Introduction
Somatically acquired mutations are important to the
development of cancer. It has been estimated that 1–2% of all human
genes are implicated in cancer via mutation (1). Of these, ~90% have somatic mutations,
20% bear germline mutations that predispose to cancer and 10% show
somatic and germline mutations (http://www.sanger.ac.uk/genetics/CGP/Census). In
unbiased surveys, protein kinases have emerged as one of the most
frequently mutated gene families in cancer (1–3).
Additional genome sequencing efforts in diverse cancers have
focused specifically on the protein kinase gene family, leading to
a large database of known mutations (4). In numerous cases, the potential
functional effects of these mutations are unknown. Thus, it is
important to conduct follow-up biochemical studies to identify
which mutants may represent the drivers that increase cell
proliferation.
A recent study of genomic DNA from colorectal cancer
patients identified a novel gene fusion event between the
anaplastic lymphoma kinase (ALK) and a previously uncharacterized
gene, chromosome 2, open reading frame 44 (C2orf44)
(5). ALK is a receptor-type
tyrosine kinase that has been implicated in several gene fusion
events in human cancer (6–10). A chromosomal translocation
associated with anaplastic large-cell lymphomas produces a fusion
protein between ALK and nucleophosmin (NPM) (10). A fusion between the echinoderm
microtubule-associated protein-like 4 (EML4) and ALK was identified
in a subset of non-small cell lung cancer (11). The C2orf44-ALK fusion results
from a large (5 million base pair) tandem duplication and results
in ALK kinase overexpression (5).
The function of the C2orf44-encoded protein in normal or
cancer cells is unknown.
Our previous study conducted a proteomic screen to
identify signaling components in U937 human monocytic cells that
interact with the non-receptor tyrosine kinase (NRTK) hematopoietic
cell kinase (Hck) (12). The screen
was based on the ability of Hck substrates and binding proteins to
interact with the Src homology 3 (SH3) domain of the enzyme, a
critical regulatory region. In addition to identifying several
regulators of phagocytosis, migration and the actin cytoskeleton,
an interaction between Hck and the C2orf44 protein was found
(12). In the present study, the
C2orf44 protein was cloned and characterized as a first step in
understanding its cellular function. Due to the presence of
conserved sequence elements, the C2orf44 protein was designated as
WD repeat and coiled coil containing protein (WDCP). WDCP was
demonstrated to bind tightly and specifically to the SH3 domain of
Hck through a proline-rich motif at the C-terminus. WDCP was also
shown to exist as an oligomer when expressed in mammalian cells.
These results indicate that the C2orf44-ALK fusion in
colorectal cancer may activate ALK through increased kinase
oligomerization and trans-phosphorylation.
Materials and methods
Materials
Antibodies against phosphotyrosine (4G10, cat. no.
05-321) and Hck (cat. no. 06-833) were from EMD Millipore
(Billerica, MA, USA). M45 antibody was a gift from Dr Pat Hearing
(Stony Brook University, NY, USA). Protein A, buffers, antibiotics
and other chemicals were from Sigma-Aldrich (St. Louis, MO, USA).
Glutathione agarose was purchased from Molecular Probes/Life
Technologies (Carlsbad, CA, USA). The mammalian expression vector
for Hck was as described previously (12).
Cloning WDCP
Polymerase chain reaction (PCR) was used to amplify
the WDCP coding sequence from human cDNA, clone HEP04702 (obtained
from the Human Genome Center, Institute of Medical Science,
University of Tokyo, Tokyo, Japan). The primers used were: forward,
5′, GGATCCTGA TGGAGTTGGGAAAAGGAAAACTACTCAGG; and reverse 3′,
AAGCTTTCAAGCCATGCCATCTACATGGTT ACAACAGCC. The WDCP cDNA was cloned
into the BamHI and HindIII sites of plasmid pCM45 (a
gift from Dr Pat Hearing, Stony Brook University) to produce an
N-terminal M45-tagged version. Protein features were predicted with
the Universal Protein Resource UniProt (13).
Cell culture, transfections,
immunoprecipitations and western blotting
Human embryonic kidney (HEK) 293 cells were
maintained in Dulbecco's modified Eagle's medium with 10% fetal
bovine serum, 100 U/ml streptomycin sulfate and 100 µg/ml
amphotericin B. Transfections were carried out with Mirus TransIT
(Mirus Corp., Madison, WI, USA) using HEK293 cells, which had been
plated 24 h previously. The cells were harvested 48 h after
transfection and were lysed in buffer containing 20 mmol/l Tris (pH
8.0), 5 mmol/l EDTA, 1% Nonidet P-40, 150 mmol/l NaCl and 2 mmol/l
sodium orthovanadate, with protease inhibitors (5 mg/l aprotinin, 5
mg/l leupeptin and 0.1 mmol/l phenylmethylsulfonyl fluoride) at 4°C
for 30 min. The cell lysates were centrifuged at 14,000 x g for 15
min at 4°C. Following protein concentration determination, 1 mg/ml
concentrations of the cell lysates were used for
immunoprecipitation experiments. The lysates were first precleared
with protein A beads for 1 h at 4°C. The protein A beads were
discarded following centrifugation. Subsequently, 1 µg antibody (or
the immunoglobulin G control) and 15 µl protein A beads were added
to the precleared cell lysates and incubated for 1 h at 4°C. The
beads were washed three times with lysis buffer and
immunoprecipitated proteins were resolved by SDS-PAGE. The proteins
were transferred to polyvinylidene difluoride membranes and western
blotting was carried out with the appropriate antibodies. The
proteins were visualized using horseradish peroxidase-conjugated
secondary antibody and an enhanced chemiluminescent detection kit
(GE Life Sciences, Pittsburgh, PA, USA).
Binding assays
Glutathione S-transferase (GST) or GST-SH3 fusions
(Hck, Crk, Grb2 N-terminal SH3 and Nck) were immobilized on
glutathione-agarose. HEK293 cell lysates were added to the resins
and agitated at 4°C for 30 min. The resins were washed extensively
with lysis buffer and bound proteins were eluted with SDS sample
buffer and analyzed by SDS-PAGE and western blotting.
Gel filtration
HEK293 lysates were fractionated on a Superdex-200
fast protein liquid chromatography column (GE Life Sciences,
Pittsburgh, PA, USA) pre-equilibrated with 20 mmol/l Tris (pH 8.0),
0.2 M NaCl, 10% glycerol and 0.2 mmol/l
Na3VO4. The proteins were eluted from the
column at a flow rate of 0.15 ml/min. Fractions of 0.15 ml were
collected. The column fractions were subjected to anti-M45
immunoprecipitation, and assayed for WDCP using anti-M45 western
blotting. The molecular weight calibration was performed using
protein standards (Sigma-Aldrich) and by constructing a standard
curve of retention time vs. molecular weight.
Results
C2orf44 protein
We previously conducted a proteomic screen for the
binding partners of the SH3 domain of the Hck non-receptor tyrosine
in the U937 human monocytic cell line. One of the proteins
identified was the product of the human C2orf44 gene
(12). PCR was used to amplify the
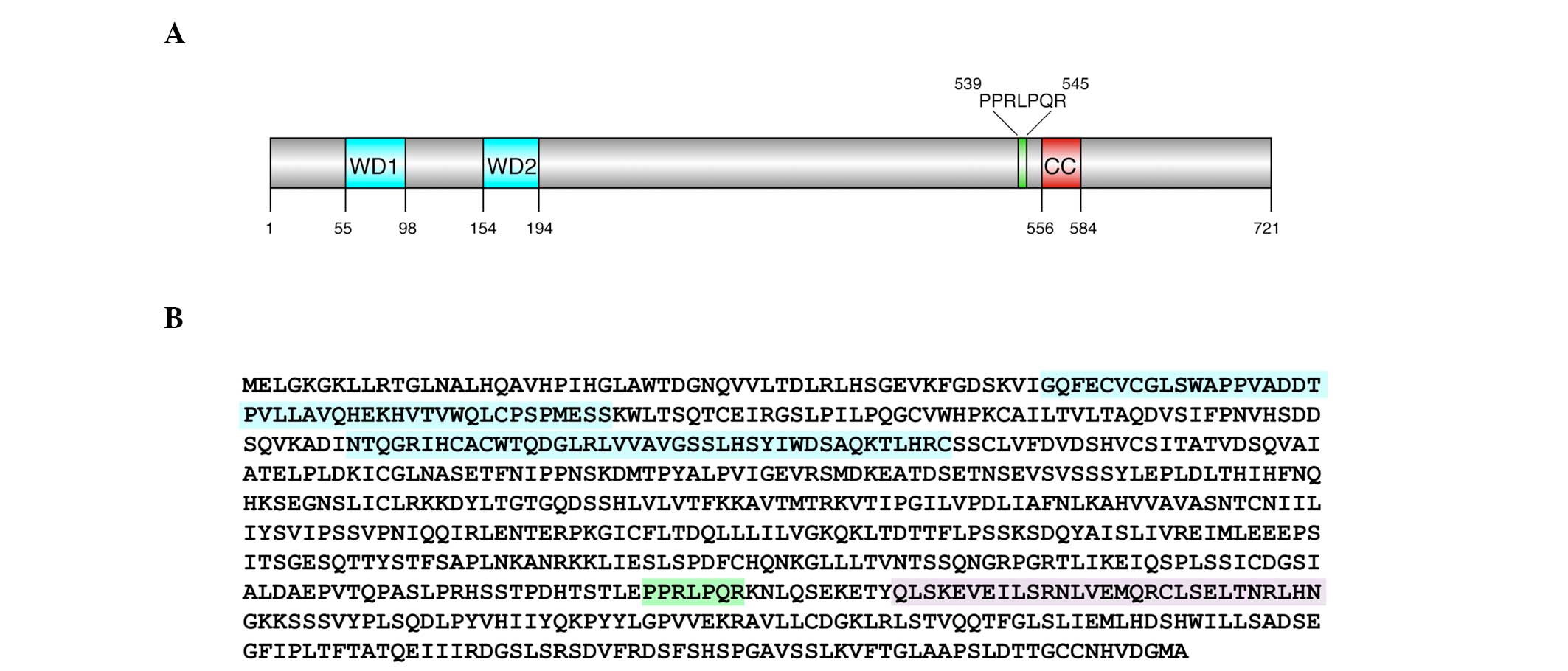
full-length cDNA encoding the C2orf44 protein. The protein consists
of 721 amino acids (Fig. 1). The
amino-terminal half of the protein contains two WD repeats (WD1 and
WD2); these units are involved in a range of biological functions,
including apoptosis, transcriptional regulation and signal
transduction (14). The
carboxy-terminal half of the protein contains a proline-rich
sequence (PPRLPQR) that is similar to known ligands for the SH3
domains (15). The C-terminus is
also predicted to have a leucine-rich coiled coil region (CC). In
view of these sequence elements, the C2orf44-encoded protein
was designated as WDCP.
Western blotting for WDCP
analysis
CC regions are frequently involved in protein
oligomerization, often through the formation of amphiphilic
structures. WD repeats have also been observed to mediate
oligomerization (14). The
C2orf44-ALK gene fusion in colorectal cancer could
potentially be activated by the inappropriate oligomerization of
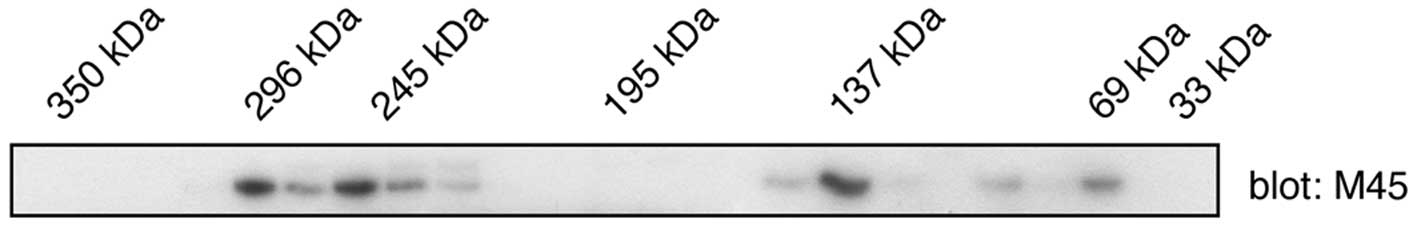
the ALK tyrosine kinase. To test whether WDCP exists as an
oligomer, a mammalian expression vector encoding WDCP with an
N-terminal M45 tag was produced. The protein was expressed in
HEK293 cells and cell lysates were fractionated on a Superdex-200
gel filtration column that had been calibrated with molecular
weight standards. WDCP was visualized in column fractions by
western blotting with anti-M45 antibodies (Fig. 2). Although the predicted molecular
weight of WDCP is 79 kilodaltons (kDa), relatively low amounts of
the protein were detected in this mass range. Instead, the majority
of the proteins co-eluted in the same region as higher (130–140 and
>200 kDa) molecular weight standards. These results are
consistent with the formation of WDCP oligomers in mammalian
cells.
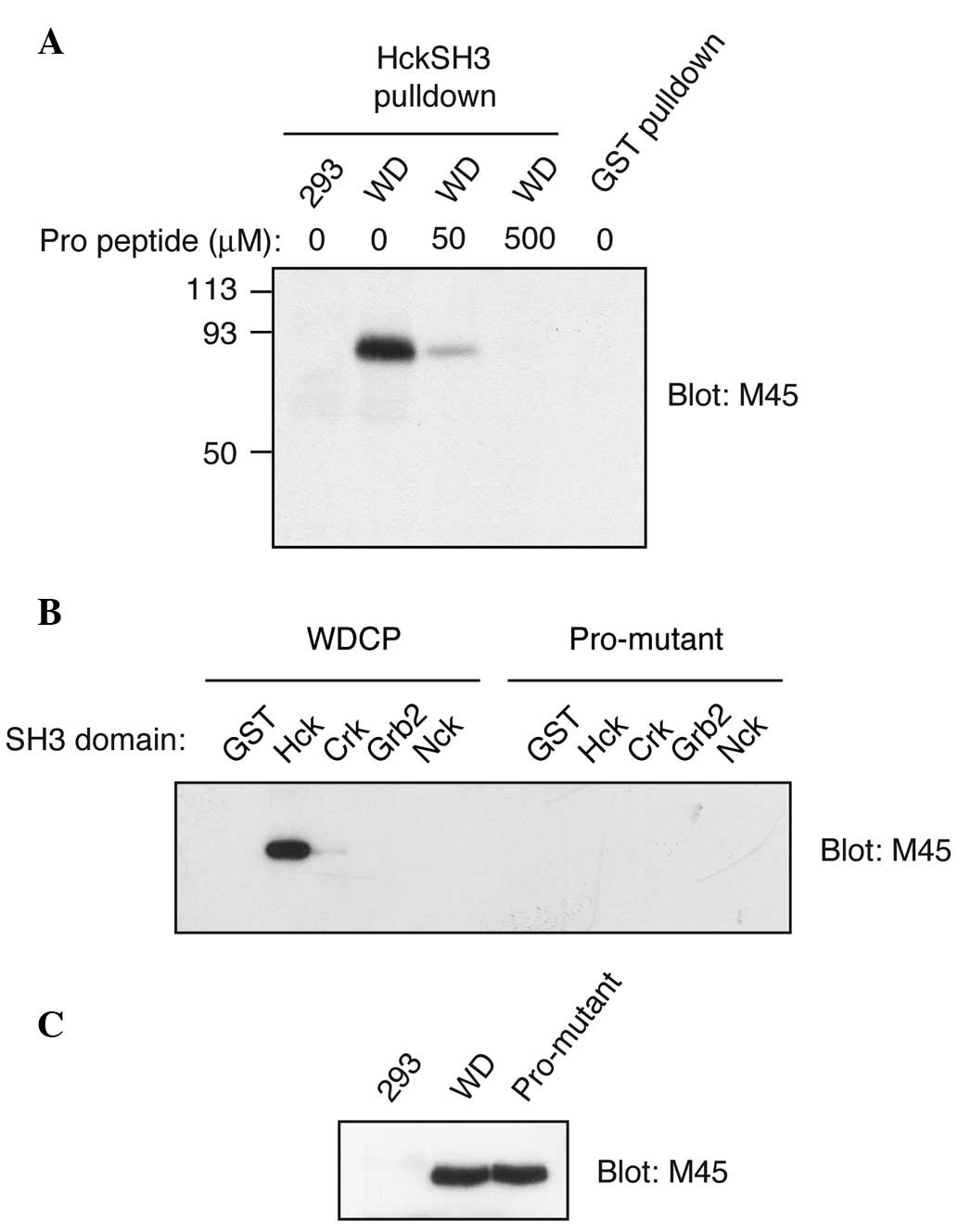
Subsequently, the ability of WDCP to interact with
SH3 domains was tested. M45-tagged WDCP was expressed in HEK293
cells and incubated lysates with the GST-tagged Hck SH3 domain,
immobilized on glutathione agarose. In certain reactions, a
Pro-rich competitor peptide (YEVPPPVPPRRR, a peptide we previously
showed binds tightly to the Hck SH3 domain) was included (16). WDCP bound to the immobilized Hck SH3
domain in the absence of the competitor peptide (Fig. 3A). Binding was substantially reduced
in the presence of 50 µM Pro-rich peptide and eliminated altogether
in the presence of 500 µM peptide (Fig.
3A). WDCP did not bind to control beads containing immobilized
GST (Fig. 3A). These experiments
validated the results of the proteomic screen and indicated that
WDCP binds to the typical ligand-binding site on the Hck SH3
domain, which recognizes proline-rich sequences.
WDCP contains one predicted SH3 ligand sequence
between amino acids 539 and 545. This Pro-rich sequence is
predicted to be a class 2 motif, with the consensus sequence PXXPXR
(15). The two proline residues
within this sequence (Pro 540 and Pro 543) were mutated to alanine
and the wild-type and mutant forms were expressed in HEK293 cells.
Binding experiments were carried out using cell lysates and a
variety of immobilized SH3 domains. Wild-type WDCP showed a strong
preference for the SH3 domain of Hck; there were trace amounts of
binding to the Crk SH3 domain and undetectable binding to the SH3
domains of Grb2 (N-SH3) and Nck (Fig.
3B). The proline to alanine mutations eliminated binding to the
SH3 domain of Hck (Fig. 3B). The
expression of the Pro-mutant form of WDCP was confirmed to be
equally strong as the wild-type (Fig.
3C). Thus, the SH3 binding site was localized on WDCP to the
Pro-rich sequence between 539–545 and it was demonstrated that WDCP
has selectivity with respect to the SH3-mediated protein-protein
interactions in which it is involved.
Interaction of WDCP and Hck
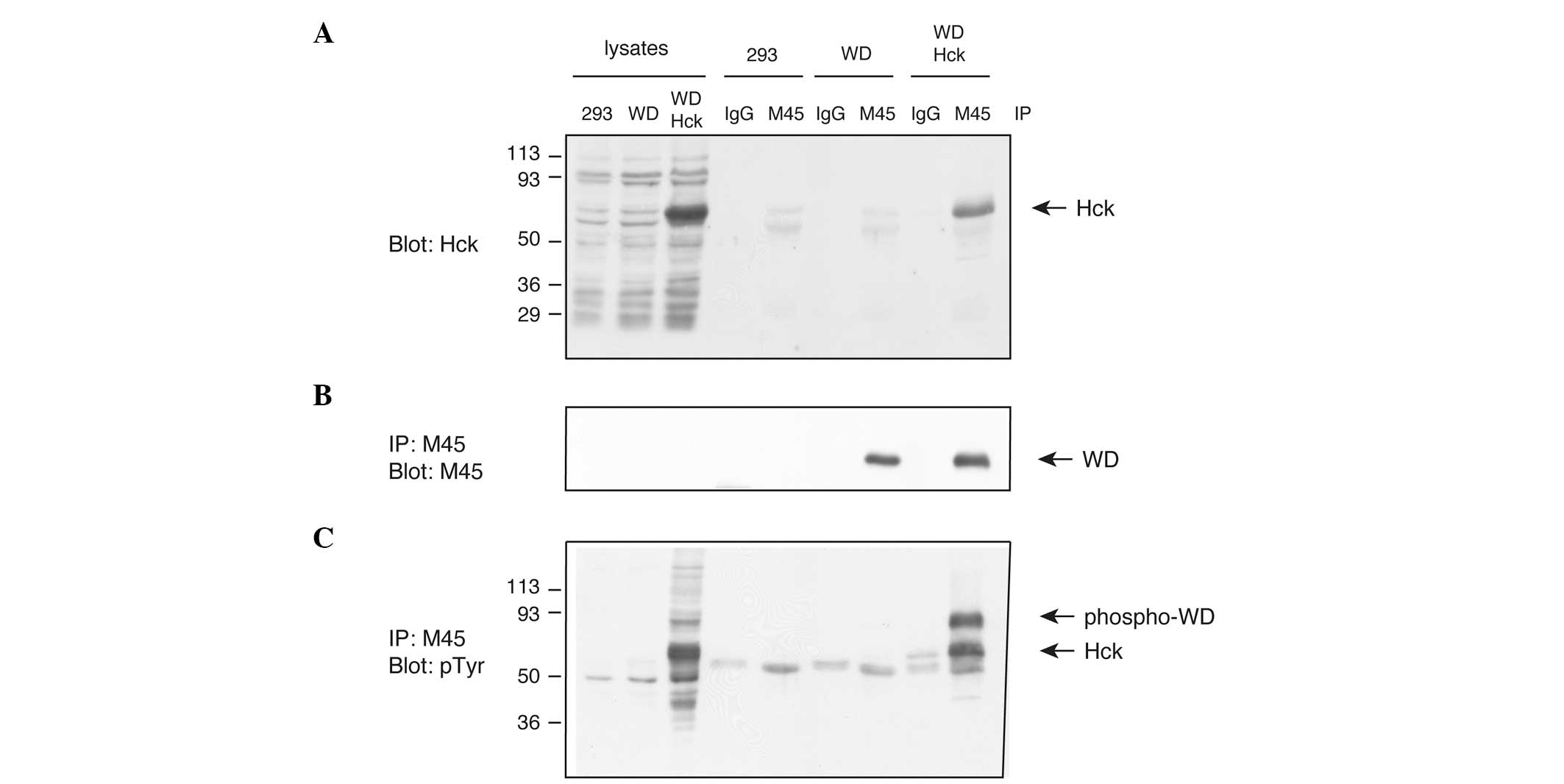
Subsequently, the interaction of WDCP with
full-length Hck kinase in mammalian cells was tested. M45-tagged
WDCP was expressed in HEK293 cells, either alone or together with
Hck. Following immunoprecipitation of WDCP, anti-Hck western
blotting experiments were performed to show that the two proteins
interact (Fig. 4A). The levels of
precipitated WDCP were similar in the presence or absence of Hck
(Fig. 4B). The blot was stripped
and reprobed with anti-phosphotyrosine antibodies (Fig. 4C). These experiments showed that
WDCP is tyrosine phosphorylated when co-expressed with Hck, but not
when expressed alone. Thus, Hck promotes WDCP tyrosine
phosphorylation, either directly (via an enzyme-substrate
interaction) or indirectly (for example, by stimulating another
kinase that directly phosphorylates WDCP).
Discussion
ALK is a member of the superfamily of RTKs (6,9). The
ALK gene is localized on chromosome 2p23.1. ALK knockout
mice develop normally, with a normal lifespan, exhibiting only
behavioral and neurochemical alterations (17). Similar to other RTKs, ALK contains
an extracellular ligand binding domain, a single-pass transmembrane
sequence and an intracellular tyrosine kinase catalytic domain.
Ligand binding to the extracellular portion of ALK triggers
trans-autophosphorylation of tyrosine residues within the kinase
domain. Once phosphorylated, the ALK kinase domain is fully active
and phosphorylates downstream substrates to promote cell growth and
survival. The downstream signaling partners activated by ALK
include phospholipase Cγ, mitogen-activated protein kinase,
phosphoinositide 3′-kinase/AKT and signal transducer and activator
of transcription (STAT) proteins (6,9).
The ALK gene is involved in a number of
chromosomal rearrangements in human cancers (6,7).
Frequently, these events produce fusion proteins containing the
intracellular tyrosine kinase domain of ALK; the fusion proteins
dimerize to give constitutive kinase activation. These ALK fusions
have been described as oncogenic drivers in solid tumors and
hematopoietic cancers (7–9). In non-small cell lung cancer, multiple
EML4 breakpoints fuse in frame with exon 20 of ALK to
produce activated EML4-ALK fusions. These fusions are oncogenic in
cell lines (11) and produce lung
adenocarcinoma in transgenic mice (18). The Food and Drug
Administration-approved ALK small molecule kinase inhibitor
crizotinib is a clinically effective therapy for patients harboring
the EML4-ALK translocation; a response rate of 57% and a disease
control rate of 90% were reported in a group of 82 patients
(7–9). The fusion between ALK and NPM in
anaplastic large-cell lymphoma was the first chromosomal
translocation to be reported involving the ALK gene
(10). Similar to the situation
with the EML4-ALK fusion, this translocation leads to constitutive
ALK kinase activity and stimulation of downstream signaling
pathways. In addition to chromosomal translocations, somatic and
germline point mutations in the kinase or juxtamembrane domains of
ALK have been reported in neuroblastoma and anaplastic thyroid
cancer (6,9). Whereas the biochemical effects of all
these mutations have not been investigated, they also presumably
lead to enhanced/constitutive ALK kinase activity.
In the present study, a novel fusion partner for ALK
was characterized: The protein encoded by the C2orf44 gene,
for which we propose to be known as WDCP. This gene fusion was
identified in a colorectal cancer patient. The resulting fusion
protein contains the N-terminal 680 amino acid residues fused
in-frame to ALK residues 1061–1620 (5). This portion of ALK comprises the
entire tyrosine kinase catalytic domain and C-terminal region. The
attachment of the WDCP residues presumably leads to increased ALK
activity by promoting oligomerization, which has been observed for
other ALK fusions. C2orf44 cDNA was cloned and the WDCP
protein was expressed in mammalian cells. WDCP was demonstrated to
exist as a high-molecular weight protein, supporting this idea. A
proline-rich segment within WDCP (residues 539–545, contained in
the C2orf44-ALK fusion) was also shown to bind specifically to the
SH3 domain of the Src family kinase Hck. Furthermore, WDCP serves
as a substrate for Hck. This is reminiscent of the situation in
anaplastic large-cell lymphoma, in which the NMP-ALK fusion serves
as a Src kinase substrate (19).
Furthermore, treatment of anaplastic large-cell lymphoma cells with
Src kinase inhibitors or Src-specific small interfering RNA
decreases their proliferation rate (19). In conclusion, these results on WDCP
indicate that such treatments may also be successful against the
C2orf44-ALK fusion.
Acknowledgements
The present study was supported by the National
Institutes of Health (grant CA no. 58530) to W.T.M.
References
|
1
|
Futreal PA, Coin L, Marshall M, et al: A
census of human cancer genes. Nat Rev Cancer. 4:177–183. 2004.
View Article : Google Scholar
|
|
2
|
Pleasance ED, Cheetham RK, Stephens PJ, et
al: A comprehensive catalogue of somatic mutations from a human
cancer genome. Nature. 463:191–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pleasance ED, Stephens PJ, O'Meara S, et
al: A small-cell lung cancer genome with complex signatures of
tobacco exposure. Nature. 463:184–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greenman C, Stephens P, Smith R, et al:
Patterns of somatic mutation in human cancer genomes. Nature.
446:153–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lipson D, Capelletti M, Yelensky R, et al:
Identification of new ALK and RET gene fusions from colorectal and
lung cancer biopsies. Nat Med. 18:382–384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiarle R, Voena C, Ambrogio C, Piva R and
Inghirami G: The anaplastic lymphoma kinase in the pathogenesis of
cancer. Nat Rev Cancer. 8:11–23. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shackelford RE, Vora M, Mayhall K and
Cotelingam J: ALK-rearrangements and testing methods in non-small
cell lung cancer: a review. Genes Cancer. 5:1–14. 2014.PubMed/NCBI
|
|
8
|
Morales La Madrid A, Campbell N, Smith S,
Cohn SL and Salgia R: Targeting ALK: a promising strategy for the
treatment of non-small cell lung cancer, non-Hodgkin's lymphoma,
and neuroblastoma. Target Oncol. 7:199–210. 2012.PubMed/NCBI
|
|
9
|
Hallberg B and Palmer RH: Mechanistic
insight into ALK receptor tyrosine kinase in human cancer biology.
Nat Rev Cancer. 13:685–700. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morris SW, Kirstein MN, Valentine MB, et
al: Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM,
in non-Hodgkin's lymphoma. Science. 263:1281–1284. 1994. View Article : Google Scholar
|
|
11
|
Soda M, Choi YL, Enomoto M, et al:
Identification of the transforming EML4-ALK fusion gene in
non-small-cell lung cancer. Nature. 448:561–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scott MP, Zappacosta F, Kim EY, Annan RS
and Miller WT: Identification of novel SH3 domain ligands for the
Src family kinase Hck. Wiskott-Aldrich syndrome protein (WASP),
WASP-interacting protein (WIP), and ELMO1. J Biol Chem.
277:28238–28246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
UniProt Consortium, . Activities at the
Universal Protein Resource (UniProt). Nucleic Acids Res.
42:(Database issue). D191–D198. 2014.PubMed/NCBI
|
|
14
|
Smith TF, Gaitatzes C, Saxena K and Neer
EJ: The WD repeat: a common architecture for diverse functions.
Trends Biochem Sci. 24:181–185. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mayer BJ and Eck MJ: SH3 domains. Minding
your p's and q's. Curr Biol. 5:364–367. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nguyen JT, Porter M, Amoui M, Miller WT,
Zuckermann RN and Lim WA: Improving SH3 domain ligand selectivity
using a non-natural scaffold. Chem Biol. 7:463–473. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bilsland JG, Wheeldon A, Mead A, et al:
Behavioral and neurochemical alterations in mice deficient in
anaplastic lymphoma kinase suggest therapeutic potential for
psychiatric indications. Neuropsychopharmacology. 33:685–700. 2008.
View Article : Google Scholar
|
|
18
|
Soda M, Takada S, Takeuchi K, et al: A
mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci
USA. 105:19893–19897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cussac D, Greenland C, Roche S, et al:
Nucleophosmin-anaplastic lymphoma kinase of anaplastic large-cell
lymphoma recruits, activates, and uses pp60c-src to mediate its
mitogenicity. Blood. 103:1464–1471. 2004. View Article : Google Scholar : PubMed/NCBI
|