Introduction
Obesity is associated with several chronic diseases.
Environmental changes are not sufficient to control obesity;
however, there is overwhelming evidence that certain
pharmacological agents may act as therapeutic targets for obesity.
One such agent is topiramate (TPM), initially used for epilepsy
treatment and migraine prophylaxis. TPM is a glutamate
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate
receptor agonist and enhances the inhibitory effects mediated by
γ-aminobutyric acid (1). One of the
side effects of TPM is weight loss, which makes this drug a
possible option for obesity treatment. The central mechanisms
suggested for TPM-induced weight loss include a reduction in energy
efficiency, influence on the hypothalamus and alteration of
neuropeptides (1). However, the direct
action of TPM on adipose tissue, particularly on lipolysis, has not
been observed.
Two essential enzymes for lipid hydrolysis are
adipocyte triglyceride lipase (ATGL) and hormone-sensitive lipase
(HSL). ATGL hydrolyzes triacylglycerols into a fatty acid and a
diacylglycerol (2). To fully activate,
ATGL must interact with its cofactor, comparative genetic
identification 58 (CGI-58) (3). In
adipocytes without lipolytic stimuli, CGI-58 is strongly bound to
lipid droplets, interacting with perilipin A (2). However, perilipin A and CGI-58 dissociate
when lipolytic activity is stimulated by the activation of
β-adrenergic receptors, which causes an increase in the cyclic
adenosine monophosphate and consequent activation of protein kinase
A (PKA). This process releases CGI-58 to associate with ATGL.
Perilipin A is a structural protein that coats lipid droplets and
protects triglycerides (TAG) molecules from basal enzymatic
hydrolysis (4). Additionally, in
stimulated cells, perilipin A is phosphorylated and facilitates the
HSL translocation from the fat vesicles surface with consequent
access to its diacylglycerol substrate (5). Together, the phosphorylation of HSL and
its translocation to the lipid droplets surface, coupled with the
activation of ATGL by CGI-58, results in the hydrolysis of 90% of
TAG.
TPM treatment has been shown to reduce adiposity in
humans and rodents (6,7). TPM is assumed to act directly on
lipolysis, however, this has not been explicitly described in
vivo, once TPM acts on the central nervous system (CNS). Thus,
in the present study, 3T3-L1 adipocytes were used and TPM was
demonstrated to have a direct effect on lipolysis.
Materials and methods
Cell culture and measurement of
lipolysis
3T3-L1 cells were obtained from the American Type
Culture Collection and cultured at 37°C in 5% CO2 in
Dulbecco's modified Eagle's medium supplemented with 25 mmol/l
glucose, 1.0 mmol/l pyruvate, 4.02 mmol/l L-alanyl-glutamine and
10% fetal bovine serum (Gibco, New York, NY, USA). Cell
differentiation began 24 h after confluency and occurred over 4
days in a medium containing 0.25 µM dexamethasone, 0.5 mmol/l
3-isobutyl-1-methylxanthine and 5 µg/ml insulin (Sigma, St. Louis,
MO, USA). Following differentiation, the cells were cultured for 10
days in growth medium containing 5 µg/ml of insulin. Each parameter
was evaluated using a 6-well aliquot from this culture. On day 10
after differentiation, the cells were incubated for 24 h in a
medium containing 0.5% fetal bovine serum. Cells were treated with
10 µl of TPM (50 µM) or isoproterenol (20 µM) for 30 min. At the
end of the incubation, the glycerol and non-esterified fatty acid
(NEFA) contents (Wako Chemical, Richmond, Inc., VA, USA) of the
incubation medium were used as an index of lipolysis and were
measured using enzymatic-colorimetric kits. In addition, the
release of lactate dehydrogenase (LDH) induced by plasma membrane
disruption in culture medium was used as a cellular viability
index.
Incorporation of
[1-14C]-palmitate into lipids
Cells were incubated in Krebs/Ringer/phosphate
buffer (pH 7.4), supplemented with 1% bovine serum albumin and 1
mmol/l palmitate, at 37°C under a CO2 (5%)/O2
(95%) gas mixture. Aliquots (450 µl) were transferred to
polypropylene test tubes containing 5 µl of
[1-14C]-palmitate, in the presence or absence of insulin
(10 nmol/l), and in the presence or absence of TPM (50 µM). These
samples were subsequently incubated for 1 h at 37°C in a water
bath. Following incubation, the mixture was acidified with 0.2 ml
H2SO4 (8 N) and incubated for an additional
30 min. At the end of the incubation, the reaction mixture was
treated with 2.5 ml of Dole's reagent
(isopropanol:n-heptane:H2SO4,
4:1:0.25, v/v/v) for lipid extraction.
Immunoblotting
The whole cell extracts were prepared by lysis of
the cells in extraction buffer [1% Triton X-100, 100 mmol/l Tris
(pH 7.4), containing 100 mmol/l sodium pyrophosphate, 100 mmol/l
sodium fluoride, 10 mmol/l EDTA, 10 mmol/l sodium vanadate, 2
mmol/l phenylmethylsulphonyl fluoride and 0.1 mg of aprotinin/ml]
at 4°C. The extracts were centrifuged at 9,000 × g at 4°C (5804R;
Eppendorf AG, Hamburg, Germany) for 40 min to remove insoluble
material, and the supernatants of these tissues were used for
protein quantification, according to the Bradford method. Proteins
were denatured by boiling in Laemmli sample buffer containing 100
mM dithiothreitol, run on SDS-PAGE and transferred to
nitrocellulose membranes. Membranes were blocked, probed and
blotted with primary antibodies. Antibodies used for immunoblotting
were phospho HSLser660, phospho HSLser563,
HSL total (Cell Signaling Technology, Beverly, MA, USA), and
phospho PKAThr198, PKA total, CGI-58, perilipin A (Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), phospho
ATGLSer406, ATGL total, β-actin (Abcam, Eugene, OR,
USA), and phospho perilipin A (Vala Sciences, Inc., San Diego, CA,
USA). Chemiluminescent detection was performed with horseradish
peroxidase-conjugated secondary antibodies (Thermo Scientific,
Rockford, IL, USA). Autoradiographs of membranes were obtained for
visualization of protein bands. The results of the blots are
presented as direct comparisons of the area of the apparent bands
in autoradiographs and quantified by densitometry using the Scion
Image software (Scion Image software; Scion Corp., Frederick, MD,
USA).
Statistical analysis
Bars represent 6 different experiments. Differences
between the groups were evaluated using one-way analysis of
variance followed by the Bonferroni post hoc test. P<0.05 was
considered to indicate a statistically significant difference. The
software used for data analysis was the Statistical Package for the
Social Sciences (SPSS) version 17.0 for Windows (SPSS, Inc.,
Chicago, IL, USA).
Results
Effects of TPM on lipolysis in 3T3-L1
adipocytes
To evaluate TPM-induced physiological alterations in
lipolysis, the glycerol and NEFA contents were assayed in the
incubation medium. The contents of glycerol and NEFA in
isoproterenol-treated cells were higher compared with the control
group. Similar results were observed for the isoproterenol group
(Fig. 1A and B). To analyze
lipogenesis, insulin-induced [1-14C]-palmitate
incorporation into lipids was assayed. These results show that the
use of TPM decreased palmitate incorporation into lipids compared
to the control group regardless of insulin stimulus (Fig. 1C). To evaluate whether TPM exposure
effects cellular viability, the cytosolic enzyme LDH release was
quantified. The analyzed groups did not demonstrate significant
differences, suggesting that TPM was not cytotoxic for the times
and dosages used (Fig. 1D).
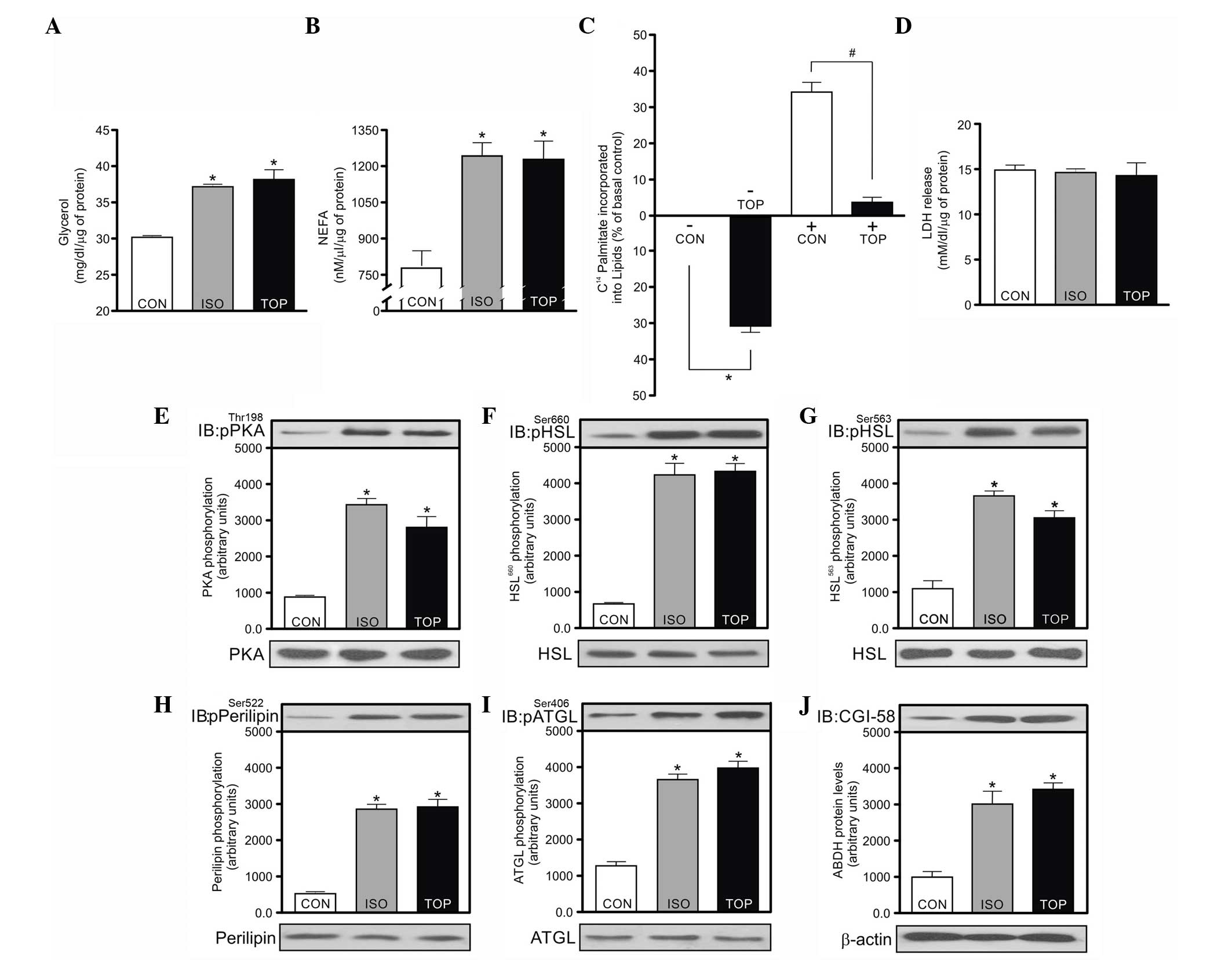 | Figure 1.Effects of topiramate on the lipolysis
and lipogenesis in 3T3-L1 adipocytes. Adipocytes were incubated for
30 min at 37°C in the presence or absence of topiramate or
isoproterenol. (A) Release of glycerol and (B) non-esterified fatty
acid (NEFA) into culture medium was measured and expressed as
mg/dl/µg of protein and nM/µl/µg of protein, respectively.
Isoproterenol was the positive control. (C) The incorporation of
[14C]-palmitate into lipids in the presence or absence
of insulin (10 nmol/l) and presence or absence of topiramate (50
µM) was evaluated. The results are expressed as percentage of basal
control. Cellular viability was evaluated by (D) monitoring lactate
dehydrogenase (LDH) and expressed as mmol/l/dl/µg of protein.
Phosphorylation of (E) protein kinase A (PKA), (F)
hormone-sensitive lipaseSer660 (HSLSer660),
(G) HSLSer563, (H) perilipin, (I) ATGLSer406
and (J) cofactor comparative genetic identification 58 (CGI-58)
protein levels, respectively. The lower panel shows bands
representative of PKA, HSL, perilipin, adipocyte triglyceride
lipase (ATGL) and protein levels β-actin. Bars represent the mean ±
standard error of the mean of 6 different experiments. *P<0.05,
vs. control group. In Fig. 1C, *P<0.05 vs. control group without
insulin (-) and #P<0.05 for topiramate vs. control
group with insulin (+). CON, control; ISO, isoproterenol; TOP,
topiramate. |
Based on the current findings, we hypothesize that
TPM treatment could increase the phosphorylation of the main
lipolytic enzymes. Subsequently, the phosphorylation of
PKAThr198, HSLSer660, HSLSer563,
ATGLSer406 and perilipin A, as well as the protein
levels of CGI-58, PKAThr198, HSLSer660,
HSLSer563, ATGLSer406 and perilipin A were
analyzed (Fig. 1E–J). TPM treatment
led to a higher enzymatic phosphorylation compared to the control
group. Therefore, this result may explain, at least in part, why
the glycerol and NEFA levels were increased. Notably, the analyzed
protein phosphorylation and the CGI-58 protein levels were similar
for cells treated with TPM or isoproterenol (Fig. 1E–J).
Discussion
As increased lipolysis and decreased lipogenesis
cause fat loss in obese individuals, the present study tested
whether the use of TPM increases lipolysis in 3T3-L1 cells. The
3T3-L1 cells treated with TPM presented high phosphorylation of
lipolytic enzymes and subsequent lipolysis, without alteration of
cell viability. In addition, the data showed that TPM modulated
lipogenesis. A previous 6-month randomized human study indicated
that TPM led to marked weight loss compared to the placebo
(8). Another recent study showed that
co-treatment with phentermine and TPM also induced weight loss in
obese patients (9). A study in which
animals were fed with a high fat diet showed that the concurrent
TPM use at 50 mg/kg led to body weight reduction and insulin
sensitivity improvement (10).
Caricilli et al (11) observed
that TPM improved insulin and leptin sensitivity in the
hypothalamus of obese mice, which can contribute to the reduction
of food intake and adiposity. However, these in vivo studies
did not exclude the TPM effects on CNS.
In the present study, TPM treatment increased the
release of NEFA and glycerol in the culture medium. In addition,
decreased [14C]-palmitate incorporation was observed in
adipocytes exposed to TPM. One of the proposed mechanisms for
lipogenesis reduction can be the carbonic anhydrase enzyme
inhibition, which performs the first step of de novo
lipogenesis. Although this enzyme was not analyzed in the present
study, a previous study suggested that TPM can inhibit the
cytosolic and mitochondrial levels of this enzyme, leading to
weight loss (12).
Subsequent to observing that NEFA and glycerol
levels were changed, five molecules that have a crucial role in the
triacylglycerol hydrolysis were analyzed. TPM treatment increased
the phosphorylation of PKA, HSL, ATGL and perilipin A, as well as
the protein levels of CGI-58 compared to control cells. However,
the TPM-induced phosphorylation degrees were similar to those using
isoproterenol. The present data did not allow the proposition of
the mechanism responsible by the increase of these molecules
induced by TPM. Taken together, these results demonstrate that TPM
treatment led to lipolysis independently of its action on the CNS.
These data increase the understanding of the processes involved on
the TPM-induced weight loss and suggest a direct action of this
drug on the adipose tissue.
Acknowledgements
The present study was supported by grants from
Conselho Nacional de Desenvolvimento Científico e Tecnológico
(CNPq) and University of Extremo Sul Catarinense (UNESC).
Glossary
Abbreviations
Abbreviations:
|
ATGL
|
adipocyte triglyceride lipase
|
|
CGI-58
|
cofactor comparative genetic
identification 58
|
|
CNS
|
central nervous system
|
|
HSL
|
hormone-sensitive lipase
|
|
LDH
|
lactate dehydrogenase
|
|
NEFA
|
non-esterified fatty acid
|
|
PKA
|
protein kinase A
|
|
TAG
|
triglycerides
|
|
TPM
|
topiramate
|
References
|
1
|
Verrotti A, Scaparrotta A, Agostinelli S,
Di Pillo S, Chiarelli F and Grosso S: Topiramate-induced weight
loss: A review. Epilepsy Res. 95:189–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zechner R, Kienesberger PC, Haemmerle G,
Zimmermann R and Lass A: Adipose triglyceride lipase and the
lipolytic catabolism of cellular fat stores. J Lipid Res. 50:3–21.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lass A, Zimmermann R, Haemmerle G,
Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG,
Gorkiewicz G and Zechner R: Adipose triglyceride lipase-mediated
lipolysis of cellular fat stores is activated by CGI-58 and
defective in Chanarin-Dorfman Syndrome. Cell Metab. 3:309–319.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sztalryd C, Xu G, Dorward H, Tansey JT,
Contreras JA, Kimmel AR and Londos C: Perilipin A is essential for
the translocation of hormone-sensitive lipase during lipolytic
activation. J Cell Biol. 161:1093–1103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brasaemle DL: Thematic review series:
Adipocyte biology. The perilipin family of structural lipid droplet
proteins: Stabilization of lipid droplets and control of lipolysis.
J Lipid Res. 48:2547–2559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ben Menachem, Axelsen M, Johanson EH,
Stagge A and Smith U: Predictors of weight loss in adults with
topiramate-treated epilepsy. Obes Res. 11:556–562. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Picard F, Deshaies Y, Lalonde J, Samson P
and Richard D: Topiramate reduces energy and fat gains in lean
(Fa/?) and obese (fa/fa) Zucker rats. Obes Res. 8:656–663. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilding J, Van Gaal L, Rissanen A,
Vercruysse F and Fitchet M: OBES-002 Study Group: A randomized
double-blind placebo-controlled study of the long-term efficacy and
safety of topiramate in the treatment of obese subjects. Int J Obes
Relat Metab Disord. 28:1399–1410. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gadde KM, Allison DB, Ryan DH, Peterson
CA, Troupin B, Schwiers ML and Day WW: Effects of low-dose,
controlled-release, phentermine plus topiramate combination on
weight and associated comorbidities in overweight and obese adults
(CONQUER): A randomised, placebo-controlled, phase 3 trial. Lancet.
377:1341–1352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abo-Elmatty DM and Zaitone SA: Topiramate
induces weight loss and improves insulin sensitivity in dietary
obese rats: Comparison to sibutramine. Eur Rev Med Pharmacol Sci.
15:1187–1195. 2011.PubMed/NCBI
|
|
11
|
Caricilli AM, Penteado E, de Abreu LL,
Quaresma PG, Santos AC, Guadagnini D, Razolli D, Mittestainer FC,
Carvalheira JB, Velloso LA, et al: Topiramate treatment improves
hypothalamic insulin and leptin signaling and action and reduces
obesity in mice. Endocrinology. 153:4401–4411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Supuran CT, Di Fiore A and De Simone G:
Carbonic anhydrase inhibitors as emerging drugs for the treatment
of obesity. Expert Opin Emerg Drugs. 13:383–392. 2008. View Article : Google Scholar : PubMed/NCBI
|















