Introduction
Coronary artery disease (CAD) is a complex disease
that develops due to environmental, lifestyle and genetic factors.
Clinical and epidemiological studies have demonstrated an inverse
association between high-density lipoprotein cholesterol (HDL-C)
concentrations and cardiovascular disease risk (1,2). This
association is supported by the anti-atherogenic properties of
HDL-C; in particular, HDL-C functions in reverse cholesterol
transport (RCT) (3). The initial
step in RCT is regulated by adenosine triphosphate (ATP)-binding
cassette A1 (ABCA1), which participates in the
apolipoprotein-mediated efflux of cholesterol and phospholipid from
peripheral cells (3). The
identification of mutations in the ABCA1 gene in patients
with Tangier disease, a rare disorder characterized by the absence
of HDL (4), suggests a significant
role for ABCA1 in regulating HDL-C levels. ABCA1 is a transmembrane
protein that mediates the efflux of cholesterol and phospholipid to
apolipoprotein A-I (Apo AI). This is the first step in RCT, the
process by which cholesterol from peripheral macrophages is
transferred back to the liver. Thus, ABCA1 availability is
considered the rate-limiting step in HDL production (5).
Although HDL is involved in numerous
atheroprotective mechanisms, the relative activity of ABCA1 plays a
significant role (6). Evaluation of
common genetic variations in the ABCA1 gene may be critical to
understanding the contribution of ABCA1 to inter-individual
variability in plasma lipid levels and susceptibility to CHD in the
general population. Several common polymorphisms of the ABCA1 gene
may differentially affect HDL-C levels and may be of clinical
importance (7–12). The common rs2230806
single-nucleotide polymorphism (SNP) of the ABCA1 gene has been
associated with an effect on HDL-C concentrations in some
populations, suggesting that this SNP is of clinical importance
(13–16). However, the presence of this
polymorphism did not result in a consistent effect on HDL-C and
other circulating lipids. The aim of the present study was to
assess the impact of a common SNP of the ABCA1 gene (rs2230806) on
the HDL-C concentration and the related plasma lipid profile in the
Saudi Arabian population.
Materials and methods
Patient population
This study was approved by the Clinical Research
Ethics Committee of the Institutional Review Board of King Saud
University. In total, 120 Saudi patients with CAD (68 male, 52
female; mean age ± standard deviation, 39.8±15.2 years) and 100
ethnically matched, healthy controls from Riyadh, Saudi Arabia (60
males, 40 females; age-matched) were included. At least 95% of the
patients were previously diagnosed with CAD based on their
electrocardiographic profiles. None of the subjects had a history
of diabetes, hypertension or hypercholesterolemia. None of the
subjects recruited for the study were ingesting lipid-lowering
drugs, and those with triglyceride levels ≥400 mg/dl were excluded
from the analysis. All of the study subjects provided informed
consent as per the protocol approved by the Ethics Review
Board.
Determination of lipid parameters
Serum total cholesterol, HDL-C and triglyceride
levels were measured by standard enzymatic methods, including the
cholesterol oxidase-peroxidase-amidopyrine method (Roche
Diagnostics, Mannheim, Germany) and the glycerol phosphate
oxidase-peroxidase-amidopyrine method (Roche Diagnostics).
Low-density lipoprotein cholesterol (LDL-C) was calculated using
the Friedewald formula if total cholesterol, triglyceride and HDL-C
values were available. Serum samples were separated by
centrifugation and stored at −70°C until determination of the lipid
parameters.
Genotyping the rs2230806 polymorphism by
restriction fragment length polymorphism (RFLP)
DNA was extracted using a Qiagen kit according to
the manufacturer’s instructions. The presence of SNP rs2230806
(R219K variant) in exon 7 of the ABCA1 gene (c.969A→G) was
determined for each sample. The presence of the missense mutation
at nucleotide 969 (AGG to AAG), which leads to the replacement of
arginine with lysine at codon 219, was evaluated in genomic DNA by
polymerase chain reaction (PCR)-RFLP analysis, according to methods
described by Cook et al(17). A 333-bp PCR product was generated
using forward 5′-TCC AAAAGACTTCAAGGACCCAGCT-3′ and reverse 5′-AAGT
CATGCTGTCCAAGGAAAA-3′ primers. PCR was performed in a 96-well
microtiter plate in a 25-μl volume containing 50 ng of
genomic DNA, 5 μM of each primer, 2.5 μl of 10X
buffer, 20 nM each of dATP, dCTP, dGTP, dTTP and 1 unit of
Taq polymerase (HotStarTaq; Applied Biosystems, Carlsbad,
CA, USA). Initial denaturation at 94°C for 2 min was followed by 40
cycles of denaturation at 94°C for 15 sec, annealing at 55°C for 30
sec and extension at 72°C for 1 min, with a final extension at 72°C
for 2 min.
The PCR products were digested with Cac81 (New
England Biolabs, Ipswich, MA, USA) for 4 h and separated by
electrophoresis on a 2% agarose gel containing ethidium bromide.
The 333-bp PCR product was not digested in homozygous wild-type
samples (AA), whereas samples from individuals heterozygous for the
missense mutation caused by rs2230806 (AG) demonstrated 333-, 189-
and 144-bp bands. Samples from individuals homozygous for the
missense mutation (GG) were cleaved into two fragments of 189 and
144 bp. Subsets of the samples were also directly sequenced.
Direct sequencing
Briefly, purified PCR products were directly
sequenced using the dideoxynucleotide chain-termination method with
an ABI PRISM Big Dye Terminator v3.1 Cycle Sequencing kit,
following the manufacturer’s instructions and processed on an ABI
3730×l capillary sequencer (Applied Biosystems). Sequence analysis
was performed using the SeqMan 6.1 module of the Lasergene software
package (DNAStar Inc., Madison, WI, USA), and the sequences were
compared with the reference GenBank sequence.
Statistical analysis
Data are presented as means ± SD. The association
between CAD occurrence and the SNP genotype and allelic frequencies
were measured by the odds ratio (OR) with its confidence interval
(CI). The degree of significance was calculated using the
Chi-square method. P≤0.05 was considered to indicate a
statistically significant difference. The Hardy-Weinberg
equilibrium (HWE) and deviation from HWE for genotype distribution
were tested for each SNP for statistically significant association
with CAD in order to examine the effects of genotype frequencies in
the population by comparing the observed to the expected genotype
frequencies.
Results
The characteristics of the patients and control
subjects, including the presence of traditional risk factors for
CAD, are provided in Table I. The
mean age of the control subjects was 39.8±15.2 years. There was a
significant difference in the mean age between the two groups. To
assess the important genotype-phenotype associations, plasma lipid
profile determination and genotyping were performed for the control
(n=100) and patient (n=120) samples. A comparison of the plasma
lipid parameters and the genotypic data revealed significant
differences between the control and patient groups. The lipid
profiles for CAD patients and healthy control subjects are provided
in Table I. The results indicated
that the mean levels of TC and LDL-C in the CAD group were
significantly higher than those observed in the control group. CAD
patients had significantly higher TG levels (P<0.001), and HDL-C
levels were significantly lower in CAD patients as compared to the
controls. All of the subjects selected for the study were
non-smokers.
 | Table IClinical and biochemical
characteristics in coronary artery disease (CAD) patients and
control subjects. |
Table I
Clinical and biochemical
characteristics in coronary artery disease (CAD) patients and
control subjects.
| Characteristics | Controlsa (n=100) | CAD groupa (n=120) | P-valueb |
|---|
| Age, years | 39.8±15.2 | 38.8±16.2 | <0.001c |
| Gender |
| Male (%) | 60 (60) | 68 (56.66) | <0.001c |
| Female, (%) | 40 (40) | 52 (43.33) | |
| TG, mmol/l |
| Mean ± SD | 1.06±0.28 | 1.56±0.70 | <0.0001c |
| Range | (0.53–1.72) | (0.57–3.77) | |
| TC, mmol/l |
| Mean ± SD | 3.80±0.48 | 4.24±1.09 | 0.0002c |
| Range | (3.01–5.11) | (0.77–7.5) | |
| HDL-C, mmol/l |
| Mean ± SD | 1.70±0.39 | 1.11±0.88 | <0.0001c |
| Range | (0.76–2.11) | (0.53–5.1) | |
| LDL-C, mmol/l |
| Mean ± SD | 1.65±0.33 | 2.53±0.91 | <0.0001c |
| Range | (1.0–2.5) | (1.01–4.89) | |
Distribution of the rs2230806 genotypes
in the CAD and control groups
The observed genotype frequencies in each case
demonstrated Hardy-Weinberg equilibrium, as indicated by the
P-values (Table II). The genotype
distribution and allelic frequency of exon 7 rs2230806 variants of
ABCA1 are provided in Table III
for CAD patients and control subjects. The frequencies of the
rs2230806-associated genotypes GG, GA and AA were 84, 33 and 3% for
CAD patients and 60, 35 and 5% for control subjects, respectively.
Moreover, the allelic frequency of the G allele was significantly
higher in CAD patients compared to the controls, suggesting that
the variant confers a higher risk for developing disease. The crude
OR for CAD conferred by carrying the ABCA1 GG genotype was 2.333
(95% CI 0.537–10.140), P<0.05 (Table III).
 | Table IIGenotype distributions and allelic
frequency of rs2230806 in the ATP-binding cassette A1 (ABCA1)
gene. |
Table II
Genotype distributions and allelic
frequency of rs2230806 in the ATP-binding cassette A1 (ABCA1)
gene.
| rs2230806 | Genotypes | In HWE
chi-squarea | P-valueb | Deviation from HWE
chi-squarea | P-valueb |
|---|
|
|---|
| Wild-type | Heterozygote | Homozygote |
|---|
| CAD patients
(n=120) | | | | | | | |
| Observed (n) | 84 | 33 | 3 | 0.90 | NSc | 0.16 | NSb |
| Expected (n) | 84.17 | 32.66 | 3.17 | | | | |
| Control subjects
(n=100) | | | | | | | |
| Observed (n) | 60 | 35 | 5 | 0.97 | NSb | 0.23 | NSb |
| Expected (n) | 60.06 | 34.88 | 5.06 | | | | |
 | Table IIIGenotype and allelic distributions
with odds ratios (ORs) of the assessed polymorphisms. |
Table III
Genotype and allelic distributions
with odds ratios (ORs) of the assessed polymorphisms.
| Genotype and
allele | CAD (120) | Control (100) | OR (95% CI) | P-valuea |
|---|
| G/G | 84 | 60 | 2.333
(0.537–10.140) | 0.05 |
| G/A | 33 | 35 | 2.053
(0.478–8.810) | 0.24 |
| A/A | 3 | 5 | | |
| G | 201 | 155 | 1.496
(0.928–2.411) | 0.32 |
| A | 39 | 45 | | |
Association of the rs2230806 polymorphism
with CAD and plasma lipid profiles
Controls and patients were divided into two
subgroups with normal and high observed values for serum lipids
(Table IV). In each of the
subgroups, individuals carrying the K allele presented higher
triglyceride levels; however, there was no evidence of a more
pronounced phenotype in the older patients. There was no
significant difference in the correlation between age and HDL and
triglyceride levels in individuals carrying different rs2230806
(R219K) genotypes (data not shown). An analysis of the relationship
between plasma lipid profiles and genotypic background demonstrated
an association between the G allele and decreased HDL levels. The K
variant of the G allele was detected at a higher frequency in CAD
patients compared to the controls, suggesting that the K variant of
the G allele is likely a genetic risk factor for CAD. The multiple
logistic regression analysis of the association of the ABCA1
rs2230806 G allele with HDL yielded an OR of 1.73 (95% CI,
1.026–2.864) (Table IV),
suggesting that individuals carrying the G allele may have a low
HDL-C profile and thus, an increased relative risk of developing
CAD.
 | Table IVOdds ratios (ORs) and confidence
intervals (CIs) for carriers of rs2230806. |
Table IV
Odds ratios (ORs) and confidence
intervals (CIs) for carriers of rs2230806.
| | | | Alleles |
|---|
| | | |
|
|---|
| Metabolic
profile | AA | AG | GG | A | G |
|---|
| Controls, TG
(mmol/l) |
| Normal (%) | 30 (30) | 17 (17) | 10 (10) | 0.385 | 0.185 |
| High (%) | 23 (23) | 18 (18) | 2 (2) | 0.32 | 0.11 |
| Patients, TG
(mmol/l) |
| Normal | 32 (26.6) | 51 (42.5) | 9 (0.075) | 0.479 | 0.287 |
| High | 17 (14.1) | 11 (9.1) | | 0.187 | 0.045 |
| OR of GG genotypes
vs AA+AG, (95% CI), P-value | 2.362
(0.9055–6.161), 0.08 | | | | |
| Controls, TC
(mmol/l) |
| Normal (%) | 14 (14) | 35 (35) | 26 (26) | 0.216 | 0.381 |
| High (%) | 8 (8) | 9 (9) | 8 (8) | 0.125 | 0.125 |
| Patients, TC
(mmol/l) |
| Normal (%) | 53 (44.1) | 21 (17.5) | 22 (18.2) | 0.528 | 0.27 |
| High (%) | 13 (10.8) | 3 (2.5) | 8 (6.6) | 0.12 | 0.079 |
| OR of GG genotypes
vs. AA+AG, (95% CI), P-value | 0.6182
(0.3073–1.244), 0.2158 | | | | |
| Controls, HDL-C
(mmol/l) |
| Normal (%) | 53 (53) | 26 (26) | 7 (7) | 0.66 | 0.2 |
| High (%) | 5 (5) | 5 (5) | 4 (4) | 0.075 | 0.065 |
| Patients, HDL-C
(mmol/l) |
| Normal (%) | 20 (16.6) | 73 (60.8) | 13 (10.7) | 0.48 | 0.411 |
| High (%) | 6 (5) | 5 (4.1) | 3 (2.5) | 0.07 | 0.045 |
| OR of GG genotypes
vs. AA+AG, (95%CI), P-value | 1.730
(0.7222–4.143), 0.0247 | | | | |
| Controls, LDL-C
(mmol/) |
| Normal (%) | 23 (23) | 60 (60) | 5 (5) | 0.475 | 0.455 |
| High (%) | 8 (8) | 4 (4) | 0 (0) | 0.10 | 0.04 |
| Patients, LDL-C
(mmol/l) |
| Normal (%) | 65 (45.1) | 19 (15.7) | 9 (7.5) | 0.62 | 0.153 |
| High (%) | 8 (6.6) | 15 (12.5) | 4 (3.3) | 0.129 | 0.095 |
| OR of GG genotypes
vs. AA+AG, (95% CI), P-value | 0.2043
(0.06718–0.6215), 0.0039 | | | | |
The results of this study suggest that the GG
genotype for the ABCA1 gene is associated with CAD in the Saudi
population. However, when gender was analyzed separately, the K
allele was present less frequently in females compared to males
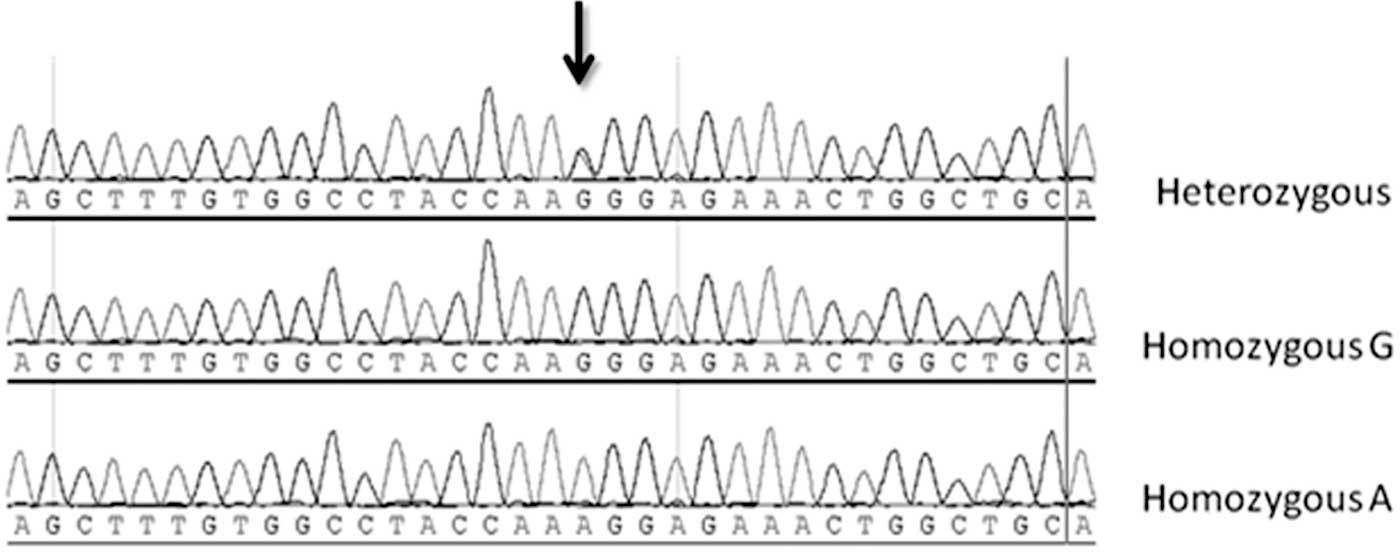
(data not shown). In a subset of the samples, sequencing was used
to validate the GG, AA and GA genotypes of the R219K polymorphism
that were identified by RFLP (Fig.
1).
Discussion
ABCA1 mediates the cellular efflux of cholesterol
via the transfer of cholesterol from the inner to the outer layer
of the cell membrane, thereby regulating extracellular cholesterol
levels in the heart and central nervous system. Attention has been
focused on the association between ABCA1 gene polymorphisms and
different phenotypes, including lipid variables and clinical
endpoints (18–21). Our results showed a significant
association of rs2230806 with CAD, which is consistent with studies
conducted on other populations and ethnicities (9,20,21).
We also observed an association between triglyceride levels and the
R219K polymorphism, but no significant associations were observed
for other lipid parameters. The most common missense polymorphism
in the coding region of the ABCA1 gene is R219K, with an allelic
frequency of 25–46% in the Caucasian population. Two large studies
including 2,028 and 794 individuals resulted in contradictory
conclusions regarding the possible role of rs2230806 (R219K) in
arteriosclerosis. One study reported an elevated R219K allelic
frequency in patients with CHD and a low HDL level compared to
disease-free individuals, suggesting that the mutant allele is
likely associated with decreased HDL levels, the promotion of
arteriosclerosis and the subsequent development of CHD (11). The other study reported a decreased
R219K allelic frequency in patients with CHD in conjunction with
high observed levels of HDL, suggesting that the mutant allele
conferred a protective effect (22). The reason for the inconsistency
between the various studies remains to be determined. However, the
frequency of ABCA1 G allele is variable between different races and
ethnic groups. This polymorphism has been shown to be associated
with triglyceride levels (22), but
not with HDL-C levels (9,21). Our results are consistent with the
observations concerning triglyceride levels reported in those
studies. However, to the best of our knowledge, the present study
provides the first evidence for an association between an ABCA1
gene polymorphism and decreased HDL-C levels.
In this study, we observed a correlation between the
R219K allele and lipid parameters, although the observed
associations were not very significant, except for the associations
with HDL-C levels (P=0.02). Total HDL-C levels were low in the CAD
patient group and the presence of the rs2230806 polymorphism was
correlated with these lower levels, suggesting that this
polymorphism is a genetic risk factor for CAD in the Saudi
population. Lutucuta et al(23) reported the effects of polymorphisms
in the promoter of the ABCA1 gene on coronary atherosclerosis.
However, findings of this study provide evidence for the importance
of rs2230806 in the ABCA1 gene in the development of CAD with low
levels of HDL-C within a specific population. Thus, this SNP is a
potential genetic biomarker that may be used to screen individuals
at high risk of CAD.
In conclusion, we identified significant allelic
frequency increases in the K allele in the patient group,
suggesting that theR219K polymorphism is a potential risk factor
for CAD in the Saudi Arabian population. The K allele was highly
associated with high triglyceride and low HDL-C levels in the CAD
patient group, suggesting a possible role for the ABCA1 gene in the
promotion of CAD via its function in decreasing HDL-C levels.
However, future investigations should focus on the association
between the K allele and CAD in other populations, as the disease
association of this polymorphism may be population-dependent.
Acknowledgements
This study was supported by a grant from the
‘Research Center of the Center for Female Scientific and Medical
Colleges’, Deanship of Scientific Research, King Saud
University.
References
|
1
|
Bruckert E, Baccara-Dinet M and Eschwege
E: Low HDL-cholesterol is common in European Type 2 diabetic
patients receiving treatment for dyslipidaemia: data from a
pan-European survey. Diabet Med. 24:388–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldbourt U, Yaari S and Medalie JH:
Isolated low HDL cholesterol as a risk factor for coronary heart
disease mortality. A 21-year follow-up of 8000 men. Arterioscler
Thromb Vasc Biol. 17:107–113. 1997.PubMed/NCBI
|
|
3
|
Fredenrich A and Bayer P: Reverse
cholesterol transport, high density lipoproteins and HDL
cholesterol: recent data. Diabetes Metab. 29:201–205. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brooks-Wilson A, Marcil M, Clee SM, Zhang
LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO,
Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen
CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K,
Koop B, Pimstone S, Kastelein JJ, Genest J Jr and Hayden MR:
Mutations in ABC1 in Tangier disease and familial high-density
lipoprotein deficiency. Nat Genet. 22:336–345. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benton JL, Ding J, Tsai MY, Shea S, Rotter
JI, Burke GL and Post W: Associations between two common
polymorphisms in the ABCA1 gene and subclinical atherosclerosis:
Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis.
193:352–360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doosti M, Najafi M, Reza JZ and Nikzamir
A: The role of ATP-binding-cassette-transporter-A1 (ABCA1) gene
polymorphism on coronary artery disease risk. Transl Res.
155:185–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clee SM, Kastelein JJ, van Dam M, Marcil
M, Roomp K, Zwarts KY, Collins JA, Roelants R, Tamasawa N, Stulc T,
Suda T, Ceska R, Boucher B, Rondeau C, DeSouich C, Brooks-Wilson A,
Molhuizen HO, Frohlich J, Genest J Jr and Hayden MR: Age and
residual cholesterol efflux affect HDL cholesterol levels and
coronary artery disease in ABCA1 heterozygotes. J Clin Invest.
106:1263–1270. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pullinger CR, Hakamata H, Duchateau PN,
Eng C, Aouizerat BE, Cho MH, Fielding CJ and Kane JP: Analysis of
hABC1 gene 5′ end: additional peptide sequence, promoter region,
and four polymorphisms. Biochem Bibiophys Res Comm. 271:451–455.
2000.PubMed/NCBI
|
|
9
|
Brousseau ME, Bodzioch M, Schaefer EJ,
Goldkamp AL, Kielar D, Probst M, Ordovas JM, Aslanidis C, Lackner
KJ, Bloomfield Rubins H, Collins D, Robins SJ, Wilson PW and
Schmitz G: Common variants in the gene encoding ATP-binding
cassette transporter 1 in men with low HDL cholesterol levels and
coronary heart disease. Atherosclerosis. 154:607–611. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singaraja RR, Brunham LR, Visscher H,
Kastelein JJ and Hayden MR: Efflux and atherosclerosis: the
clinical and biochemical impact of variations in the ABCA1 gene.
Arterioscler Thromb Vasc Biol. 23:1322–1332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frikke-Schmidt R, Nordestgaard BG, Jensen
GB and Tybjaerg-Hansen A: Genetic variation in ABC transporter A1
contributes to HDL cholesterol in the general population. J Clin
Invest. 114:1343–1353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Howard BV, Ruotolo G and Robbins DC:
Obesity and dyslipidemia. Endocrinol Metab Clin North Am.
32:855–867. 2003. View Article : Google Scholar
|
|
13
|
Qi LP, Yan XW, Ye P and Dang AM:
Association between two common polymorphisms in ATP-binding
cassette A1 gene and coronary heart disease complicated with
diabetes in Chinese Han people. Chin Med. 5:295–297. 2010.(In
Chinese).
|
|
14
|
Shi WY, Zhao ZZ, Xiao DM, Tang CK, Xu GZ
and Yang YZ: Study on single nucleotide polymorphisms of ABCA1R219K
in Han Population. Pract Prev Med. 16:1057–1060. 2009.(In
Chinese).
|
|
15
|
Frikke-Schmidt R, Nordestgaard BG, Jensen
GB, Steffensen R and Tybjaerg-Hansen A: Genetic variation in ABCA1
predicts ischemic heart disease in the general population.
Arterioscler Thromb Vasc Biol. 28:180–186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tregouet DA, Ricard S, Nicaud V, Arnould
I, Soubigou S, Rosier M, Duverger N, Poirier O, Mace S, Kee F,
Morrison C, Denefle P, Tiret L, Evans A, Deleuze JF and Cambien F:
In-depth haplotype analysis of ABCA1 gene polymorphisms in relation
to plasma ApoA1 levels and myocardial infarction. Arterioscler
Thromb Vasc Biol. 24:775–781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cook LJ, Ho LW, Wang L, Terrenoire E,
Brayne C, Evans JG, Xuereb J, Cairns NJ, Turic D, Hollingworth P,
Moore PJ, Jehu L, Archer N, Walter S, Foy C, Edmondson A, Powell J,
Lovestone S, Williams J and Rubinsztein DC: Candidate gene
association studies of genes involved in neuronal cholinergic
transmission in Alzheimer’s disease suggests choline
acetyltransferase as a candidate deserving further study. Am J Med
Genet B Neuropsychiatr Genet. 132B:5–8. 2005.PubMed/NCBI
|
|
18
|
Tan JH, Low PS, Tan YS, Tong MC, Saha N,
Yang H and Heng CK: ABCA1 gene polymorphisms and their associations
with coronary artery disease and plasma lipids in males from three
ethnic populations in Singapore. Hum Genet. 113:106–117.
2003.PubMed/NCBI
|
|
19
|
Clee SM, Zwinderman AH, Engert JC, Zwarts
KY, Molhuizen HO, Roomp K, Jukema JW, van Wijland M, van Dam M,
Hudson TJ, Brooks-Wilson A, Genest J Jr, Kastelein JJ and Hayden
MR: Common genetic variation in ABCA1 is associated with altered
lipoprotein levels and a modified risk for coronary artery disease.
Circulation. 103:1198–1205. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Burnett JR, Near S, Young K,
Zinman B, Hanley AJ, Connelly PW, Harris SB and Hegele RA: Common
and rare ABCA1 variants affecting plasma HDL cholesterol.
Arterioscler Thromb Vasc Biol. 20:1983–1989. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cenarro A, Artieda M, Castillo S, Mozas P,
Reyes G, Tejedor D, Alonso R, Mata P, Pocovi M and Civeira F: A
common variant in the ABCA1 gene is associated with a lower risk
for premature coronary heart disease in familial
hypercholesterolaemia. J Med Genet. 40:163–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Talmud PJ: Genetic determinants of plasma
triglycerides: impact of rare and common mutations. Curr
Atheroscler Rep. 3:191–199. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lutucuta S, Ballantyne CM, Elghannam H,
Gotto AM Jr and Marian AJ: Novel polymorphisms in promoter region
of atp binding cassette transporter gene and plasma lipids,
severity, progression, and regression of coronary atherosclerosis
and response to therapy. Circ Res. 88:969–973. 2001. View Article : Google Scholar
|