Introduction
Hypoxia-ischemic brain damage (HIBD) refers to the
fetal/neonatal brain damage caused by partial or complete cerebral
hypoxia, cerebral blood flow reduction or suspension (1,2). HIBD is a
major cause of acute mortality and subsequent central nervous
system sequelae. These serious complications include learning
disability, epilepsy and even cerebral palsy and mental retardation
(3,4).
Regardless of the improvements in obstetric and neonatal care, HIBD
with severe neurological disability remains a clinical issue.
Presently, all the clinically available therapies are ineffective
at reducing the neurodevelopmental disorders identified in the
surviving infants. Recently, therapeutic measures of HIBD have been
investigated in clinical as well as animal studies (5). In demonstrating the mechanisms underlying
the HI injury in the neonatal brain to devise effective therapeutic
strategies, several methods have been used to establish HIBD
models, such as unilateral common carotid artery (CCA) ligation
with hypoxia (6,7), transient cerebral ischemia/reperfusion
(8) and intrauterine hypoxia and
ischemia (9,10). Among those methods, the ‘Rice method’
is the most popular. The present study was designed to set up a
reliable model of severe HIBD in neonatal rats and several methods
were used to identify whether this was successful. Neonatal
hypoxia-ischemia brain injury models were generated by referring to
the Rice method (6) and adopting the
methods of improved arterial ligation by Nakajima et al
(11). The influence of the recent and
long-term neurological pathology and the behavioral changes were
observed in the perinatal hypoxic ischemia of newborn rats, and
cognitive functions were studied in each group.
Materials and methods
Animals
A total of 40 healthy 7-day-old Sprague-Dawley rats
(weighing 12–20 g) were obtained from the Experimental Animal
Centre of Zhejiang University (Zhejiang, China) along with their
mother for breastfeeding. They were randomly divided into 2 groups:
The sham-surgery group (n=18) and the HIBD model group (n=22). The
study was carried out in strict accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The animal use protocol has been
reviewed and approved by the Institutional Animal Care and Use
Committee of Zhejiang University.
Improvement of the HIBD animal
model
The experiment was approved by the University Animal
Ethics Committee according to the local government legislation. All
the animals were weighed prior to surgery at room temperature
(20±5°C). To generate the HIBD model, the Rice method (6) was referred to and the methods of improved
arterial ligation were adopted from the study by Nakajima et
al (11) (the left CCA was
isolated, double-ligated and cut between the ligatures). After
anesthesia with 0.2 ml of ether in an airtight box for 1 min, the
rat limbs were fixed on the minor board in the supine position. The
skin was disinfected with 75% alcohol, and following the anterior
portion of the incision, the left CCA underwent sterile separation,
and was double-ligated with a 7–0 surgical silk suture and
subsequently cut in the middle. The skin incision was subsequently
sutured and disinfected again. The pups were permitted to recover
for 2 h in a temperature-controlled incubator (32.5°C). The rats
were subsequently placed in a 2-litre volume airtight chamber with
soda lime at the bottom (to absorb the CO2 and water
vapor), which was maintained at 37°C in a thermostatic water bath.
HIBD was generated by exposure to a rate of 1–2 l/min of 8%
O2+92% N2 for 2 h. The oxygen concentration
was monitored with an oxygen monitor and maintained at ~8% (7–9%).
At the end of the 2 h of hypoxia, the pups were returned to their
mother for nursing. The surgery lasted <15 min, avoiding an
anesthesia time that was too long. The surgery appeared to minimize
bleeding and stimulate the surrounding tissues. Bloodstains
following the surgery were removed to minimize the refusal of the
mother to feed the pups due to any changes in odor. The control
group rats only received the ether anesthesia and exposure of the
left CCA without ligation, cutting or hypoxia following skin
suture. All the pups were returned to their cages following the
surgery.
Behavioral observation
A state of consciousness, general behavior and
physical activity in the HIBD-model and sham-surgery groups were
observed prior to the experiment, following anesthesia and surgery,
in the process of anaerobic and at 1, 4, 12, 24 and 72 h after
anoxia, respectively.
Collection and processing of brain
tissue
The rats were ether anesthetized and their limbs
fixed on the minor board in the supine position 7 days after the HI
insult. The chest was opened, and 20 ml of a medical sterile
syringe needle was carefully inserted into the left ventricle, the
right auricle was simultaneously cut and sterilized saline was
injected. Until the fluid was clear, 10–20 ml of 4%
paraformaldehyde was injected. The brains were removed from the
skulls and it was observed by eye that the limbs and liver of the
rats were white. The brains were post-fixed in the 4%
paraformaldehyde for 24–48 h. Subsequently, the brains were placed
in 10, 20 and 30% sucrose solution in turn until they sank.
Preparation of the frozen section
For the slide processing, the slides were soaked in
concentrated sulfuric acid for 24 h, and were subsequently rinsed
gently with running water, and dried in a temperature-controlled
incubator (37°C). Slides were dried briefly to remove excess
liquid. Following this, the slides were immersed in a mixed liquor
[10 ml mix of 3-aminopropyltriethoxysilane (APES) with 500 ml
acetone] for 2 min. Finally, unbound APES was washed with acetone
and dried in the 37°C temperature-controlled incubator for use.
The dehydrated brain specimens were embedded in
optimal cutting temperature compound. Coronal serials sections
(20-µm) containing the hippocampus were cut by freezing microtome,
and washed with phosphate-buffered saline. Tissue sections were
mounted on the treated glass slides with a brush. The group and
slice number were marked on the frosted side of the slides, and
Nissl staining continued following drying.
Nissl staining
Slides were placed in distilled water with gentle
agitation for 2 min. The slides were subsequently placed in Nissl
staining fluid for 5–10 min. Following this the slides were washed
with distilled water twice (for several sec each time). The slides
were dehydrated in 95% ethanol for 2 min twice, and subsequently
they were cleared in xylene for 5 min twice. Finally, the slides
were mounted with neutral gum. The tissue structures and the
morphology and arrangement of neurons were observed in the cerebral
cortex and hippocampus by light microscopy (CX-21 DIN; Olympus,
Tokyo, Japan).
Learning and memory ability
assessment
The Morris water maze test was used to examine the
long-term learning and memory abilities at the age of 28 days
(12,13).
The experimental apparatus consisted of a circular
water pool (diameter, 120 cm; height, 60 cm) filled with carbon
ink-clouded water and the temperature was controlled at 22±0.5°C.
The maze was divided geographically into four equal quadrants and
included release points in each quadrant (marked as N, E, S and W).
A plexiglas hidden platform (10×50 cm) situated in the center of
the target quadrant was submerged 1-cm below the surface of the
water (thereby making it invisible) for testing of spatial
learning. A camera was mounted above the center of the maze. The
motion of the animals could be recorded and sent to a computer. A
tracking system was used to measure the escape latency (EL),
traveled path and swimming speed (14,15).
Throughout the tests, the investigator was always placed in the
same position. Care was taken to maintain the location of the water
maze relative to other objects in the laboratory for consistency
and so that prominent visual clues would not be disturbed during
the test.
Maze adaptation training and swimming
ability test
One day before the spatial training in the water
maze was initiated, the platform was removed and the rat was placed
into the pool from a fixed starting point and was allowed to swim
freely for a duration of 2 min. This was the first contact with the
water (water adaptation trial) for the animal. The aim was for the
rats to adapt to the environment, and to allow a preliminary
inspection of the swimming ability of rats, and to eliminate
evident abnormalities.
Acquisition trials
Acquisition trials were used to the testing the
object recognition memory of the animals. All the rats performed a
block of four trials during four daily sessions. The hidden
platform position remained stable during the 4 days of the
assessment. Each rat was placed in the water gently while facing
the wall, at every quadrant each day, and rats were allowed a ≤120
sec at each trial to find the hidden platform. The duration to find
the platform was called the EL. When the rat succeeded to escape,
it was allowed to stay on the platform for an additional 30 sec
before starting the next trial, in order to help the rat to
recognize its orientation cues. If the rat failed to find and reach
the platform within 120 sec, it was gently guided onto the platform
by the investigator with 120 sec scored, and allowed to remain
there for 30 sec. Subsequently, the rat was removed from the
platform and the next trial was initiated. Following completion of
the fourth trial, the rats were gently dried with a towel, kept
warm for 1 h, and returned to their home cage. The mean data of EL
from four trials each day was taken as the daily average of the
aforementioned parameters and used to form cognitive function-time
curves.
Retrieval trial
A retrieval trial was used to measure the accurate
memory of the platform space position subsequent to the rats
learning to search platform, namely memory retention. The platform
was removed following the place navigation (day 5 of the water
maze). The rat was placed into the pool facing the wall from a
optional fixed starting point and was allowed to swim freely for a
duration of 120 sec. The traveled path and the number of times
crossing the former platform location were recorded as an index of
retrieval.
Retention trial
The rats had a rest of 3 days after the retrieval
trial. Susbequently, on day 8, the Morris water maze assessment was
repeated to obtain retention memory data. The method was similar
with the acquisition trials, in order to assess their long-term
memory retention.
Statistical analysis
Experimental data are presented as mean ± standard
deviation (SD), using SPSS 20.0 statistical software (IBM Corp.,
Armonk, NY, USA). Repeated measures analyses of variance [one-way
repeated measures analysis of variance (ANOVA)] were used to
compare the EL of the experiment. The process of multifactor ANOVA
made comparisons between the groups at each time point. Statistical
differences between multiple groups were compared using one-way
ANOVA and with least SD post hoc tests used for pairwise
comparison. Comparison between the two groups used independent
sample t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Behavior observation
All the neonatal rats had normal behaviors prior to
the experiment. The rats gradually ceased activity and their
respiratory rate decreased following ether anesthesia, and became
sober quickly following surgery. During hypoxia, all the animals
exhibited different levels of behavioral change, including behaving
with intermittent agitation, rolling for 5–10 min or even 3 min as
the earliest following exposure in the hypoxic device. Following
this, those animals exhibited shortness of breath, gradual
cyanoderma was observed in their faces, ears, limbs and tails,
their muscle twitched, they were restless, exhibited head tremors,
stay downed and exhibited disturbances in their consciousness (such
as drowsiness, lethargy, confusion, and in certain cases, even
coma). Those symptoms were alternating, and fatality occurred in
the low oxygen tank in severe cases. Following hypoxia, 90% (18/20)
showed turning to the left spontaneously or when the tail was
clamped the tail, 85% (17/20) showed a poor turn over ability, 55%
(11/20) exhibited limb shaking and 45% (9/20) showed convulsion.
When exposed to normal oxygen, the animals awoke gradually, resumed
sucking ability, and increased the activity of the limbs. Activity
gradually increased after 1 h, and the majority of the
neurobehavioral abnormalities disappeared 4 h later. However,
features such as turning to the left spontaneously or when the tail
was clamped remained for >1 day (fixed rotation to the left is
due to cerebral cortex motor center damage caused by HI, leading to
imbalance. Paralysis of the ligated limbs is the typical behavioral
changes of the model). Two rats succumbed to hypoxia during the
modeling, and the survival rate was 90.91%. In the latter feeding
process, HIBD rats showed slow weight gain, and the weight of
certain individuals remained stable or even reduced. The opening of
the left eye was delayed and eye fissure was smaller compared with
the opposite side.
Among the 18 rats in the sham-surgery group, 1
succumbed due to over-medication of the anesthesia and was excluded
from the study. There were no significantly different findings in
the other animals at any time-point.
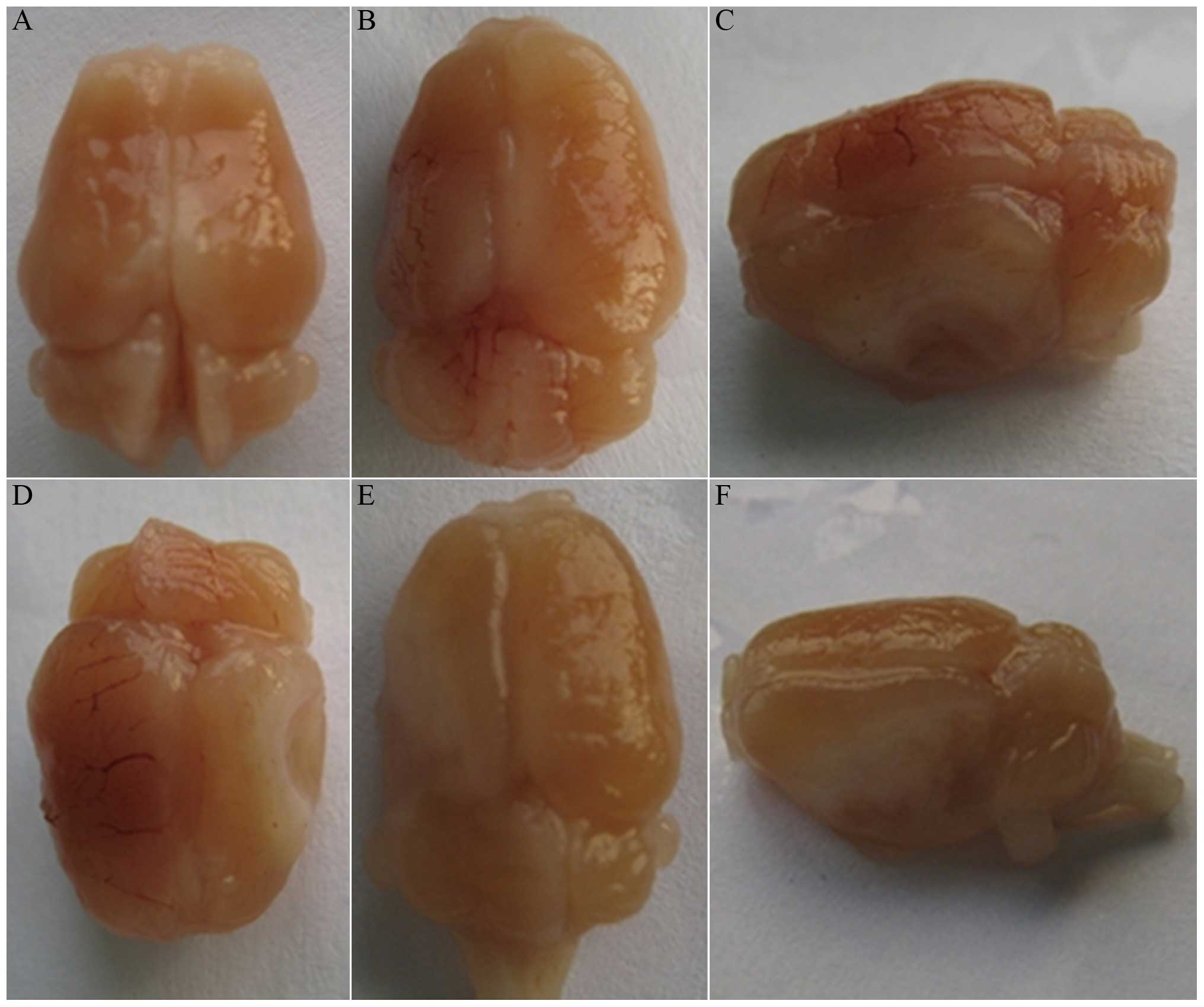
Gross anatomy observation
A total of 9 pups from each group were sacrificed
and their brains were removed after 7 days of HI exposure. The
general features of the brain in the HIBD group were as follows:
Atrophy of the left brain, 89% (8/9); softening or liquefaction of
the left brain, 67% (6/9); and even cavum brain (the walls were
smooth-filled with liquid but there was no colloid filling in the
cavity), 33% (3/9). There was no evident abnormity identified in
the right brain, brain stem or cerebellum of the HIBD group and the
cerebral hemispheres of the sham-surgery group (Fig. 1).
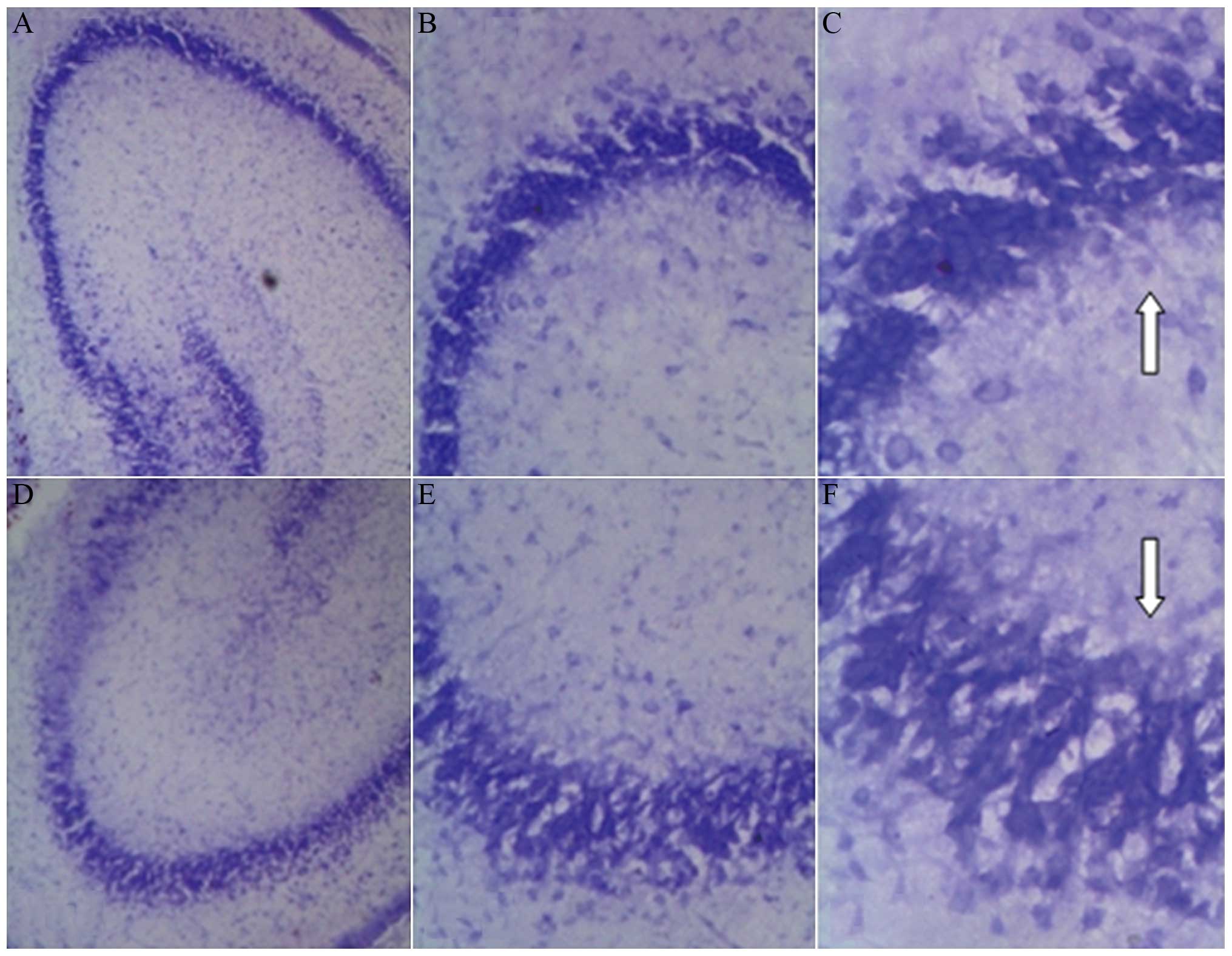
Pathological examination of brain
tissue (Nissl staining by light microscopy)
Normal hippocampal cells were arranged in neat rows
of 3–4 layers, and were large and round. Nissl bodies were deeply
stained. There was no evident neuronal damage in the cerebrals of
the sham-surgery group and the contralateral cerebral hemisphere of
the HIBD group. Morphology and the levels of the cells were clear
and complete with clearly visible nucleoli. Nissl bodies were
equidistributed around the nucleus, with rules and without
cavitation.
The pathological alterations were prominent in the
left hemisphere (modeling side) of the HIBD group, characterized by
a large number of nucleur pyknosis and nuclear fragmentation. The
Nissl body was blurred or disappeared, with vacuolation, a
disordered arrangement and formation of a network. The morphology
of pyramidal cells of the HIBD group changed significantly compared
with the sham-surgery group. Morphological features included a
disordered arrangement of the cells, significant loss in volume,
light staining of the Nissl body, deformation or disappearance of
the nucleus. There was even apparent cell death and cell loss, and
the normal pyramidal cells were scattered within the background of
the dead cells (Fig. 2).
Swimming ability test
All the rats passed the swimming ability test on day
1, and the swimming ability in all rats was normal.
Acquisition trials
Repeated measure ANOVA results were as follows: The
tests of within-subjects effects showed that the time factor (day)
was statistically significant (HIBD group: F=0.057, P<0.01; sham
group: F=3.043, P=0.046). This denoted that the EL of the rats in
each group had a variation trend over the time, but was more
evident in the sham group. The day and group interaction (day ×
group) was also statistically significant (F=3.410, P=0.025). This
indicated that the role of the time factor varies within the
groups. After 4 days of the hidden platform training, the EL curves
showed a tendency to decrease in each group of rats; however, the
EL values of the sham-surgery group exhibited a more evident
reduction, and showed the ability to maintain a good memory by day
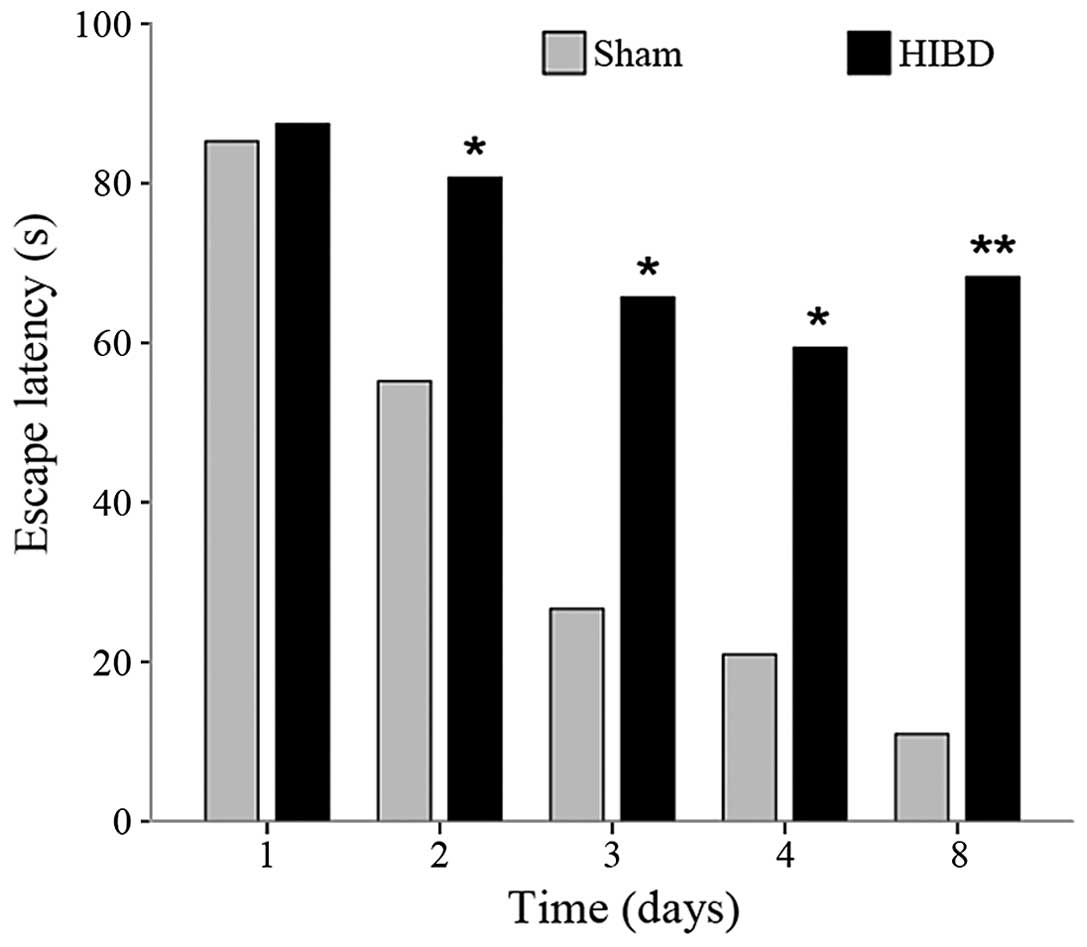
8 (Fig. 3).
Between-group comparison at the same time were as
follows: There was no significant difference between the average EL
in the groups on day 1 (t=0.169; P=0.868). From day 2, the EL value
of the HIBD group was higher compared to the sham-surgery group,
and the gap increased with time, which indicated that spatial
learning and short-term memory ability were damaged in the rats
following HI. On day 8, the difference of the EL values between
groups were the most evident (t=5.111, P<0.001) (Table I).
 | Table I.Average escape latency at the
different time-points in each group. |
Table I.
Average escape latency at the
different time-points in each group.
|
|
| Escape latency, mean
± standard deviation |
|---|
|
|
|
|
|---|
| Group | Total, n | Day 1 | Day 2 | Day 3 | Day 4 | Day 8 |
|---|
| Sham-operation | 8 | 85.25±22.19 | 55.19±33.47 | 26.64±28.24 | 20.93±22.14 | 10.93±5.52 |
| HIBD | 10 | 87.46±31.02 | 80.69±12.86 | 65.72±35.76 | 59.41±35.59 | 68.25±31.14 |
| t-test |
| 0.169 | 2.227 | 2.521 | 2.676 | 5.111 |
| P-value |
| 0.868 | 0.041 | 0.023 | 0.017 | <0.001 |
On day 8, the sham-surgery group rats were able to
rapidly identify the hidden platform, and the swimming path was
short and targeted. By contrast, the EL time increased rather than
decreased for the HIBD group, indicating the loss of spatial memory
ability and positioning capabilities following HI injury, and
long-term memory defects (Fig. 4).
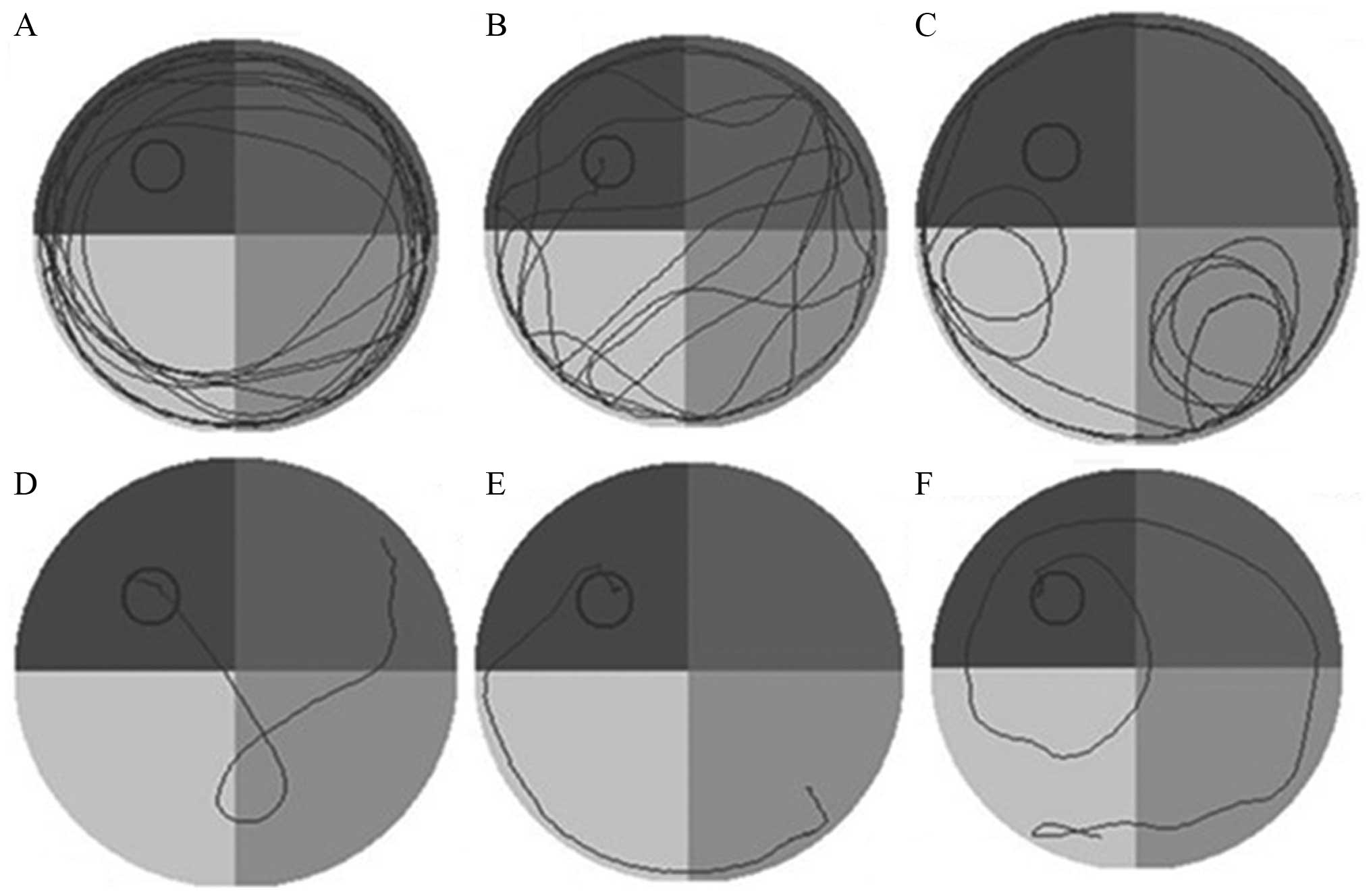
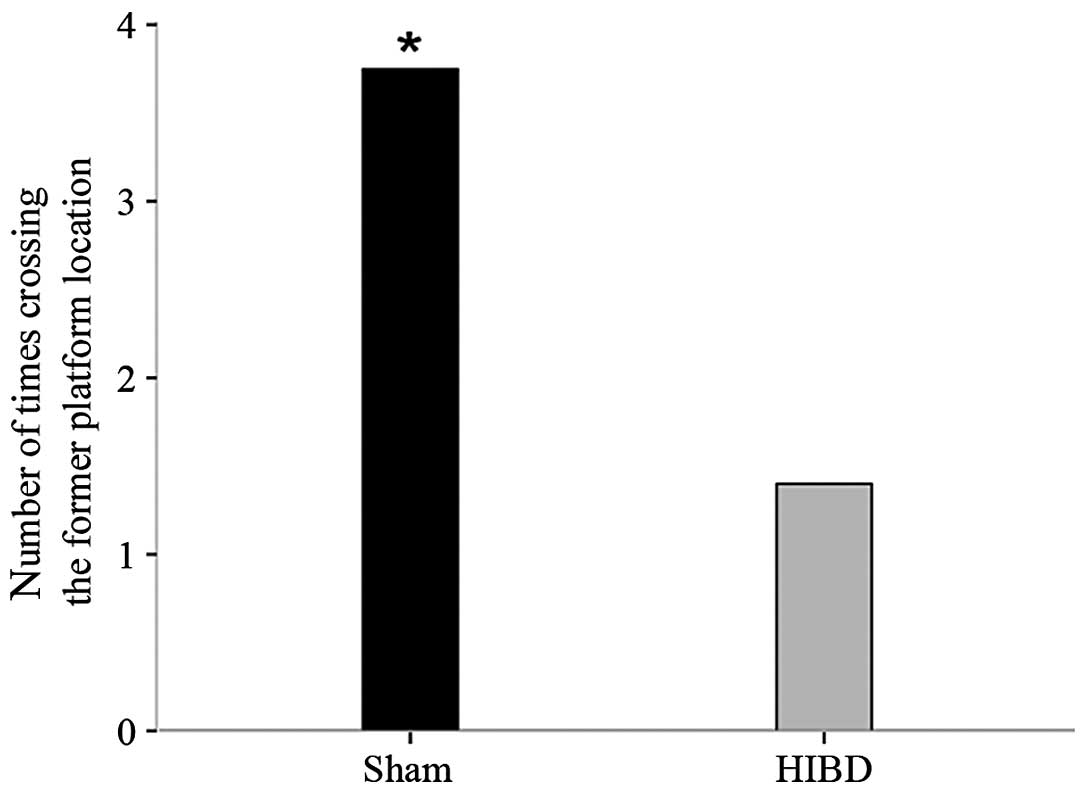
Spatial probe
On day 5 of the water maze, there were significant
differences in the number of times for crossing the former platform
location between the two groups (t=2.756, P=0.022). This indicated
that the sham-surgery group rats had an improved spatial memory
ability and positioning capabilities compared to that of the HIBD
group (Fig. 5).
Discussion
In experimental animals, structural brain damage
from HI has been produced in immature rats, rabbits, sheep and
monkeys. These models have provided the basis for investigations to
clarify not only the physiological and biochemical mechanisms of
tissue injury, but also the efficacy of specific management
strategies. Among those models, the fetal and newborn rhesus monkey
and immature rat have been studied most extensively due to their
similarities to humans in respect to the physiology of reproduction
and their neuroanatomy. Newborn rats have more advantages, such as
easiness to obtain, short pregnancy and fast breeding, and a nest
of rats can produce 8–15 newborn rats. The advantages also include
the low cost, and the favorable control of the experimental
conditions. The sources of newborn pigs, monkeys and sheep are more
difficult, and the mortality and costs are higher. Therefore,
newborn rats are the preferred experimental animals for neonatal
HIBD research by medical workers from domestic and foreign medical
institutions. Studies have shown that the degrees of the rat brains
are different at distinct ages. The 7-day postnatal rat was
originally chosen for study as at this stage of development its
brain is histologically similar to that of a 32- to 34-week
gestation human fetus or newborn infant (16,17). The
characteristic features (18) include
completion of cerebral cortical layering, involution of germinal
matrix, the presence of little myelinated white matter,
neurotransmitters and immune inflammatory changes. Cerebral blood
flow and metabolic correlates have been fully characterized. The
immature rat model has been proved and applied for studies of HIBD
(19). Consequently, at present, the
7-day postnatal rat was originally utilized to produce the HIBD
model, of which the most popular method is based on the Rice method
(6). The specific surgical procedure
is as follows: The left carotid artery was exposed and permanently
legated under the mixture of halothane anesthesia. The rat pups
were returned to their home cage for 4 h followed by exposure to
hypoxia (92% N2+8% O2) for 3.5 h by placing
them in an airtight chamber partially submersed in a 37°C water
bath. The oxygen concentration was maintained at 8±0.1%. This model
of HIBD was successful and is cheap, easily duplicated and has a
higher successful rate. Additionally, there were significant
differences in terms of long-term behavioral and morphological
changes in the HIBD group compared with the control group. This
study demonstrated that it is the ideal model for the research of
HI brain injury on athletic ability, the brain maturation process
and other mechanisms of short- and long-term effects, and is
therefore a good model of HIBD. However, there are numerous defects
in the conventional Rice method. First, the use of halothane during
anesthesia can inhibit the respiration and circulation, while the
neonatal rat is small in size and lightweight, and prone to
overdose fatalities. Second, only the left CCA was ligated instead
of being cut completely. Therefore, the ligated artery may require
recanalization and bypass formation with the growth of animals to
influence the degree of brain injury.
Therefore, certain minor modifications of the Rice
method were performed as described previously. First, anesthesia
was improved. In the present study, the anesthesia ether was
selected instead of halothane. Although it takes a longer time
compared to halothane in the induction of anesthesia, muscle
relaxation is good, and relatively safe, avoiding inhibition of the
circulation and respiration. Second, arterial ligation was
improved. The present study improved artery ligation using the
study by Nakajima et al (11).
The left CCA was isolated, double-ligated and cut between the
ligatures, to avoid the formation of blood reperfusion. One key to
success lies in the accuracy of unilateral carotid artery ligation.
In order to avoid mistaking the neck muscles for vascular, the
blood flow must be identified and the arterial pulse can be
observed prior to making a permanent ligation and cut. The second
was that the reasonable ambient temperature in the airtight chamber
should be maintained at 37±0.5°C. If the temperature is too high or
too low it can increase the mortality rate in rats. Additionally,
low or moderate hypothermia has a protective effect on HIBD to
impact model preparation effect. Therefore, it is crucial to
control the temperature with a water bath to ensure the modeling
effort.
Indicators of successful HIBD model preparation are
as follows: Slow weight gain, neurobehavioral abnormalities,
general and microscopic abnormalities of the lesion side, increased
brain water content and decreased brain blood volume. The present
study identified that all the animals have different levels of
behavioral change during hypoxia, mainly as irritability, head
tremor, intermittent agitation, rolling, convulsions, sleepiness,
skin bruising and urine and feces incontinence. Following hypoxia,
the animals awoke gradually, resumed sucking and the activity of
their limbs increased. The minority exhibited head tremor,
convulsion and could not turnover. Certain rats showed a
spontaneously turn round to the left and right limb movement
disorder. Mortality in the HIBD group rats was 9.1%, and 90% had
abnormal neurological behavior, particularly during the course of
hypoxia, which disappeared 4 h after oxygen was supplied. In the
latter feeding process, the HIBD group rats showed slow weight
gain, and the weight of certain individuals stayed constant or even
reduced. The opening of the left eye was delayed and the eye
fissure was smaller compared with the contralateral side. It showed
that compared with the sham-surgery group, behaviors were
significantly different in the HIBD group, which indicates HI
induced behavioral changes in rats. On this basis, the general and
microscopic pathology of the brain tissues were observed. The
pathological features in the HIBD group were as follows: Atrophy,
softening and liquefaction of left brain, and even cavum brain in
severe cases. Analysis by light microscopy observed cellular
swelling and degeneration, nuclear condensation and fragmentation,
the Nissl body was blurred or disappeared, cytoplasm was vacuolated
and the endoplasmic reticulum was disarranged. Morphological
alterations were more prominent in the cerebral cortex and
hippocampus neurons. Further validation from morphological and
histological analysis indicated that this method successfully
established the model of HIBD in the neonatal rats.
Learning-memory is an advanced feature of the brain.
Learning-memory disabilities, cognitive function deficit or even
loss are important signs of brain injury causing mental decline,
and may also be the core symptoms of HIBD. Manifestations are as
follows: Memory and cognitive decline, decrease in spatial
orientation and learning ability, and even cerebral palsy. The
integrity of the hippocampus is extremely important for normal
development of spatial learning and memory. Other regions cannot
compensate the hippocampal spatial learning and memory ability
following neonatal injury. There were pathological impairments in
the hippocampus of HIBD rats. The Morris water maze was also
adopted to assess the learning and memory performance. The HIBD
rats were significantly worse in the learning/memory acquisition
and memory retention ability compared to the sham-surgery group.
These findings demonstrate that HI brain injury can cause
learning/memory and cognitive function defects.
Therefore, according to the aforementioned method
using 7-day-old Sprague-Dawley rats, the HIBD animal model can be
established with morphological structure and function injury of the
brain. This experiment established the neonatal rat HIBD model that
is similar to a clinical environment, and thus can contribute to
perform future associated studies.
References
|
1
|
Dammann O, Ferriero D and Gressens P:
Neonatal encephalopathy or hypoxic-ischemic encephalopathy?
Appropriate terminology matters. Pediatr Res. 70:1–2. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wachtel EV and Hendricks-Muñoz KD: Current
management of the infant who presents with neonatal encephalopathy.
Curr Probl Pediatr Adolesc Health Care. 41:132–153. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gulczyńska E and Gadzinowski J:
Therapeutic hypothermia for neonatal hypoxic-ischemic
encephalopathy. Ginekol Pol. 83:214–218. 2012.(In Polish).
PubMed/NCBI
|
|
4
|
Yang LJ, Ma DQ and Cui H: Proteomic
analysis of immature rat pups brain in response to hypoxia and
ischemia challenge. Int J Clin Exp Pathol. 7:4645–4660.
2014.PubMed/NCBI
|
|
5
|
Donega V, van Velthoven CT, Nijboer CH,
van Bel F, Kas MJ, Kavelaars A and Heijnen CJ: Intranasal
mesenchymal stem cell treatment for neonatal brain damage:
Long-term cognitive and sensorimotor improvement. PLoS One.
8:e512532013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rice JE III, Vannucci RC and Brierley JB:
The influence of immaturity on hypoxic-ischemic brain damage in the
rat. Ann Neurol. 9:131–141. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dingley J, Tooley J, Porter H and Thoresen
M: Xenon provides short-term neuroprotection in neonatal rats when
administered after hypoxia-ischemia. Stroke. 37:501–506. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sola A, Rogido M, Lee BH, Genetta T and
Wen TC: Erythropoietin after focal cerebral ischemia activates the
Janus kinase-signal transducer and activator of transcription
signaling pathway and improves brain injury in postnatal day 7
rats. Pediatr Res. 57:481–487. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harris AP, Helou S, Gleason CA, Traystman
RJ and Koehler RC: Fetal cerebral and peripheral circulatory
responses to hypoxia after nitric oxide synthase inhibition. Am J
Physiol Regul Integr Comp Physiol. 281:R381–R390. 2001.PubMed/NCBI
|
|
10
|
Gonzalez H, Hunter CJ, Bennet L, Power GG
and Gunn AJ: Cerebral oxygenation during postasphyxial seizures in
near-term fetal sheep. J Cereb Blood Flow Metab. 25:911–918. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakajima W, Ishida A, Lange MS, Gabrielson
KL, Wilson MA, Martin LJ, Blue ME and Johnston MV: Apoptosis has a
prolonged role in the neurodegeneration after hypoxic ischemia in
the newborn rat. J Neurosci. 20:7994–8004. 2000.PubMed/NCBI
|
|
12
|
Morris R: Developments of a water-maze
procedure for studying spatial learning in the rat. J Neurosci
Methods. 11:47–60. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao D, He X, Wang JH and Zhao ZY: Effects
of PI3K/Akt signaling pathway on learning and memory abilities in
neonatal rats with hypoxic-ischemic brain damage. Zhongguo Dang Dai
Er Ke Za Zhi. 13:424–427. 2011.(In Chinese). PubMed/NCBI
|
|
14
|
Luine VN, Jacome LF and Maclusky NJ: Rapid
enhancement of visual and place memory by estrogens in rats.
Endocrinology. 144:2836–2844. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Monteiro SC, Matté C, Bavaresco CS, Netto
CA and Wyse AT: Vitamins E and C pretreatment prevents
ovariectomy-induced memory deficits in water maze. Neurobiol Learn
Mem. 84:192–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang W, Duong TM and de Lanerolle NC: The
neuropathology of hyperthermic seizures in the rat. Epilepsia.
40:5–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clancy B, Darlington RB and Finlay BL:
Translating developmental time across mammalian species.
Neuroscience. 105:7–17. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vannucci RC: Experimental models of
perinatal hypoxic-ischemic brain damage. APMIS Suppl. 40:89–95.
1993.PubMed/NCBI
|
|
19
|
Vannucci RC and Vannucci SJ: A model of
perinatal hypoxic-ischemic brain damage. Ann N Y Acad Sci.
835:234–249. 1997. View Article : Google Scholar : PubMed/NCBI
|