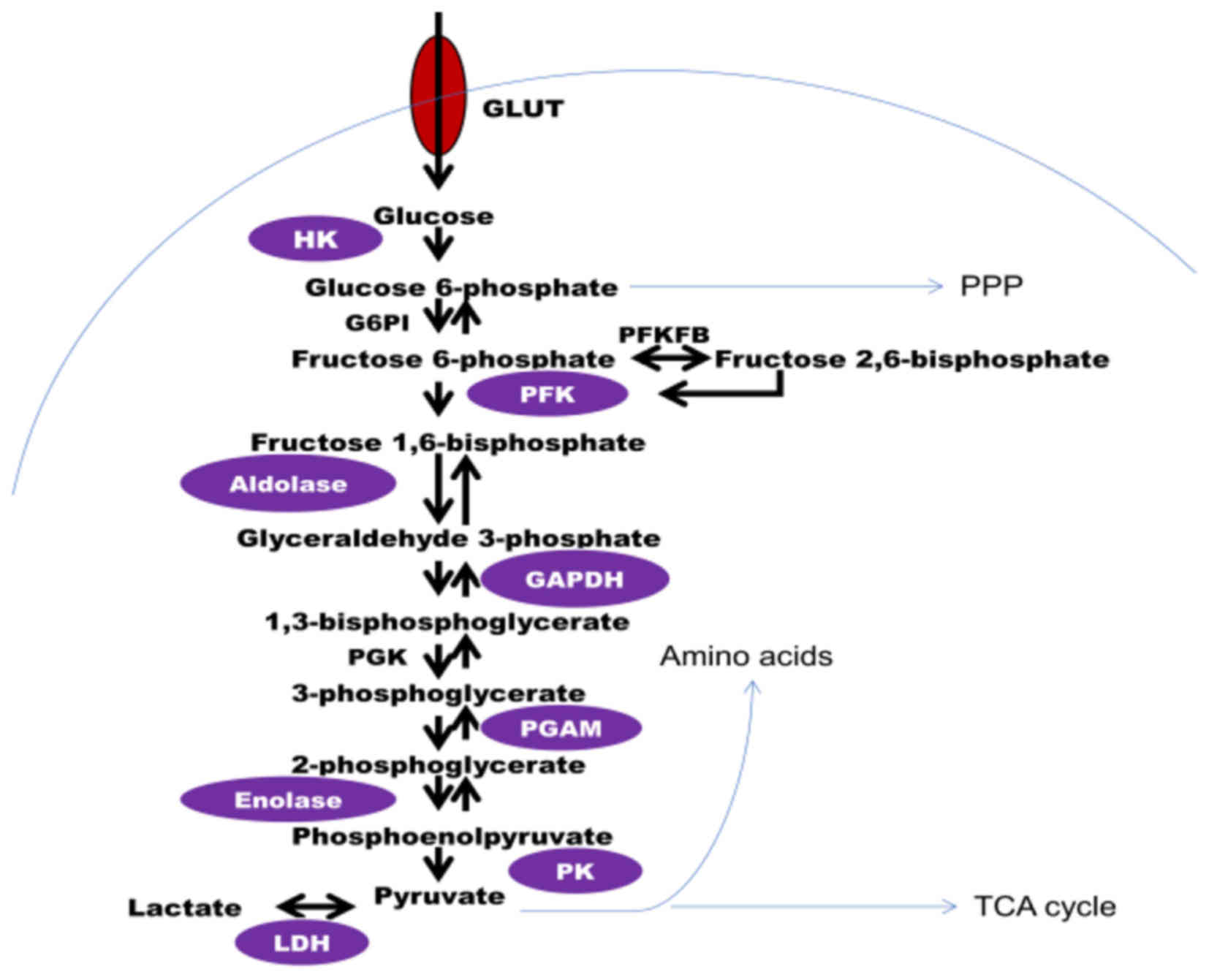
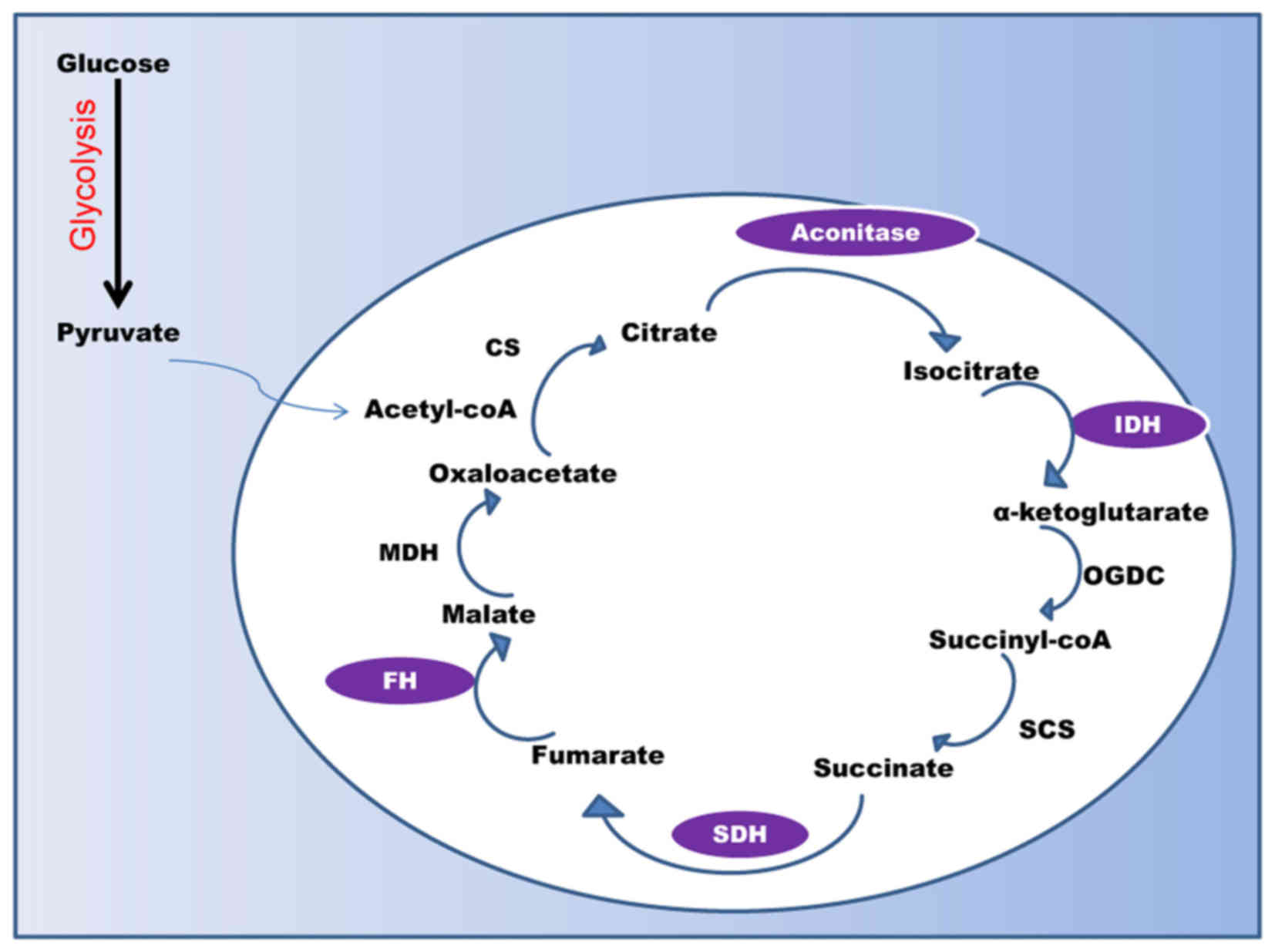
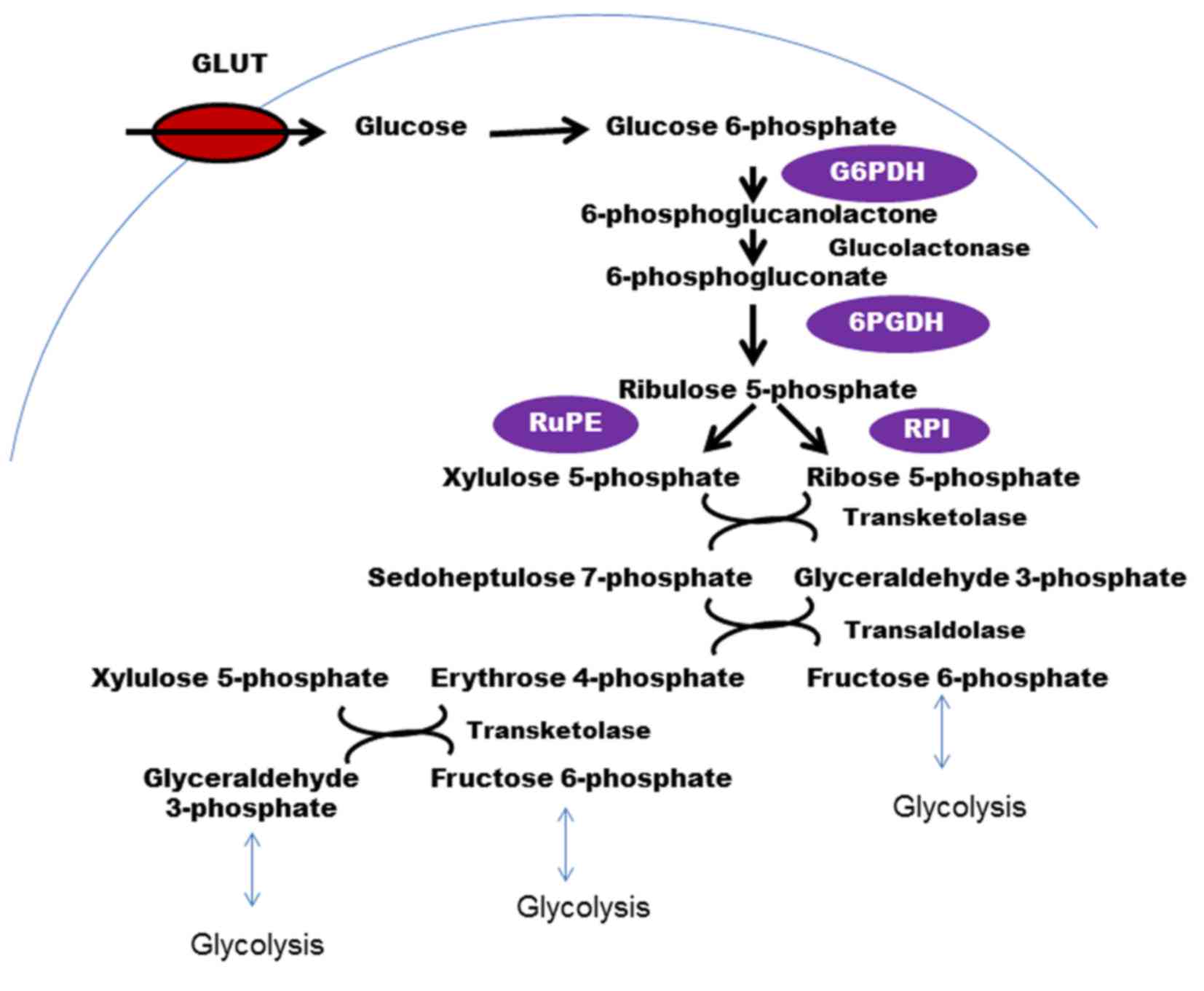
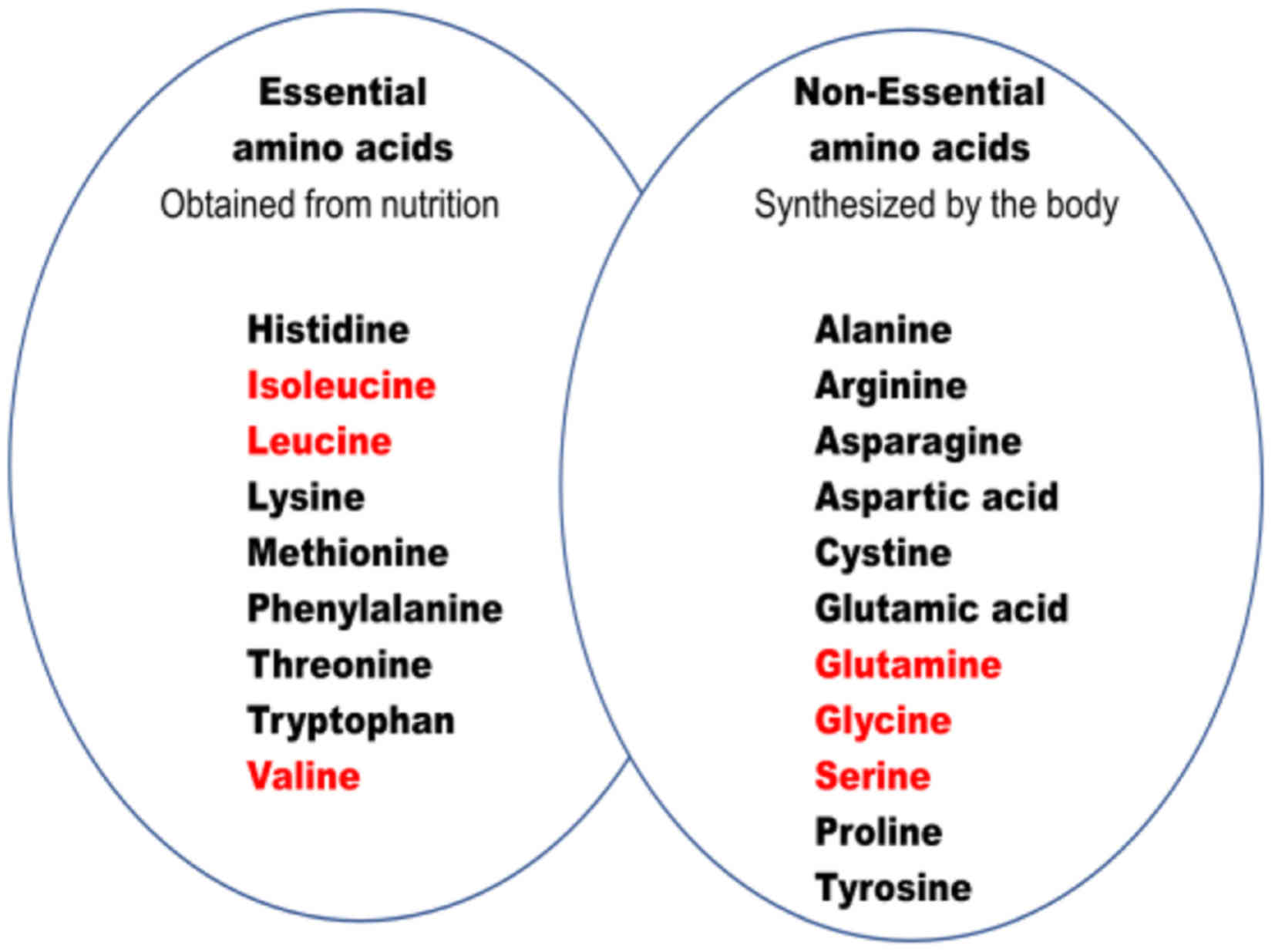
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dang CV: Links between metabolism and
cancer. Genes Dev. 26:877–890. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Newmeyer DD and Ferguson-Miller S:
Mitochondria: Releasing power for life and unleashing the
machineries of death. Cell. 112:481–490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
5
|
Detmer SA and Chan DC: Functions and
dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol.
8:870–879. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
McBride HM, Neuspiel M and Wasiak S:
Mitochondria: More than just a powerhouse. Curr Biol. 16:R551–R560.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wallace DC: Mitochondria and cancer. Nat
Rev Cancer. 12:685–698. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weinberg SE and Chandel NS: Targeting
mitochondria metabolism for cancer therapy. Nat Chem Biol. 11:9–15.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wen S, Zhu D and Huang P: Targeting cancer
cell mitochondria as a therapeutic approach. Future Med Chem.
5:53–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang F, Ogasawara MA and Huang P: Small
mitochondria-targeting molecules as anti-cancer agents. Mol Aspects
Med. 31:75–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carew JS and Huang P: Mitochondrial
defects in cancer. Mol Cancer. 1:92002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DeBerardinis RJ: Is cancer a disease of
abnormal cellular metabolism? New angles on an old idea. Genet Med.
10:767–777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seyfried TN and Shelton LM: Cancer as a
metabolic disease. Nutr Metab (Lond). 7:72010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pelicano H, Martin DS, Xu RH and Huang P:
Glycolysis inhibition for anticancer treatment. Oncogene.
25:4633–4646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niederacher D and Entian KD:
Characterization of Hex2 protein, a negative regulatory element
necessary for glucose repression in yeast. FEBS J. 200:311–319.
1991.
|
|
18
|
Herrero P, Galíndez J, Ruiz N,
Martínez-Campa C and Moreno F: Transcriptional regulation of the
Saccharomyces cerevisiae HXK1, HXK2 and GLK1 genes. Yeast.
11:137–144. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rempel A, Mathupala SP, Griffin CA,
Hawkins AL and Pedersen PL: Glucose catabolism in cancer cells:
Amplification of the gene encoding type II hexokinase. Cancer Res.
56:2468–2471. 1996.PubMed/NCBI
|
|
20
|
Bustamante E and Pedersen PL: High aerobic
glycolysis of rat hepatoma cells in culture: Role of mitochondrial
hexokinase. Proc Natl Acad Sci USA. 74:pp. 3735–3739. 1977;
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Bacha T, de Freitas MS and Sola-Penna
M: Cellular distribution of phosphofructokinase activity and
implications to metabolic regulation in human breast cancer. Mol
Genet Metab. 79:294–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zancan P, Sola-Penna M, Furtado CM and Da
Silva D: Differential expression of phosphofructokinase-1 isoforms
correlates with the glycolytic efficiency of breast cancer cells.
Mol Genet Metab. 100:372–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clem BF, O'Neal J, Tapolsky G, Clem AL,
Imbert-Fernandez Y, Kerr DA II, Klarer AC, Redman R, Miller DM,
Trent JO, et al: Targeting 6-phosphofructo-2-kinase (PFKFB3) as a
therapeutic strategy against cancer. Mol Cancer Ther. 12:1461–1470.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Atsumi T, Chesney J, Metz C, Leng L,
Donnelly S, Makita Z, Mitchell R and Bucala R: High expression of
inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase
(iPFK-2; PFKFB3) in human cancers. Cancer Res. 62:5881–5887.
2002.PubMed/NCBI
|
|
25
|
Moon JS, Jin WJ, Kwak JH, Kim HJ, Yun MJ,
Kim JW, Park SW and Kim KS: Androgen stimulates glycolysis for de
novo lipid synthesis by increasing the activities of hexokinase 2
and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2 in
prostate cancer cells. Biochem J. 433:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okar DA, Manzano A, Navarro-Sabatè A,
Riera L, Bartrons R and Lange AJ: PFK-2/FBPase-2: Maker and breaker
of the essential biofactor fructose-2,6-bisphosphate. Trends
Biochem Sci. 26:30–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li C, Xiao Z, Chen Z, Zhang X, Li J, Wu X,
Li X, Yi H, Li M, Zhu G, et al: Proteome analysis of human lung
squamous carcinoma. Proteomics. 6:547–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tokunaga K, Nakamura Y, Sakata K, Fujimori
K, Ohkubo M, Sawada K and Sakiyama S: Enhanced expression of a
glyceraldehyde-3-phosphate dehydrogenase gene in human lung
cancers. Cancer Res. 47:5616–5619. 1987.PubMed/NCBI
|
|
29
|
Schek N, Hall BL and Finn OJ: Increased
glyceraldehyde-3-phosphate dehydrogenase gene expression in human
pancreatic adenocarcinoma. Cancer Res. 48:6354–6359.
1988.PubMed/NCBI
|
|
30
|
Epner DE, Partin AW, Schalken JA, Isaacs
JT and Coffey DS: Association of glyceraldehyde-3-phosphate
dehydrogenase expression with cell motility and metastatic
potential of rat prostatic adenocarcinoma. Cancer Res.
53:1995–1997. 1993.PubMed/NCBI
|
|
31
|
Krasnov GS, Dmitriev AA, Snezhkina AV and
Kudryavtseva AV: Deregulation of glycolysis in cancer:
Glyceraldehyde-3-phosphate dehydrogenase as a therapeutic target.
Expert Opin Ther Targets. 17:681–693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng C, Gao Y, Wang C, Yu X, Zhang W, Guan
H, Shan Z and Teng W: Aberrant overexpression of pyruvate kinase M2
is associated with aggressive tumor features and the BRAF mutation
in papillary thyroid cancer. J Clin Endocrinol Metab.
98:E1524–E1533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Azoitei N, Becher A, Steinestel K, Rouhi
A, Diepold K, Genze F, Simmet T and Seufferlein T: PKM2 promotes
tumor angiogenesis by regulating HIF-1α through NF-κB activation.
Mol Cancer. 15:32016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu W, Cao Y, Zhang Y, Li S, Gao J, Wang
XA, Mu J, Hu YP, Jiang L, Dong P, et al: Up-regulation of PKM2
promote malignancy and related to adverse prognostic risk factor in
human gallbladder cancer. Sci Rep. 6:263512016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wittwer JA, Robbins D, Wang F, Codarin S,
Shen X, Kevil CG, Huang TT, Van Remmen H, Richardson A and Zhao Y:
Enhancing mitochondrial respiration suppresses tumor promoter
TPA-induced PKM2 expression and cell transformation in skin
epidermal JB6 cells. Cancer Prev Res (Phila). 4:1476–1484. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Le A, Cooper CR, Gouw AM, Dinavahi R,
Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL and Dang
CV: Inhibition of lactate dehydrogenase A induces oxidative stress
and inhibits tumor progression. Proc Natl Acad Sci USA. 107:pp.
2037–2042. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Linnane AW, Marzuki S, Ozawa T and Tanaka
M: Mitochondrial DNA mutations as an important contributor to
ageing and degenerative diseases. Lancet. 1:642–645. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taylor RW and Turnbull DM: Mitochondrial
DNA mutations in human disease. Nat Rev Genet. 6:389–402. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fliss MS, Usadel H, Caballero OL, Wu L,
Buta MR, Eleff SM, Jen J and Sidransky D: Facile detection of
mitochondrial DNA mutations in tumors and bodily fluids. Science.
287:2017–2019. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cardaci S and Ciriolo MR: TCA cycle
defects and cancer: When metabolism tunes redox state. Int J Cell
Biol. 2012:1618372012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rustin P, Bourgeron T, Parfait B, Chretien
D, Munnich A and Rötig A: Inborn errors of the Krebs cycle: A group
of unusual mitochondrial diseases in human. Biochim Biophys Acta.
1361:185–197. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Singh KK, Desouki MM, Franklin RB and
Costello LC: Mitochondrial aconitase and citrate metabolism in
malignant and nonmalignant human prostate tissues. Mol Cancer.
5:142006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Parsons DW, Jones S, Zhang X, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et
al: An integrated genomic analysis of human glioblastoma
multiforme. Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yan H, Parsons DW, Jin G, McLendon R,
Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ,
et al: IDH1 and IDH2 mutations in gliomas. N Engl J Med.
360:765–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dang L, White DW, Gross S, Bennett BD,
Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et
al: Cancer-associated IDH1 mutations produce 2-hydroxyglutarate.
Nature. 462:739–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Toro JR, Nickerson ML, Wei MH, Warren MB,
Glenn GM, Turner ML, Stewart L, Duray P, Tourre O, Sharma N, et al:
Mutations in the fumarate hydratase gene cause hereditary
leiomyomatosis and renal cell cancer in families in North America.
Am J Hum Genet. 73:95–106. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen YB, Brannon AR, Toubaji A, Dudas ME,
Won HH, Al-Ahmadie HA, Fine SW, Gopalan A, Frizzell N, Voss MH, et
al: Hereditary leiomyomatosis and renal cell carcinoma
syndrome-associated renal cancer: Recognition of the syndrome by
pathologic features and the utility of detecting aberrant
succination by immunohistochemistry. Am J Surg Pathol. 38:627–637.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Frezza C, Zheng L, Folger O, Rajagopalan
KN, MacKenzie ED, Jerby L, Micaroni M, Chaneton B, Adam J, Hedley
A, et al: Haem oxygenase is synthetically lethal with the tumour
suppressor fumarate hydratase. Nature. 477:225–228. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gaude E and Frezza C: Defects in
mitochondrial metabolism and cancer. Cancer Metab. 2:102014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Neumann HP, Pawlu C, Pęczkowska M, Bausch
B, McWhinney SR, Muresan M, Buchta M, Franke G, Klisch J, Bley TA,
et al: European-American Paraganglioma Study Group: Distinct
clinical features of paraganglioma syndromes associated with SDHB
and SDHD gene mutations. JAMA. 292:943–951. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pollard PJ, Wortham NC and Tomlinson IP:
The TCA cycle and tumorigenesis: The examples of fumarate hydratase
and succinate dehydrogenase. Ann Med. 35:632–639. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pollard PJ, Brière JJ, Alam NA, Barwell J,
Barclay E, Wortham NC, Hunt T, Mitchell M, Olpin S, Moat SJ, et al:
Accumulation of Krebs cycle intermediates and over-expression of
HIF1α in tumours which result from germline FH and SDH mutations.
Hum Mol Genet. 14:2231–2239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Habano W, Sugai T, Nakamura S, Uesugi N,
Higuchi T, Terashima M and Horiuchi S: Reduced expression and loss
of heterozygosity of the SDHD gene in colorectal and gastric
cancer. Oncol Rep. 10:1375–1380. 2003.PubMed/NCBI
|
|
54
|
Selak MA, Armour SM, MacKenzie ED,
Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB
and Gottlieb E: Succinate links TCA cycle dysfunction to
oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell.
7:77–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Patra KC and Hay N: The pentose phosphate
pathway and cancer. Trends Biochem Sci. 39:347–354. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deberardinis RJ, Sayed N, Ditsworth D and
Thompson CB: Brick by brick: Metabolism and tumor cell growth. Curr
Opin Genet Dev. 18:54–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Riganti C, Gazzano E, Polimeni M, Aldieri
E and Ghigo D: The pentose phosphate pathway: An antioxidant
defense and a crossroad in tumor cell fate. Free Radic Biol Med.
53:421–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jiang P, Du W and Wu M: Regulation of the
pentose phosphate pathway in cancer. Protein Cell. 5:592–602. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jonas SK, Benedetto C, Flatman A, Hammond
RH, Micheletti L, Riley C, Riley PA, Spargo DJ, Zonca M and Slater
TF: Increased activity of 6-phosphogluconate dehydrogenase and
glucose-6-phosphate dehydrogenase in purified cell suspensions and
single cells from the uterine cervix in cervical intraepithelial
neoplasia. Br J Cancer. 66:185–191. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lucarelli G, Galleggiante V, Rutigliano M,
Sanguedolce F, Cagiano S, Bufo P, Lastilla G, Maiorano E, Ribatti
D, Giglio A, et al: Metabolomic profile of glycolysis and the
pentose phosphate pathway identifies the central role of
glucose-6-phosphate dehydrogenase in clear cell-renal cell
carcinoma. Oncotarget. 6:13371–13386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
D'Alessandro A, Amelio I, Berkers CR,
Antonov A, Vousden KH, Melino G and Zolla L: Metabolic effect of
TAp63α: Enhanced glycolysis and pentose phosphate pathway,
resulting in increased antioxidant defense. Oncotarget.
5:7722–7733. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sukhatme VP and Chan B: Glycolytic cancer
cells lacking 6-phosphogluconate dehydrogenase metabolize glucose
to induce senescence. FEBS Lett. 586:2389–2395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nishimura M and Uyeda K: Purification and
characterization of a novel xylulose 5-phosphate-activated protein
phosphatase catalyzing dephosphorylation of
fructose-6-phosphate,2-kinase:fructose-2,6-bisphosphatase. J Biol
Chem. 270:26341–26346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wise DR and Thompson CB: Glutamine
addiction: A new therapeutic target in cancer. Trends Biochem Sci.
35:427–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
DeBerardinis RJ and Cheng T: Q's next: The
diverse functions of glutamine in metabolism, cell biology and
cancer. Oncogene. 29:313–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dang CV: Glutaminolysis: Supplying carbon
or nitrogen or both for cancer cells? Cell Cycle. 9:3884–3886.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Altman BJ, Stine ZE and Dang CV: From
Krebs to clinic: Glutamine metabolism to cancer therapy. Nat Rev
Cancer. 16:619–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hensley CT, Wasti AT and DeBerardinis RJ:
Glutamine and cancer: Cell biology, physiology, and clinical
opportunities. J Clin Invest. 123:3678–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wise DR, DeBerardinis RJ, Mancuso A, Sayed
N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon
SB, et al: Myc regulates a transcriptional program that stimulates
mitochondrial glutaminolysis and leads to glutamine addiction. Proc
Natl Acad Sci USA. 105:pp. 18782–18787. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Stepulak A, Luksch H, Gebhardt C,
Uckermann O, Marzahn J, Sifringer M, Rzeski W, Staufner C, Brocke
KS, Turski L, et al: Expression of glutamate receptor subunits in
human cancers. Histochem Cell Biol. 132:435–445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Durán RV, Oppliger W, Robitaille AM,
Heiserich L, Skendaj R, Gottlieb E and Hall MN: Glutaminolysis
activates Rag-mTORC1 signaling. Mol Cell. 47:349–358. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jain M, Nilsson R, Sharma S, Madhusudhan
N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB and Mootha
VK: Metabolite profiling identifies a key role for glycine in rapid
cancer cell proliferation. Science. 336:1040–1044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Amelio I, Cutruzzolá F, Antonov A,
Agostini M and Melino G: Serine and glycine metabolism in cancer.
Trends Biochem Sci. 39:191–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hasegawa S, Ichiyama T, Sonaka I, Ohsaki
A, Okada S, Wakiguchi H, Kudo K, Kittaka S, Hara M and Furukawa S:
Cysteine, histidine and glycine exhibit anti-inflammatory effects
in human coronary arterial endothelial cells. Clin Exp Immunol.
167:269–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Alarcon-Aguilar FJ, Almanza-Perez J,
Blancas G, Angeles S, Garcia-Macedo R, Roman R and Cruz M: Glycine
regulates the production of pro-inflammatory cytokines in lean and
monosodium glutamate-obese mice. Eur J Pharmacol. 599:152–158.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cruz M, Maldonado-Bernal C,
Mondragón-Gonzalez R, Sanchez-Barrera R, Wacher NH,
Carvajal-Sandoval G and Kumate J: Glycine treatment decreases
proinflammatory cytokines and increases interferon-γ in patients
with type 2 diabetes. J Endocrinol Invest. 31:694–699. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang WC, Shyh-Chang N, Yang H, Rai A,
Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, et al: Glycine
decarboxylase activity drives non-small cell lung cancer
tumor-initiating cells and tumorigenesis. Cell. 148:259–272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Locasale JW: Serine, glycine and
one-carbon units: Cancer metabolism in full circle. Nat Rev Cancer.
13:572–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Possemato R, Marks KM, Shaul YD, Pacold
ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, et
al: Functional genomics reveal that the serine synthesis pathway is
essential in breast cancer. Nature. 476:346–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Locasale JW, Grassian AR, Melman T,
Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen
T, Sharfi H, et al: Phosphoglycerate dehydrogenase diverts
glycolytic flux and contributes to oncogenesis. Nat Genet.
43:869–874. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mattaini KR, Sullivan MR and Vander Heiden
MG: The importance of serine metabolism in cancer. J Cell Biol.
214:249–257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Baenke F, Peck B, Miess H and Schulze A:
Hooked on fat: The role of lipid synthesis in cancer metabolism and
tumour development. Dis Model Mech. 6:1353–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Santos CR and Schulze A: Lipid metabolism
in cancer. FEBS J. 279:2610–2623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Currie E, Schulze A, Zechner R, Walther TC
and Farese RV Jr: Cellular fatty acid metabolism and cancer. Cell
Metab. 18:153–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Vance JE and Vance DE: Biochemistry of
lipids, lipoproteins and membranes. Elsevier; Amsterdam: 2002,
View Article : Google Scholar
|
|
87
|
Bauer DE, Hatzivassiliou G, Zhao F,
Andreadis C and Thompson CB: ATP citrate lyase is an important
component of cell growth and transformation. Oncogene.
24:6314–6322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Qian X, Hu J, Zhao J and Chen H: ATP
citrate lyase expression is associated with advanced stage and
prognosis in gastric adenocarcinoma. Int J Clin Exp Med.
8:7855–7860. 2015.PubMed/NCBI
|
|
89
|
Xin M, Qiao Z, Li J, Liu J, Song S, Zhao
X, Miao P, Tang T, Wang L, Liu W, et al: miR-22 inhibits tumor
growth and metastasis by targeting ATP citrate lyase: Evidence in
osteosarcoma, prostate cancer, cervical cancer and lung cancer.
Oncotarget. 7:44252–44265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lucenay KS, Doostan I, Karakas C, Bui T,
Ding Z, Mills GB, Hunt KK and Keyomarsi K: Cyclin E associates with
the lipogenic enzyme ATP-citrate lyase to enable malignant growth
of breast cancer cells. Cancer Res. 76:2406–2418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Su YW, Lin YH, Pai MH, Lo AC, Lee YC, Fang
IC, Lin J, Hsieh RK, Chang YF and Chen CL: Association between
phosphorylated AMP-activated protein kinase and acetyl-CoA
carboxylase expression and outcome in patients with squamous cell
carcinoma of the head and neck. PLoS One. 9:e961832014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang MD, Wu H, Fu GB, Zhang HL, Zhou X,
Tang L, Dong LW, Qin CJ, Huang S, Zhao LH, et al: Acetyl-coenzyme A
carboxylase alpha promotion of glucose-mediated fatty acid
synthesis enhances survival of hepatocellular carcinoma in mice and
patients. Hepatology. 63:1272–1286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bauerschlag DO, Maass N, Leonhardt P,
Verburg FA, Pecks U, Zeppernick F, Morgenroth A, Mottaghy FM, Tolba
R, Meinhold-Heerlein I, et al: Fatty acid synthase overexpression:
Target for therapy and reversal of chemoresistance in ovarian
cancer. J Transl Med. 13:1462015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ogino S, Kawasaki T, Ogawa A, Kirkner GJ,
Loda M and Fuchs CS: Fatty acid synthase overexpression in
colorectal cancer is associated with microsatellite instability,
independent of CpG island methylator phenotype. Hum Pathol.
38:842–849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gong J, Shen S, Yang Y, Qin S, Huang L,
Zhang H, Chen L, Chen Y, Li S, She S, et al: Inhibition of FASN
suppresses migration, invasion and growth in hepatoma carcinoma
cells by deregulating the HIF-1α/IGFBP1 pathway. Int J Oncol.
50:883–892. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Carracedo A, Cantley LC and Pandolfi PP:
Cancer metabolism: Fatty acid oxidation in the limelight. Nat Rev
Cancer. 13:227–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ito K and Suda T: Metabolic requirements
for the maintenance of self-renewing stem cells. Nat Rev Mol Cell
Biol. 15:243–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zaugg K, Yao Y, Reilly PT, Kannan K,
Kiarash R, Mason J, Huang P, Sawyer SK, Fuerth B, Faubert B, et al:
Carnitine palmitoyltransferase 1C promotes cell survival and tumor
growth under conditions of metabolic stress. Genes Dev.
25:1041–1051. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
McGarry JD and Brown NF: The mitochondrial
carnitine palmitoyltransferase system. From concept to molecular
analysis. Eur J Biochem. 244:1–14. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Coller HA: Is cancer a metabolic disease?
Am J Pathol. 184:4–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tan DJ, Bai RK and Wong LJ: Comprehensive
scanning of somatic mitochondrial DNA mutations in breast cancer.
Cancer Res. 62:972–976. 2002.PubMed/NCBI
|
|
102
|
Liu VW, Shi HH, Cheung AN, Chiu PM, Leung
TW, Nagley P, Wong LC and Ngan HY: High incidence of somatic
mitochondrial DNA mutations in human ovarian carcinomas. Cancer
Res. 61:5998–6001. 2001.PubMed/NCBI
|
|
103
|
Richard SM, Bailliet G, Páez GL, Bianchi
MS, Peltomäki P and Bianchi NO: Nuclear and mitochondrial genome
instability in human breast cancer. Cancer Res. 60:4231–4237.
2000.PubMed/NCBI
|
|
104
|
Ishikawa K, Takenaga K, Akimoto M,
Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y and Hayashi
J: ROS-generating mitochondrial DNA mutations can regulate tumor
cell metastasis. Science. 320:661–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Swalwell H, Kirby DM, Blakely EL, Mitchell
A, Salemi R, Sugiana C, Compton AG, Tucker EJ, Ke BX, Lamont PJ, et
al: Respiratory chain complex I deficiency caused by mitochondrial
DNA mutations. Eur J Hum Genet. 19:769–775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kwong JQ, Henning MS, Starkov AA and
Manfredi G: The mitochondrial respiratory chain is a modulator of
apoptosis. J Cell Biol. 179:1163–1177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Osellame LD, Blacker TS and Duchen MR:
Cellular and molecular mechanisms of mitochondrial function. Best
Pract Res Clin Endocrinol Metab. 26:711–723. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shen YH, Wang XL and Wilcken DE: Nitric
oxide induces and inhibits apoptosis through different pathways.
FEBS Lett. 433:125–131. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Seiler N and Raul F: Polyamines and
apoptosis. J Cell Mol Med. 9:623–642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Agostinelli E, Tempera G, Molinari A,
Salvi M, Battaglia V, Toninello A and Arancia G: The physiological
role of biogenic amines redox reactions in mitochondria. New
perspectives in cancer therapy. Amino Acids. 33:175–187. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Grancara S, Ohkubo S, Artico M,
Ciccariello M, Manente S, Bragadin M, Toninello A and Agostinelli
E: Milestones and recent discoveries on cell death mediated by
mitochondria and their interactions with biologically active
amines. Amino Acids. 48:2313–2326. 2016. View Article : Google Scholar : PubMed/NCBI
|