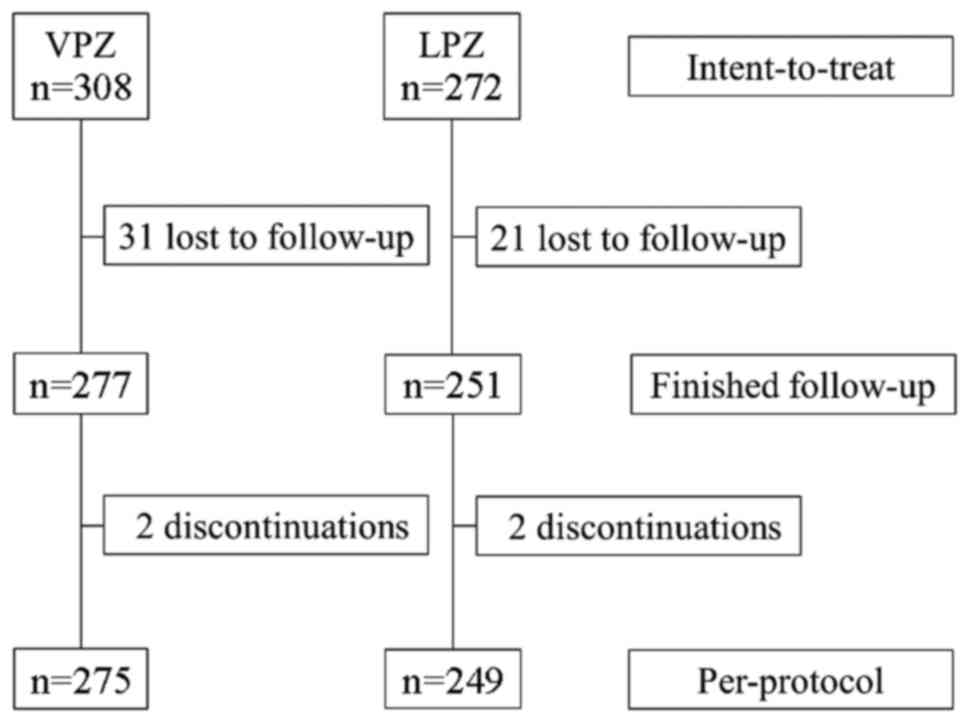
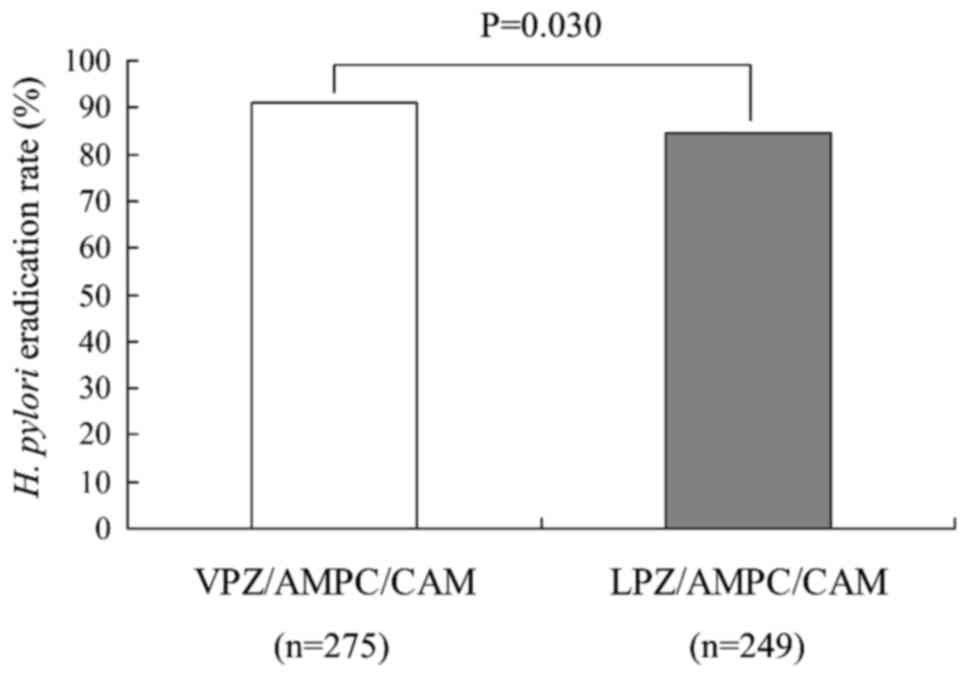
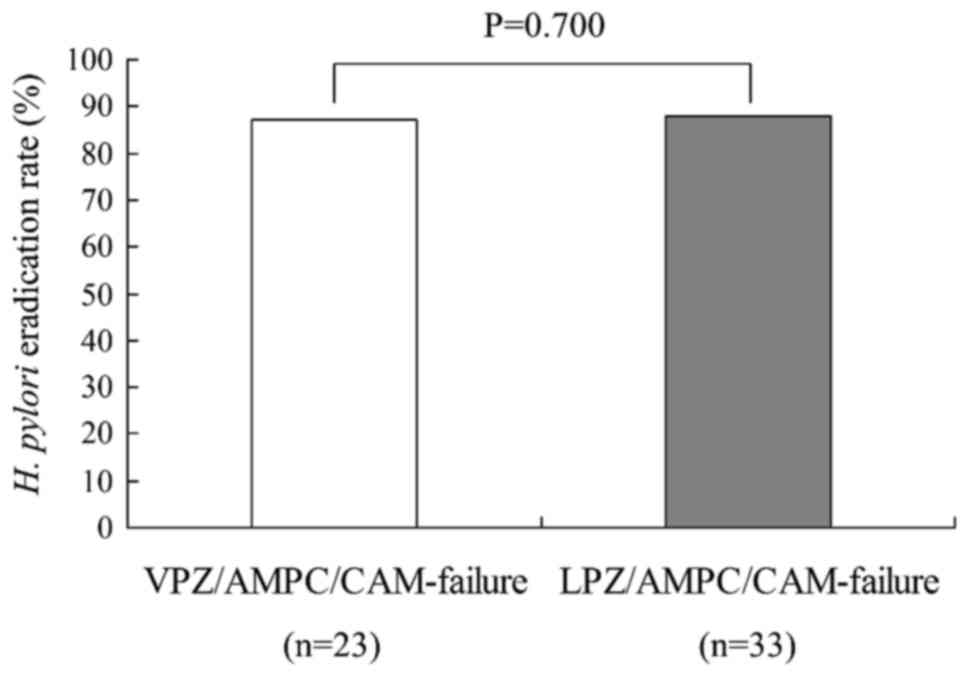
|
1
|
Murakami K, Sakurai Y, Shiino M, Funao N,
Nishimura A and Asaka M: Vonoprazan, a novel potassium-competitive
acid blocker, as a component of first-line and second-line triple
therapy for Helicobacter pylori eradication: A phase III,
randomised, double-blind study. Gut. 65:1439–1446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McColl KE: Clinical practice.
Helicobacter pylori infection. N Engl J Med. 362:1597–1604.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsujimae M, Yamashita H, Hashimura H, Kano
C, Shimoyama K, Kanamori A, Matsumoto K, Koizumi A, Momose K,
Eguchi T, et al: A comparative study of a new class of gastric acid
suppressant agent named vonoprazan versus esomeprazole for the
eradication of Helicobacter pylori. Digestion. 94:240–246.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okamura T, Suga T, Nagaya T, Arakura N,
Matsumoto T, Nakayama Y and Tanaka E: Antimicrobial resistance and
characteristics of eradication therapy of Helicobacter
pylori in Japan: A multi-generational comparison. Helicobacter.
19:214–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Graham DY and Shiotani A: New concepts of
resistance in the treatment of Helicobacter pylori
infections. Nat Clin Pract Gastroenterol Hepatol. 5:321–331. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hunt RH: pH and Hp-gastric acid secretion
and Helicobacter pylori: Implications for ulcer healing and
eradication of the organism. Am J Gastroenterol. 88:481–483.
1993.PubMed/NCBI
|
|
7
|
Sakurai Y, Shiino M, Okamoto H, Nishimura
A, Nakamura K and Hasegawa S: Pharmacokinetics and safety of triple
therapy with vonoprazan, amoxicillin, and clarithromycin or
metronidazole: A phase 1, open-label, randomized, crossover study.
Adv Ther. 33:1519–1535. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jung YS, Kim EH and Park CH: Systematic
review with meta-analysis: The efficacy of vonoprazan-based triple
therapy on Helicobacter pylori eradication. Aliment
Pharmacol Ther. 46:106–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanabe H, Ando K, Sato K, Ito T, Goto M,
Sato T, Fujinaga A, Kawamoto T, Utsumi T, Yanagawa N, et al:
Efficacy of vonoprazan-based triple therapy for Helicobacter
pylori eradication: A multicenter study and a review of the
literature. Dig Dis Sci. 64:3069–3076. 2017. View Article : Google Scholar
|
|
10
|
International Conference on Harmonisation
of Technical Requirements for Pharmaceuticals for Human Use: ICH
Harmonised Tripartite Guideline: Guideline for good clinical
Practice E6 (R1). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf
|
|
11
|
National Cancer Institute, National
Institutes of Health, US Department of Health and Human Services:
Common Terminology criteria for adverse events (CTCAE). Version
4.0.
|
|
12
|
Grayson ML, Eliopoulos GM, Ferraro MJ and
Moellering RC Jr: Effect of varying pH on the susceptibility of
Campylobacter pylori to antimicrobial agents. Eur J Clin
Microbiol Infect Dis. 8:888–889. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sachs G, Meyer-Rosberg K, Scott DR and
Melchers K: Acid, protons and Helicobacter pylori. Yale J
Biol Med. 69:301–316. 1996.PubMed/NCBI
|
|
14
|
Shin JM, Inatomi N, Munson K, Strugatsky
D, Tokhtaeva E, Vagin O and Sachs G: Characterization of a novel
potassium-competitive acid blocker of the gastric H,K-ATPase,
1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine
monofumarate (TAK-438). J Pharmacol Exp Ther. 339:412–420. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hori Y, Imanishi A, Matsukawa J, Tsukimi
Y, Nishida H, Arikawa Y, Hirase K, Kajino M and Inatomi N:
1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine
monofumarate (TAK-438), a novel and potent potassium-competitive
acid blocker for the treatment of acid-related diseases. J
Pharmacol Exp Ther. 335:231–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsukawa J, Hori Y, Nishida H, Kajino M
and Inatomi N: A comparative study on the modes of action of
TAK-438, a novel potassium-competitive acid blocker, and
lansoprazole in primary cultured rabbit gastric glands. Biochem
Pharmacol. 81:1145–1151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hori Y, Matsukawa J, Takeuchi T, Nishida
H, Kajino M and Inatomi N: A study comparing the antisecretory
effect of TAK-438, a novel potassium-competitive acid blocker, with
lansoprazole in animals. J Pharmacol Exp Ther. 337:797–804. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakurai Y, Mori Y, Okamoto H, Nishimura A,
Komura E, Araki T and Shiramoto M: Acid-inhibitory effects of
vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10
mg in healthy adult male subjects-a randomised open-label
cross-over study. Aliment Pharmacol Ther. 42:719–730. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashida K, Sakurai Y, Nishimura A, Kudou K,
Hiramatsu N, Umegaki E, Iwakiri K and Chiba T: Randomised clinical
trial: A dose-ranging study of vonoprazan, a novel
potassium-competitive acid blocker, vs. lansoprazole for the
treatment of erosive oesophagitis. Aliment Pharmacol Ther.
42:685–695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ashida K, Sakurai Y, Hori T, Kudou K,
Nishimura A, Hiramatsu N, Umegaki E and Iwakiri K: Randomised
clinical trial: Vonoprazan, a novel potassium-competitive acid
blocker, vs. lansoprazole for the healing of erosive oesophagitis.
Aliment Pharmacol Ther. 43:240–251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miwa H, Uedo N, Watari J, Mori Y, Sakurai
Y, Takanami Y, Nishimura A, Tatsumi T and Sakaki N: Randomised
clinical trial: Efficacy and safety of vonoprazan vs. lansoprazole
in patients with gastric or duodenal ulcers - results from two
phase 3, non-inferiority randomised controlled trials. Aliment
Pharmacol Ther. 45:240–252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kagawa T, Iwamuro M, Ishikawa S, Ishida M,
Kuraoka S, Sasaki K, Sakakihara I, Izumikawa K, Yamamoto K,
Takahashi S, et al: Vonoprazan prevents bleeding from endoscopic
submucosal dissection-induced gastric ulcers. Aliment Pharmacol
Ther. 44:583–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishimura N, Owada Y, Aimi M, Oshima T,
Kamada T, Inoue K, Mikami H, Takeuchi T, Miwa H, Higuchi K, et al:
No increase in gastric acid secretion in healthy Japanese over the
past two decades. J Gastroenterol. 50:844–852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishioka H, Mizuno M, Take S, Ishiki K,
Nagahara Y, Yoshida T, Okada H, Yokota K and Oguma K: A better cure
rate with 800 mg than with 400 mg clarithromycin regimens in
one-week triple therapy for Helicobacter pylori infection in
cigarette-smoking peptic ulcer patients. Digestion. 75:63–68. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Francesco V, Margiotta M, Zullo A,
Hassan C, Troiani L, Burattini O, Stella F, Di Leo A, Russo F,
Marangi S, et al: Clarithromycin-resistant genotypes and
eradication of Helicobacter pylori. Ann Intern Med.
144:94–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kato M, Yamaoka Y, Kim JJ, Reddy R, Asaka
M, Kashima K, Osato MS, El-Zaatari FA, Graham DY and Kwon DH:
Regional differences in metronidazole resistance and increasing
clarithromycin resistance among Helicobacter pylori isolates
from Japan. Antimicrob Agents Chemother. 44:2214–2216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katayama Y, Toyoda K, Kusano Y, Suda T,
Adachi S, Terauchi I, Oka S, Takahashi M and Tamano M: Efficacy of
vonoprazan-based second-line Helicobacter pylori eradication
therapy in patients for whom vonoprazan-based first-line treatment
failed. Gut. 66:752–753. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakurai K, Suda H, Ido Y, Takeichi T,
Okuda A, Hasuda K and Hattori M: Comparative study: Vonoprazan and
proton pump inhibitors in Helicobacter pylori eradication
therapy. World J Gastroenterol. 23:668–675. 2017. View Article : Google Scholar : PubMed/NCBI
|