Introduction
In recent decades, stem cells have attracted
increasing attention due to their capacity to self-replicate or
produce specific differentiated cell types and their potential as
cell therapies for human disease (1). Neural stem cells (NSCs) that reside
within the central nervous system (CNS) can differentiate into
neurons, astrocytes or oligodendrocytes under specific conditions,
which can help to develop new therapies for CNS disease (2–4). Bone
marrow derived-mesenchymal stem cells (BMSCs) are multipotent stem
cells that are capable of differentiating into a variety of
lineages (5–7). Through stress signals, BMSCs show
significant tropism for the injured site to manage the regenerative
process via direct or indirect interactions (8–10).
BMSCs are easily obtained and cultured and they can avoid the
immune rejection through autologous transplantation. Therefore,
BMSCs have emerged as ideal cellular material for cell and gene
therapies in regenerative medicine (11, 12).
However, it requires more effort to achieve the aim
of cell and gene therapies using NSCs or BMSCs clinically. One main
unsolved problem is how to modulate NSCs or BMSCs to differentiate
into the required specific types of cells, which is the premise of
a safe and effective treatment. In addition, the interaction
between them is not yet clear. In the present study, the Transwell
co-culture system was used to mimic the in vivo environment
and the differentiation of NSCs or BMSCs was observed to
investigate the interaction between them.
Materials and methods
Cell culture Preparation and culture
of human embryo NSCs
Primary dissociated cell cultures were prepared from
the periventricular region of the telencephalon of a 15 weeks
gestation, as described previously (13). A suspension of primary neural cells
at a density of 5×105/ml was plated on uncoated tissue
culture dishes (Corning Life Sciences, Glendale, AZ, USA) in the
following growth medium: Dulbecco's modified Eagle's medium/F12
(1:1) supplemented with N2 (1:50), B27 (1:100) (Invitrogen, Life
Technologies, Carlsbad, CA, USA), basic fibroblast growth factor
(Millipore, Billerica, MA, USA), heparin and epidermal growth
factor (Sigma-Aldrich, St. Louis, MO, USA). Medium was changed
every 3–4 days. Cell aggregates were dissociated into passage
culture with 0.25% trypsin/1 mmol/1 EDTA (Sigma-Aldrich) when the
neurospheres were >10 cell diameters in size and replated in
growth medium at a density of 5×105/ml. Cells from
passages 3 to 7 were used.
Preparation and culture of human bone
marrow-derived BMSCs
Bone marrow cells were harvested from volunteer
donors who had provided informed consent and the study was carried
out according to local ethical guidelines. Briefly, primary BMSCs
were generated as previously described (14). Cultures were maintained at 37˚C in a
humidified atmosphere of 5% CO2. Culture medium was
replaced twice a week. BMSCs were harvested after reaching ≥90%
confluence by digestion with 0.25% trypsin/1 mmol/1 EDTA and
replated into passage culture at a density of
1.0×106/ml. All the BMSCs were cultured in <8
passages.
Transwell co-culture
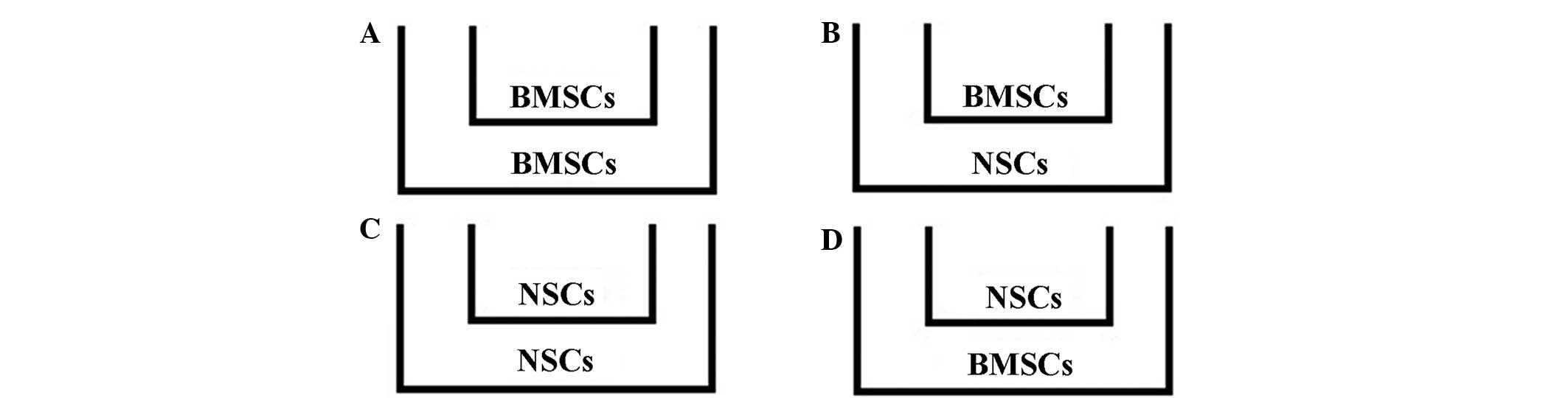
To investigate the interaction of BMSCs and NSCs
during differentiation, the Transwell chambers were used to
co-culture BMSCs and NSCs and four experimental groups were
established (Fig. 1). According to
the experimental groups, BMSCs or NSCs were seeded onto the upper
insert of a 6-well Transwell (with 0.4 µm pores; Millipore) at a
density of 5×105/ml placed above the NSCs or BMSCs, with
the different culture medium. After 1, 4, 7, 10 and 14 days of
culture, brain-derived neurotrophic factor (BDNF) and nerve growth
factor (NGF) were measured in the culture medium as described
below. After 14-day culture, expression of neural markers was
analyzed by immunofluorescence as described below.
Identifying the differentiation by
immunofluorescence
For immunofluorescence, cells cultured in the
Transwell chambers were fixed with 4% paraformaldehyde and
permeabilized using 0.3% Triton X-100 in phosphate-buffered saline
prior to blocking in 10% normal goat serum. Subsequently, the
samples were incubated with primary antibodies at 4˚C overnight.
Fluorochrome-conjugated species-specific secondary antibodies were
used for immunodetection. Nuclei were stained with
4′,6-diamidino-2-phenylindole (1 µg/ml; Sigma-Aldrich). The
following antibodies and final dilutions were used: Rabbit
anti-Nestin (1:250; cat. no. AB5922; Millipore), rabbit
anti-microtubule-associated protein 2 (MAP-2) (1:200; cat. no.
AB5622; Millipore) and goat anti-rabbit immunoglobulin G-cyanine 3
(1:1,000; cat. no. A10520; Invitrogen). The positive cells were
counted per high-power field under a fluorescence microscope (Carl
Zeiss Axiovert 200; Carl Zeiss Microscopy GmbH, Gottingen,
Germany). Each sample was counted randomly in ten separated
high-power fields.
Enzyme-linked immunosorbent assay
(ELISA)
At the different time points of the co-culture, the
media were collected and centrifuged at 10,000 x g at 4˚C for 5 min
and the supernatants were stored at −20˚C. The immunoreactive
levels of BDNF and NGF were measured by ELISA kits (Promega,
Madison, WI, USA) according to the manufacturer's instructions.
Absorbance at 450 nm was measured with a microplate reader
(Bio-Rad, Hercules, CA, USA).
Statistical analysis
The results obtained were expressed as mean ±
standard deviation of triplicate or quadruplicate cultures. One-way
analysis of variance was performed for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Distribution of NSCs in human embryo
brain
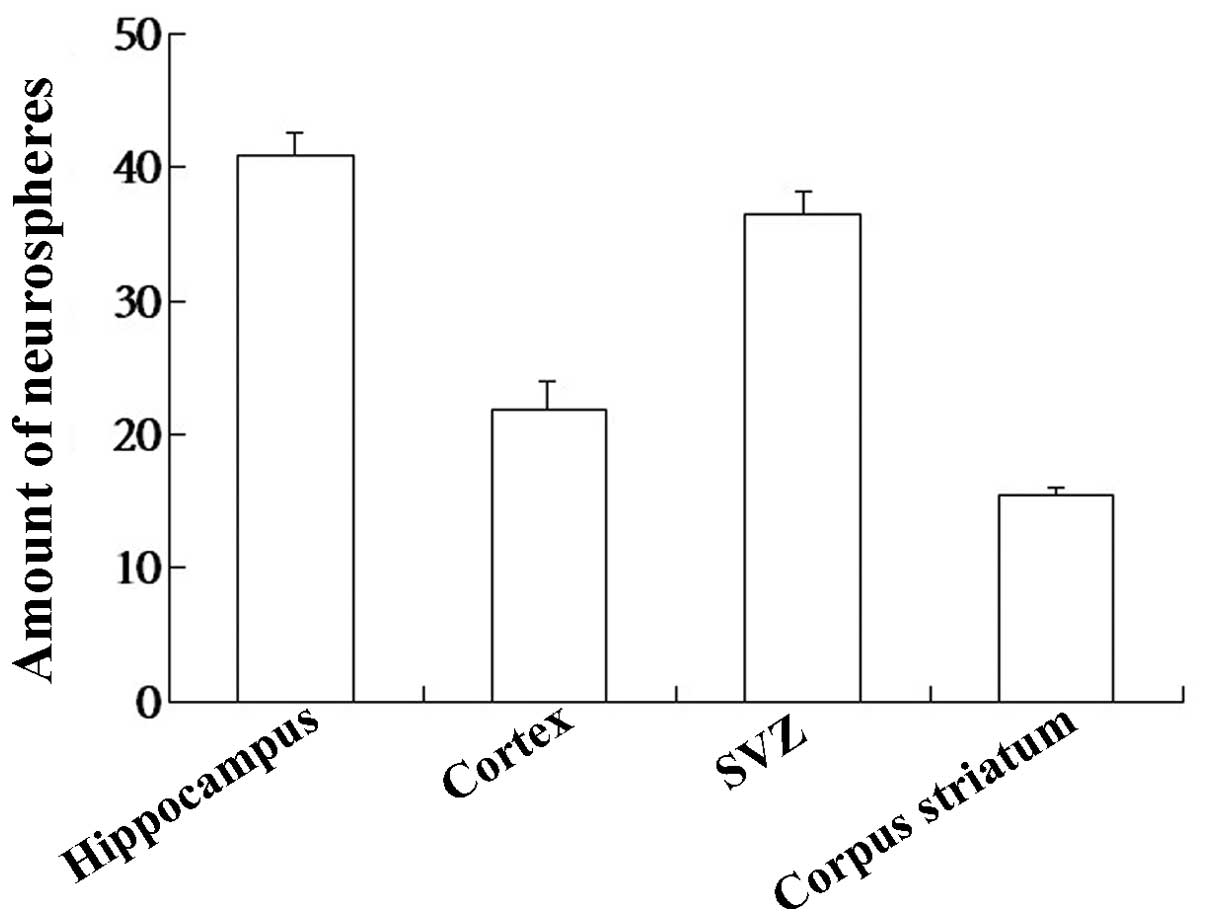
In the present study, t he human embryo NSCs were
cultured and distributions of NSCs varied in different regions of
the human embryo brain. The number of NSCs in the hippocampus was
higher than other regions (P<0.05; Fig. 2).
Interaction between NSCs and BMSCs
during differentiation
Previous studies indicated that NSCs and BMSCs
alleviated the damage of brain injury as they could differentiate
into neural cells following transplantation (8, 15,
16). However, the interaction
between them during differentiation remains unknown. To assess the
interaction, the advantage of the Transwell co-culture system was
used.
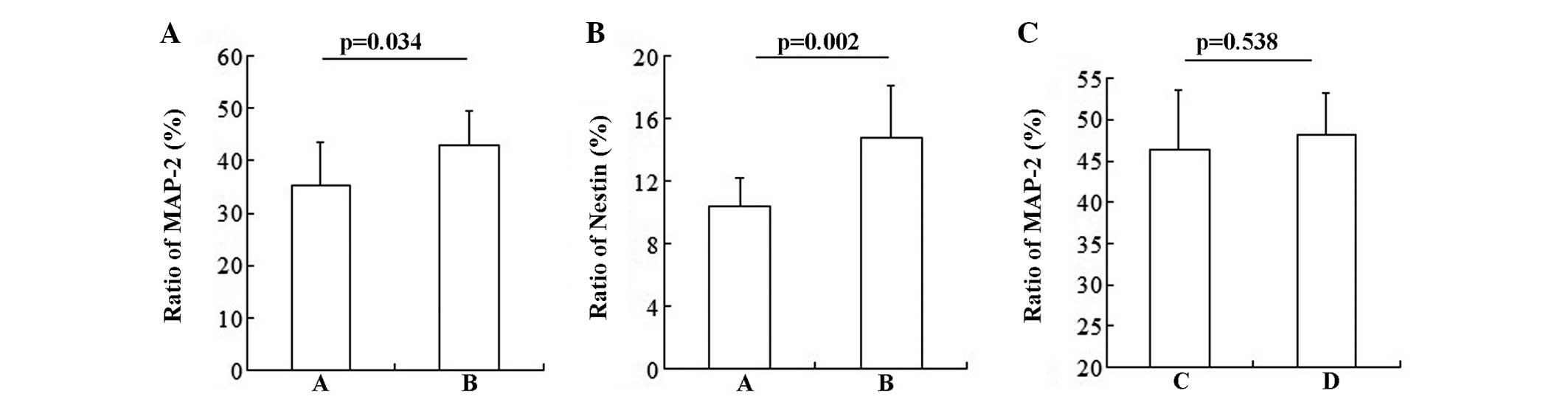
NSCs and BMSCs in the co-culture system were found
to differentiate into neurons. The statistical result showed that
NSCs promoted BMSCs to differentiate into neurons (Fig. 3A; group A vs. group B: 36.368±7.94
vs. 42.887±6.64%; P=0.034) and NSCs (Fig. 3B; group A vs. group B: 10.368±1.83
vs. 14.775±3.33%; P=0.002). However, BMSCs had no influence on the
differentiation of NSCs (Fig. 3C;
group C vs. group D: 46.377±7.15 vs. 48.116±5.07%; P=0.538).
Secretion of BDNF and NGF in
co-culture
As known, NSCs and BMSCs secrete neurotrophic
factors, including BDNF and NGF (17, 18).
The contribution of BDNF and NGF to a range of cell responses has
been confirmed, including cell differentiation (19–21).
Therefore, the level of BDNF and NGF in different experimental
groups was detected. As the detection range of the ELISA kits is
7.8-500 ng/l, the concentration of BDNF and NGF at days 7, 10 and
14 was in the range (detection occurred at days 1, 4, 7, 10 and
14). The concentration of BDNF and NGF in the four groups increased
with the prolonged time (P<0.05; Tables I and II). Compared to the mono-culture groups
(groups A and C), a higher concentration of BDNF and NGF was
observed in the two co-culture groups (groups B and D) (P<0.05;
Tables I and II). In addition, there was no significant
difference in the concentration of BDNF and NGF between groups B
and D (P>0.05).
 | Table I.Concentration of brain-derived
neurotrophic factor at different time points in the four
experimental groups. |
Table I.
Concentration of brain-derived
neurotrophic factor at different time points in the four
experimental groups.
| Variables | 7 days | 10 days | 14 days | F | P-value |
|---|
| Groups |
| A | 8.272±0.208 | 9.518±0.479 | 10.572±0.350 | 45.229 | <0.001 |
| B |
9.004±0.286a, b |
11.046±0.551a, b |
12.282±0.698a, b | 47.161 | <0.001 |
| C | 8.248±0.274 | 9.610±0.642 | 10.726±0.253 | 41.890 | <0.001 |
| D |
8.763±0.438a, b |
10.702±0.514a |
11.838±0.575a, b | 47.033 | <0.001 |
| F | 6.695 | 9.813 | 14.28 | | |
| P-value | 0.003 | 0.001 | 0.000 | | |
 | Table II.Concentration of nerve growth factor
at different time points in the four experimental groups. |
Table II.
Concentration of nerve growth factor
at different time points in the four experimental groups.
| Variables | 7 days | 10 days | 14 days | F | P-value |
|---|
| Groups |
| A | 9.808±0.329 | 12.146±0.865 | 14.852±0.502 | 86.139 | <0.001 |
| B |
11.492±0.582a, b |
14.168±0.322a, b |
16.070±0.390a, b | 133.383 | <0.001 |
| C | 9.106±0.527 | 11.950±0.159 | 14.574±0.745 | 130.655 | <0.001 |
| D |
10.850±0.454a, b |
13.860±0.249a, b |
15.828±0.451a, b | 199.619 | <0.001 |
| F | 24.262 | 27.895 | 9.143 | | |
| P-value | 0.000 | 0.000 | 0.01 | | |
Discussion
NSCs and BMSCs possess a significant expansion and
differentiation potential, placing themselves as a potential source
of material for cell transplantation therapies. Previous studies
identified that transplanting BMSCs and NSCs decreased the
apoptosis of neurons and improved the prognosis (10, 15,
22, 23). However, the mechanisms are
complicated and modulated by a variety of factors, such as
cytokines, neurotrophic factors, inflammation and apoptosis
(24–26). The interaction of NSCs with
transplanted BMSCs and whether the interaction is beneficial to the
treatment remains to be determined. Confirming these may aid in
improving the understanding of the potential of cell therapy and
improving the efficacy.
In the present study, the interaction between NSCs
and BMSCs during differentiation was investigated and the following
findings are considered: i) NSCs promoted BMSCs to differentiate
into neurons and NSCs, although they do not come into direct
contact; and ii) co-culture increased the level of BDNF and NGF.
Similarly, certain studies also identified that NSCs induced the
differentiation of BMSCs. Sanchez-Ramos et al (27) observed that the amount of
neuron-specific nuclear protein-positive cells differentiated from
BMSCs co-cultured with the brain tissue of fetal rat was
approximately two-fold higher compared to the induced group of
mono-cultured BMSCs.
In the present study, BMSCs did not affect the
differentiation of NSCs; however, other studies exhibited
conflicting results. Lou et al (28) reported that BMSCs induced NSCs to
differentiate into more MAP-2 positive cells and Wang et al
(29) also found that the
interactions between MSCs and NSCs are involved in specifying
neuronal fate.
Co-culture with Transwell chambers circumvents the
contact of NSCs and BMSCs, however, the small soluble factors can
pass through. Therefore, these small soluble factors may be
involved in the interplay of NSCs and BMSCs during differentiation.
NSCs and BMSCs can secrete BDNF and NGF, which play the crucial
roles in cell differentiation and the concentration of the two
neurotrophic factors was increased in the present study. Through
binding to the tropomyosin-receptor-kinase (Trk) receptors (TrkA
and TrkB), BDNF and NGF activated the downstream signaling.
Previous studies indicated that BDNF and NGF modulated cell
differentiation through the protein kinase B/mitogen-activated
protein kinase pathway (21). BDNF
has also been reported to possibly contribute to the
differentiation of NSCs by triggering the Wnt/β-catenin signaling
pathway (30). However, the
mechanisms of BDNF and NGF in modulating the differentiation of
NSCs and BMSCs requires further confirmation.
Taken together, the interaction of NSCs and BMSCs
promoted BMSCs to differentiate into neurons and NSCs, which may be
associated with BDNF and NGF. However, the underlying mechanism
requires further clarification. To understand the interplay of NSCs
and BMSCs during differentiation is likely to aid in obtaining new
information for cell transplantation therapy in CNS disease.
References
|
1
|
Hess DC and Borlongan CV: Stem cells and
neurological diseases. Cell Prolif. 41 (Suppl 1):94–114. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song H, Stevens CF and Gage FH: Astroglia
induce neurogenesis from adult neural stem cells. Nature.
417:39–44. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johansson CB, Momma S, Clarke DL, Risling
M, Lendahl U and Frisen J: Identification of a neural stem cell in
the adult mammalian central nervous system. Cell. 96:25–34. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Temple S: The development of neural stem
cells. Nature. 414:112–117. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Woodbury D, Schwarz EJ, Prockop DJ and
Black IB: Adult rat and human bone marrow stromal cells
differentiate into neurons. J Neurosci Res. 61:364–370. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hermann A, Gastl R, Liebau S, et al:
Efficient generation of neural stem cell-like cells from adult
human bone marrow stromal cells. J Cell Sci. 117:4411–4422. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin W, Chen X, Wang X, Liu J and Gu X:
Adult rat bone marrow stromal cells differentiate into Schwann
cell-like cells in vitro. In Vitro Cell Dev Biol Anim. 44:31–40.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao LR, Duan WM, Reyes M, Keene CD,
Verfaillie CM and Low WC: Human bone marrow stem cells exhibit
neural phenotypes and ameliorate neurological deficits after
grafting into the ischemic brain of rats. Exp Neurol. 174:11–20.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Novikova LN, Brohlin M, Kingham PJ,
Novikov LN and Wiberg M: Neuroprotective and growth-promoting
effects of bone marrow stromal cells after cervical spinal cord
injury in adult rats. Cytotherapy. 13:873–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hawryluk GW, Mothe A, Wang J, Wang S,
Tator C and Fehlings MG: An in vivo characterization of trophic
factor production following neural precursor cell or bone marrow
stromal cell transplantation for spinal cord injury. Stem Cells
Dev. 21:2222–2238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamada H, Kobune M, Nakamura K, et al:
Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene
therapy. Cancer Sci. 96:149–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
El-Badri NS, Hakki A, Saporta S, et al:
Cord blood mesenchymal stem cells: Potential use in neurological
disorders. Stem Cells Dev. 15:497–506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flax JD, Aurora S, Yang C, et al:
Engraftable human neural stem cells respond to development cues,
replace neurons, and express foreign genes. Nat Biotechnol.
16:1033–1039. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kafienah W, Mistry S, Williams C and
Hollander AP: Nucleostemin is a marker of proliferating stromal
stem cells in adult human bone marrow. Stem Cells. 24:1113–1120.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen JR, Cheng GY, Sheu CC, Tseng GF, Wang
TJ and Huang YS: Transplanted bone marrow stromal cells migrate,
differentiate and improve motor function in rats with
experimentally induced cerebral stroke. J Anat. 213:249–258. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chu K, Kim M, Park KI, et al: Human neural
stem cells improve sensorimotor deficits in the adult rat brain
with experimental focal ischemia. Brain Res. 1016:145–153. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crigler L, Robey RC, Asawachaicharn A,
Gaupp D and Phinney DG: Human mesenchymal stem cell subpopulations
express a variety of neuro-regulatory molecules and promote
neuronal cell survival and neuritogenesis. Exp Neurol. 198:54–64.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Llado J, Haenggeli C, Maragakis NJ, Snyder
EY and Rothstein JD: Neural stem cells protect against
glutamate-induced excitotoxicity and promote survival of injured
motor neurons through the secretion of neurotrophic factors. Mol
Cell Neurosci. 27:322–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Piirsoo M, Kaljas A, Tamm K and Timmusk T:
Expression of NGF and GDNF family members and their receptors
during peripheral nerve development and differentiation of Schwann
cells in vitro. Neurosci Lett. 469:135–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Razavi S, Razavi MR,
Kheirollahi-Kouhestani M, Mardani M and Mostafavi FS: Co-culture
with neurotrophic factor secreting cells induced from
adipose-derived stem cells: Promotes neurogenic differentiation.
Biochem Biophys Res Commun. 440:381–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan J, Huang G, Xiao Z, Lin L and Han T:
Overexpression of β-NGF promotes differentiation of bone marrow
mesenchymal stem cells into neurons through regulation of AKT and
MAPK pathway. Mol Cell Biochem. 383:201–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng YB, Ye WB, Hu ZZ, et al:
Intravenously administered BMSCs reduce neuronal apoptosis and
promote neuronal proliferation through the release of VEGF after
stroke in rats. Neurol Res. 32:148–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen CC, Lin CH, Yang YC, Chiao MT, Cheng
WY and Ko JL: Intravenous implanted neural stem cells migrate to
injury site, reduce infarct volume, and improve behavior after
cerebral ischemia. Curr Neurovasc Res. 7:167–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walker PA, Letourneau PA, Bedi S, Shah SK,
Jimenez F and Cox CS Jr: Progenitor cells as remote ‘bioreactors’:
Neuroprotection via modulation of the systemic inflammatory
response. World J Stem Cells. 3:9–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hokari M, Kuroda S, Shichinohe H, Yano S,
Hida K and Iwasaki Y: Bone marrow stromal cells protect and repair
damaged neurons through multiple mechanisms. J Neurosci Res.
86:1024–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Walker PA, Harting MT, Jimenez F, et al:
Direct intrathecal implantation of mesenchymal stromal cells leads
to enhanced neuroprotection via an NFkabba B-mediated increase in
interleukin-6 production. Stem Cells Dev. 19:867–876. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanchez-Ramos J, Song S, Cardozo-Pelaez F,
et al: Adult bone marrow stromal cells differentiate into neural
cells in vitro. Exp Neurol. 164:247–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lou SJ, Gu P, Chen F, He C, Wang MW and Lu
CL: The effect of bone marrow stromal cells on neuronal
differentiation of mesencephalic neural stem cells in
Sprague-Dawley rats. Brain Res. 968:114–121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Tu W, Lou Y, et al: Mesenchymal
stem cells regulate the proliferation and differentiation of neural
stem cells through Notch signaling. Cell Biol Int. 33:1173–1179.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen BY, Wang X, Wang ZY, Wang YZ, Chen LW
and Luo ZJ: Brain-derived neurotrophic factor stimulates
proliferation and differentiation of neural stem cells, possibly by
triggering the Wnt/β-catenin signaling pathway. J Neurosci Res.
91:30–41. 2013.PubMed/NCBI
|