Introduction
Vascular endothelial growth factor (VEGF), an
important angiogenic rate limiting factor, combined with its
specific receptors, VEGFR-1 and VEGFR-2, may stimulate vascular
endothelial cell proliferation promoting the formation of blood and
lymphatic vessels, tumor growth and metastasis (1). Being a vascular solid malignant tumor,
the biological behaviors of gastric cancer progression and
metastasis depend on neovascularity (2). High levels of VEGF protein expressions
have been confirmed in the human gastric cancer MGC-803 cell line
(3). Endostar (ES), an
anti-angiogenetic drug, inhibits tumor angiogenesis by reducing
VEGF protein levels. This study aimed to examine the effects of ES
on the VEGF expression in MGC-803 cells.
Materials and methods
Study approval
The present study was approved by the ethics
committee of the First Affiliated Hospital of the Liaoning Medical
University. The subjects enrolled in the study provided written
formal consent.
Materials
The MGC-803 cell line was purchased from the Cell
Bank, Shanghai Institute of Life Sciences, Chinese Academy of
Sciences (Shanghai, China). RPMI-1640 culture medium and fetal
bovine serum (FBS) were purchased from Gibco (Carlsbad, CA, USA).
TRIzol reagent and the RT-PCR kit were purchased from Takara
(Shiga, Japan). PV two-step immunocytochemistry kit was purchased
from Zhongshan Golden Bridge (Beijing, China). Rabbit anti-human
VEGF monoclonal antibody, horseradish peroxidase-labeled secondary
antibody (goat anti-rabbit HRP-IgG) and β-actin were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). ES (15 mg/3
ml/ampoule containing 3×105 units) was donated by
Maidejing Company (Yantai, China). Primers were designed and
synthesized by Takara.
Cell culture
MGC-803 cells were cultured in RPMI-1640 culture
medium containing 10% of FBS at 37°C in a 5% CO2
atmosphere. When the growing cells covered 80% of the bottom area
of the culture bottle, the cells were digested with 0.25% of
trypsin at 37°C for 1 min. The cells were washed with
phosphate-buffered saline (PBS) followed by the addition of fresh
culture medium. Mechanical blow allowed cells into single cell
suspension for passage. The cells of passage 5 were stored until
use.
VEGF expression examined using
immunocytochemistry
The cells were cultured until cell growth reached a
single-layer, and then fixed with cold acetone. The primary
antibody, rabbit anti-human VEGF monoclonal antibody, was diluted
according to 1:50 with PBS. At the same time, PBS served as the
negative control instead of VEGF. The secondary antibody, goat
anti-rabbit HRP-IgG was diluted according to 1:200. Staining was
performed with DAB. The slides were then sealed with gum. In case
of brown particles in the cytoplasm and cell membrane, VEGF protein
was considered present in gastric cancer cells.
VEGF mRNA expression detected with
RT-PCR
MGC-803 cells were divided into 4 groups
(n=8/group). The MGC-803 cells in the 4 groups were cultured in
RPMI-1640 culture medium, respectively, containing 1, 10 or 20
μg/ml of ES and ES-free RPMI-1640 culture medium at 37°C in a 5%
CO2 atmosphere for 24 h. After cells were collected,
total RNA was extracted and RT-PCR was performed, according to the
manufacturer’s instructions. RT-PCR conditions were as follows:
predenaturation at 95°C for 10 min, denaturation at 94°C for 1 min,
reannealing at 60°C for 1 min, elongation at 72°C for 2 min, 30
cycles, and a final elongation at 72°C for 8 min. VEGF primers used
were: upstream: 5′-GAAGTGGTGAAGTTCATGGATGTC-3′ and downstream:
5′-CGATCGTTCTGTATCACTCTTTCC-3′. PCR products underwent agarose gel
electrophoresis and image scanning was performed, while analysis
was carried out using the Lab Works software. The average gray
values of positive electrophoretic bands were determined, and the
average gray value sample:β-actin ratio was determined as the
relative expression level of VEGF mRNA.
Western blotting used to determine VEGF
protein
MGC-803 cells were divided into 4 groups
(n=8/group). The MGC-803 cells in the 4 groups were cultured at
37°C in a 5% CO2 atmosphere for 24 h, in RPMI-1640
culture medium containing 1, 10 or 20 μg/ml of ES and ES-free
RPMI-1640 culture medium, respectively. The cells were collected,
washed three times with cold PBS followed by the addition of 400 μl
of lysate, and placed on ice for 30 min. Subsequent to
centrifugation at a rate of 14,000 rpm for 5 min at 4°C, the
supernatant was collected to determine the protein concentration
followed by SDS-PAGE and the transmembrane, and then sealed with
tris-buffered saline tween-20 (TBST) containing 5% of bovine serum
albumin (BSA) at room temperature for 1 h. The antibodies of VEGF
and β-actin were added, respectively, at 4°C overnight, followed by
the secondary antibody, HRP-IgG (1:1,000). The films were then
washed and the relative expression level of VEGF protein was
analyzed using the gel imaging system.
Statistical analysis
Statistical analysis was carried out using the SPSS
16.0 software. The measurement data were indicated as the mean ±
SD. Single factor analysis was used for the comparison between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
VEGF protein expression in MGC-803
cells
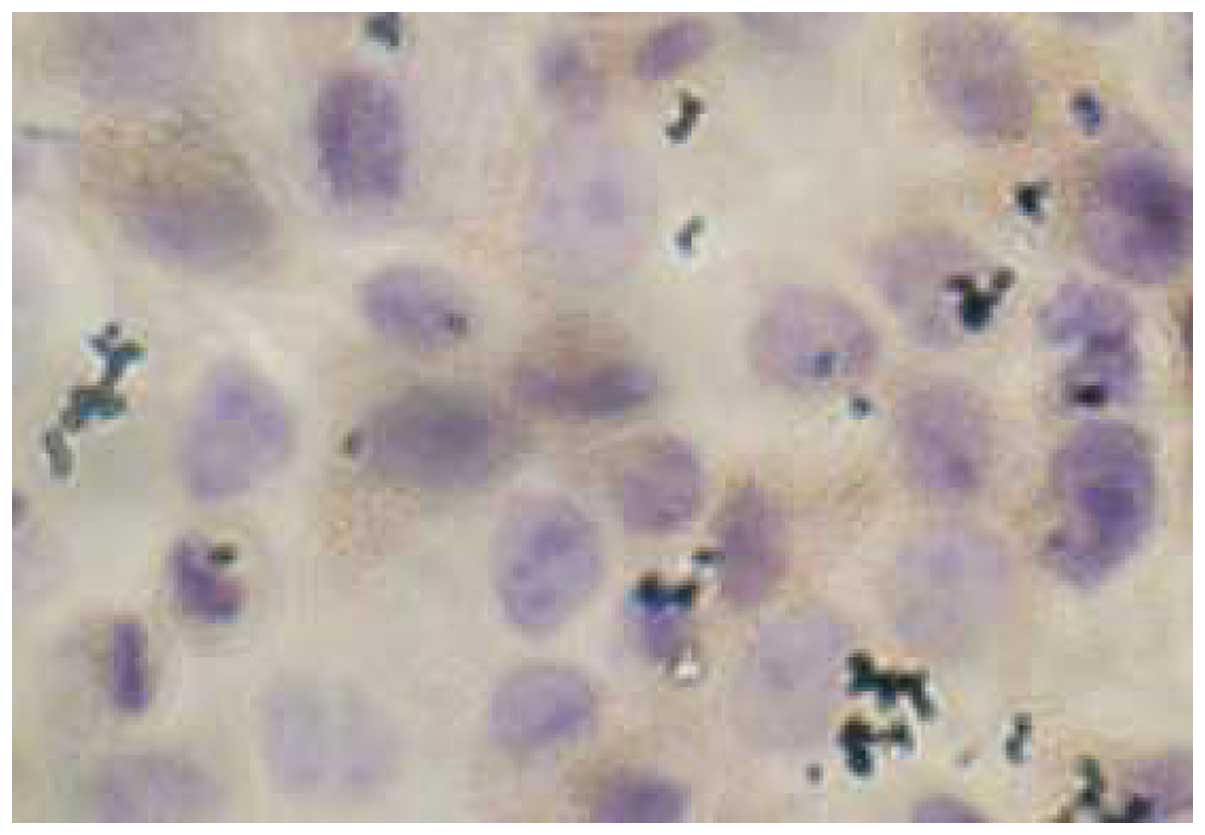
Immunohisto-chemistry indicated a high level of VEGF
protein expression in cytoplasm in the MGC-803 cells (Fig. 1).
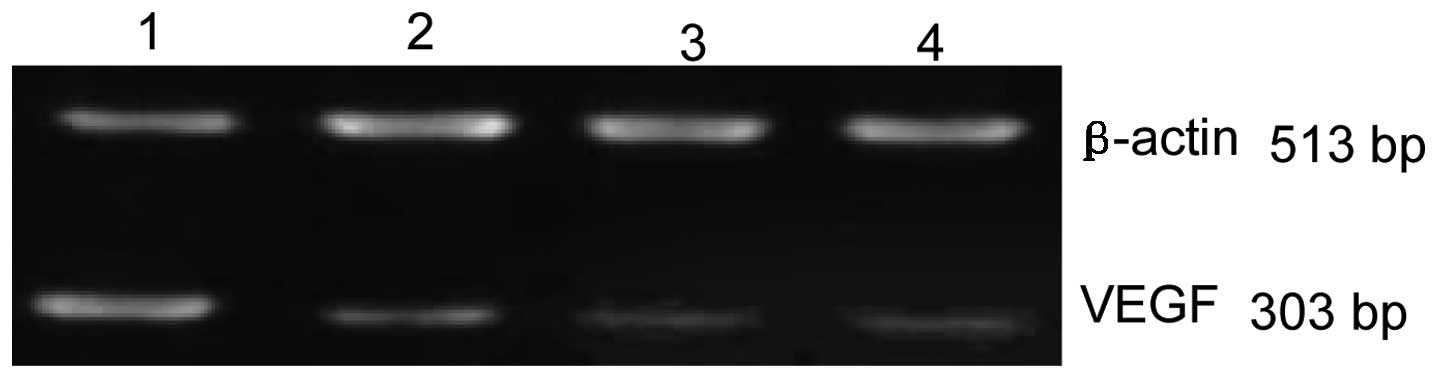
Effects of ES on VEGF mRNA
RT-PCR indicated that the relative expression levels
of VEGF mRNA in 1, 10 and 20 μg/ml of ES and the control groups
were 0.47±0.06, 0.24±0.03, 0.17±0.05 and 0.92±0.03, respectively.
Statistically significant differences were detected in ES and the
control groups (all P<0.05), demonstrating that ES markedly
decreased the level of VEGF mRNA in MGC-803 cells. Statistically
significant differences were detected in the ES groups (all
P<0.05), demonstrating that the level of VEGF mRNA expression is
negatively associated with the dose of ES (Fig. 2).
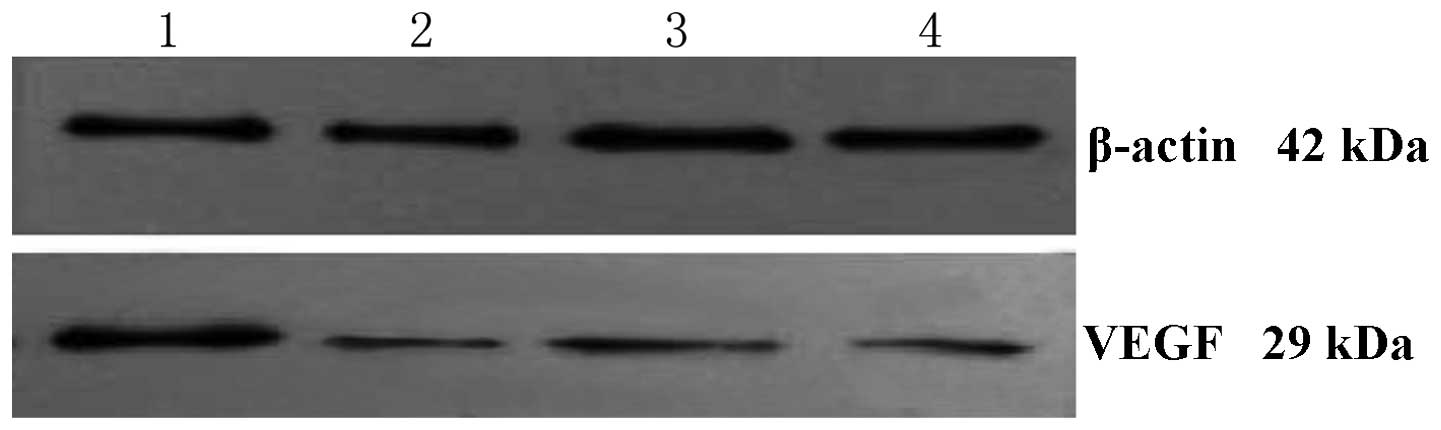
Effects of ES on VEGF protein
Result of the western blotting indicated that the
relative expression levels of VEGF protein in 1, 10 and 20 μg/ml of
ES and the control groups were 0.75±0.03, 0.37±0.07, 0.21±0.06 and
2.23±0.14, respectively. Statistically significant differences were
detected in the ES and the control groups (all P<0.05),
demonstrating that ES markedly decreased the level of VEGF protein
in MGC-803 cells. Statistically significant differences were
detected in ES groups (P<0.05), demonstrating that the level of
VEGF protein is negatively associated with the dose of ES (Fig. 3).
Discussion
Tumor growth and metastasis are based on
angiogenesis, while VEGF is an important regulatory factor of
neovascularization. In various types of gastric cancer tissues, the
mRNA and VEGF protein expressions are positive or strongly
positive, higher compared to healthy gastric tissues (4). Microvascular density is significantly
higher in gastric cancer tissues, especially in strongly
VEGF-positive tissues, compared to healthy gastric tissues,
demonstrating that VEGF is closely correlated with
neovascularization in gastric cancer tissues and promotes gastric
cancer growth and metastasis. In this study, MGC-803 cells derived
from poorly-differentiated gastric adenocarcinoma were used, due to
their high malignancy and VEGF expression level.
ES, a broad-spectrum angiogenesis inhibitor
(5), blocks the combination of VEGF
through its receptors and inhibits VEGF biological activities, such
as signal transduction of blood and lymphatic vessel formation and
the survival, proliferation and migration of epithelial cells
(6). Therefore, neovascularization
was decreased, whereas the blood supply to the tumor was reduced,
inhibiting tumor growth (7).
In this study, after MGC-803 cells were directly
treated with ES, immunohistochemistry indicated the presence of a
large amount of VEGF protein in MGC-803 cells. The results of
RT-PCR suggested that ES significantly decreased VEGF mRNA, and
with the increase in ES, VEGF mRNA was decreased. The decreased
VEGF mRNA affected the downstream protein translation and reduced
the specific protein confirmed by western blotting. ES also reduced
VEGF protein, thus increasing ES. VEGF is mainly secreted by tumor
and neovascular endothelial cells, while being markedly involved in
neovascularization, tumor growth and metastasis. Therefore, in
theory, blocking neovascularization inhibits tumor growth, while
improving the general status of patients and extending their
survival period. ES has been confirmed to interfere with the
combination of VEGF through its receptors to inhibit tumor growth
and metastasis (8). ES also
exhibited positive effects in II or III clinical trials of breast
and hepatic cancer. In MGC-803 cells, ES inhibited tumor growth and
metastasis possibly through a reduction of the formation of blood
and lymphatic vessels, requiring additional in vivo
experiments to be confirmed.
Acknowledgements
This study was financed by grants from
the Department of Education of Liaoning (no. 2009A443).
References
|
1.
|
He XW, Yu X, Liu T, Yu SY and Chen DJ:
Vector-based RNA interference against vascular endothelial growth
factor C inhibits tumor lymph angiogenesis and growth of colorectal
cancer in vivo in mice. Chin Med J (Engl). 121:439–444. 2008.
|
|
2.
|
Satchi-Fainoaro R, Puder M, Davies JW,
Tran HT, Sampson DA, Greene AK, Corfas G and Folkman J: Targeting
angiogenesis with a conjugate of H PMA copolymer and TNP-470. Nat
Med. 10:255–261. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Tian XJ, Wu J and Meng L: Preparation of
monoclonal antibodies to human vascular endothelial growth factor
(VEGF 121) and identification of its expression on gastric
carcinoma cell line MGC803. Zhonghua Zhong Liu Za Zhi. 21:93–95.
1999.(In Chinese).
|
|
4.
|
Luo XD, Xin Y and Xiao YP: Expression of
VEGF and its receptors Flt-1 and KDR in gastric carcinoma and its
significance. J Chin Med Univ. 37:760–763. 2008.(In Chinese).
|
|
5.
|
Xu HR, Tian W, Niu XH, Yuan RY, Zhang Q,
Chen DF and Liu WF: In vitro study of recombinant human endostatin
in combination with adriamycin on human osteosarcoma cell OS-732.
Chin J Bone Tumor Bone Dis. 7:129–132. 2008.(In Chinese).
|
|
6.
|
Neskey DM, Ambesi A, Pumiglia KM and
McKeown-Longo PJ: Endostatin and anastellin inhibit distinct
aspects of the angiogenic process. J Exp Clin Cancer Res.
27:612008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wu XZ: New strategy of antiangiogenic
therapy for hepatocellular carcinoma. Neoplasma. 55:472–481.
2008.PubMed/NCBI
|
|
8.
|
Franco TH, Khan A, Joshi V and Thomas B:
Takotsubo cardiomyopathy in two men receiving bevacizumab for
metastatic cancer. Ther Clin Risk Manag. 4:1367–1370.
2008.PubMed/NCBI
|