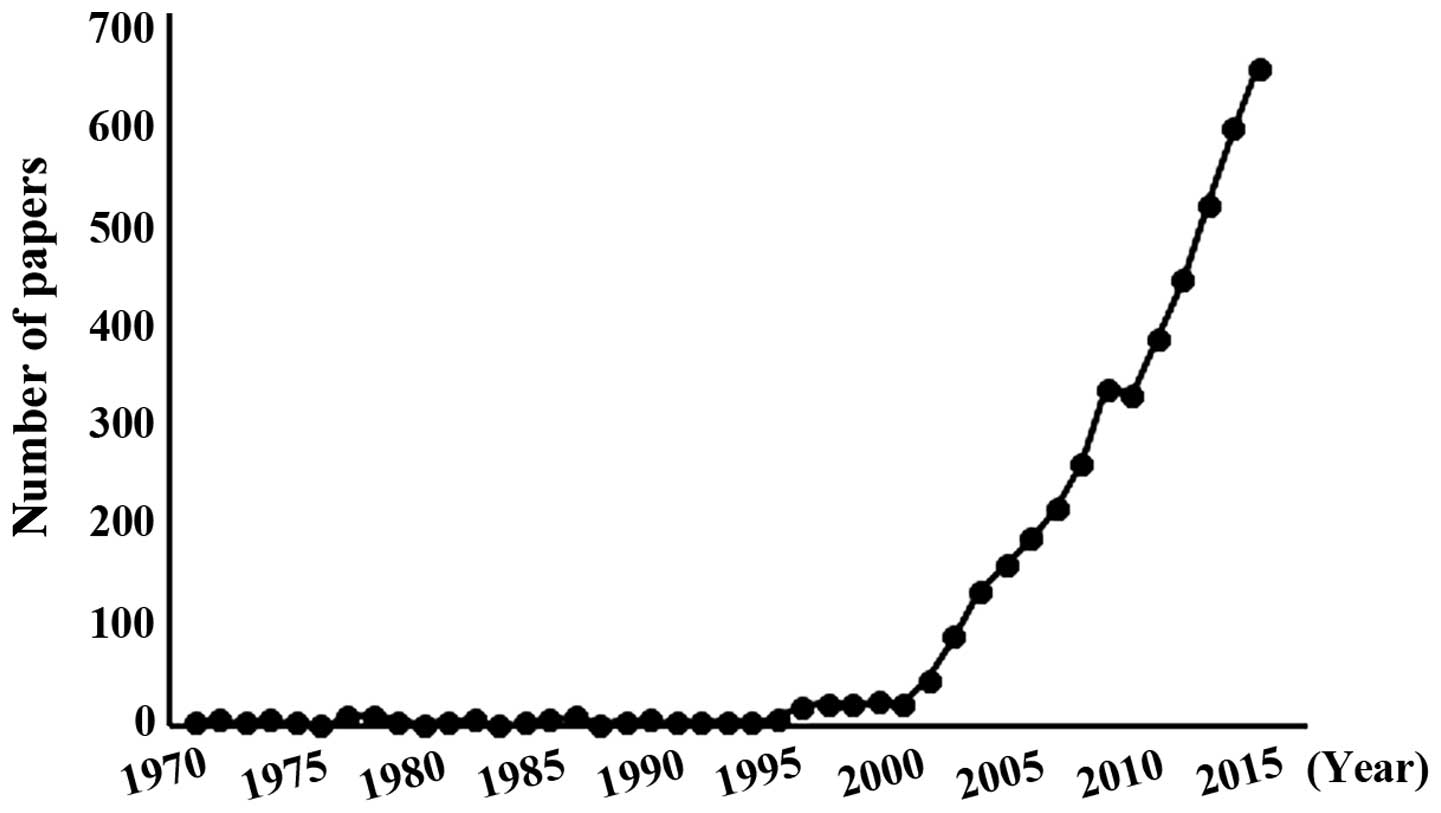
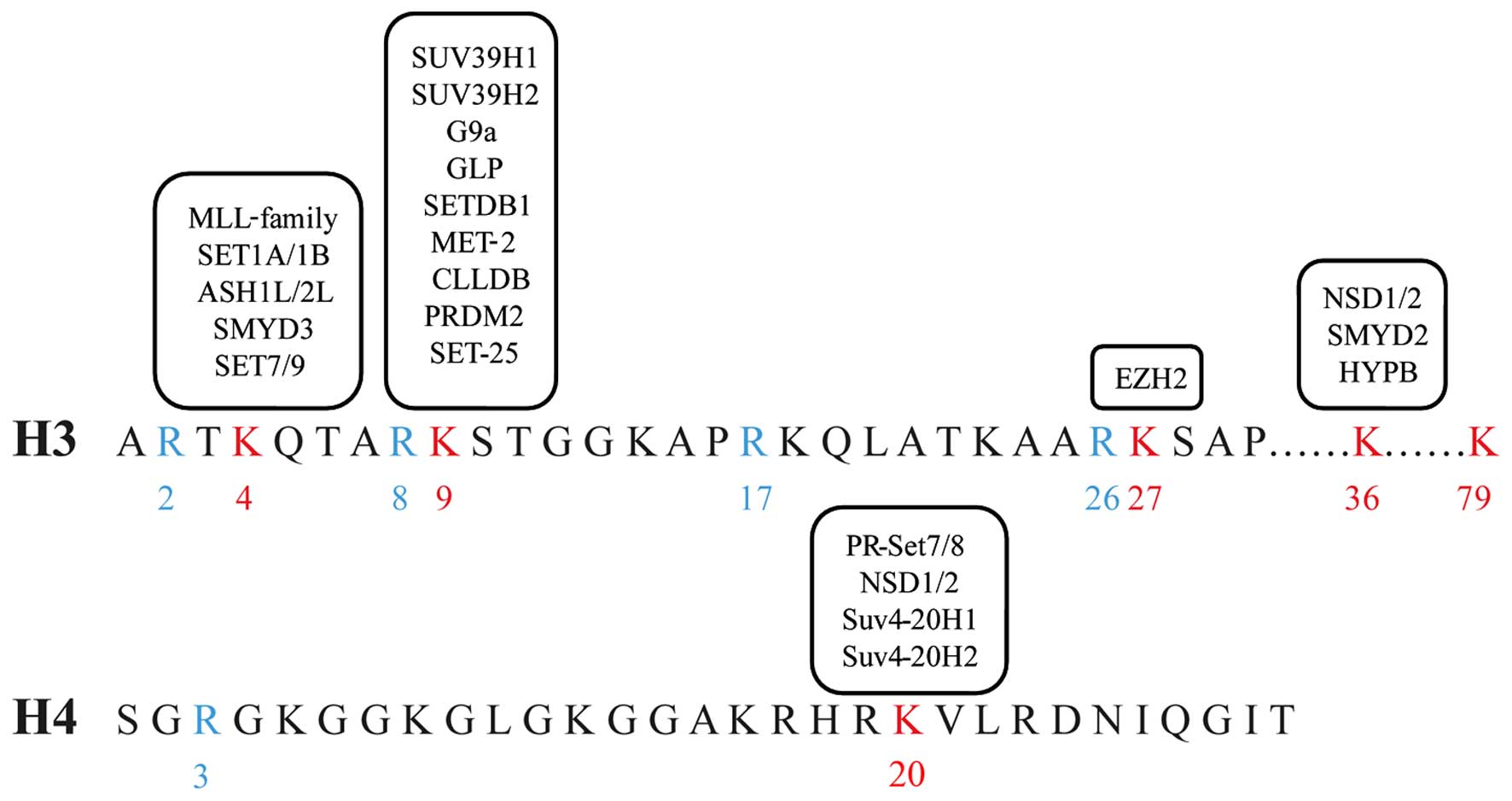
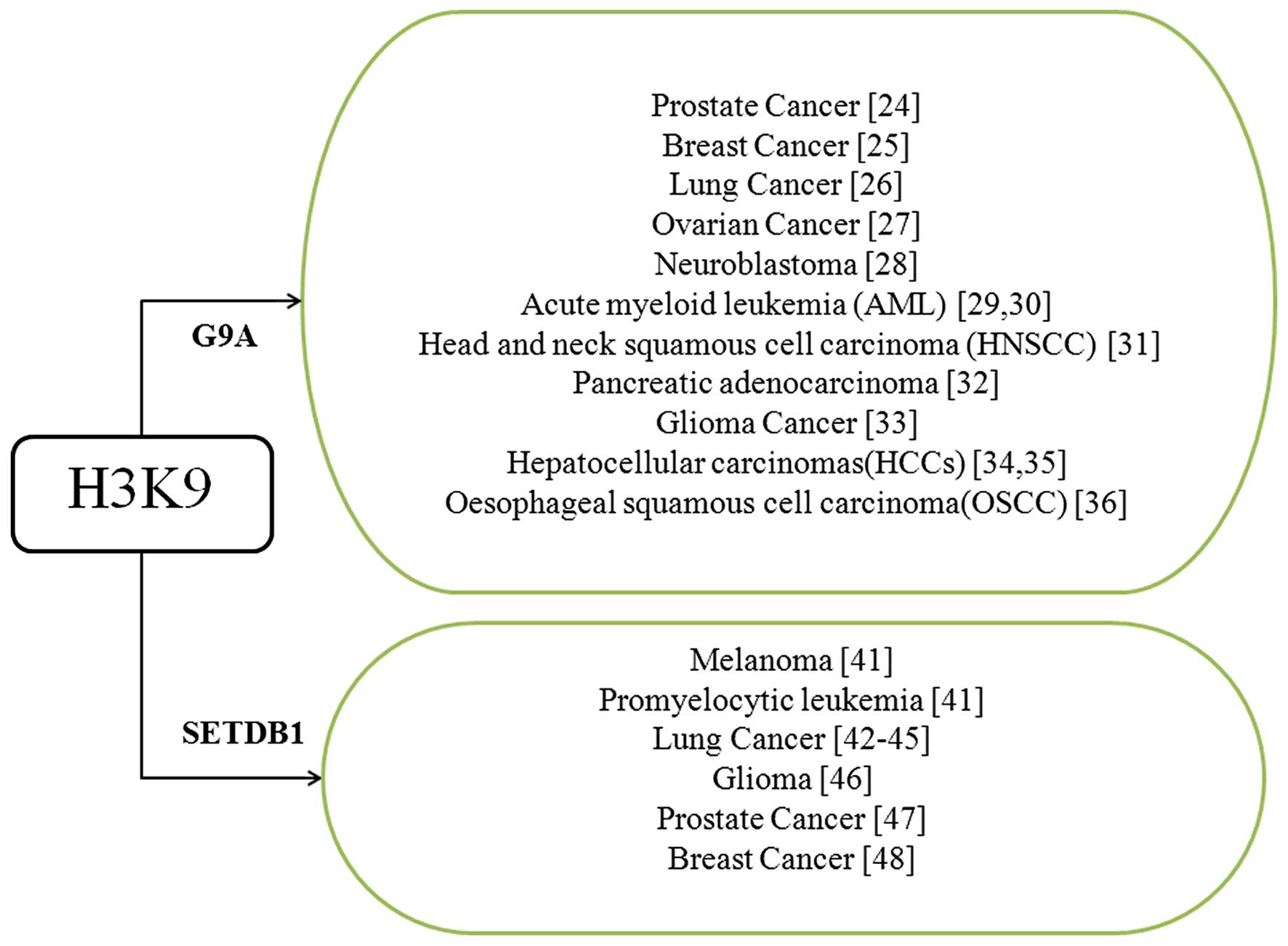
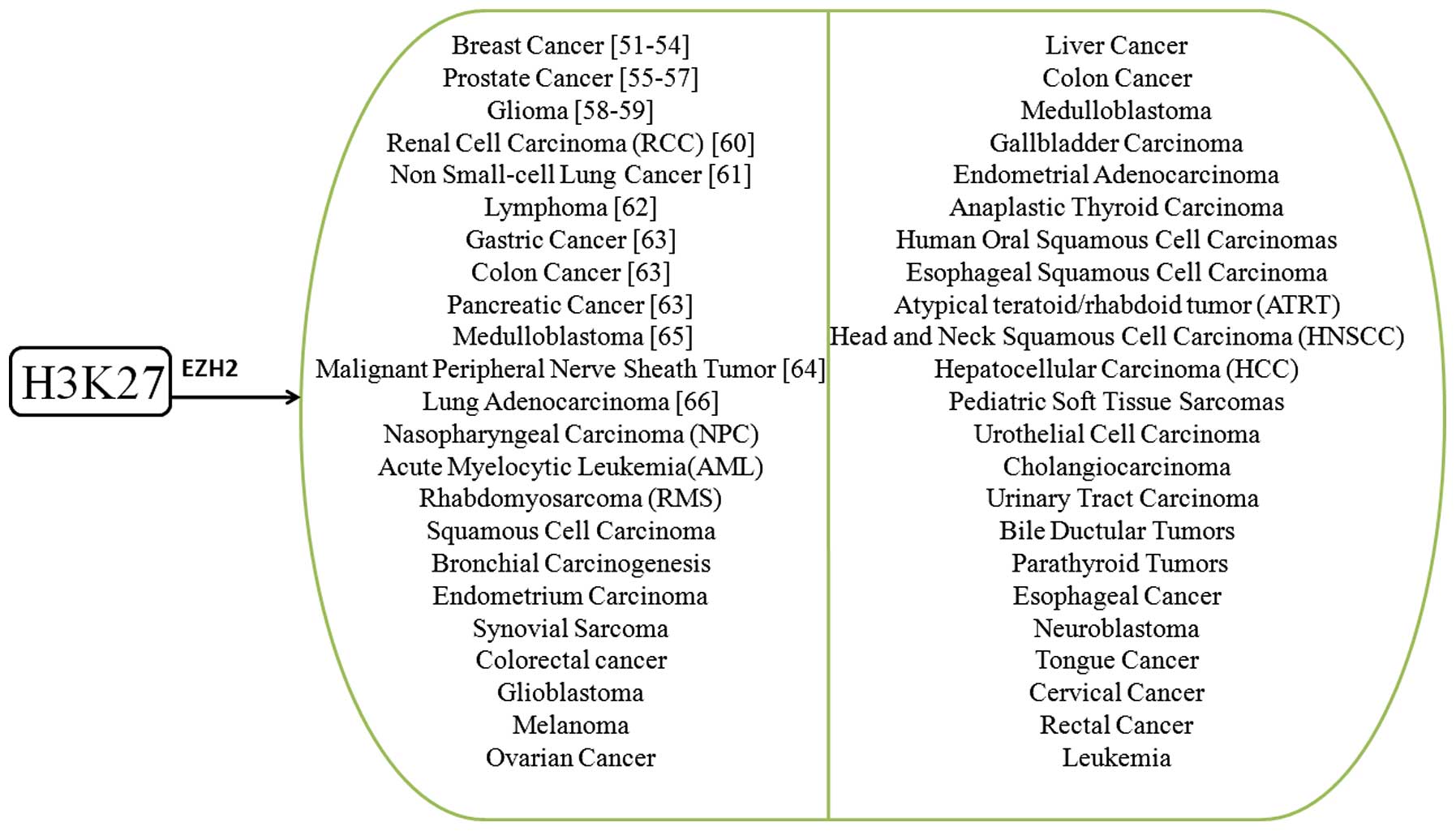
|
1
|
Klener P: Epigenetic cancer drugs and
their role in anticancer therapy. Vnitr Lek. 59:463–465. 2013.(In
Czech).PubMed/NCBI
|
|
2
|
Murray K: The occurrence of E-N-methyl
lysine in histones. Biochemistry. 3:10–15. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yun M, Wu J, Workman JL and Li B: Readers
of histone modifications. Cell Res. 21:564–578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Biancotto C, Frigè G and Minucci S:
Histone modification therapy of cancer. Adv Genet. 70:341–386.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kouzarides T: Chromatin modifications and
their function. Cell. 128:693–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JC, Kang SU, Jeon Y, Park JW, You JS,
Ha SW, Bae N, Lubec G, Kwon SH, Lee JS, et al: Protein
L-isoaspartyl methyltransferase regulates p53 activity. Nat Commun.
3:9272012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verma M and Srivastava S: Epigenetics in
cancer: Implications for early detection and prevention. Lancet
Oncol. 3:755–763. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chaib H, Prébet T, Vey N and Collette Y:
Histone methyltransferases: A new class of therapeutic targets in
cancer treatment? Med Sci (Paris). 27:725–732. 2011.In French.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X and Zhu WG: Advances in histone
methyltransferases and histone demethylases. Ai Zheng.
27:1018–1025. 2008.(In Chinese). PubMed/NCBI
|
|
10
|
Collazo E, Couture JF, Bulfer S and
Trievel RC: A coupled fluorescent assay for histone
methyltransferases. Anal Biochem. 342:86–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Campagna-Slater V, Mok MW, Nguyen KT,
Feher M, Najmanovich R and Schapira M: Structural chemistry of the
histone methyltransferases cofactor binding site. J Chem Inf Model.
51:612–623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qian C and Zhou MM: SET domain protein
lysine methyltransferases: Structure, specificity and catalysis.
Cell Mol Life Sci. 63:2755–2763. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vermeulen M, Eberl HC, Matarese F, Marks
H, Denissov S, Butter F, Lee KK, Olsen JV, Hyman AA, Stunnenberg
HG, et al: Quantitative interaction proteomics and genome-wide
profiling of epigenetic histone marks and their readers. Cell.
142:967–980. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peterson CL and Laniel MA: Histones and
histone modifications. Curr Biol. 14:R546–R551. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stancheva I: Caught in conspiracy:
Cooperation between DNA methylation and histone H3K9 methylation in
the establishment and maintenance of heterochromatin. Biochem Cell
Biol. 83:385–395. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Towbin BD, González-Aguilera C, Sack R,
Gaidatzis D, Kalck V, Meister P, Askjaer P and Gasser SM: Step-wise
methylation of histone H3K9 positions heterochromatin at the
nuclear periphery. Cell. 150:934–947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krouwels IM, Wiesmeijer K, Abraham TE,
Molenaar C, Verwoerd NP, Tanke HJ and Dirks RW: A glue for
heterochromatin maintenance: Stable SUV39H1 binding to
heterochromatin is reinforced by the SET domain. J Cell Biol.
170:537–549. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Carroll D, Scherthan H, Peters AH,
Opravil S, Haynes AR, Laible G, Rea S, Schmid M, Lebersorger A,
Jerratsch M, et al: Isolation and characterization of Suv39h2, a
second histone H3 methyltransferase gene that displays
testis-specific expression. Mol Cell Biol. 20:9423–9433. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tachibana M, Sugimoto K, Fukushima T and
Shinkai Y: Set domain-containing protein, G9a, is a novel
lysine-preferring mammalian histone methyltransferase with
hyperactivity and specific selectivity to lysines 9 and 27 of
histone H3. J Biol Chem. 276:25309–25317. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tachibana M, Matsumura Y, Fukuda M, Kimura
H and Shinkai Y: G9a/GLP complexes independently mediate H3K9 and
DNA methylation to silence transcription. EMBO J. 27:2681–2690.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Rauch T, Chen ZX, Szabó PE, Riggs AD
and Pfeifer GP: The histone methyltransferase SETDB1 and the DNA
methyltransferase DNMT3A interact directly and localize to
promoters silenced in cancer cells. J Biol Chem. 281:19489–19500.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steele-Perkins G, Fang W, Yang XH, Van
Gele M, Carling T, Gu J, Buyse IM, Fletcher JA, Liu J, Bronson R,
et al: Tumor formation and inactivation of RIZ1, an Rb-binding
member of a nuclear protein-methyltransferase superfamily. Genes
Dev. 15:2250–2262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Falandry C, Fourel G, Galy V, Ristriani T,
Horard B, Bensimon E, Salles G, Gilson E and Magdinier F:
CLLD8/KMT1F is a lysine methyltransferase that is important for
chromosome segregation. J Biol Chem. 285:20234–20241. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding J, Li T, Wang X, Zhao E, Choi JH,
Yang L, Zha Y, Dong Z, Huang S, Asara JM, et al: The histone H3
methyltransferase G9A epigenetically activates the serine-glycine
synthesis pathway to sustain cancer cell survival and
proliferation. Cell Metab. 18:896–907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kondo Y, Shen L, Ahmed S, Boumber Y,
Sekido Y, Haddad BR and Issa JP: Downregulation of histone H3
lysine 9 methyltransferase G9a induces centrosome disruption and
chromosome instability in cancer cells. PLoS One. 3:e20372008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong C, Wu Y, Yao J, Wang Y, Yu Y,
Rychahou PG, Evers BM and Zhou BP: G9a interacts with Snail and is
critical for Snail-mediated E-cadherin repression in human breast
cancer. J Clin Invest. 122:1469–1486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen MW, Hua KT, Kao HJ, Chi CC, Wei LH,
Johansson G, Shiah SG, Chen PS, Jeng YM, Cheng TY, et al: H3K9
histone methyltransferase G9a promotes lung cancer invasion and
metastasis by silencing the cell adhesion molecule Ep-CAM. Cancer
Res. 70:7830–7840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hua KT, Wang MY, Chen MW, Wei LH, Chen CK,
Ko CH, Jeng YM, Sung PL, Jan YH, Hsiao M, et al: The H3K9
methyltransferase G9a is a marker of aggressive ovarian cancer that
promotes peritoneal metastasis. Mol Cancer. 13:1892014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ke XX, Zhang D, Zhu S, Xia Q, Xiang Z and
Cui H: Inhibition of H3K9 methyltransferase G9a repressed cell
proliferation and induced autophagy in neuroblastoma cells. PLoS
One. 9:e1069622014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li KC, Hua KT, Lin YS, Su CY, Ko JY, Hsiao
M, Kuo ML and Tan CT: Inhibition of G9a induces DUSP4-dependent
autophagic cell death in head and neck squamous cell carcinoma. Mol
Cancer. 13:1722014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan Y, Tang AJ, Castoreno AB, Kuo SY,
Wang Q, Kuballa P, Xavier R, Shamji AF, Schreiber SL and Wagner BK:
Gossypol and an HMT G9a inhibitor act in synergy to induce cell
death in pancreatic cancer cells. Cell Death Dis. 4:e6902013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Son HJ, Kim JY, Hahn Y and Seo SB:
Negative regulation of JAK2 by H3K9 methyltransferase G9a in
leukemia. Mol Cell Biol. 32:3681–3694. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lehnertz B, Pabst C, Su L, Miller M, Liu
F, Yi L, Zhang R, Krosl J, Yung E, Kirschner J, et al: The
methyltransferase G9a regulates HoxA9-dependent transcription in
AML. Genes Dev. 28:317–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tao H, Li H, Su Y, Feng D, Wang X, Zhang
C, Ma H and Hu Q: Histone methyltransferase G9a and H3K9
dimethylation inhibit the self-renewal of glioma cancer stem cells.
Mol Cell Biochem. 394:23–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hung SY, Lin HH, Yeh KT and Chang JG:
Histone-modifying genes as biomarkers in hepatocellular carcinoma.
Int J Clin Exp Pathol. 7:2496–2507. 2014.PubMed/NCBI
|
|
36
|
Wu H, Zhang H, Wang P, Mao Z, Feng L, Wang
Y, Liu C, Xia Q, Li B, Zhao H, et al: Short-Form CDYLb but not
long-form CDYLa functions cooperatively with histone
methyltransferase G9a in hepatocellular carcinomas. Genes
Chromosomes Cancer. 52:644–655. 2013.PubMed/NCBI
|
|
37
|
Zhong X, Chen X, Guan X, Zhang H, Ma Y,
Zhang S, Wang E, Zhang L and Han Y: Overexpression of G9a and MCM7
in oesophageal squamous cell carcinoma is associated with poor
prognosis. Histopathology. 66:192–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schultz DC, Ayyanathan K, Negorev D, Maul
GG and Rauscher FJ III: SETDB1: A novel KAP-1-associated histoneH3,
lysine 9-specific methyltransferase that contributes to
HP1-mediated silencing of euchromatic genes by KRAB zinc-finger
proteins. Genes Dev. 16:919–932. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harte PJ, Wu W, Carrasquillo MM and Matera
AG: Assignment of a novel bifurcated SET domain gene, SETDB1, to
human chromosome band 1q21 by in situ hybridization and radiation
hybrids. Cytogenet Cell Genet. 84:83–86. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Frietze S, O'Geen H, Blahnik KR, Jin VX
and Farnham PJ: ZNF274 recruits the histone methyltransferase
SETDB1 to the 3 ends of ZNF genes. PLoS One. 5:e150822010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cho S, Park JS and Kang YK: Dual functions
of histone-lysine N-methyltransferase Setdb1 protein at
promyelocytic leukemia-nuclear body (PML-NB): Maintaining PML-NB
structure and regulating the expression of its associated genes. J
Biol Chem. 286:41115–41124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ceol CJ, Houvras Y, Jane-Valbuena J,
Bilodeau S, Orlando DA, Battisti V, Fritsch L, Lin WM, Hollmann TJ,
Ferré F, et al: The histone methyltransferase SETDB1 is recurrently
amplified in melanoma and accelerates its onset. Nature.
471:513–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee JK and Kim KC: DZNep, inhibitor of
S-adenosylhomocysteine hydrolase, down-regulates expression of
SETDB1 H3K9me3 HMTase in human lung cancer cells. Biochem Biophys
Res Commun. 438:647–652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rodriguez-Paredes M, de Paz Martinez A,
Simó-Riudalbas L, Sayols S, Moutinho C, Moran S, Villanueva A,
Vázquez-Cedeira M, Lazo PA, Carneiro F, et al: Gene amplification
of the histone methyltransferase SETDB1 contributes to human lung
tumorigenesis. Oncogene. 33:2807–2813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun QY, Ding LW, Xiao JF, Chien W, Lim SL,
Hattori N, Goodglick L, Chia D, Mah V, Alavi M, et al: SETDB1
accelerates tumourigenesis by regulating the WNT signalling
pathway. J Pathol. 235:559–570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu PC, Lu JW, Yang JY, Lin IH, Ou DL, Lin
YH, Chou KH, Huang WF, Wang WP, Huang YL, et al: H3K9 histone
methyltransferase, KMT1E/SETDB1, cooperates with the SMAD2/3
pathway to suppress lung cancer metastasis. Cancer Res.
74:7333–7343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Spyropoulou A, Gargalionis A, Dalagiorgou
G, Adamopoulos C, Papavassiliou KA, Lea RW, Piperi C and
Papavassiliou AG: Role of histone lysine methyltransferases SUV39H1
and SETDB1 in gliomagenesis: Modulation of cell proliferation,
migration, and colony formation. Neuromolecular Med. 16:70–82.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun Y, Wei M, Ren SC, Chen R, Xu WD, Wang
FB, Lu J, Shen J, Yu YW, Hou JG, et al: Histone methyltransferase
SETDB1 is required for prostate cancer cell proliferation,
migration and invasion. Asian J Androl. 16:319–324. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang H, Cai K, Wang J, Wang X, Cheng K,
Shi F, Jiang L, Zhang Y and Dou J: MiR-7, inhibited indirectly by
lincRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of
breast cancer stem cells by downregulating the STAT3 pathway. Stem
Cells. 32:2858–2868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kouzarides T: Histone methylation in
transcriptional control. Curr Opin Genet Dev. 12:198–209. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jansen MP, Reijm EA, Sieuwerts AM,
Ruigrok-Ritstier K, Look MP, Rodríguez-González FG, Heine AA,
Martens JW, Sleijfer S, Foekens JA, et al: High miR-26a and low
CDC2 levels associate with decreased EZH2 expression and with
favorable outcome on tamoxifen in metastatic breast cancer. Breast
Cancer Res Treat. 133:937–947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Raaphorst FM, Meijer CJ, Fieret E,
Blokzijl T, Mommers E, Buerger H, Packeisen J, Sewalt RA, Otte AP
and van Diest PJ: Poorly differentiated breast carcinoma is
associated with increased expression of the human polycomb group
EZH2 gene. Neoplasia. 5:481–488. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mu Z, Li H, Fernandez SV, Alpaugh KR,
Zhang R and Cristofanilli M: EZH2 knockdown suppresses the growth
and invasion of human inflammatory breast cancer cells. J Exp Clin
Cancer Res. 32:702013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zeidler M, Varambally S, Cao Q, Chinnaiyan
AM, Ferguson DO, Merajver SD and Kleer CG: The Polycomb group
protein EZH2 impairs DNA repair in breast epithelial cells.
Neoplasia. 7:1011–1019. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hoffmann MJ, Engers R, Florl AR, Otte AP,
Muller M and Schulz WA: Expression changes in EZH2, but not in
BMI-1, SIRT1, DNMT1 or DNMT3B are associated with DNA methylation
changes in prostate cancer. Cancer Biol Ther. 6:1403–1412. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, et al: The polycomb group protein EZH2 is involved in
progression of prostate cancer. Nature. 419:624–629. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bryant RJ, Cross NA, Eaton CL, Hamdy FC
and Cunliffe VT: EZH2 promotes proliferation and invasiveness of
prostate cancer cells. Prostate. 67:547–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim SH, Joshi K, Ezhilarasan R, Myers TR,
Siu J, Gu C, Nakano-Okuno M, Taylor D, Minata M, Sulman EP, et al:
EZH2 protects glioma stem cells from radiation-induced cell death
in a MELK/FOXM1-dependent manner. Stem Cell Rep. 4:226–238. 2015.
View Article : Google Scholar
|
|
59
|
Zhang W, Lv S, Liu J, Zang Z, Yin J, An N,
Yang H and Song Y: PCI-24781 down-regulates EZH2 expression and
then promotes glioma apoptosis by suppressing the PIK3K/Akt/mTOR
pathway. Genet Mol Biol. 37:716–724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu ZQ, Zhang L, Gao BS, Wan YG, Zhang XH,
Chen B, Wang YT, Sun N and Fu YW: EZH2 promotes tumor progression
by increasing VEGF expression in clear cell renal cell carcinoma.
Clin Transl Oncol. 17:41–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xia H, Zhang W, Li Y, Guo N and Yu C: EZH2
silencing with RNA interference induces G2/M arrest in
human lung cancer cells in vitro. BioMed Res Int. 2014:3487282014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Guo SQ and Zhang YZ: Overexpression of
enhancer of zests homolog 2 in lymphoma. Chin Med J (Engl).
125:3735–3739. 2012.PubMed/NCBI
|
|
63
|
Fujii S, Ito K, Ito Y and Ochiai A:
Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by
increasing histone H3 methylation. J Biol Chem. 283:17324–17332.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang P, Yang X, Ma X, Ingram DR, Lazar
AJ, Torres KE and Pollock RE: Antitumor effects of pharmacological
EZH2 inhibition on malignant peripheral nerve sheath tumor through
the miR-30a and KPNB1 pathway. Mol Cancer. 14:552015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dubuc AM, Remke M, Korshunov A, Northcott
PA, Zhan SH, Mendez-Lago M, Kool M, Jones DT, Unterberger A,
Morrissy AS, et al: Aberrant patterns of H3K4 and H3K27 histone
lysine methylation occur across subgroups in medulloblastoma. Acta
Neuropathol. 125:373–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wan J, Zhan J, Li S, Ma J, Xu W, Liu C,
Xue X, Xie Y, Fang W, Chin YE, et al: PCAF-primed EZH2 acetylation
regulates its stability and promotes lung adenocarcinoma
progression. Nucleic Acids Res. 43:3591–3604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Pekowska A, Benoukraf T, Zacarias-Cabeza
J, Belhocine M, Koch F, Holota H, Imbert J, Andrau JC, Ferrier P
and Spicuglia S: H3K4 tri-methylation provides an epigenetic
signature of active enhancers. EMBO J. 30:4198–4210. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang P, Lin C, Smith ER, Guo H, Sanderson
BW, Wu M, Gogol M, Alexander T, Seidel C, Wiedemann LM, et al:
Global analysis of H3K4 methylation defines MLL family member
targets and points to a role for MLL1-mediated H3K4 methylation in
the regulation of transcriptional initiation by RNA polymerase II.
Mol Cell Biol. 29:6074–6085. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wu M, Wang PF, Lee JS, Martin-Brown S,
Florens L, Washburn M and Shilatifard A: Molecular regulation of
H3K4 trimethylation by Wdr82, a component of human Set1/COMPASS.
Mol Cell Biol. 28:7337–7344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gregory GD, Vakoc CR, Rozovskaia T, Zheng
X, Patel S, Nakamura T, Canaani E and Blobel GA: Mammalian ASH1L is
a histone methyltransferase that occupies the transcribed region of
active genes. Mol Cell Biol. 27:8466–8479. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Stoller JZ, Huang L, Tan CC, Huang F, Zhou
DD, Yang J, Gelb BD and Epstein JA: Ash2l interacts with Tbx1 and
is required during early embryogenesis. Exp Biol Med (Maywood).
235:569–576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hamamoto R, Furukawa Y, Morita M, Iimura
Y, Silva FP, Li M, Yagyu R and Nakamura Y: SMYD3 encodes a histone
methyltransferase involved in the proliferation of cancer cells.
Nat Cell Biol. 6:731–740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tao Y, Neppl RL, Huang ZP, Chen J, Tang
RH, Cao R, Zhang Y, Jin SW and Wang DZ: The histone
methyltransferase Set7/9 promotes myoblast differentiation and
myofibril assembly. J Cell Biol. 194:551–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sirinupong N, Brunzelle J, Ye J, Pirzada
A, Nico L and Yang Z: Crystal structure of cardiac-specific histone
methyltransferase SmyD1 reveals unusual active site architecture. J
Biol Chem. 285:40635–40644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Peserico A, Germani A, Sanese P, Barbosa
AJ, di Virgilio V, Fittipaldi R, Fabini E, Bertucci C, Varchi G,
Moyer MP, et al: A SMYD3 small-molecule inhibitor impairing cancer
cell growth. J Cell Physiol. 230:2447–2460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xu JY, Chen LB, Xu JY, Yang Z, Wei HY and
Xu RH: Inhibition of SMYD3 gene expression by RNA interference
induces apoptosis in human hepatocellular carcinoma cell line
HepG2. Ai Zheng. 25:526–532. 2006.(In Chinese). PubMed/NCBI
|
|
77
|
Dong SW, Zhang H, Wang BL, Sun P, Wang YG
and Zhang P: Effect of the downregulation of SMYD3 expression by
RNAi on RIZ1 expression and proliferation of esophageal squamous
cell carcinoma. Oncol Rep. 32:1064–1070. 2014.PubMed/NCBI
|
|
78
|
Hamamoto R, Silva FP, Tsuge M, Nishidate
T, Katagiri T, Nakamura Y and Furukawa Y: Enhanced SMYD3 expression
is essential for the growth of breast cancer cells. Cancer Sci.
97:113–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang SZ, Luo XG, Shen J, Zou JN, Lu YH and
Xi T: Knockdown of SMYD3 by RNA interference inhibits cervical
carcinoma cell growth and invasion in vitro. BMB Rep. 41:294–299.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu C, Wang C, Wang K, Liu L, Shen Q, Yan
K, Sun X, Chen J, Liu J, Ren H, et al: SMYD3 as an oncogenic driver
in prostate cancer by stimulation of androgen receptor
transcription. J Natl Cancer Inst. 105:1719–1728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Morishita M and di Luccio E: Cancers and
the NSD family of histone lysine methyltransferases. Biochim
Biophys Acta. 1816:158–163. 2011.PubMed/NCBI
|
|
82
|
Duns G, van den Berg E, van Duivenbode I,
Osinga J, Hollema H, Hofstra RM and Kok K: Histone
methyltransferase gene SETD2 is a novel tumor suppressor gene in
clear cell renal cell carcinoma. Cancer Res. 70:4287–4291. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Maltby VE, Martin BJ, Schulze JM, Johnson
I, Hentrich T, Sharma A, Kobor MS and Howe L: Histone H3 lysine 36
methylation targets the Isw1b remodeling complex to chromatin. Mol
Cell Biol. 32:3479–3485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sampson ER, Yeh SY, Chang HC, Tsai MY,
Wang X, Ting HJ and Chang C: Identification and characterization of
androgen receptor associated coregulators in prostate cancer cells.
J Biol Regul Homeost Agents. 15:123–129. 2001.PubMed/NCBI
|
|
85
|
Bianco-Miotto T, Chiam K, Buchanan G,
Jindal S, Day TK, Thomas M, Pickering MA, O'Loughlin MA, Ryan NK,
Raymond WA, et al: Global levels of specific histone modifications
and an epigenetic gene signature predict prostate cancer
progression and development. Cancer Epidemiol Biomarkers Prev.
19:2611–2622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Berdasco M, Ropero S, Setien F, Fraga MF,
Lapunzina P, Losson R, Alaminos M, Cheung NK, Rahman N and Esteller
M: Epigenetic inactivation of the Sotos overgrowth syndrome gene
histone methyltransferase NSD1 in human neuroblastoma and glioma.
Proc Natl Acad Sci USA. 106:21830–21835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhao F, Chen Y, Zeng L, Li R, Zeng R, Wen
L, Liu Y and Zhang C: Role of triptolide in cell proliferation,
cell cycle arrest, apoptosis and histone methylation in multiple
myeloma U266 cells. Eur J Pharmacol. 646:1–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Thanasopoulou A, Tzankov A and Schwaller
J: Potent co-operation between the NUP98-NSD1 fusion and the
FLT3-ITD mutation in acute myeloid leukemia induction.
Haematologica. 99:1465–1471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ostronoff F, Othus M, Gerbing RB, Loken
MR, Raimondi SC, Hirsch BA, Lange BJ, Petersdorf S, Radich J,
Appelbaum FR, et al: NUP98/NSD1 and FLT3/ITD coexpression is more
prevalent in younger AML patients and leads to induction failure: A
COG and SWOG report. Blood. 124:2400–2407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Job B, Bernheim A, Beau-Faller M,
Camilleri-Broët S, Girard P, Hofman P, Mazières J, Toujani S,
Lacroix L, Laffaire J, et al: LG Investigators: Genomic aberrations
in lung adenocarcinoma in never smokers. PLoS One. 5:e151452010.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Seiwert TY, Zuo Z, Keck MK, Khattri A,
Pedamallu CS, Stricker T, Brown C, Pugh TJ, Stojanov P, Cho J, et
al: Integrative and comparative genomic analysis of HPV-positive
and HPV-negative head and neck squamous cell carcinomas. Clin
Cancer Res. 21:632–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Deardorff MA, Maisenbacher M and Zackai
EH: Ganglioglioma in a Sotos syndrome patient with an NSD1
deletion. Am J Med Genet A. 130A:393–394. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang Y, Thomas A, Lau C, Rajan A, Zhu Y,
Killian JK, Petrini I, Pham T, Morrow B, Zhong X, et al: Mutations
of epigenetic regulatory genes are common in thymic carcinomas. Sci
Rep. 4:73362014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Gossage L, Murtaza M, Slatter AF,
Lichtenstein CP, Warren A, Haynes B, Marass F, Roberts I, Shanahan
SJ, Claas A, et al: Clinical and pathological impact of VHL, PBRM1,
BAP1, SETD2, KDM6A, and JARID1c in clear cell renal cell carcinoma.
Genes Chromosomes Cancer. 53:38–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hao C, Wang L, Peng S, Cao M, Li H, Hu J,
Huang X, Liu W, Zhang H, Wu S, et al: Gene mutations in primary
tumors and corresponding patient-derived xenografts derived from
non-small cell lung cancer. Cancer Lett. 357:179–185. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Huether R, Dong L, Chen X, Wu G, Parker M,
Wei L, Ma J, Edmonson MN, Hedlund EK, Rusch MC, et al: The
landscape of somatic mutations in epigenetic regulators across
1,000 paediatric cancer genomes. Nat Commun. 5:36302014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang Q, Xue P, Li H, Bao Y, Wu L, Chang
S, Niu B, Yang F and Zhang T: Histone modification mapping in human
brain reveals aberrant expression of histone H3 lysine 79
dimethylation in neural tube defects. Neurobiol Dis. 54:404–413.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Martin C and Zhang Y: The diverse
functions of histone lysine methylation. Nat Rev Mol Cell Biol.
6:838–849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kim W, Choi M and Kim JE: The histone
methyltransferase Dot1/DOT1L as a critical regulator of the cell
cycle. Cell Cycle. 13:726–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chang MJ, Wu H, Achille NJ, Reisenauer MR,
Chou CW, Zeleznik-Le NJ, Hemenway CS and Zhang W: Histone H3 lysine
79 methyltransferase Dot1 is required for immortalization by MLL
oncogenes. Cancer Res. 70:10234–10242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kim W, Kim R, Park G, Park JW and Kim JE:
Deficiency of H3K79 histone methyltransferase Dot1-like protein
(DOT1L) inhibits cell proliferation. J Biol Chem. 287:5588–5599.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Oda H, Okamoto I, Murphy N, Chu J, Price
SM, Shen MM, Torres-Padilla ME, Heard E and Reinberg D:
Monomethylation of histone H4-lysine 20 is involved in chromosome
structure and stability and is essential for mouse development. Mol
Cell Biol. 29:2278–2295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Qin Y, Ouyang H, Liu J and Xie Y: Proteome
identification of proteins interacting with histone
methyltransferase SET8. Acta Biochim Biophys Sin (Shanghai). 2013.
View Article : Google Scholar
|
|
104
|
Jørgensen S, Schotta G and Sørensen CS:
Histone H4 lysine 20 methylation: Key player in epigenetic
regulation of genomic integrity. Nucleic Acids Res. 41:2797–2806.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wu S and Rice JC: A new regulator of the
cell cycle: The PR-Set7 histone methyltransferase. Cell Cycle.
10:68–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Morishita M and di Luccio E: Structural
insights into the regulation and the recognition of histone marks
by the SET domain of NSD1. Biochem Biophys Res Commun. 412:214–219.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yang P, Guo L, Duan ZJ, Tepper CG, Xue L,
Chen X, Kung HJ, Gao AC, Zou JX and Chen HW: Histone
methyltransferase NSD2/MMSET mediates constitutive NF-κB signaling
for cancer cell proliferation, survival, and tumor growth via a
feed-forward loop. Mol Cell Biol. 32:3121–3131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Beck DB, Oda H, Shen SS and Reinberg D:
PR-Set7 and H4K20me1: At the crossroads of genome integrity, cell
cycle, chromosome condensation, and transcription. Genes Dev.
26:325–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yokoyama Y, Matsumoto A, Hieda M, Shinchi
Y, Ogihara E, Hamada M, Nishioka Y, Kimura H, Yoshidome K,
Tsujimoto M, et al: Loss of histone H4K20 trimethylation predicts
poor prognosis in breast cancer and is associated with invasive
activity. Breast Cancer Res. 16:R662014. View Article : Google Scholar : PubMed/NCBI
|