Introduction
Constipation can contribute to health care costs and
cause a decline in work productivity and the quality of life, as
well as result in disability (1).
Therefore, an effective treatment for constipation is important;
however, only a few treatment options exist for constipation and
there is limited evidence of suitable treatments.
Daiokanzoto (DKT) is a Kampo medicine often used
clinically to treat constipation. DKT is composed of rhubarb and
glycyrrhiza. Sennoside A is the active ingredient in DKT
responsible for its effects in constipation. However, DKT has
demonstrated efficacy in patients with constipation who were
intolerant to treatment with senna extract. According to the
guidelines set by Lindberg et al (2), senna extract is recommended for the
treatment of constipation. Magnesium oxide (MgO) is commonly used
as an osmotic laxative (3). Senna
extract and MgO are therefore used as first-line treatments in
numerous cases of constipation; however, patients who were
intolerant to these two common laxatives were encountered. Since
there is limited information on other laxatives, it is difficult to
effectively treat such patients. Therefore, the present study
examined the laxative effects of DKT in patients intolerant to the
first-line treatments.
Materials and methods
Background
The present study was approved by the Ogaki Civilian
Hospital Ethics Committee and was performed at Ogaki Municipal
Hospital (Ogaki, Japan) between 1st May 2012 and 30th April 2015.
Patients who used DKT, but were intolerant to either MgO (MgO
group) or senna extract (Senna group) were included in the present
study. Patients were administered either MgO tablets or senna
extract tablets for 1 week prior to the initiation of DKT
treatment.
The present study retrospectively reviewed the
following details of the patients from the electronic medical
records: Age, height, weight, dose of DKT, concomitant medications
and medical history. The increased total bilirubin (T-Bil) and
blood urea nitrogen (BUN) levels were also recorded, as well as the
serum levels of aspartate aminotransferase (AST), alanine
transaminase (ALT), creatinine (Cre), Na, K and Cl. Other data that
was deemed relevant was also recorded.
Comparison of the effects of DKT in
the MgO and Senna groups
From the medical records of the patients, the
present study obtained and compared the frequencies of their bowel
movements during the 1 week prior to and following DKT
administration.
Adverse events from DKT
administration
The present study investigated the incidence of
adverse events following treatment with DKT. The predominant
adverse events were diarrhea and abdominal pain, which were
evaluated using the Common Terminology Criteria for Adverse Events
(version 4.0; http://evs.nci.nih.gov/ftp1/CTCAE/About.html). In
addition, the presents study also compared the changes in clinical
laboratory data prior to, 3 days and 1 week after DKT
administration.
Statistical analysis
Statistical analyses were performed using EZR
software v1.26 (4). The effects of DKT
in the groups were compared using Wilcoxon signed-rank test at a
significance level of 5%. Differences in the laboratory test
results, 3 days and 1 week after DKT administration, were evaluated
using the Friedman test. The significance level was also set at
5%.
Results
Background
A total of 16 patients (11 male and 5 female) were
enrolled into the MgO group and their median age was 71 years
(interquartile range, 65–77 years). A total of 26 patients (14 male
and 12 female) were enrolled in the Senna group and their median
age was 72 years (interquartile range, 68–78 years). In both the
MgO and Senna groups, the last bowel movements were 3.5
(interquartile range, 2–6 days) and 4.0 days (interquartile range,
2–6 days), respectively, prior to DKT administration. Baseline
characteristics of the patients are shown in Table I.
 | Table I.Baseline characteristics of the
patients. |
Table I.
Baseline characteristics of the
patients.
| Characteristics | MgO resistance group
(n=16) | Senna resistance
group (n=26) |
|---|
| Median age, years
(interquartile range) |
71
(65–77) |
72
(68–78) |
| No. of males | 11 | 14 |
| No. of females | 5 | 12 |
| Median eight, kg
(interquartile range) |
53
(49–64) |
51
(42–60) |
| Median height, cm
(interquartile range) |
160
(154–166) |
157
(151–163) |
| Median days since
last bowel movement (interquartile range) |
3.5 (2–6) |
4.0 (2–6) |
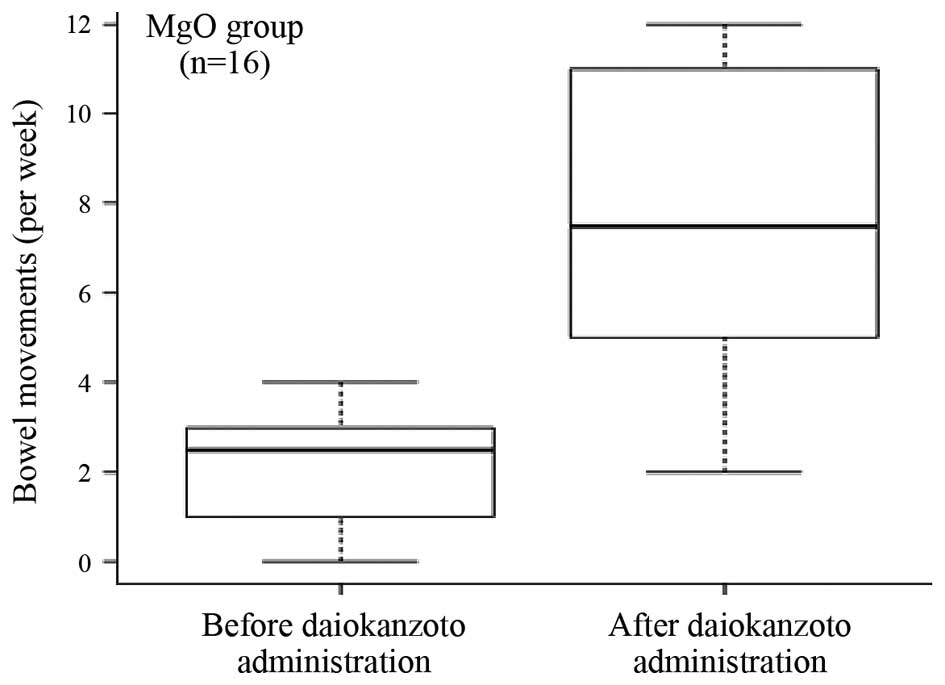
Effect of DKT in the MgO group
Following the administration of DKT, 93.8% of
patients in the MgO group evacuated their bowels within 24 h. The
median bowel movement frequency 1 week prior to DKT administration
was 2.5 (interquartile range, 1–3). However, 1 week after DKT
administration, the frequency significantly (P<0.001) increased
to 7.5 (interquartile range, 5–11), as shown in Fig. 1.
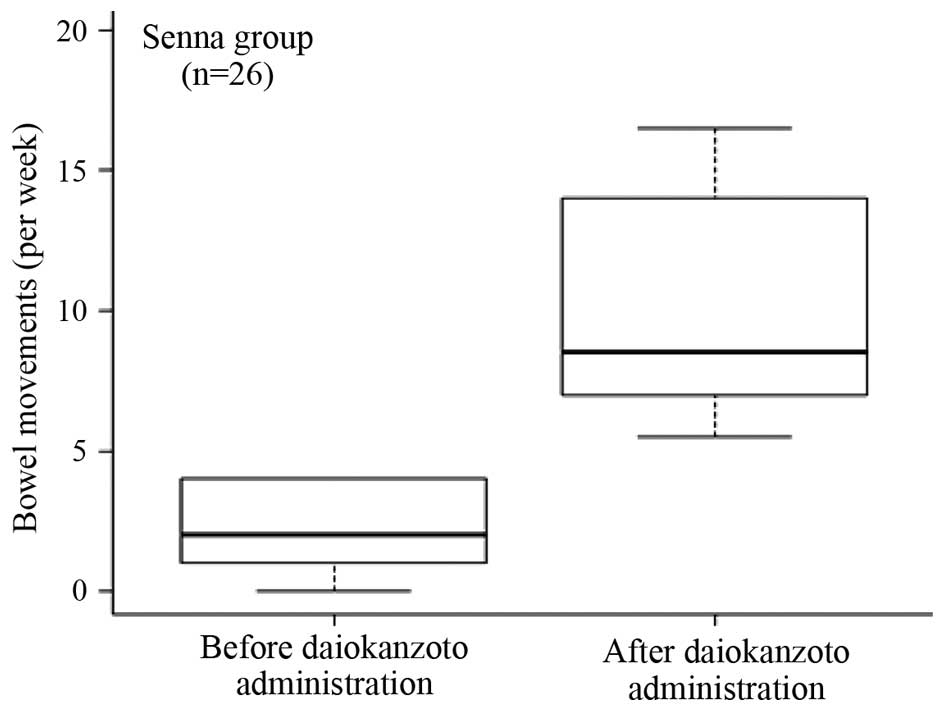
Effect of DKT in the Senna group
Following the administration of DKT, 80.8% of the
patients in the Senna group evacuated their bowels within 24 h. The
median bowel movement frequency 1 week prior to DKT treatment was
2.0 (interquartile range, 1–4), which significantly (P<0.001)
increased to 8.5 (interquartile range, 7–14) 1 week after the
administration of DKT (Fig. 2).
Adverse events from DKT
administration
In the Senna group, there were four cases of
diarrhea (15.4%) and one case of abdominal pain (3.8%). Both
adverse events were mild (Grade 1) in each group of patients.
In the MgO group, no statistically significant
differences were observed among the clinical laboratory data
obtained prior to, 3 days and 1 week after DKT administration
(Table II). By contrast, in the Senna
group, the differences among the levels of ALT prior to, 3 days and
1 week after DKT administration were statistically significant
(P<0.05 for each pair compared) (Table III). It was observed that ALT
decreased with increasing duration following treatment with
DKT.
 | Table II.Clinical laboratory data in the MgO
group. |
Table II.
Clinical laboratory data in the MgO
group.
|
| MgO resistance
group |
|
|---|
|
|
|
|
|---|
| Median levels | Prior to
administration | Administered 3 days
after | Administered 1 week
after | P-value |
|---|
| Na, mEq/l
(interquartile range) | 136.5
(135.75–139.25) | 136 (135.5–139) | 137 (136.5–138) | 0.618 |
| K, mEq/l
(interquartile range) | 4.25 (4.075–4.5) | 4.4 (4.1–4.55) | 4.3 (4.1–4.45) | 0.846 |
| Cl, mEq/l
(interquartile range) | 102.5
(99.75–106) | 104 (100.5–106) | 104 (102–105.5) | 0.88 |
| AST, IU/l
(interquartile range) | 20.5
(14.75–26.75) | 18.5
(12.5–26.75) | 18 (13.5–25) | 0.782 |
| ALT, IU/l
(interquartile range) | 19 (13.75–26) | 18 (15.5–32.5) | 15 (11–30) | 0.607 |
| T-Bil, mg/dl
(interquartile range) | 0.6
(0.525–0.775) | 0.6 (0.5–0.8) | 0.5 (0.4–0.6) | 0.0861 |
| BUN, mg/dl
(interquartile range) | 17.1
(14.225–19.725) | 16.7
(14.625–22.1) | 14.5
(12.8–18.35) | 0.119 |
| Cre, mg/dl
(interquartile range) | 0.625
(0.59–0.9325) | 0.705
(0.6125–0.9925) | 0.72 (0.64–0.92) | 0.185 |
 | Table III.Clinical laboratory data of the Senna
group. |
Table III.
Clinical laboratory data of the Senna
group.
|
| Senna resistance
group |
|
|---|
|
|
|
|
|---|
| Median levels | Prior to
administration | Administered 3 days
after | Administered 1 week
after | P-value |
|---|
| Na, mEq/l
(interquartile range) | 138 (135.25–140) | 138 (134–140) | 138.5
(137.75–141) | 0.127 |
| K, mEq/l
(interquartile range) | 4.1
(3.725–4.475) | 4.1 (3.8–4.6) | 4.1 (3.575–4.55) | 0.565 |
| Cl, mEq/l
(interquartile range) | 101.5
(100–105.75) | 102 (99–106) | 103.5
(101.5–105.25) | 0.558 |
| AST, IU/l
(interquartile range) | 23 (19–33.75) | 20 (15–34) | 20 (13–30) | 0.0739 |
| ALT, IU/l
(interquartile range) | 21 (12.75–32.75) | 18 (12–28) | 15 (11–34) | <0.05 |
| T-Bil, mg/dl
(interquartile range) | 0.5 (0.4–0.8) | 0.5
(0.375–0.625) | 0.4 (0.3–0.7) | 0.984 |
| BUN, mg/dl
(interquartile range) | 18.6 (14.7–23) | 15.3 (10.9–22.4) | 15 (9.7–19.2) | 0.148 |
| Cre, mg/dl
(interquartile range) | 0.72
(0.5525–0.945) | 0.64 (0.53–0.86) | 0.62 (0.51–0.88) | 0.952 |
Discussion
It is known that ~14% of the global adult population
suffer from chronic constipation (5).
In a previous systematic review, it was stated that chronic
constipation lowers the quality of life (5). In addition, individuals with chronic
constipation have an increased risk of developing colon cancer. The
risk increases with an increasing degree of constipation (6).
In a previous study, it was observed that preventing
constipation improved multi-organ failure in critically ill
patients (7). Therefore, it is
important that evidence of the clinical effects of the treatment of
constipation are accumulated to improve the care of patients.
Usually, clinical treatment of constipation with a single laxative
is ineffective. DKT is a laxative predominantly used in Japan;
however, clinical data on its efficacy and safety is lacking.
In the present study, DKT was considered beneficial
in constipation refractory to MgO treatment. Although MgO is often
prescribed for constipation, it is not effective in certain
individuals, particularly elderly patients. MgO is an osmotic
laxative; it is converted into magnesium carbonate in the
intestine, which inhibits water reabsorption from the intestine. As
a result, the volume of intestinal contents increases, which
stimulates motility of the intestinal tract, thereby facilitating
defecation. Elderly patients have reduced intestinal motility,
which results in reduced bowel movements. Therefore, MgO has
minimal efficacy in treating constipation in the elderly.
The mechanism by which sennoside A in DKT improves
constipation is via enhanced peristalsis. A senna extract tablet
contains 6.8 mg sennoside A. In the present study, two senna
extract tablets (13.6 mg sennoside A) were administered to the
patients. By contrast, one package of DKT contains 2.9 mg sennoside
A. In the present study, patients were administered three packages
of DKT (8.7 mg sennoside A). Although the administered DKT
contained a lesser quantity of sennoside A, it was observed that
DKT was more effective compared with the senna extract tablets,
regarding the frequency of bowel movements.
The predominant components of rhubarb and
glycyrrhiza are sennoside A and glycyrrhizin, respectively.
Sennoside A is metabolically activated to rheinanthrone, which is
responsible for the laxative action of sennoside A (8–10).
Glycyrrhiza contains liquiritin, which has been reported to promote
the metabolic activation sennoside A into rheinanthrone (11,12). In
addition, Takayama et al (13)
reported that rhein in rhubarb promotes the metabolic activation
sennoside A to rheinanthrone.
Although patients exhibited inadequate bowel
movements after taking senna extract tablets, switching from the
senna extract tablets to DKT was effective in relieving
constipation. It was therefore hypothesized that reason for this
was the presence of glycyrrhiza and rhubarb in the DKT.
The adverse events from DKT treatment were mild and
controllable. Therefore, properly adjusting the doses of DKT
according to patients' requirements may suitably reduce the
occurrence of adverse events.
The results from the present study indicated that
DKT may be a suitable second-line treatment for chronic
constipation, particularly in elderly patients.
References
|
1
|
Sun SX, Dibonaventura M, Purayidathil FW,
Wagner JS, Dabbous O and Mody R: Impact of chronic constipation on
health-related quality of life, work productivity, and healthcare
resource use: An analysis of the National Health and Wellness
Survey. Dig Dis Sci. 56:2688–2695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lindberg G, Hamid SS, Malfertheiner P,
Thomsen OO, Fernandez LB, Garisch J, Thomson A, Goh KL, Tandon R,
Fedail S, et al: World Gastroenterology Organisation: World
Gastroenterology Organisation global guideline: Constipation - a
global perspective. J Clin Gastroenterol. 45:483–487. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roque M Vazquez and Bouras EP:
Epidemiology and management of chronic constipation in elderly
patients. Clin Interv Aging. 10:919–930. 2015.PubMed/NCBI
|
|
4
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gürsen C, Günel M Kerem, Kaya S, Kav T and
Akbayrak T: Effect of connective tissue manipulation on symptoms
and quality of life in patients with chronic constipation: A
randomized controlled trial. J Manipulative Physiol Ther.
38:335–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guérin A, Mody R, Fok B, Lasch KL, Zhou Z,
Wu EQ, Zhou W and Talley NJ: Risk of developing colorectal cancer
and benign colorectal neoplasm in patients with chronic
constipation. Aliment Pharmacol Ther. 40:83–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Azevedo RP, Freitas FG, Ferreira EM, de
Azevedo LC Pontes and Machado FR: Daily laxative therapy reduces
organ dysfunction in mechanically ventilated patients: A phase II
randomized controlled trial. Crit Care. 19:3292015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sasaki K, Yamauchi K and Kuwano S:
Metabolic activation of sennoside A in mice. Planta Med.
37:370–378. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kobashi K, Nishimura T, Kusaka M, Hattori
M and Namba T: Metabolism of sennosides by human intestinal
bacteria. Planta Med. 40:225–236. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lemli J and Lemmens L: Metabolism of
sennosides and rhein in the rat. Pharmacology. 20 Suppl 1:50–57.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsui E, Takayama K, Sato E and Okamura
N: The influence of glycyrrhiza and antibiotics on the purgative
action of sennoside a from Daiokanzoto in mice. Biol Pharm Bull.
34:1438–1442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takayama K, Matsui E, Kobayashi T, Inoue
H, Tsuruta Y and Okamura N: High-performance liquid chromatographic
determination and metabolic study of sennoside a in daiokanzoto by
mouse intestinal bacteria. Chem Pharm Bull (Tokyo). 59:1106–1109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takayama K, Morita T, Tabuchi N, Fukunaga
M and Okamura N: The effect of anthraquinones in daiokanzoto on
increasing the synthesis of sennoside A-metabolic enzyme derived
from bifidobacteria. J Trad Med. 30:215–220. 2013.
|