Introduction
Type 2 diabetes mellitus (DM2) is a systemic,
chronic-degenerative disease with a global prevalence of 9% in
adults (1). It has been reported that
22–33% of adults >65 years of age in the United States have DM2
(1,2).
Metabolic, vascular and neurologic complications are common in
patients with DM2 and it is the most frequent cause of lower limb
amputation (3). Approximately 25% of
diabetic patients develop foot ulcers, which, if left untreated,
may result in amputation (4).
Delayed wound healing in the diabetic foot is due to
a number of factors, including elevated blood glucose levels,
immune system deficiencies, peripheral arterial disease, peripheral
neuropathy, foot deformity and secondary bacterial infection
(4). In addition, the
microenvironment of lesions in patients with diabetes is abnormal
and pathogenic factors result in delayed closure of the ulcer and
deficient formation of granulation tissue (5). Specifically, a persistent inflammatory
infiltrate associated with bacterial colonization in the lesion may
contribute to delay (5).
Despite recent advances in antimicrobial therapy
(6,7),
diabetic foot lesions continue to be a serious problem. Foot ulcer
treatments are lengthy, costly and require intensive care (8,9).
Alternative therapies, including topical treatments, have therefore
been adopted to treat wounds (6–10). The use
of several topical and systemic antibiotic agents has been halted
due to the emergence of resistant strains (11). Given the increased prevalence of
antibiotic-resistant pathogens, the use of mineral substances with
antimicrobial activity, including potassium permanganate, may have
potential as alternative treatments (12–15).
Potassium permanganate solution is a strong
oxidizing agent that alters the cell walls of pathogenic organisms,
interfering with their DNA structure and exerting potent
microbicidal activity on bacteria, fungi, viruses and protozoa
(13). It acts as an astringent and
has a strongly alkaline pH, producing immediate oxidation (13). In addition, it promotes the formation
of granulation tissue and collagen synthesis, which are essential
for the healing process (13,15).
Potassium permanganate has previously been used to
treat exuding wounds in dermatology and there is evidence that it
acts on microbial species, fungi and the human immunodeficiency
virus (13,15). Despite its growing popularity in the
treatment of exuding lesions and its contributions to their
healing, to the best of our knowledge, there are limited studies on
the effect of potassium permanganate on diabetic foot ulcers have
been performed. The aim of the present study was to determine
whether the topical application of 5% potassium permanganate
solution could increase the efficacy of the current standard
treatment for chronic diabetic foot ulcers.
Patients and methods
Patients
Adult patients with Wagner stage I (uninfected
superficial ulcer) or II (deep ulcer, often infected, no bone
involvement or abscesses) diabetic foot ulcers were enrolled in the
present study (5,11). The study was a simple-blind,
randomized, controlled clinical trial conducted from March 2015 to
November 2015. All patients had DM2 and presented with a chronic
ulcer with a history of progression >3 months. Patients were
recruited from an outpatient setting at the Medical Specialties
Unit for Chronic Diseases at the Department of Health (Colima,
Mexico) for diabetes control. A total of 25 patients (age range,
18–65 years; male-to-female ratio, 1:1.5) were enrolled in the
present study. The clinical characteristics of the patients are
presented in Table I. All patients
signed statements of informed consent, and the present study was
approved by the Ethics Committee of the Instituto Estatal de
Cancerología (Colima, Mexico).
 | Table I.Clinical characteristics of the study
subjects. |
Table I.
Clinical characteristics of the study
subjects.
|
| Treatment |
|
|---|
|
|
|
|
|---|
| Clinical
characteristics | Standard (n=10) | Experimental
(n=14)a | P-value |
|---|
| Men (%) | 50.0 | 35.71 | 0.50 |
| Age (years) | 58±4.70 | 53.50±2.34 | 0.36 |
| Diabetes duration
(years) | 12.81±3.78 | 12.14±3.43 | 0.90 |
| High blood pressure
(%) | 50.0 | 21.40 | 0.10 |
| Hyperlipidemia
(%) | 10.0 | 21.40 | 0.54 |
| Alcoholism (%) | 10.0 | 21.40 | 0.54 |
| Smoking (%) | 10.0 | 7.14 | 0.75 |
| Fasting glucose
(mg/dl) | 140.11±19.81 | 161.50±15.40 | 0.40 |
| HbA1c (%) | 6.65±0.42 | 7.83±1.35 | 0.38 |
| Body mass index
(kg/m2) | 30.61±1.74 | 28.02±1.09 | 0.20 |
| Ulcer area
(mm2) | 5.38±1.24 | 6.20±1.23 | 0.65 |
| Days with ulcer | 114.00±61.95 | 169.16±58.39 | 0.56 |
| Wagner stage I
(%) | 50.0 | 50.0 | 0.82 |
| Wagner stage II
(%) | 50.0 | 50.0 | 0.82 |
| Local infection
(%) | 20.0 | 64.28 | 0.03 |
Groups and treatments
The 25 participating patients were randomly divided
into 2 groups: The standard treatment group (control) and the
experimental treatment group (intervention). The control group
(n=10) received the standard treatment for diabetic foot ulcers
administered by the Colima State Health Services at the Department
of Health (Colima). The standard treatment comprises measures for
reducing pressure on the ulcerated area, daily cleansing of the
ulcer with potable water and antiseptic wash solution, and the
application of a super-oxidized disinfectant solution (Microdacyn™;
TeArai BioFarma, Auckland, New Zealand) on the entire surface area
of the ulcer. The patients were assessed every 7 days to evaluate
the wound and debride the lesion if necessary. The intervention
group (n=15) received the same treatment as the control group
except that 5% potassium permanganate solution (Vikút, Mexico City,
Mexico) was used as a substitute for Microdacyn™. The potassium
permanganate solution was applied once daily to the entire surface
area of the ulcer with the wound as dry as possible. The wound was
not washed or rinsed after the application of the potassium
permanganate solution. In deep ulcers, excess solution was removed
with dry, sterile gauze. The concentration of the potassium
permanganate solution was chosen as 5% as this is the commercially
available pharmacologic concentration for use as a topical
antiseptic. Metabolic control measures for diabetes were continued
as usual in all the patients.
Ulcer assessment
The ulcer area was measured upon admission (day 0)
and on days 7, 14 and 21 by placing a piece of transparent acetate
over the ulcer and outlining it with a permanent ink marker. The
contour of the ulcer was digitalized as previously described
(16,17) and the area calculations were made
using ImageJ v1.51 software following the manufacturer's
instructions (National Institutes of Health, Bethesda, MD, USA).
The ulcer area at day 0 was recorded as 100%. The physician who
assessed ulcer areas was blinded to the patient group.
Statistical analysis
Normal distribution of data was confirmed using the
Shapiro-Wilk test. The Student's t-test was used to make
comparisons between groups. Categorical values were compared using
Fisher's exact test. Relative risk (RR) was calculated to determine
the probability of a ≥50% reduction of the ulcer area at day 21 in
the intervention group compared with the control. The
number-needed-to-treat (NNT) was defined as the number of
individuals receiving the experimental treatment necessary to give
an additional beneficial effect (a 50% ulcer reduction on day 21)
compared with the control group. The 95% confidence interval (CI)
was calculated for the RR and NNT. Statistical analysis was
performed using SPSS software, version 20 (IBM Corp., Armonk, NY,
USA) with the exception of the NNT, which was calculated using
MedCalc v17.7.2 software (MedCalc Software bvba, Ostend, Belgium).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline characteristics
Patient characteristics, pathological history, ulcer
presentation and size were similar between the two groups (Table I). Only the infected ulcer percentage
was significantly higher in the intervention group (P=0.03;
Table I).
Treatment tolerance
On the first day of the trial, 1 patient complained
of pain and intolerance upon application of the potassium
permanganate solution. Its application was suspended, and there
were no subsequent adverse effects. The patient was withdrawn from
the study and continued to receive standard treatment outside of
the clinical trial, leaving a total of 14 patients in the
intervention group. All patients in the intervention group stated
that the potassium permanganate solution produced a warm sensation,
however this was well tolerated.
Clinical efficacy of potassium
permanganate solution
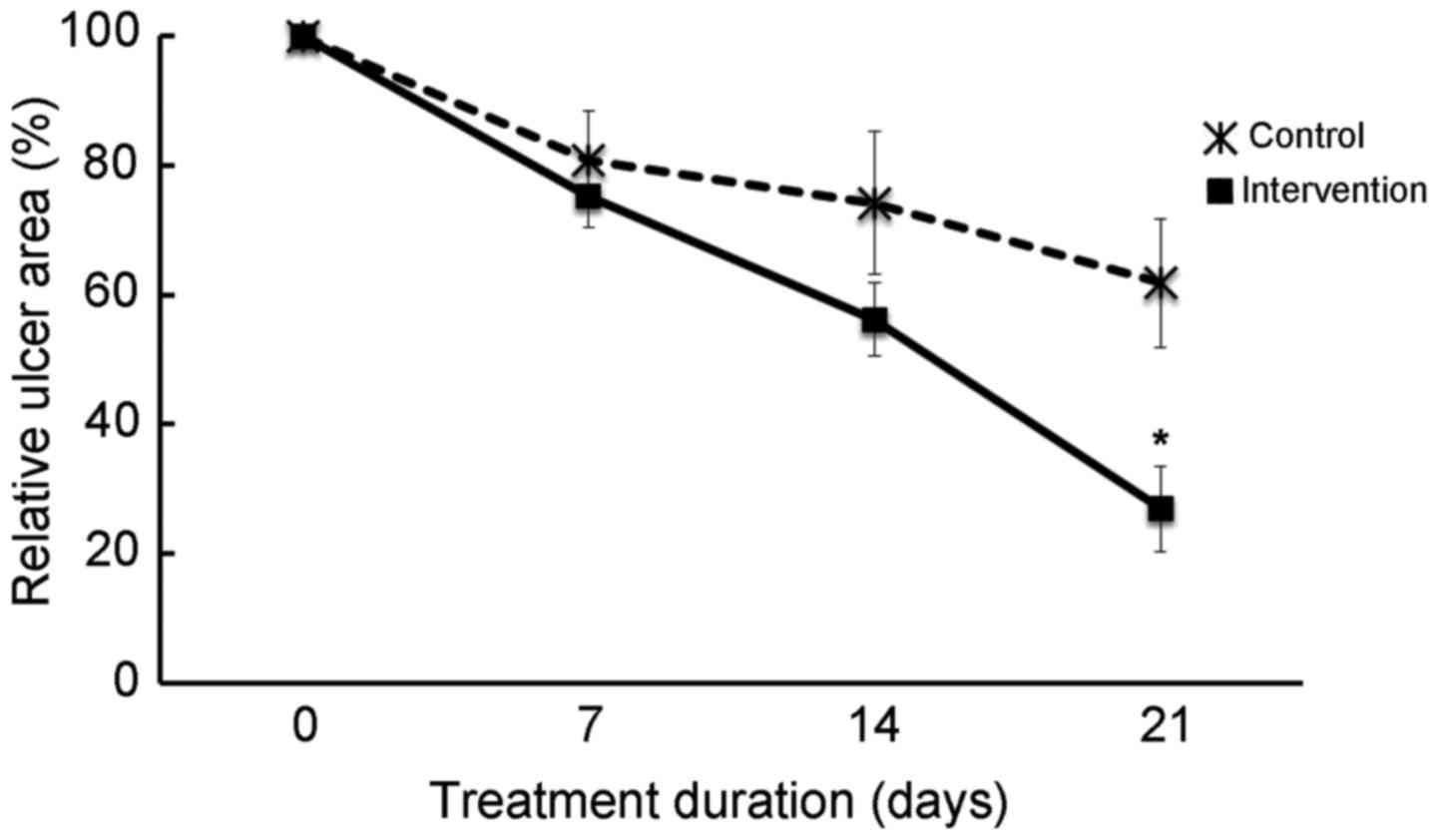
Ulcer reduction was observed in the intervention
group from day 7, and at day 21 the ulcer area was significantly
decreased compared with the control group (P<0.009); at day 21
the mean reduction in ulcer size was 73% in the intervention group
and 38% in the standard treatment group (Fig. 1). Furthermore, at day 21, the ulcer
size in 86% of intervention group patients was reduced by at ≥50%;
however, in the control group only 40% of patients achieved ≥50%
reduction (RR, 3; 95% CI, 1.1–7.6; P=0.02; data not shown). The NNT
with potassium permanganate to produce the benefit of ≥50% reduced
ulcer size at day 21 was 2.18 (95% CI, 1.26–8.25; data not
shown).
Complete healing of the ulcer occurred in 4 of the
14 patients (29%) in the intervention group following 3 weeks of
treatment, whereas complete healing was not observed in any control
patients during the study period (data not shown). Progression
after day 21 was not analyzed.
Discussion
Topical application of 5% potassium permanganate
solution, in addition to the standard treatment and cleansing
regimen, accelerated the healing process of chronic diabetic foot
ulcers compared with standard treatment alone. A ≥50% reduction in
ulcer size was observed in 86% of patients following 21 days of
potassium permanganate treatment, compared with 40% of patients
receiving standard treatment. The complementary use of potassium
permanganate for wound treatment has not been widely studied and is
generally limited to clinical case studies. To the best of our
knowledge, the present study is the first demonstrating the
efficacy of potassium permanganate as a treatment for diabetic foot
ulcers. Previous studies have demonstrated that potassium
permanganate solution is an effective auxiliary treatment for
weeping varicose eczema (18,19). One study investigated patients with
gas gangrene as a complication of trauma wounds, and the results
indicated that potassium permanganate treatment helped to eliminate
the anaerobic microenvironment and had a therapeutic effect on
wound healing (20). In another
study, 1% potassium permanganate solution was applied 3 or 4 times
a day to infected and fetid ulcerations in advanced tumors
(21). No quantitative data on
patient improvement was included, however the authors reported a
clinical improvement of the infection and fetidness without the use
of local or systemic antibiotics (21).
The benefits of potassium permanganate include lower
cost, a reduced rate of allergies and a significantly higher
healing rate compared with other medications (18–20).
However, the 5% concentration of potassium permanganate used in the
present study was higher than other reported concentrations (0.01
and 1%) (20–22), which should be considered in future
studies or comparisons. The patients in the intervention group had
a 3-fold greater probability of a ≥50% ulcer reduction following 3
weeks of treatment compared with patients receiving standard
treatment. The standard treatment used in the control group
included a disinfectant (a super-oxidized solution with a neutral
pH) that has previously been reported to effectively improve
granulation and ameliorate ulcer infections in the diabetic food
(22), making it a good reference for
evaluating the effectiveness of 5% potassium permanganate
solution.
Amputations are used to delimit systemic damage
caused by gangrene or infection. Complicated diabetic foot ulcers
often result in major or minor amputations that greatly impact
patient life expectancy and quality of life (20), as well as having serious economic
repercussions (9,22). Potassium permanganate is a strong
oxidizing agent that may help to eliminate the anaerobic
microenvironment necessary for the growth of bacteria, including
those of the genus Clostridia and other pathogenic bacteria
(20). It has previously been
demonstrated that lavages with potassium permanganate solution have
a therapeutic effect, even in mixed infections (20). The application of potassium
permanganate may therefore be beneficial for fighting infections,
possibly reducing their progression in addition to accelerating the
healing process of diabetic foot ulcers. The present study included
patients with superficial and deep ulcers with no abscesses,
classified as Wagner stages I and II. One limitation was that ulcer
depth was not assessed. Future studies should include this
measurement and also investigate the effect of potassium
permanganate solution on severely infected ulcers with concomitant
abscesses or gangrene (Wagner stages III or IV).
In the intervention group, 1/15 patients did not
tolerate the potassium permanganate treatment, however they
experienced no adverse effects once the application was stopped. A
high tolerance for topical potassium permanganate treatment has
been described in previous studies (18–21). There
is no evidence that topical application of potassium permanganate
raises plasma potassium levels (20).
However, when used at higher concentrations than described in the
present study, topical application may cause chemical burns and
there have been studies of harmful effects associated with the
accidental ingestion of potassium permanganate solution (23,24).
Studies on homemade potassium permanganate solutions, prepared by
dissolving commercially available tablets or crystals intended for
nonmedical use, have indicated that solid fragments of potassium
permanganate may come into contact with the skin and cause caustic
burns if the tablets or crystals are not completely dissolved
(24).
The present study had several limitations, including
the small sample size and the fact that ulcer progression was not
analyzed beyond day 21. Future studies should include a larger
number of patients with a longer follow-up period to further
investigate the treatment response of diabetic foot ulcers.
In conclusion, the present study demonstrated that
5% potassium permanganate solution as a complementary treatment for
diabetic foot ulcers is well tolerated and viable, effectively
accelerating the healing process of diabetic foot ulcers of Wagner
stages I and II. These data support the use of 5% potassium
permanganate as a topical alternative to conventional antibiotics
and antiseptic agents.
Acknowledgements
The present study was supported by grants
INFRAESTRUCTURA-CONACYT-2016 (grant no. 270485) and
FOSISS-CONACYT-2016 (grant no. 272792).
References
|
1
|
Yakaryılmaz FD and Öztürk ZA: Treatment of
type 2 diabetes mellitus in the elderly. World J Diabetes.
8:278–285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villalpando S, de la Cruz V, Rojas R,
Shamah-Levy T, Avila MA, Gaona B, Rebollar R and Hernández L:
Prevalence and distribution of type 2 diabetes mellitus in Mexican
adult population: A probabilistic survey. Salud Publica Mex. 52
Suppl 1:S19–S26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hernández-Ávila M, Gutiérrez JP and
Reynoso-Noverón N: Diabetes mellitus in Mexico. Status of the
epidemic. Salud Publica Mex. 55(Suppl 2): S129–S136. 2013.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escobedo-de la Peña J and Rico-Verdín B:
Incidence and fatality of the acute and chronic complications of
diabetes mellitus in Mexico. Salud Publica Mex. 38:236–242.
1996.(In Spanish). PubMed/NCBI
|
|
5
|
Cavanagh PR, Lipsky BA, Bradbury AW and
Botek G: Treatment for diabetic foot ulcers. Lancet. 366:1725–1735.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adeghate J, Nurulain S, Tekes K, Fehér E,
Kalász H and Adeghate E: Novel biological therapies for the
treatment of diabetic foot ulcers. Expert Opin Biol Ther.
17:979–987. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dalla Paola L, Carone A, Boscarino G,
Scavone G and Vasilache L: Combination of open subtotal
calcanectomy and stabilization with external fixation as limb
salvage procedure in hindfoot-infected diabetic foot ulcers. Int J
Low Extrem Wounds. 15:332–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joret MO, Dean A, Cao C, Stewart J and
Bhamidipaty V: The financial burden of surgical and endovascular
treatment of diabetic foot wounds. J Vasc Surg. 64:648–655. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Danmusa UM, Terhile I, Nasir IA, Ahmad AA
and Muhammad HY: Prevalence and healthcare costs associated with
the management of diabetic foot ulcer in patients attending Ahmadu
Bello University Teaching Hospital, Nigeria. Int J Health Sci
(Qassim). 10:219–228. 2016.PubMed/NCBI
|
|
10
|
Bowling FL, Rashid ST and Boulton AJ:
Preventing and treating foot complications associated with diabetes
mellitus. Nat Rev Endocrinol. 11:606–616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Falanga V: Wound healing and its
impairment in the diabetic foot. Lancet. 366:1736–1743. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Payne DJ, Gwynn MN, Holmes DJ and
Pompliano DL: Drugs for bad bugs: Confronting the challenges of
antibacterial discovery. Nat Rev Drug Discov. 6:29–40. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sánchez-Saldaña L and Sáenz Anduaga E:
Antisépticos y desinfectantes. Dermatol Peru. 15:82–103.
2005.http://metabase.uaem.mx/bitstream/handle/123456789/1468/280_4.pdf
|
|
14
|
Majtan J: Methylglyoxal - a potential risk
factor of manuka honey in healing of diabetic ulcers. Evid Based
Complement Alternat Med 2011. 2954942011.
|
|
15
|
Anderson I: Should potassium permanganate
be used in wound care? Nurs Times. 99:612003.PubMed/NCBI
|
|
16
|
Liu R, Li L, Yang M, Boden G and Yang G:
Systematic review of the effectiveness of hyperbaric oxygenation
therapy in the management of chronic diabetic foot ulcers. Mayo
Clin Proc. 88:pp. 166–175. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu L, McLennan SV, Lo L, Natfaji A, Bolton
T, Liu Y, Twigg SM and Yue DK: Bacterial load predicts healing rate
in neuropathic diabetic foot ulcers. Diabetes Care. 30:378–380.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quartey-Papafio CM: Lesson of the week:
Importance of distinguishing between cellulitis and varicose eczema
of the leg. BMJ. 318:1672–1673. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Biswas M, Gibby O, Ivanova-Stoilova T and
Harding K: Cushing's syndrome and chronic venous ulceration - a
clinical challenge. Int Wound J. 8:99–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu N, Wu XH, Liu R, Yang SH, Huang W,
Jiang DM, Wu Q, Xia T, Shao ZW and Ye ZW: Novel application of
vacuum sealing drainage with continuous irrigation of potassium
permanganate for managing infective wounds of gas gangrene. J
Huazhong Univ Sci Technolog Med Sci. 35:563–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dulciné-Roque R, Pullés-González V and
Gutierrez-González L: Eficiencia del Permanganato de Potasio
aplicado en las heridas septicas en el servicio de cirugia
cervico-facial. Santiago de Cuba, Centro Provincial de Información
de Ciencias Médicas de Santiago de Cuba. 1:1–10. 1995.
|
|
22
|
Martínez-De Jesús FR, Ramos-De la Medina
A, Remes-Troche JM, Armstrong DG, Wu SC, Lázaro Martínez JL and
Beneit-Montesinos JV: Efficacy and safety of neutral pH
superoxidised solution in severe diabetic foot infections. Int
Wound J. 4:353–362. 2007.PubMed/NCBI
|
|
23
|
Johnson TB and Cassidy DD: Unintentional
ingestion of potassium permanganate. Pediatr Emerg Care.
20:185–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baron S and Moss C: Caustic burn caused by
potassium permanganate. Arch Dis Child. 88:962003. View Article : Google Scholar : PubMed/NCBI
|