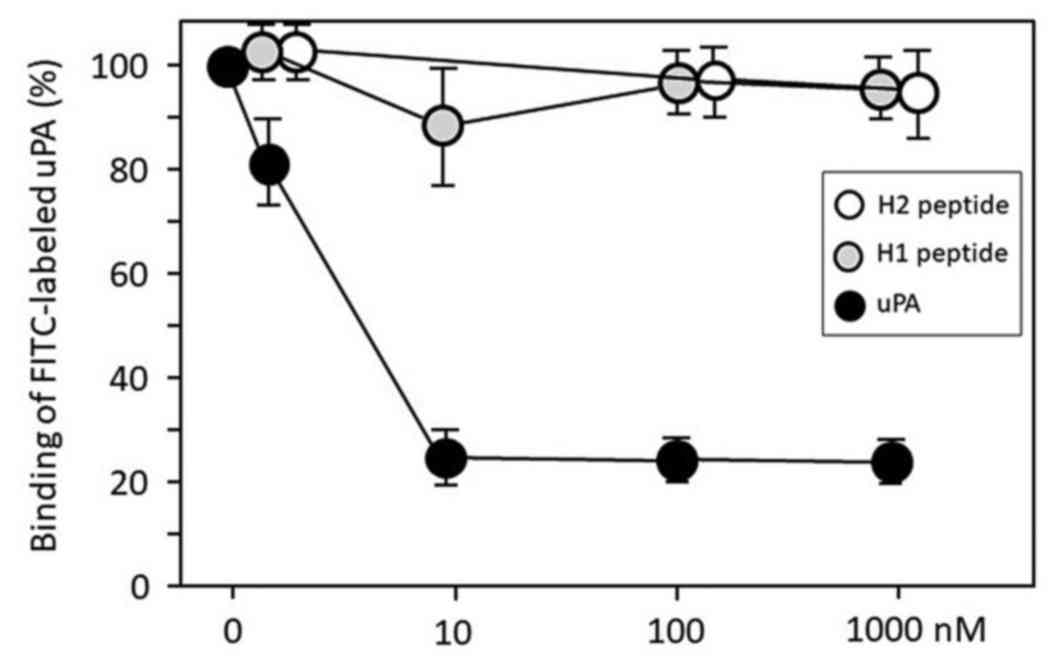
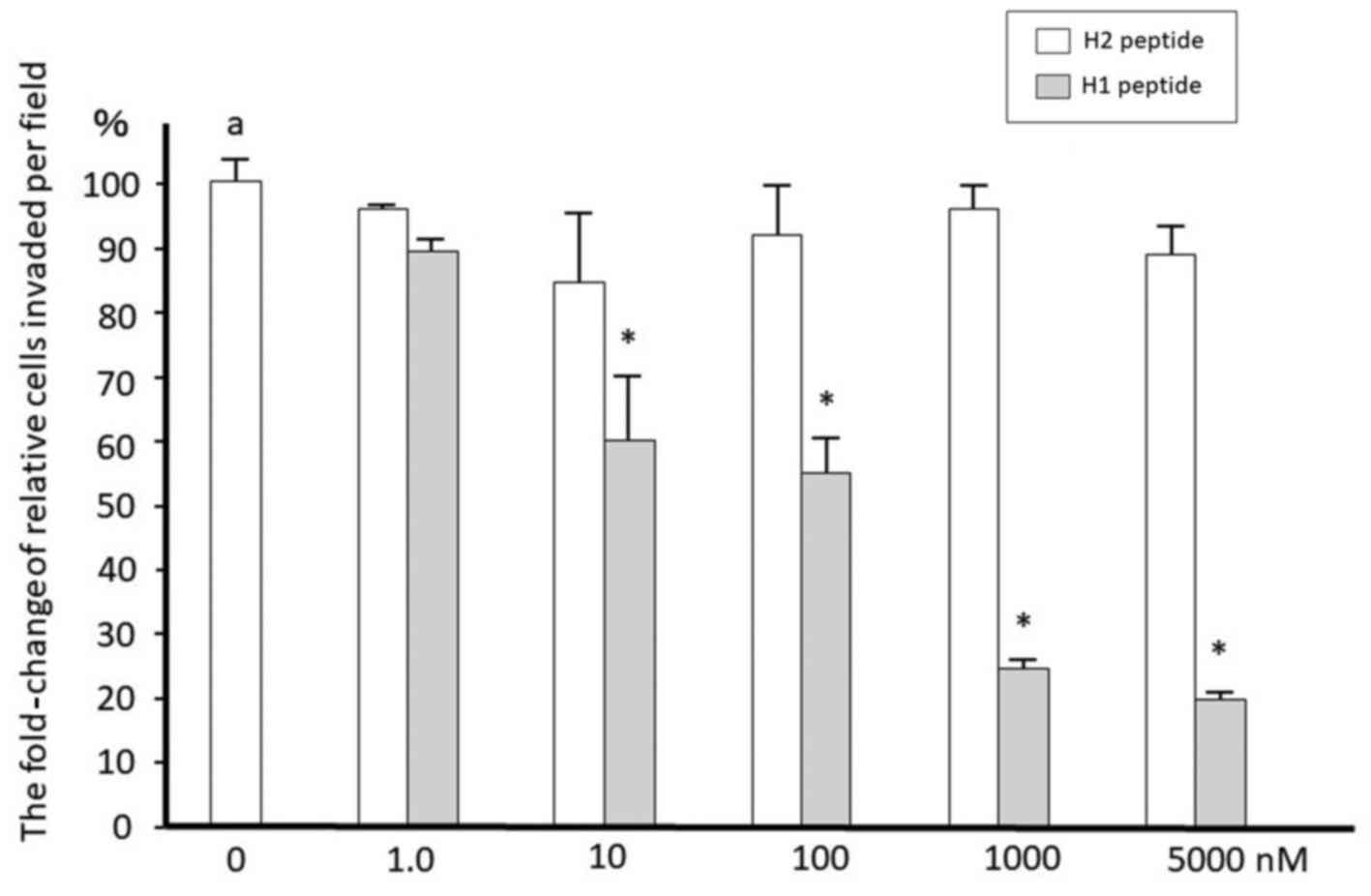
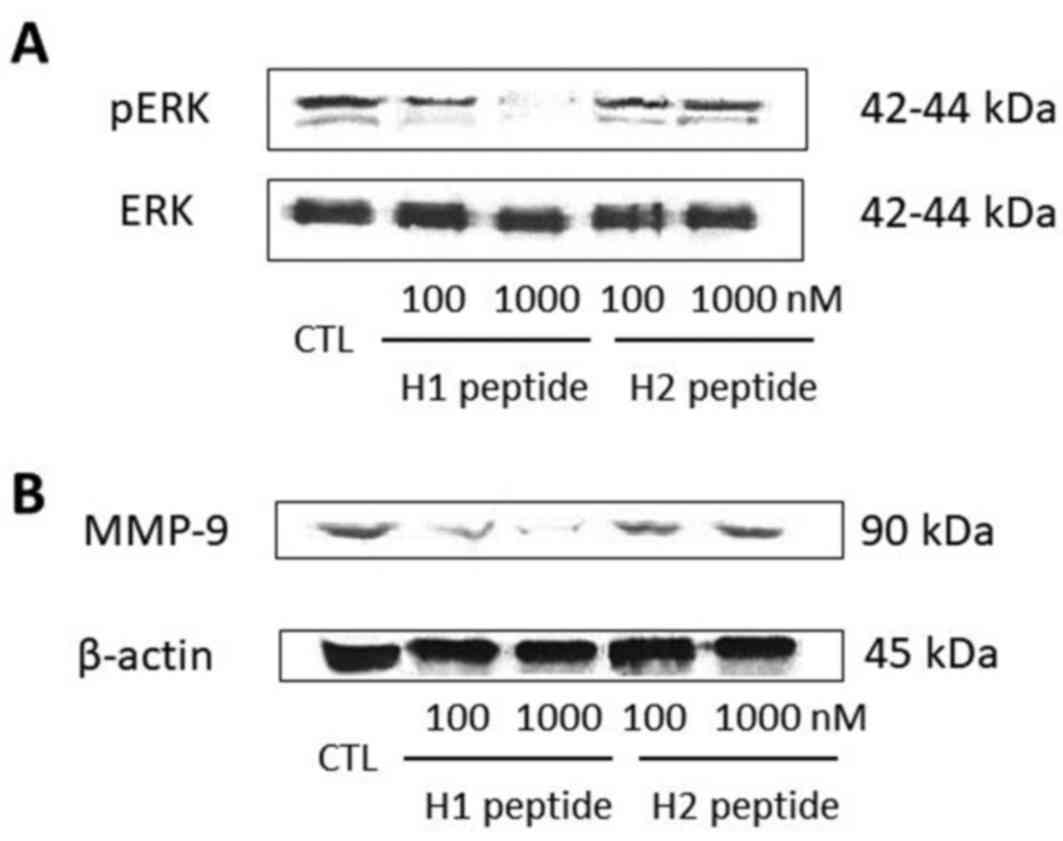
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bifulco K, Votta G, Ingangi V, Di
Carluccio G, Rea D, Losito S, Montuori N, Ragno P, Stoppelli MP,
Arra C, et al: Urokinase receptor promotes ovarian cancer cell
dissemination through its 84–95 sequence. Oncotarget. 5:4154–4169.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noh H, Hong S and Huang S: Role of
urokinase receptor in tumor progression and development.
Theranostics. 3:487–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmad A, Kong D, Sarkar SH, Wang Z,
Banerjee S and Sarkar FH: Inactivation of uPA and its receptor uPAR
by 3,3′-diindolylmethane (DIM) leads to the inhibition of prostate
cancer cell growth and migration. J Cell Biochem. 107:516–527.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gondi CS, Lakka SS, Dinh DH, Olivero WC,
Gujrati M and Rao JS: Downregulation of uPA, uPAR and MMP-9 using
small, interfering, hairpin RNA (siRNA) inhibits glioma cell
invasion, angiogenesis and tumor growth. Neuron Glia Biol.
1:165–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghamande SA, Silverman MH, Huh W, Behbakht
K, Ball G, Cuasay L, Würtz SO, Brunner N and Gold MA: A phase 2,
randomized, double-blind, placebo-controlled trial of clinical
activity and safety of subcutaneous A6 in women with asymptomatic
CA125 progression after first-line chemotherapy of epithelial
ovarian cancer. Gynecol Oncol. 111:89–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi H, Ohi H, Sugimura M, Shinohara
H, Fujii T and Terao T: Inhibition of in vitro ovarian cancer cell
invasion by modulation of urokinase-type plasminogen activator and
cathepsin B. Cancer Res. 52:3610–3614. 1992.PubMed/NCBI
|
|
8
|
Towle MJ, Lee A, Maduakor EC, Schwartz CE,
Bridges AJ and Littlefield BA: Inhibition of urokinase by
4-substituted benzo[b]thiophene-2-carboxamidines: An important new
class of selective synthetic urokinase inhibitor. Cancer Res.
53:2553–2559. 1993.PubMed/NCBI
|
|
9
|
Todaro LB, Ladeda V, de Bal Kier Joffé E
and Farías EF: Combined treatment with verapamil, a calcium channel
blocker, and B428, a synthetic uPA inhibitor, impairs the
metastatic ability of a murine mammary carcinoma. Oncol Rep.
10:725–732. 2003.PubMed/NCBI
|
|
10
|
Ertongur S, Lang S, Mack B, Wosikowski K,
Muehlenweg B and Gires O: Inhibition of the invasion capacity of
carcinoma cells by WX-UK1, a novel synthetic inhibitor of the
urokinase-type plasminogen activator system. Int J Cancer.
110:815–824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Evans DM and Sloan-Stakleff K: Suppression
of the invasive capacity of human breast cancer cells by inhibition
of urokinase plasminogen activator via amiloride and B428. Am Surg.
66:460–464. 2000.PubMed/NCBI
|
|
12
|
Henneke I, Greschus S, Savai R, Korfei M,
Markart P, Mahavadi P, Schermuly RT, Wygrecka M, Stürzebecher J and
Seeger W: Inhibition of urokinase activity reduces primary tumor
growth and metastasis formation in a murine lung carcinoma model.
Am J Respir Crit Care Med. 181:611–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lund IK, Illemann M, Thurison T,
Christensen IJ and Høyer-Hansen G: uPAR as anti-cancer target:
Evaluation of biomarker potential, histological localization, and
antibody-based therapy. Curr Drug Targets. 12:1744–1760. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohanam S, Chandrasekar N, Yanamandra N,
Khawar S, Mirza F, Dinh DH, Olivero WC and Rao JS: Modulation of
invasive properties of human glioblastoma cells stably expressing
amino-terminal fragment of urokinase-type plasminogen activator.
Oncogene. 21:7824–7830. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bürgle M, Koppitz M, Riemer C, Kessler H,
König B, Weidle UH, Kellermann J, Lottspeich F, Graeff H and
Schmitt M: Inhibition of the interaction of urokinase-type
plasminogen activator (uPA) with its receptor (uPAR) by synthetic
peptides. Biol Chem. 378:231–237. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato S, Kopitz C, Schmalix WA, Muehlenweg
B, Kessler H, Schmitt M, Krüger A and Magdolen V: High-affinity
urokinase-derived cyclic peptides inhibiting urokinase/urokinase
receptor-interaction: effects on tumor growth and spread. FEBS
Lett. 528:212–216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mani T, Wang F, Knabe WE, Sinn AL, Khanna
M, Jo I, Sandusky GE, Sledge GW Jr, Jones DR and Khanna R:
Small-molecule inhibition of the uPAR·uPA interaction: Synthesis,
biochemical, cellular, in vivo pharmacokinetics and efficacy
studies in breast cancer metastasis. Bioorg Med Chem. 21:2145–2155.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilhelm O, Weidle U, Höhl S, Rettenberger
P, Schmitt M and Graeff H: Recombinant soluble urokinase receptor
as a scavenger for urokinase-type plasminogen activator (uPA).
Inhibition of proliferation and invasion of human ovarian cancer
cells. FEBS Lett. 337:131–134. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kobayashi H, Gotoh J, Fujie M, Shinohara
H, Moniwa N and Terao T: Inhibition of metastasis of Lewis lung
carcinoma by a synthetic peptide within growth factor-like domain
of urokinase in the experimental and spontaneous metastasis model.
Int J Cancer. 57:727–733. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Min HY, Doyle LV, Vitt CR, Zandonella CL,
Stratton-Thomas JR, Shuman MA and Rosenberg S: Urokinase receptor
antagonists inhibit angiogenesis and primary tumor growth in
syngeneic mice. Cancer Res. 56:2428–2433. 1996.PubMed/NCBI
|
|
21
|
Weidle UH and König B: Urokinase receptor
antagonists: Novel agents for the treatment of cancer. Expert Opin
Investig Drugs. 7:391–403. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo Y, Higazi AA, Arakelian A, Sachais BS,
Cines D, Goldfarb RH, Jones TR, Kwaan H, Mazar AP and Rabbani SA: A
peptide derived from the nonreceptor binding region of urokinase
plasminogen activator (uPA) inhibits tumor progression and
angiogenesis and induces tumor cell death in vivo. FASEB J.
14:1400–1410. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Soria C, Griscelli F, Opolon P,
Soria J, Yeh P, Legrand C, Vannier JP, Belin D and Perricaudet M:
Amino-terminal fragment of urokinase inhibits tumor cell invasion
in vitro and in vivo: Respective contribution of the urokinase
plasminogen activator receptor-dependent or-independent pathway.
Hum Gene Ther. 16:1157–1167. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu D, Zhou D, Wang B, Knabe WE and
Meroueh SO: A new class of orthosteric uPAR·uPA small-molecule
antagonists are allosteric inhibitors of the uPAR·vitronectin
interaction. ACS Chem Biol. 10:1521–1534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khanna M, Wang F, Jo I, Knabe WE, Wilson
SM, Li L, Bum-Erdene K, Li J, W Sledge G and Khanna R: Targeting
multiple conformations leads to small molecule inhibitors of the
uPAR·uPA protein-protein interaction that block cancer cell
invasion. ACS Chem Biol. 6:1232–1243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kook YH, Adamski J, Zelent A and Ossowski
L: The effect of antisense inhibition of urokinase receptor in
human squamous cell carcinoma on malignancy. EMBO J. 13:3983–3991.
1994.PubMed/NCBI
|
|
27
|
Ahmed N, Oliva K, Wang Y, Quinn M and Rice
G: Downregulation of urokinase plasminogen activator receptor
expression inhibits Erk signalling with concomitant suppression of
invasiveness due to loss of uPAR-β1 integrin complex in colon
cancer cells. Br J Cancer. 89:374–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nozaki S, Endo Y, Nakahara H, Yoshizawa K,
Hashiba Y, Kawashiri S, Tanaka A, Nakagawa K, Matsuoka Y and Kogo
M: Inhibition of invasion and metastasis in oral cancer by
targeting urokinase-type plasminogen activator receptor. Oral
Oncol. 41:971–977. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kondraganti S, Gondi CS, McCutcheon I,
Dinh DH, Gujrati M, Rao JS and Olivero WC: RNAi-mediated
downregulation of urokinase plasminogen activator and its receptor
in human meningioma cells inhibits tumor invasion and growth. Int J
Oncol. 28:1353–1360. 2006.PubMed/NCBI
|
|
30
|
Kunigal S, Lakka SS, Gondi CS, Estes N and
Rao JS: RNAi-mediated downregulation of urokinase plasminogen
activator receptor and matrix metalloprotease-9 in human breast
cancer cells results in decreased tumor invasion, angiogenesis and
growth. Int J Cancer. 121:2307–2316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kargiotis O, Chetty C, Gogineni V, Gondi
CS, Pulukuri SM, Kyritsis AP, Gujrati M, Klopfenstein JD, Dinh DH
and Rao JS: uPA/uPAR downregulation inhibits radiation-induced
migration, invasion and angiogenesis in IOMM-Lee meningioma cells
and decreases tumor growth in vivo. Int J Oncol. 33:937–947.
2008.PubMed/NCBI
|
|
32
|
Raghu H, Gondi CS, Dinh DH, Gujrati M and
Rao JS: Specific knockdown of uPA/uPAR attenuates invasion in
glioblastoma cells and xenografts by inhibition of cleavage and
trafficking of Notch-1 receptor. Mol Cancer. 10:1302011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duriseti S, Goetz DH, Hostetter DR, LeBeau
AM, Wei Y and Craik CS: Antagonistic anti-urokinase plasminogen
activator receptor (uPAR) antibodies significantly inhibit
uPAR-mediated cellular signaling and migration. J Biol Chem.
285:26878–26888. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mohanam S, Sawaya R, McCutcheon I,
Ali-Osman F, Boyd D and Rao JS: Modulation of in vitro invasion of
human glioblastoma cells by urokinase-type plasminogen activator
receptor antibody. Cancer Res. 53:4143–4147. 1993.PubMed/NCBI
|
|
35
|
Pass J, Jögi A, Lund IK, Rønø B, Rasch MG,
Gårdsvoll H, Lund LR, Ploug M, Rømer J and Danø K: Murine
monoclonal antibodies against murine uPA receptor produced in
gene-deficient mice: Inhibitory effects on receptor-mediated uPA
activity in vitro and in vivo. Thromb Haemost. 97:1013–1022.
2007.PubMed/NCBI
|
|
36
|
Nowicki TS, Kummer NT, Iacob C, Suslina N,
Schaefer S, Schantz S, Shin E, Moscatello AL, Tiwari RK and
Geliebter J: Inhibition of uPAR and uPA reduces invasion in
papillary thyroid carcinoma cells. Laryngoscope. 120:1383–1390.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu J, Jo M, Eastman BM, Gilder AS, Bui JD
and Gonias SL: uPAR induces expression of transforming growth
factor β and interleukin-4 in cancer cells to promote
tumor-permissive conditioning of macrophages. Am J Pathol.
184:3384–3393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ragno P: The urokinase receptor: A ligand
or a receptor? Story of a sociable molecule. Cell Mol Life Sci.
63:1028–1037. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wei Y, Tang CH, Kim Y, Robillard L, Zhang
F, Kugler MC and Chapman HA: Urokinase receptors are required for
alpha 5 beta 1 integrin-mediated signaling in tumor cells. J Biol
Chem. 282:3929–3939. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Carriero MV, Del Vecchio S, Capozzoli M,
Franco P, Fontana L, Zannetti A, Botti G, D'Aiuto G, Salvatore M
and Stoppelli MP: Urokinase receptor interacts with alpha(v)beta5
vitronectin receptor, promoting urokinase-dependent cell migration
in breast cancer. Cancer Res. 59:5307–5314. 1999.PubMed/NCBI
|
|
41
|
Tang CH, Hill ML, Brumwell AN, Chapman HA
and Wei Y: Signaling through urokinase and urokinase receptor in
lung cancer cells requires interactions with beta1 integrins. J
Cell Sci. 121:3747–3756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsuji S, Kasumi T, Nagase K, Yoshikawa E,
Kobayashi H and Kurita N: The effects of amino-acid mutations on
specific interactions between urokinase-type plasminogen activator
and its receptor: Ab initio molecular orbital calculations. J Mol
Graph Model. 29:975–984. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kasumi T, Araki K, Ohyama T, Tsuji S,
Yoshikawa E, Kobayashi H and Kurita N: The effects of vitronectin
on specific interactions between urokinase-type plasminogen
activator and its receptor: Ab initio molecular orbital
calculations. Mol Simul. 39:769–779. 2013. View Article : Google Scholar
|
|
44
|
Ploug M, Østergaard S, Gårdsvoll H,
Kovalski K, Holst-Hansen C, Holm A, Ossowski L and Danø K:
Peptide-derived antagonists of the urokinase receptor. affinity
maturation by combinatorial chemistry, identification of functional
epitopes and inhibitory effect on cancer cell intravasation.
Biochemistry. 40:12157–12168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Luther T, Magdolen V, Albrecht S, Kasper
M, Riemer C, Kessler H, Graeff H, Müller M and Schmitt M:
Epitope-mapped monoclonal antibodies as tools for functional and
morphological analyses of the human urokinase receptor in tumor
tissue. Am J Pathol. 150:1231–1244. 1997.PubMed/NCBI
|
|
46
|
Wei Y, Eble JA, Wang Z, Kreidberg JA and
Chapman HA: Urokinase receptors promote beta1 integrin function
through interactions with integrin alpha3beta1. Mol Biol Cell.
12:2975–2986. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|