152:1054502020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gout PW, Buckley AR, Simms CR and
Bruchovsky N: Sulfasalazine, a potent suppressor of lymphoma growth
by inhibition of the x(c)-cystine transporter: A new action for an
old drug. Leukemia. 15:1633–1640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang W, Green M, Choi JE, Gijon M, Kennedy
PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al: CD8(+) T
cells regulate tumour ferroptosis during cancer immunotherapy.
Nature. 569:270–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ye LF, Chaudhary KR, Zandkarimi F, Harken
AD, Kinslow CJ, Upadhyayula PS, Dovas A, Higgins DM, Tan H, Zhang
Y, et al: Radiation-induced lipid peroxidation triggers ferroptosis
and synergizes with ferroptosis inducers. ACS Chem Biol.
15:469–484. 2020. View Article : Google Scholar : PubMed/NCBI
|