Introduction
As recently reviewed (1), the involvement of D-glucose in parotid
cell energetics was previously investigated with emphasis on such
variables as the overall energy status estimated by the uptake of
Tc-MIBI (2), the uptake of the
hexose by intact parotid cells (3),
its phosphorylation in cell homogenates (4,5), the
utilization and oxidation of the sugar (4,5), the
eventual accumulation of glycogen in situations of sustained
hyperglycemia (6) and the role of
mitochondrial carbonic anhydrase in the conversion of
CO2 generated by the catabolism of D-glucose to
bicarbonate anions (7). In the
framework of recent studies on the regulation of D-glucose
production by salivary glands and on the expression of several
glucose transporters possibly involved in the latter process
(8,9), the aim of the present study was to
compare the uptake of 3-O-[14C]methyl-D-glucose by
parotid cells, isolated from either control or diabetic rats, over
a 6-min incubation at 37°C to its outflow from parotid cells
prelabelled with the radioactive D-glucose analog, also over a
6-min incubation period.
Materials and methods
Animals
Control female Wistar rats and
streptozotocin-induced diabetic rats were allowed free access to
food and water up to the time of euthanasia, exsanguination and
decapitation (9). The diabetic rats
were obtained as previously described (10). The present study was approved by the
ethics committee of Brussels Free University (ULB), Brussels,
Belgium.
Experimental procedure
Parotid cells were prepared as previously described
(3). Unless otherwise mentioned,
the cells were incubated for 6 min at 37°C in groups of
160–330×103 cells placed in 100 μl of a salt-balanced
HEPES- and bicarbonate-buffered medium (3) supplemented with 1.0 mg/ml bovine serum
albumin. To measure the distribution space of 3HOH and
L-[1-14C]glucose, the incubation medium contained two
radioactive tracers, unlabelled L-glucose (0.4 mM) and
3-O-methyl-D-glucose (8.3 mM). To measure the uptake of
3-O-[14C]methyl-D-glucose, the incubation medium
contained a tracer amount of the 14C-labelled glucose
analog and unlabelled 3-O-methyl-D-glucose (8.3 mM). To measure the
efflux of 3-O-[14C]methyl-D-glucose, groups of
4–5×106 cells were preincubated for 30 min at 37°C in
1,250 μl of medium containing a tracer amount of
3-O-[14C]methyl-D-glucose and 8.3 mM unlabelled
3-O-methyl-D-glucose. The cells were then submitted to three
successive washes with 1.0 ml of iced medium containing
cytochalasin B (20 μM) and eventually incubated for 6 min at 37°C
in the presence of 8.3 mM unlabelled 3-O-methyl-D-glucose. In all
the cases, the cells were eventually separated from the incubation
medium by centrifugation through an oil layer (3).
Statistical analysis
Results were presented as means ± SEM together with
the number of separate observations (n). The statistical
significance of the differences between mean values was assessed
using the Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Intracellular water space assessment
The first objective of the present investigation was
to assess the intracellular water space of isolated parotid cells,
as estimated from the difference between the apparent distribution
space of 3HOH and L-[1-14C]glucose (used as
an extracellular marker). The results of the two experiments are
provided in Table I. The mean
values for the four variables listed in Table I, as measured in parotid cells
prepared from normal rats, did not differ significantly between the
first and second experiment (P≥0.33). In the second experiment, the
results recorded in normal rats did not differ significantly from
those recorded in diabetic animals (P≥0.44). The overall mean
values averaged 2.88±0.17 nl/103 cells for the total
3HOH space, 1.50±0.18 nl/103 cells for the
L-[1-14C]glucose distribution space, 1.37±0.18
nl/103 cells for the intracellular 3HOH space
and 49.3±5.7% for the ratio between the intracellular and total
3HOH space (n=5 per group).
 | Table I.Apparent distribution spaces in
parotid cells following a 6-min incubation at 37°C. |
Table I.
Apparent distribution spaces in
parotid cells following a 6-min incubation at 37°C.
| Spaces | Experiment 1
| Experiment 2
|
|---|
| Normal rats
(n=5) | Normal rats
(n=5) | Diabetic rats
(n=5) |
|---|
| Total 3HOH
(nl/103 cells) | 2.63±0.39 | 3.15±0.32 | 2.86±0.16 |
| L-[1–14C]glucose
(nl/103 cells) | 1.16±0.29 | 1.64±0.45 | 1.71±0.13 |
| Intracellular
3HOH (nl/103 cells) | 1.47±0.15 | 1.51±0.55 | 1.14±0.09 |
| Intracellular/total
3HOH (%) | 58.5±6.0 | 45.7±15.8 | 43.7±4.4 |
3-O-[14C]methyl-D-glucose
uptake
The second series of experiments was performed to
measure the net uptake of 3-O-[14C]methyl-D-glucose by
parotid cells from normal rats incubated for 6 min at 37°C.
Considering the radioactivity of the incubation medium, such a net
uptake corresponded, following correction for extracellular
contamination, to a mean intracellular distribution space of
0.44±0.05 nl/103 cells (n=10), representing 29.8±3.4% of
the intracellular water space measured within the same experiment
(experiment 1, Table I). The latter
value was not significantly different (P>0.33) from that
recorded in a previous study (3)
following a 10-min incubation at 37°C, i.e., 26.8±1.4% (n=46).
3-O-[14C]methyl-D-glucose
outflow
The third series of experiments was conducted to
measure the outflow of radioactivity from parotid cells prelabelled
with 3-O-[14C]methyl-D-glucose. Following a 30-min
preincubation at 37°C in the presence of
3-O-[14C]methyl-D-glucose, the cells underwent three
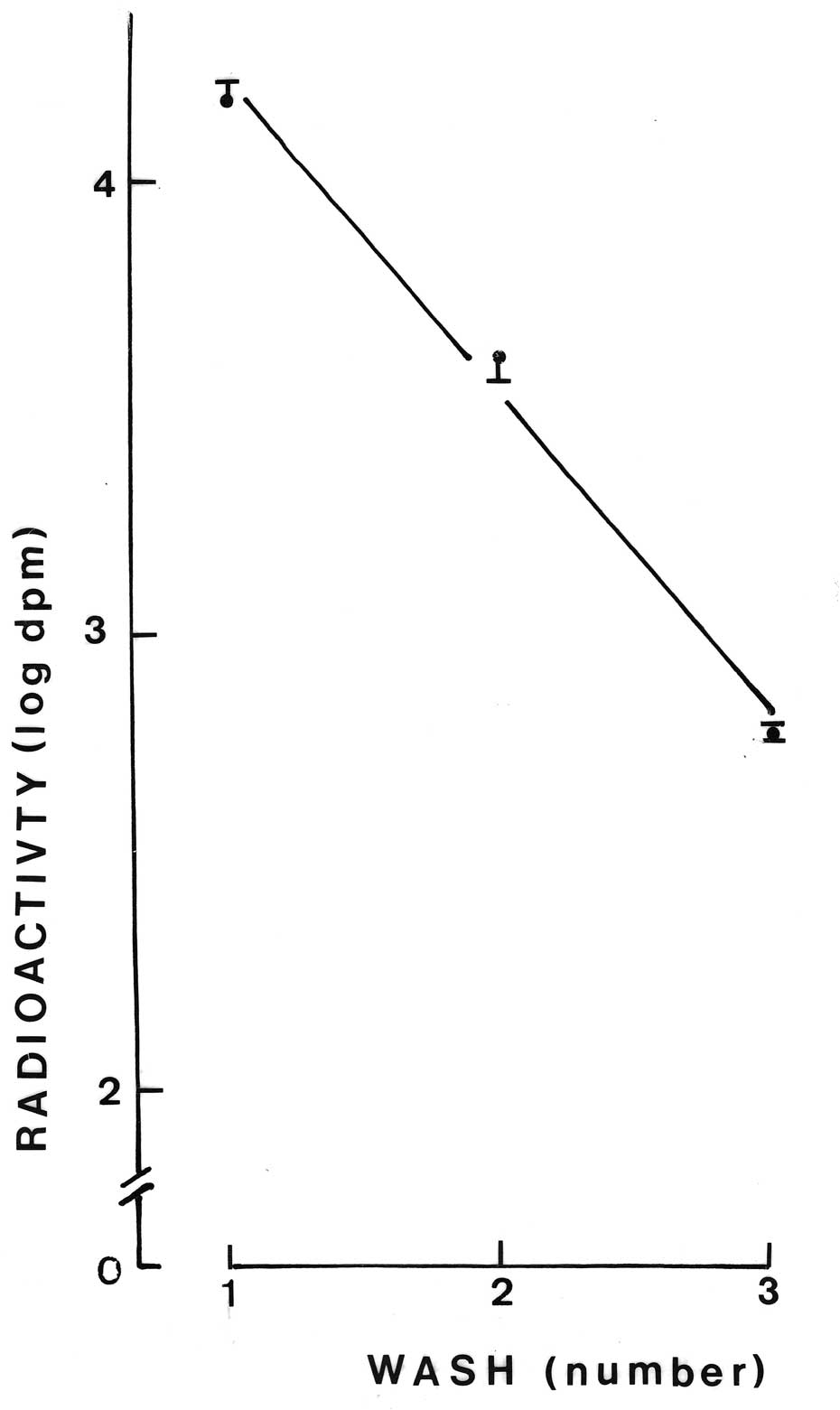
washes. As shown in Fig. 1, the
radioactive content of the washing medium decreased in an
exponential manner. The coefficient of correlation between the
logarithmic values for the radioactive content of the washing media
and the number of washes amounted to −0.9901 (n=10; P<0.001).
After the washes, the radioactive content of the parotid cells
averaged 0.47±0.05 and 0.45±0.01 dpm/103 cells in the
control and diabetic rats, respectively (n=4 in both cases). The
latter two values were in agreement with the total radioactive
content of the incubation medium and the parotid cells, as measured
following incubation of the washed cells for 6 min at 37°C. Thus,
such a total radioactive content averaged 0.54±0.03 (n=16) and
0.54±0.02 (n=18) dpm/103 cells in the control and
diabetic rats, respectively. At the end of the 6-min incubation,
the radioactive content of the incubation medium, expressed
relative to the paired value for total radioactivity (incubation
medium plus cells), averaged 82.9±4.8% (n=18) in the control rats
and 84.1±2.5% (n=18) in the diabetic animals. The radioactive
content of the cells after three washes corresponded to an apparent
distribution space of 0.38±0.04 nl/103 cells (n=4),
which was in agreement with the value recorded in the second series
of experiments after only the 6-min incubation, where the apparent
intracellular distribution space of
3-O-[14C]methyl-D-glucose averaged 0.44±0.05
nl/103 cells. This finding demonstrates the adequacy of
the washing procedure used in the third series of experiments.
Comparison of tracer uptake and
outflow
As demonstrated by the apparent distribution space
of 3-O-[14C]methyl-D-glucose in the parotid cells
preincubated for 30 min at 37°C and then submitted to three washes
(0.38±.04 nl/103 cells) and the fractional outflow of
radioactivity over an ensuing 6-min incubation period (82.5±2.7%),
the outflow of the tracer corresponded to a volume of ∼0.32±0.03
nl/103 cells, a value somewhat lower compared to the net
uptake of the D-glucose analog, as measured also over the 6-min
incubation period and corresponding to an apparent intracellular
distribution space of 0.44±0.05 nl/103 cells.
Discussion
The present study offers several new pieces of
information. First, it documented that, expressed in either
absolute terms or relative to the total water space, the
intracellular water space of parotid cells does not differ
significantly between normal and diabetic rats. Second, it extended
a prior observation indicating that the apparent intracellular
distribution space of 3-O-[14C]methyl-D-glucose at
close-to-equilibrium values does not exceed ∼30% of the
intracellular water space. Third, it revealed that the uptake of
3-O-[14C]methyl-D-glucose and its outflow from
prelabelled parotid cells do not differ significantly between
control animals and diabetic rats. Additionally, it documented
that, within a 6-min incubation period at 37°C, the majority of
3-O-[14C]methyl-D-glucose taken up during preincubation
of parotid cells, is released from those cells over an ensuing
incubation of 6 min, with no significant difference observed
between normal and diabetic rats.
Taken together, these findings suggest that the
increased production of salivary D-glucose prevailing in diabetic
subjects (8) is primarily
attributable to the increased glycemia rather than to any
significant perturbation in the intrinsic processes involved, at
least in parotid cells, in hexose handling.
Acknowledgements
This study was supported by grant
3.4520.07 (to A.S.) from the Belgian Foundation for Scientific
Medical Research.
References
|
1
|
Jurysta C, Sener A and Malaisse WJ:
Physiopathology of parotid cell energetics. Adv Biol Chem.
View Article : Google Scholar
|
|
2
|
Blocklet D, Jijakli H, Sener A, Schoutens
A and Malaisse WJ:
99mTc-sesta-(2-methoxy-isobutyl-isonitrile) uptake by
pancreatic islets, parotid cells, and mammary carcinoma cells.
Endocrine. 9:113–117. 1998. View Article : Google Scholar
|
|
3
|
Ramirez R, Courtois P, Ladrière L, Kadiata
MM, Sener A and Malaisse WJ: Uptake of D-mannoheptulose by rat
erythrocytes, hepatocytes and parotid cells. Int J Mol Med.
8:37–42. 2001.PubMed/NCBI
|
|
4
|
Scruel O, Vanhoutte C, Sener A and
Malaisse WJ: Interference of D-mannoheptulose with D-glucose
phosphorylation, metabolism and functional effects: comparison
between liver, parotid cells and pancreatic islets. Mol Cell
Biochem. 187:113–120. 1998. View Article : Google Scholar
|
|
5
|
Malaisse WJ, Kadiata MM, Scruel O and
Sener A: Esterification of D-mannoheptulose confers to the heptose
inhibitory action on D-glucose metabolism in parotid cells. Biochem
Mol Biol Int. 44:625–633. 1998.PubMed/NCBI
|
|
6
|
Ladrière L, Louchami K, Laghmich A,
Malaisse-Lagae F and Malaisse WJ: Labeling of pancreatic glycogen
by D-[U-14C]glucose in hyperglycemic rats. Endocrine.
14:383–397. 2001.
|
|
7
|
Sener A, Jijakli H, Zahedi Asl S, Courtois
P, Yates AP, Meuris S, Best LC and Malaisse WJ: Possible role of
carbonic anhydrase in pancreatic islets: enzymatic, secretory,
metabolic, ionic, and electrical aspects. Am J Physiol Endocrinol
Metab. 292:E1624–E1630. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jurysta C, Bulur N, Oguzhan B, Satman I,
Yilmaz TM, Malaisse WJ and Sener A: Salivary glucose concentration
and excretion in normal and diabetic subjects. J Biomed Biotechnol.
May 26–2009.(Epub ahead of print). View Article : Google Scholar
|
|
9
|
Jurysta C, Nicaise C, Cetik S, Louchami K,
Malaisse WJ and Sener A: Glucose transport by acinar cells in rat
parotid glands. Cell Physiol Biochem. 29:325–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Belkacemi L, Selselet-Attou G, Hupkens E,
Nguidjoe E, Louchami K, Sener A and Malaisse WJ: Intermittent
fasting modulation of the diabetic syndrome in
streptozotocin-injected rats. Int J Endocrinol. Jan 12–2012.(Epub
ahead of print). View Article : Google Scholar
|