Introduction
Epithelial ovarian cancer has the highest mortality
rate among all the gynecologic tumors (1). Recent advances in molecular technologies
have made possible the creation of personalized medicine, but
peritoneal dissemination is associated with poor overall survival
of ovarian cancer patients (1–3). Despite some improvements in treatment,
current therapy is not considered curative as the mechanism
underlying the peritoneal dissemination remains unclear. To
overcome a poor clinical prognosis, understanding the molecular and
functional mechanisms involved in the ovarian cancer cell survival,
proliferation, invasion, dissemination and metastasis is of great
physiological and clinical importance.
Members of the plasminogen activator system
including urokinase-type plasminogen activator (uPA) and its
receptor uPAR are overexpressed on the surfaces of a wide range of
invasive cancer cells, including those of the prostate, brain,
breast and colon in addition to ovarian cancer (2). uPAR may be a more aggressive phenotype in
a broad range of human various aggressive cancer types, including
ovarian cancer (3). uPAR is a glycosyl
phosphatidylinositol-anchored cell surface receptor that interacts
with uPA and other molecules, such as integrins and vitronectin
(VN). Accumulating evidence shows that the uPA-uPAR system, such as
uPAR activation and its downstream signaling, is associated with
cancer progression, invasion, metastasis and peritoneal
dissemination in a variety of tumor types (2,3). uPAR
activates diverse signaling pathways, including phosphorylation of
extracellular signal-regulated kinase (ERK) and then stimulation of
matrix metalloproteinase (MMP)-9 expression (4,5).
Notably, multiple approaches to inhibit the uPA-uPAR
system may suppress cancer cell invasion and induce cell death.
Indeed, various treatment strategies including selective inhibitors
of uPA activity (6–13), specific antagonistic peptides that
block uPA-uPAR protein-protein interaction (13–24),
shRNA-uPAR-mediated silencing (5,25–32), or inhibitory antibodies against uPA or
uPAR (33–36) have been investigated in promising
preclinical and clinical trials. Although uPAR promotes cancer
progression independently of protease activation, scavenging the
active uPA or blocking its function leads to reduced tumor
progression and may show be promising for prolonging patient
survival (37). Anti-uPAR antibodies
preventing uPA binding to uPAR can potentially lead to the
suppression of cancer invasion. Functional analysis using
shRNA-uPAR transfected to downregulate uPAR revealed a critical
role for uPAR in cancer invasion and metastasis. The synthetic
peptides that prevent uPA-uPAR interaction are a promising template
for designing novel peptide-based small compounds to provide an
effective strategy to treat ovarian cancer (17).
Furthermore, uPAR interacts with not only uPA, but
also several molecules, such as VN, integrins, caveolin-1, G
protein-coupled receptors (formyl peptide receptor 2), low density
lipoprotein receptor-related protein 1 and epidermal growth factor
receptor (38,39). Integrins are the important
transmembrane binding partners associated with uPAR, leading to
integrin-mediated intracellular signaling. Integrins also serve as
VN receptors and binding of VN to integrins results in the
uPA-mediated cell invasion via uPAR (40). uPAR-integrin signaling to ERK increases
the expression of MMPs through AP1 transcription factors (39–41).
Computer simulations can provide a dry lab
experience that may fulfil some of the objectives of the in
vitro wet labs. We reported previously that structure-based
computational docking study using ab initio molecular
orbital calculations has predicted co-binding of the omega-loop of
amino-terminal fragment of uPA with the positively charged 46, 61
and 98 Lys residues of uPAR (42,43).
Molecular dynamics computer simulation by structure-based drug
design revealed that the single 9-residue amino acid peptide with
the Gly-Lys-Gly-Glu-Gly-Glu-Gly-Lys-Gly sequence (peptide H1) has a
large binding energy to uPAR that may block the binding between
uPAR and some ligands, uPA or VN (42,43). In the
present study, we synthesized H1 as a potent uPAR inhibitor and
control peptide (an amino acid sequence-shuffled peptide).
The aims of this study were to investigate whether
cancer cells depend on the function of uPAR for their invasion and
whether H1 specifically inhibits cell invasion in vitro.
Materials and methods
Materials
The materials used in the present study and the
manufacturers providing them are as following: anti-total ERK1/2,
anti-phospho-p44/42 ERK, anti-MMP-9, anti-β-actin (no. 4967) (all
from Cell Signaling Technology, Inc., Danvers, MA, USA), the
horseradish peroxidase-conjugated secondary antibodies from
Sigma-Aldrich (St. Louis, MO, USA), human uPA from Chemicon
International, Inc. (Temecula, CA, USA) and American Diagnostica
(Lexington, MA, USA) fluorescein isothiocyanate (FITC)-conjugated
uPA from Abcam (cat. no. ab9152; Cambridge, UK) and
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT)
powder from Roche Diagnostics (Indianapolis, IN, USA). Plastic ware
was purchased from Costar (Cambridge, MA, USA). The protein content
was determined using a bicinchoninic acid assay (Pierce
Biotechnology, Inc., Rockford, IL, USA). Linear peptides, H1
(Gly-Lys-Gly-Glu-Gly-Glu-Gly-Lys-Gly) and H2
(Glu-Gly-Gly-Lys-Glu-Lys-Gly-Gly-Gly; an amino acid
sequence-shuffled control peptide), were synthesized and
HPLC-purified as described (Peptide Institute, Inc., Osaka, Japan)
(44).
Flow cytometry
Promyeloid leukemia U937 cells, stimulated for 48 h
with phorbol-12 myristate-13-acetate (PMA; 1 mmol/l), were used to
investigate binding of H1 and H2 peptides to cell-associated uPAR.
Cells (2.5×105) were washed several times with
phosphate-buffered saline (PBS) supplemented with 0.1% bovine serum
albumin (BSA) and then subjected to acidic buffer (50 mmol/l
glycine/HCI, pH 3.0, for 1 min) to remove receptor-bound endogenous
uPA, followed by a neutralization step with 0.5 mol/l HEPES/NaOH,
pH 7.5 (45). The cells were
subsequently incubated with increasing amounts of H1 peptide or H2
peptide (0 or 1,000 nM) in the presence of 2 µg of FITC-labeled uPA
for 30 min. The cells were washed again with PBS/0.1% BSA. The
cell-associated fluorescence was determined.
Cell culture
Human ovarian carcinoma cell lines SKOV3
(serous-type) and TOV21G (clear cell-type) cells were purchased
from the American Type Culture Collection (Manassas, VA, USA). The
cells were cultured in Dulbecco's modified Eagle's medium/F12,
supplemented with 10% fetal bovine serum and 100 U/ml
penicillin/streptomycin (all from Invitrogen Life Technologies,
Carlsbad, CA, USA) in a humidified incubator with 5% CO2
and 95% air at 37°C.
Western blot analysis
Cells were washed 3 times with ice-cold PBS and
extracted in ice-cold lysis buffer containing 50 mM Tris-HCl (pH
8.0), 1% Nonidet 40, 0.5 mM ethylenediaminetetraacetic acid, 100
µg/ml phenylmethysulfonyl fluoride, 2 µg/ml leupeptin, 100 µm
sodium vanadate, 1 mM dithiothreitol, 1 µg/ml aprotinin and 150 mM
NaCl for 30 min. The equal amount of protein was separated on 10%
sodium dodecyl sulfate-polyacrylamide gel and then transferred to a
nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). The
membrane was incubated with phospho-ERK, total ERK, uPAR, or MMP-9
(46). Proteins were visualized using
an enhanced chemiluminescence kit (Pierce Biotechnology, Inc.). The
blots were quantified by densitometry with the Quantity One
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
MTS cell viability assay
The effects of synthetic peptides on cell viability
were estimated using an MTS assay kit (Promega Corp., Madison, WI,
USA) (47). After the H1 or H2
treatment, MTS solution was added to each well for 2 h at 37°C in
5% CO2. The absorbance at 490 nm was recorded using a
GloMax-Multi+Microplate Multimode Reader (Promega Corp.).
Transwell invasion assay
Transwell plates (24-well) (Costar Inc., Corning,
NY, USA) were coated with 30 µg/ml of Matrigel at 4°C overnight.
After 16 h of serum starvation, 5×104 cells in 250 µl of
0.1% FBS medium containing 10 nM uPA in the presence of the
indicated H1 peptide or H2 control peptide were added to the upper
chamber and incubated at 37°C for 22 h. A total of 500 µl of 10%
FBS medium containing the same amount of compounds was
simultaneously added to the lower chamber. Non-migrated cells on
the top of the transwell were scrapped off with a cotton swab and
the number of migrated cells was counted in ten separate fields and
averaged across two independent experiments with each concentration
in triplicate. We confirmed some results of cell invasion
experiments by a novel protocol utilizing the IncuCyte ZOOM
instrument (Essen Bioscience, Ann Arbor, MI, USA).
Statistical analysis
The bar graphs are the means ± standard deviation
from at least three independent experiments. Comparison of mean
values between 2 groups was evaluated by the t-test. For all
statistical methods, a P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization of H1 peptide
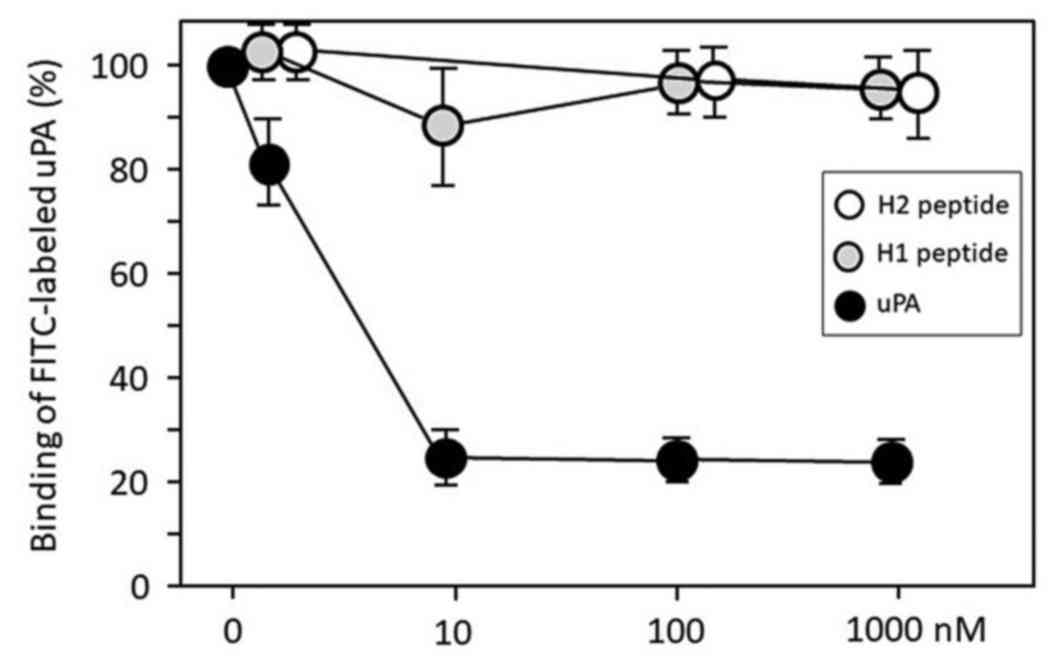
We synthesized peptides H1 and H2. H2 peptide
(control) is the amino acid sequence-shufled H1 peptide. We
employed a flow cytometry assay using FITC-conjugated uPA to assess
whether H1 blocks the uPA-uPAR protein-protein interaction in U937
cells. Free uPA protein significantly inhibited the FITC-labeled
uPA protein binding to the uPAR on the U937 cells in a
dose-dependent manner, but both H1 and H2 failed to disrupt the
interactions between FITC-labeled uPA and cellular uPAR (Fig. 1).
H1 impairs invasion of ovarian cancer
cells in vitro
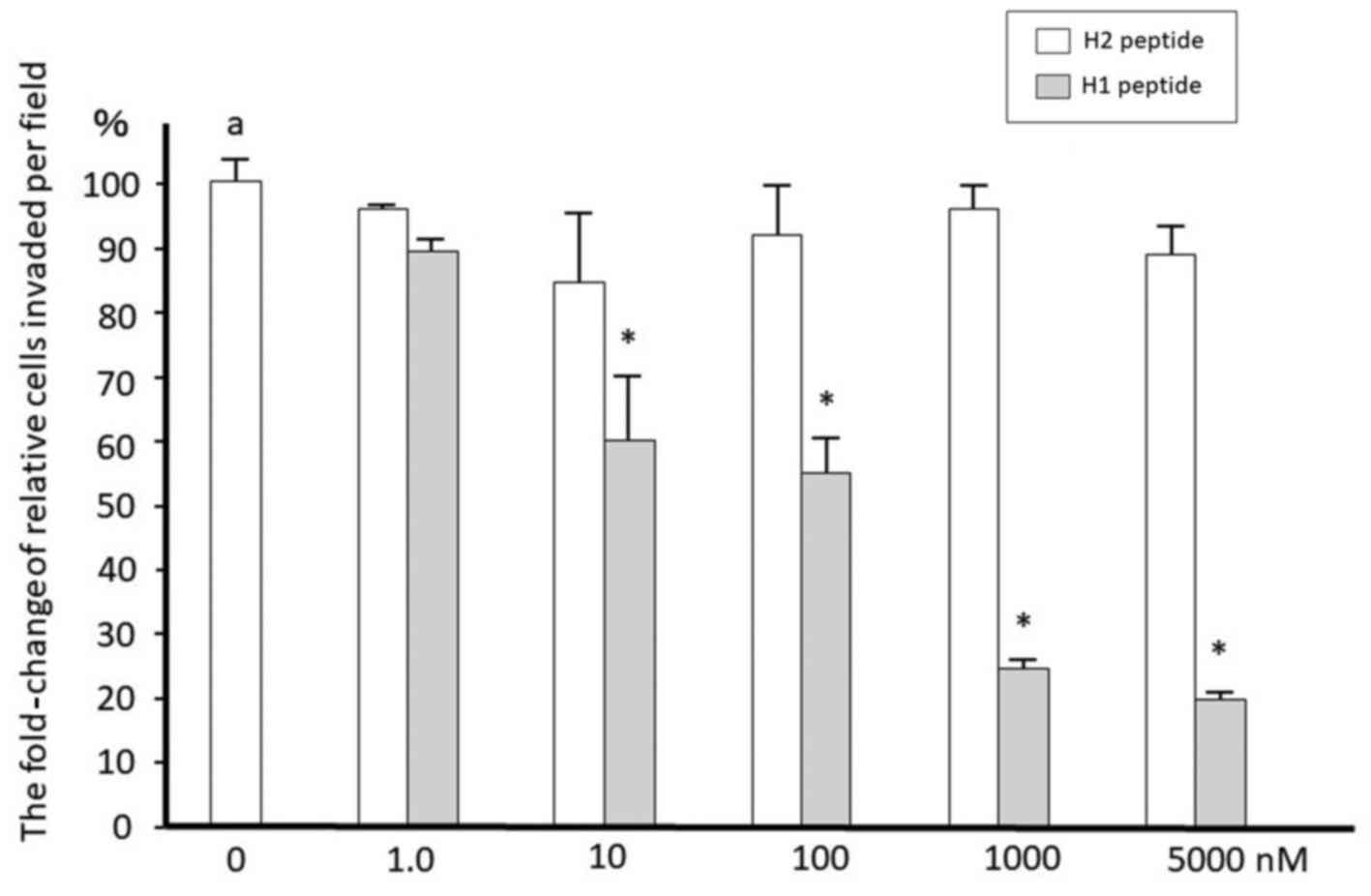
We then determined whether H1 inhibits ovarian
cancer cell invasion. Cells were serum-starved for 16 h and then
treated with 10 nM uPA at 37°C for the indicated times. SKOV3 and
TOV21G cells were treated with 10 nM uPA in the presence or absence
of increasing concentrations of H1 or H2 for 22 h. H1 was effective
in suppression of the invasion in a dose-dependent manner in cancer
cells with IC50 values of approximately 100 nM (Fig. 2). H2 showed no effect on cell invasion.
H1 has also similar inhibitory effects on TOV21G cells (data not
shown).
Cell viability
We performed MTS assays in SKOV3 and TOV21G with
increasing concentrations of H1 or H2 (1,000 nM) for 24 h. H1 and
H2 peptides did not affect the viability and growth of SKOV3 cells
(data not shown). Similar results were obtained after treatment
with each peptide in TOV21G cells.
H1 abolishes uPA-induced ERK
phosphorylation and subsequent activation of MMP-9
overexpression
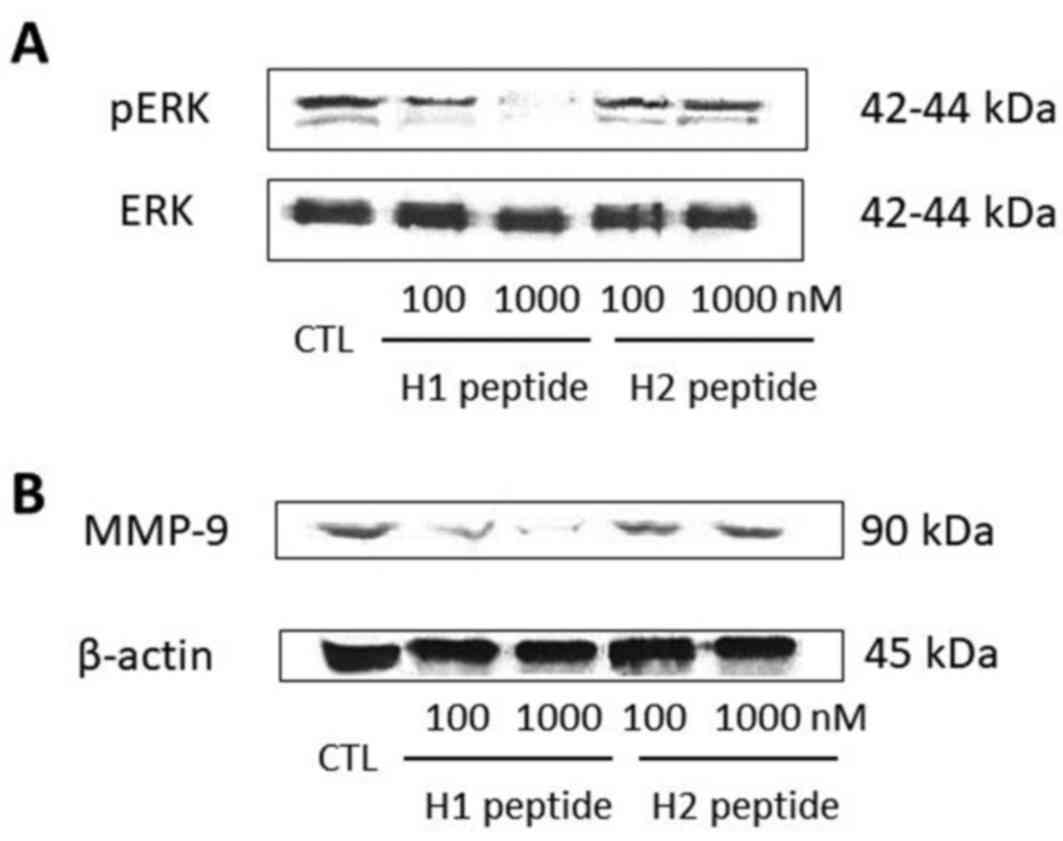
To investigate the underlying mechanism of action
for the cell invasion inhibition of H1, we selected SKOV3 cell
lines for further investigation. SKOV3 cells were serum-starved for
16 h and then pretreated with different concentrations (100 and
1,000 nM) of H1 or H2. After 30 min, the cells were incubated for
another 5 min with 10 nM uPA. Phosphorylated and total ERK were
detected by western blot analysis. H1, but not H2, significantly
suppressed the uPA-induced phosphorylation of ERK in a
dose-dependent manner (Fig. 3A).
Next we examined whether H1 was able to suppress the
uPA-induced MMP-9 expression through inactivation of the ERK
pathway. Treatment for 24 h of SKOV3 cells with H1 resulted in
dose-dependent suppression of MMP-9 expression, starting at a
concentration of 100 nM (Fig. 3B). H2
did not reduce the expression of MMP-9.
Discussion
In a previous in silico study, we designed
small molecule inhibitors of the uPAR-ligand interaction by
molecular docking and molecular dynamic simulation studies
(43). Compound H1 was selected and
identified using ab initio molecular simulation method
(42,43). Since our previous studies were fully
dependent on computational prediction algorithms, the function of
H1 was confirmed by wet lab experiments. Our results demonstrated
that H1 significantly inhibited the uPA-dependent cell invasion,
possibly though suppression of ERK-activated MMP-9 expression
(Fig. 2). The inhibition of cell
invasion occurs at high nano-molar concentrations. Importantly, the
amino acid sequence-shuffled H2 peptide exhibited no effect on
uPA-dependent cell invasion. The compound did not affect cell
viability and its potency is independent of the inhibition of cell
growth. This may provide promising evidence for the therapeutic
potential of H1 against ovarian cancer cells. Of note, H1 failed to
inhibit uPA binding to the uPAR, but mitigated the uPAR-dependent
signaling pathway. We suggested that H1 and uPA would bind at
distinct sites on uPAR molecule. However, H1 did not block the
binding of VN to uPAR protein (data not shown).
Several researchers have identified, synthesized and
preclinically examined several compounds acting as potential
inhibitors of the uPA-uPAR interaction. The following are currently
promising anti-invasive/metastatic agents: protease inhibitors
(8,13),
small molecular peptides (13–17,24),
antibodies (34,36) and siRNA/shRNA (5–25,32). Some of these have been evaluated in
in vivo pharmacokinetic and efficacy studies in an animal
cancer metastasis model. These promising findings demonstrate the
therapeutic potential of this synthetic H1 peptide against ovarian
cancer and require further preclinical investigations. However, the
effect on invasion of this active peptide was inconsistent with its
ability to inhibit the interaction between uPA and uPAR.
In conclusion, H1 is a promising template for the
development of orally bioavailable compounds with greater efficacy
on cancer cell invasion. Further studies are needed to evaluate the
molecular mechanism of H1 peptide.
Acknowledgements
The present study was supported by Grant-in-Aid for
Scientific Research from the Ministry of Education, Science and
Culture of Japan to the Department of Obstetrics and Gynecology,
Nara Medical University (awarded to H.K.).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bifulco K, Votta G, Ingangi V, Di
Carluccio G, Rea D, Losito S, Montuori N, Ragno P, Stoppelli MP,
Arra C, et al: Urokinase receptor promotes ovarian cancer cell
dissemination through its 84–95 sequence. Oncotarget. 5:4154–4169.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noh H, Hong S and Huang S: Role of
urokinase receptor in tumor progression and development.
Theranostics. 3:487–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmad A, Kong D, Sarkar SH, Wang Z,
Banerjee S and Sarkar FH: Inactivation of uPA and its receptor uPAR
by 3,3′-diindolylmethane (DIM) leads to the inhibition of prostate
cancer cell growth and migration. J Cell Biochem. 107:516–527.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gondi CS, Lakka SS, Dinh DH, Olivero WC,
Gujrati M and Rao JS: Downregulation of uPA, uPAR and MMP-9 using
small, interfering, hairpin RNA (siRNA) inhibits glioma cell
invasion, angiogenesis and tumor growth. Neuron Glia Biol.
1:165–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghamande SA, Silverman MH, Huh W, Behbakht
K, Ball G, Cuasay L, Würtz SO, Brunner N and Gold MA: A phase 2,
randomized, double-blind, placebo-controlled trial of clinical
activity and safety of subcutaneous A6 in women with asymptomatic
CA125 progression after first-line chemotherapy of epithelial
ovarian cancer. Gynecol Oncol. 111:89–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi H, Ohi H, Sugimura M, Shinohara
H, Fujii T and Terao T: Inhibition of in vitro ovarian cancer cell
invasion by modulation of urokinase-type plasminogen activator and
cathepsin B. Cancer Res. 52:3610–3614. 1992.PubMed/NCBI
|
|
8
|
Towle MJ, Lee A, Maduakor EC, Schwartz CE,
Bridges AJ and Littlefield BA: Inhibition of urokinase by
4-substituted benzo[b]thiophene-2-carboxamidines: An important new
class of selective synthetic urokinase inhibitor. Cancer Res.
53:2553–2559. 1993.PubMed/NCBI
|
|
9
|
Todaro LB, Ladeda V, de Bal Kier Joffé E
and Farías EF: Combined treatment with verapamil, a calcium channel
blocker, and B428, a synthetic uPA inhibitor, impairs the
metastatic ability of a murine mammary carcinoma. Oncol Rep.
10:725–732. 2003.PubMed/NCBI
|
|
10
|
Ertongur S, Lang S, Mack B, Wosikowski K,
Muehlenweg B and Gires O: Inhibition of the invasion capacity of
carcinoma cells by WX-UK1, a novel synthetic inhibitor of the
urokinase-type plasminogen activator system. Int J Cancer.
110:815–824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Evans DM and Sloan-Stakleff K: Suppression
of the invasive capacity of human breast cancer cells by inhibition
of urokinase plasminogen activator via amiloride and B428. Am Surg.
66:460–464. 2000.PubMed/NCBI
|
|
12
|
Henneke I, Greschus S, Savai R, Korfei M,
Markart P, Mahavadi P, Schermuly RT, Wygrecka M, Stürzebecher J and
Seeger W: Inhibition of urokinase activity reduces primary tumor
growth and metastasis formation in a murine lung carcinoma model.
Am J Respir Crit Care Med. 181:611–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lund IK, Illemann M, Thurison T,
Christensen IJ and Høyer-Hansen G: uPAR as anti-cancer target:
Evaluation of biomarker potential, histological localization, and
antibody-based therapy. Curr Drug Targets. 12:1744–1760. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohanam S, Chandrasekar N, Yanamandra N,
Khawar S, Mirza F, Dinh DH, Olivero WC and Rao JS: Modulation of
invasive properties of human glioblastoma cells stably expressing
amino-terminal fragment of urokinase-type plasminogen activator.
Oncogene. 21:7824–7830. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bürgle M, Koppitz M, Riemer C, Kessler H,
König B, Weidle UH, Kellermann J, Lottspeich F, Graeff H and
Schmitt M: Inhibition of the interaction of urokinase-type
plasminogen activator (uPA) with its receptor (uPAR) by synthetic
peptides. Biol Chem. 378:231–237. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato S, Kopitz C, Schmalix WA, Muehlenweg
B, Kessler H, Schmitt M, Krüger A and Magdolen V: High-affinity
urokinase-derived cyclic peptides inhibiting urokinase/urokinase
receptor-interaction: effects on tumor growth and spread. FEBS
Lett. 528:212–216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mani T, Wang F, Knabe WE, Sinn AL, Khanna
M, Jo I, Sandusky GE, Sledge GW Jr, Jones DR and Khanna R:
Small-molecule inhibition of the uPAR·uPA interaction: Synthesis,
biochemical, cellular, in vivo pharmacokinetics and efficacy
studies in breast cancer metastasis. Bioorg Med Chem. 21:2145–2155.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilhelm O, Weidle U, Höhl S, Rettenberger
P, Schmitt M and Graeff H: Recombinant soluble urokinase receptor
as a scavenger for urokinase-type plasminogen activator (uPA).
Inhibition of proliferation and invasion of human ovarian cancer
cells. FEBS Lett. 337:131–134. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kobayashi H, Gotoh J, Fujie M, Shinohara
H, Moniwa N and Terao T: Inhibition of metastasis of Lewis lung
carcinoma by a synthetic peptide within growth factor-like domain
of urokinase in the experimental and spontaneous metastasis model.
Int J Cancer. 57:727–733. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Min HY, Doyle LV, Vitt CR, Zandonella CL,
Stratton-Thomas JR, Shuman MA and Rosenberg S: Urokinase receptor
antagonists inhibit angiogenesis and primary tumor growth in
syngeneic mice. Cancer Res. 56:2428–2433. 1996.PubMed/NCBI
|
|
21
|
Weidle UH and König B: Urokinase receptor
antagonists: Novel agents for the treatment of cancer. Expert Opin
Investig Drugs. 7:391–403. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo Y, Higazi AA, Arakelian A, Sachais BS,
Cines D, Goldfarb RH, Jones TR, Kwaan H, Mazar AP and Rabbani SA: A
peptide derived from the nonreceptor binding region of urokinase
plasminogen activator (uPA) inhibits tumor progression and
angiogenesis and induces tumor cell death in vivo. FASEB J.
14:1400–1410. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Soria C, Griscelli F, Opolon P,
Soria J, Yeh P, Legrand C, Vannier JP, Belin D and Perricaudet M:
Amino-terminal fragment of urokinase inhibits tumor cell invasion
in vitro and in vivo: Respective contribution of the urokinase
plasminogen activator receptor-dependent or-independent pathway.
Hum Gene Ther. 16:1157–1167. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu D, Zhou D, Wang B, Knabe WE and
Meroueh SO: A new class of orthosteric uPAR·uPA small-molecule
antagonists are allosteric inhibitors of the uPAR·vitronectin
interaction. ACS Chem Biol. 10:1521–1534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khanna M, Wang F, Jo I, Knabe WE, Wilson
SM, Li L, Bum-Erdene K, Li J, W Sledge G and Khanna R: Targeting
multiple conformations leads to small molecule inhibitors of the
uPAR·uPA protein-protein interaction that block cancer cell
invasion. ACS Chem Biol. 6:1232–1243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kook YH, Adamski J, Zelent A and Ossowski
L: The effect of antisense inhibition of urokinase receptor in
human squamous cell carcinoma on malignancy. EMBO J. 13:3983–3991.
1994.PubMed/NCBI
|
|
27
|
Ahmed N, Oliva K, Wang Y, Quinn M and Rice
G: Downregulation of urokinase plasminogen activator receptor
expression inhibits Erk signalling with concomitant suppression of
invasiveness due to loss of uPAR-β1 integrin complex in colon
cancer cells. Br J Cancer. 89:374–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nozaki S, Endo Y, Nakahara H, Yoshizawa K,
Hashiba Y, Kawashiri S, Tanaka A, Nakagawa K, Matsuoka Y and Kogo
M: Inhibition of invasion and metastasis in oral cancer by
targeting urokinase-type plasminogen activator receptor. Oral
Oncol. 41:971–977. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kondraganti S, Gondi CS, McCutcheon I,
Dinh DH, Gujrati M, Rao JS and Olivero WC: RNAi-mediated
downregulation of urokinase plasminogen activator and its receptor
in human meningioma cells inhibits tumor invasion and growth. Int J
Oncol. 28:1353–1360. 2006.PubMed/NCBI
|
|
30
|
Kunigal S, Lakka SS, Gondi CS, Estes N and
Rao JS: RNAi-mediated downregulation of urokinase plasminogen
activator receptor and matrix metalloprotease-9 in human breast
cancer cells results in decreased tumor invasion, angiogenesis and
growth. Int J Cancer. 121:2307–2316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kargiotis O, Chetty C, Gogineni V, Gondi
CS, Pulukuri SM, Kyritsis AP, Gujrati M, Klopfenstein JD, Dinh DH
and Rao JS: uPA/uPAR downregulation inhibits radiation-induced
migration, invasion and angiogenesis in IOMM-Lee meningioma cells
and decreases tumor growth in vivo. Int J Oncol. 33:937–947.
2008.PubMed/NCBI
|
|
32
|
Raghu H, Gondi CS, Dinh DH, Gujrati M and
Rao JS: Specific knockdown of uPA/uPAR attenuates invasion in
glioblastoma cells and xenografts by inhibition of cleavage and
trafficking of Notch-1 receptor. Mol Cancer. 10:1302011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duriseti S, Goetz DH, Hostetter DR, LeBeau
AM, Wei Y and Craik CS: Antagonistic anti-urokinase plasminogen
activator receptor (uPAR) antibodies significantly inhibit
uPAR-mediated cellular signaling and migration. J Biol Chem.
285:26878–26888. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mohanam S, Sawaya R, McCutcheon I,
Ali-Osman F, Boyd D and Rao JS: Modulation of in vitro invasion of
human glioblastoma cells by urokinase-type plasminogen activator
receptor antibody. Cancer Res. 53:4143–4147. 1993.PubMed/NCBI
|
|
35
|
Pass J, Jögi A, Lund IK, Rønø B, Rasch MG,
Gårdsvoll H, Lund LR, Ploug M, Rømer J and Danø K: Murine
monoclonal antibodies against murine uPA receptor produced in
gene-deficient mice: Inhibitory effects on receptor-mediated uPA
activity in vitro and in vivo. Thromb Haemost. 97:1013–1022.
2007.PubMed/NCBI
|
|
36
|
Nowicki TS, Kummer NT, Iacob C, Suslina N,
Schaefer S, Schantz S, Shin E, Moscatello AL, Tiwari RK and
Geliebter J: Inhibition of uPAR and uPA reduces invasion in
papillary thyroid carcinoma cells. Laryngoscope. 120:1383–1390.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu J, Jo M, Eastman BM, Gilder AS, Bui JD
and Gonias SL: uPAR induces expression of transforming growth
factor β and interleukin-4 in cancer cells to promote
tumor-permissive conditioning of macrophages. Am J Pathol.
184:3384–3393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ragno P: The urokinase receptor: A ligand
or a receptor? Story of a sociable molecule. Cell Mol Life Sci.
63:1028–1037. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wei Y, Tang CH, Kim Y, Robillard L, Zhang
F, Kugler MC and Chapman HA: Urokinase receptors are required for
alpha 5 beta 1 integrin-mediated signaling in tumor cells. J Biol
Chem. 282:3929–3939. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Carriero MV, Del Vecchio S, Capozzoli M,
Franco P, Fontana L, Zannetti A, Botti G, D'Aiuto G, Salvatore M
and Stoppelli MP: Urokinase receptor interacts with alpha(v)beta5
vitronectin receptor, promoting urokinase-dependent cell migration
in breast cancer. Cancer Res. 59:5307–5314. 1999.PubMed/NCBI
|
|
41
|
Tang CH, Hill ML, Brumwell AN, Chapman HA
and Wei Y: Signaling through urokinase and urokinase receptor in
lung cancer cells requires interactions with beta1 integrins. J
Cell Sci. 121:3747–3756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsuji S, Kasumi T, Nagase K, Yoshikawa E,
Kobayashi H and Kurita N: The effects of amino-acid mutations on
specific interactions between urokinase-type plasminogen activator
and its receptor: Ab initio molecular orbital calculations. J Mol
Graph Model. 29:975–984. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kasumi T, Araki K, Ohyama T, Tsuji S,
Yoshikawa E, Kobayashi H and Kurita N: The effects of vitronectin
on specific interactions between urokinase-type plasminogen
activator and its receptor: Ab initio molecular orbital
calculations. Mol Simul. 39:769–779. 2013. View Article : Google Scholar
|
|
44
|
Ploug M, Østergaard S, Gårdsvoll H,
Kovalski K, Holst-Hansen C, Holm A, Ossowski L and Danø K:
Peptide-derived antagonists of the urokinase receptor. affinity
maturation by combinatorial chemistry, identification of functional
epitopes and inhibitory effect on cancer cell intravasation.
Biochemistry. 40:12157–12168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Luther T, Magdolen V, Albrecht S, Kasper
M, Riemer C, Kessler H, Graeff H, Müller M and Schmitt M:
Epitope-mapped monoclonal antibodies as tools for functional and
morphological analyses of the human urokinase receptor in tumor
tissue. Am J Pathol. 150:1231–1244. 1997.PubMed/NCBI
|
|
46
|
Wei Y, Eble JA, Wang Z, Kreidberg JA and
Chapman HA: Urokinase receptors promote beta1 integrin function
through interactions with integrin alpha3beta1. Mol Biol Cell.
12:2975–2986. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|