Introduction
Infection with Helicobacter pylori (H.
pylori) is a cause of numerous pathological conditions,
including chronic gastritis, peptic ulcers, mucosa-associated
lymphoid tissue lymphoma and gastric cancer (1,2).
Therefore, eradication of H. pylori is recommended for the
prevention of gastric diseases. The standard therapy for H.
pylori infection uses a proton pump inhibitor (PPI) with
amoxicillin (AMPC) and clarithromycin (CAM) (3), though its success rate has decreased in
multiple regions worldwide in the last 15 years. This decrease has
been attributed to an increase in the prevalence of CAM-resistant
strains of H. pylori. Between 2000 and 2013, a Japanese
study identified that the overall resistance rate to CAM was 31.1%
(2). However, in addition to
bacterial resistance to antimicrobial agents, eradication failure
may also be caused by insufficient acid inhibition during
treatment, whereby stomach acid degrades and destabilizes the
antibiotics (4). H. pylori may
have increased susceptibility to antimicrobials when the microbe
restores its replicative capability at a pH higher than 6, since
increased gastric pH may induce H. pylori to re-enter the
replicative state (5). However,
inhibition of gastric acid secretion by PPIs has been reported to
be influenced by the cytochrome P450 (CYP) 2C19 genotype and
gastric emptying (3). Therefore,
stable and strong acid inhibitors are essential for the effective
treatment of H. pylori infection.
Vonoprazan (VPZ) is a member of a novel class of
gastric acid-suppressant agents, and functions as an oral
potassium-competitive acid blocker by competitively inhibiting the
binding of potassium ions to the H+,
K+-ATPase in the final step of acid secretion in gastric
parietal cells (3). This effect on
the H+, K+-ATPase has been established to be
potent and long lasting, afforded by high-level accumulation and
slow clearance of VPZ from gastric tissues (6). Therefore, the eradication rate of H.
pylori is expected to improve with the use of VPZ-based
regimens (7).
Recently, reports have demonstrated that the
acid-inhibitory effects of VPZ are more potent than those of
conventional PPIs (8,9). A phase III randomized trial in Japan
identified that a VPZ-based triple therapy had greater efficacy as
a first- or second-line triple therapy than a lansoprazole
(LPZ)-based triple therapy (first-line eradication rate of 92.6%
with VPZ vs. 75.9% with LPZ) (1). A
recent multicenter study reported that VPZ-based triple therapy was
effective and safe for H. pylori eradication in the clinic,
yielding an eradication rate of up to 94.4% (9). However, in cases of failure of VPZ-based
triple therapy for the eradication of H. pylori, second-line
triple therapy has not been well studied in a clinical setting. The
present study aimed to investigate the efficacy and safety of VPZ
for H. pylori eradication as a first-line regimen, and the
application of a second-line regimen using metronidazole (MTZ) in
failures with a VPZ-based first-line regimen.
Materials and methods
Patients
A retrospective, open-label, single-center study
design was adopted at Mie Prefectural General Medical Center
(Yokkaichi, Japan). A total of 580 patients who were diagnosed with
H. pylori infection between January 2014 and December 2016
were enrolled in the study. Among these, 308 patients had received
VPZ-based triple therapy, while 272 patients had received LPZ-based
triple therapy during the interval from January 2014 to December
2016. Prior to treatment, demographical and clinical
characteristics including age and gender were checked. A total of
544 patients had also undergone an upper gastrointestinal endoscopy
prior to enrollment. All patients diagnosed at Mie Prefectural
General Medical Center (n=450) received an endoscopy prior to
eradication therapy as a screening process for gastric cancer.
However, a total of 36 patients who were diagnosed at affiliated
hospitals did not. The present study excluded patients with a
history of eradication therapy and gastric operations. Informed
consent was waived due to the retrospective nature of the study.
The study protocol was reviewed and approved by the Ethics
Committee of Mie Prefectural General Medical Center, and was
conducted in accordance with the Declaration of Helsinki and ICH
Guidelines for Good Clinical Practice (10).
Assessment of H. pylori infection and
eradication therapy. The presence of H. pylori infection was
detected by the rapid urease test (PyloriTek Test kit; Sekisui
Medical Co., Ltd., Tokyo, Japan) or the H. pylori stool
antigen HpSA test (ImmunoCard ST HpSA; Fujirebio, Inc., Tokyo,
Japan).
First-line eradication regimens consisted of a
combination of CAM 200 mg twice a day or 400 mg twice a day, AMPC
750 mg twice a day, and either VPZ 20 mg twice a day or LPZ 30 mg
twice a day, administered orally for 7 days. Patients were
instructed to take the triple therapy once in the morning and once
in the evening. Within 8 weeks after completion of the therapy, the
extent of eradication of H. pylori infection was assessed by
HpSA testing. If eradication failed (patients tested positive for
HpSA), the patients underwent second-line eradication treatment.
The second-line eradication regimen consisted of MTZ 250 mg twice a
day, AMPC 750 mg twice a day and rabeprazole (RPZ) 10 mg twice a
day, administered orally for 7 days. Within 8 weeks after
completion of the second-line therapy, the extent of eradication of
H. pylori infection was assessed again by the feces antigen
test. All patients were interviewed by a doctor to document adverse
events experienced (if any) and to determine the drug compliance
following completion of therapy. Adverse events were scored using
the Common Terminology Criteria for Adverse Events (CTCAE) v4.0
(11).
Data analysis
The cure rate was defined as the number of
successfully treated patients divided by the number of treated
patients. Data were expressed as median (range) or as n (%) of
subjects. Statistical analysis of eradication rate between the VPZ
and LPZ regimens was conducted using the Fisher's exact test.
Patient characteristics were compared between the VPZ and LPZ
regimen groups also via Fisher's exact test. Data analysis and
descriptive and inferential statistics were performed using Statcel
(the useful add in forms on Excel - 2nd edition; OMS Publishing,
Inc., Saitama, Japan). P<0.05 was considered to indicate
statistical significance.
Results
Characteristics of patients
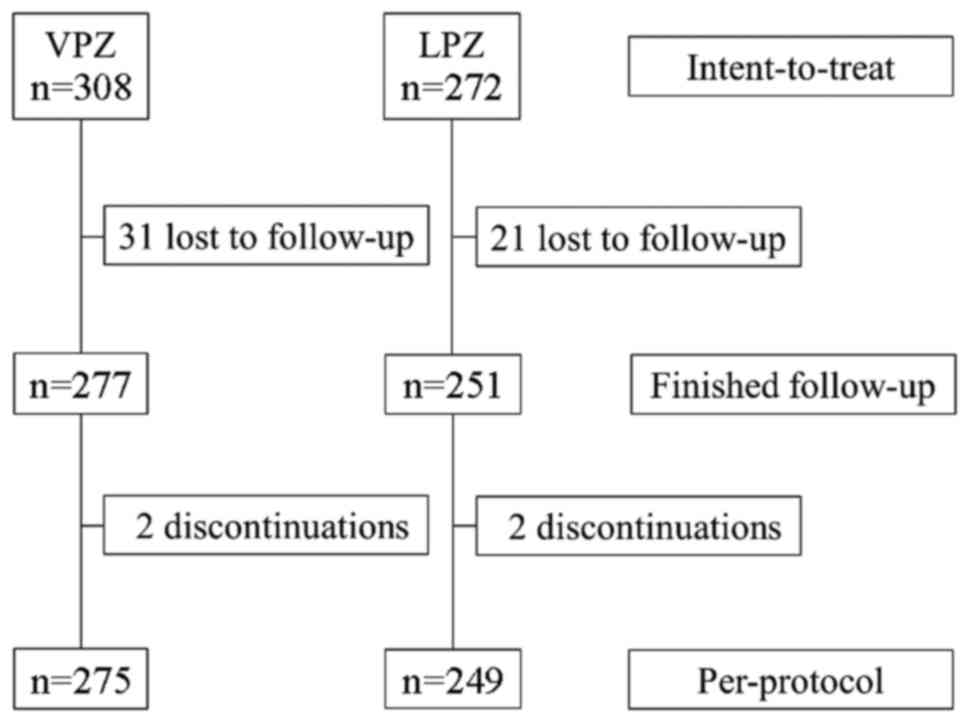
A diagram of the progression of patient inclusion
during the study is depicted in Fig.
1. A total of 580 subjects were included in the study (308
patients in the VPZ group; 272 patients in the LPZ group). In
total, 524 patients completed the first-line treatment protocol
(275 patients in the VPZ group; 249 patients in the LPZ group).
There was failure of follow-up for a total of 52 patients due to
personal reasons.
The baseline characteristics of each group are
summarized in Table I. No significant
differences were observed regarding clinical and demographic
characteristics of the patients between the two treatment
groups.
 | Table I.Baseline characteristics of enrolled
patients in each first-line treatment group. |
Table I.
Baseline characteristics of enrolled
patients in each first-line treatment group.
| Variable | VPZ/AMPC/CAM | LPZ/AMPC/CAM | P-value |
|---|
| n | 275 | 249 |
|
| Age, years, median
(range) | 62.7 (27–92) | 61.9 (26–88) | 0.572 |
| Sex, male:female,
n | 154:121 | 143:106 | 0.742 |
Comparison of first-line eradication
rate
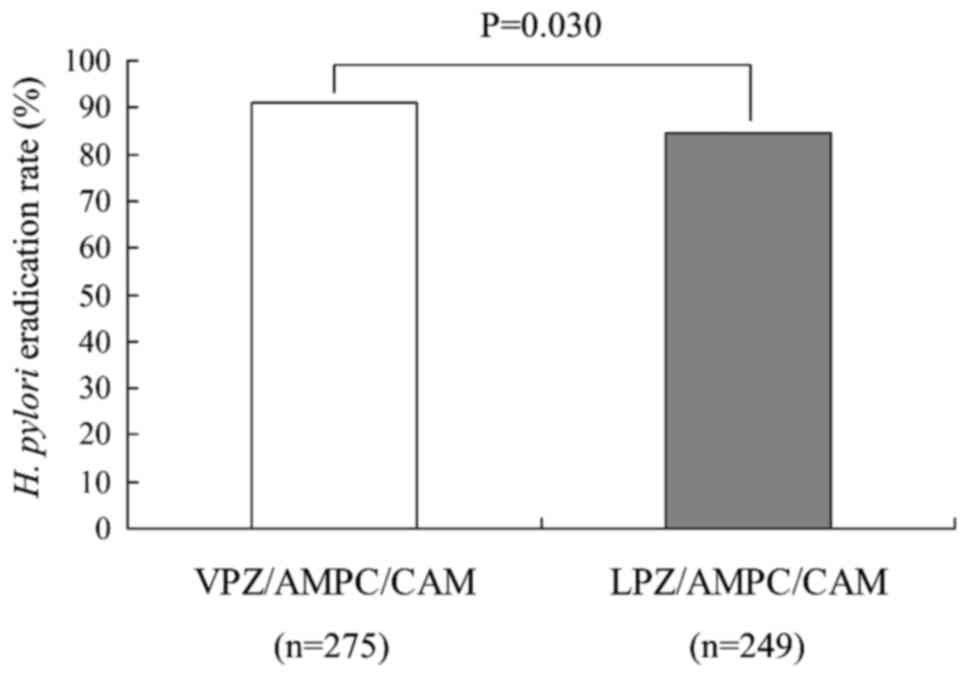
The overall first-line eradication rate was 91.0%
(250/275) for the VPZ-based triple therapy and 84.7% (211/249) for
the LPZ regimen. The eradication rate for VPZ-based triple therapy
was significantly higher than that for LPZ-based triple therapy
(P=0.030; Fig. 2). However, there was
no significant difference in the intention-to-treat eradication
rate of the treatments between the two groups (P=0.285; data not
shown), likely due to the loss at follow-up.
When comparing patient-specific eradication rates
between the regimens, a significant difference was identified in
patients who were male, and in those treated with the lower dose
(400 mg) of CAM (Table II).
Specifically, the eradication rate of the VPZ-based triple therapy
was significantly higher than that of the LPZ-based triple therapy
when assessed in males [94.1% (145/154) vs. 86.0% (123/143),
respectively, P=0.018] and in patients dosed at 400 mg CAM [96.4%
(54/56) vs. 84.9% (152/179), respectively, P=0.022]. The dose of
CAM (400 vs. 800 mg) did not affect the eradication rate in either
the VPZ or LPZ groups. In VPZ-based triple therapy, the eradication
rate in male patients was higher than that in female patients
(P=0.035; Table II).
 | Table II.Comparison of patient-specific
first-line eradication rate. |
Table II.
Comparison of patient-specific
first-line eradication rate.
|
| Eradication rate %
(n) |
|
|---|
|
|
|
|
|---|
| Variable | VPZ/AMPC/CAM | LPZ/AMPC/CAM | P-value |
|---|
| Age, years |
|
|
|
|
<70 | 91.1
(174/191)a | 84.8
(145/171)d | 0.064 |
|
≥70 | 90.5
(76/84)a | 84.6
(66/78)d | 0.185 |
| Sex |
|
|
|
|
Male | 94.1
(145/154)b | 86.0
(123/143)e | 0.018 |
|
Female | 87.4
(105/121)b | 83.0
(88/106)e | 0.429 |
| CAM, mg |
|
|
|
|
400 | 96.4
(54/56)c | 84.9
(152/179)f | 0.022 |
|
800 | 89.9
(196/219)c | 84.3
(59/70)f | 0.239 |
Adverse events
The VPZ and LPZ regimens were well tolerated by the
patients. No severe adverse effects were reported among those who
completed the protocol; however, some patients experienced
less-severe adverse effects, including fever, nausea, stomach ache
and rash. A total of 4 patients (2 receiving VPZ, 2 receiving LPZ)
did not complete the protocol due to adverse effects (Fig. 1); these were nausea (n=2 receiving
VPZ), fever (n=1 receiving LPZ) and rash (n=1 receiving LPZ).
Comparison of second-line eradication
rate
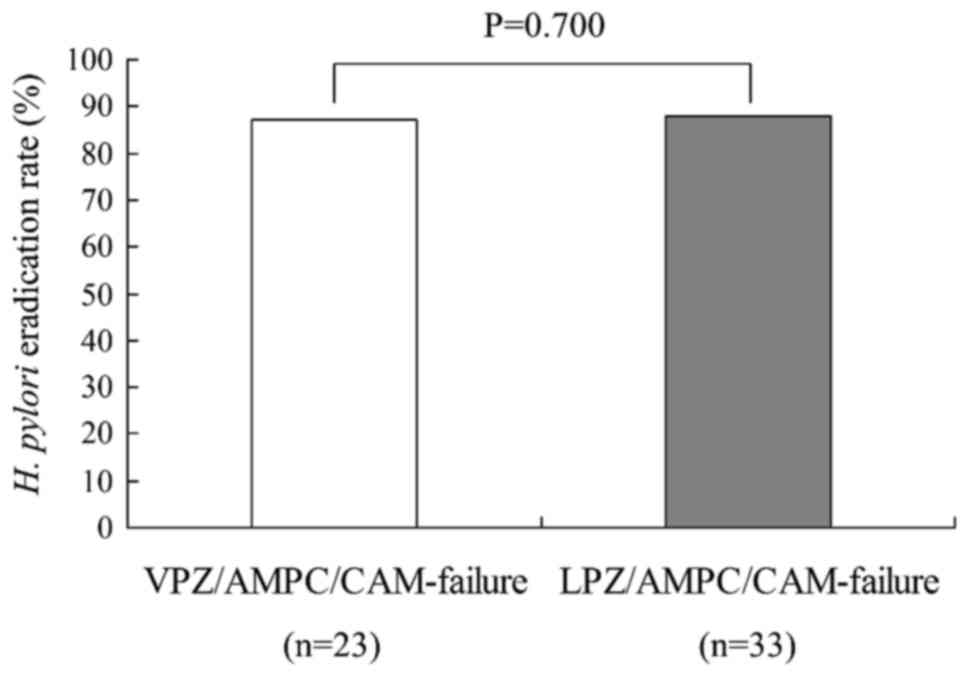
The overall second-line eradication rates with use
of MTZ instead of CAM were 87.0% (20/23) and 87.9% (29/33) for the
VPZ-failure and LPZ-failure groups, respectively; a difference that
did not achieve statistical significance (Fig. 3). There were no recurrent cases of
H. pylori infection.
Discussion
The present study demonstrated that the first-line
H. pylori eradication rate of VPZ-based triple therapy was
significantly higher than that of LPZ-based triple therapy.
A recent phase III, randomized, double-blind study
demonstrated that VPZ was non-inferior to the PPI LPZ (H.
pylori eradication rate: VPZ, 92.6%; LPZ, 75.9%) when used as a
component of first-line triple therapy (1). A meta-analysis incorporating 10 studies
demonstrated that crude H. pylori eradication rates,
determined by intention-to-treat analysis, were 87.9 and 72.8% with
VPZ-based triple therapy and PPI-based triple therapy,
respectively. The eradication rate of the VPZ-based triple therapy
was superior to that of the PPI-based triple therapy [pooled risk
ratio (95% confidence interval)=1.19 (1.15–1.24)] (8). These previous results are consistent
with those of the current study.
With respect to H. pylori eradication
therapy, both CAM and AMPC are acid-sensitive; therefore,
inhibition of gastric acid secretions must be performed to prevent
degradation of the drugs during therapy. Increased stability and
bioavailability of these acid-sensitive antibiotics has been
reported on inhibition of gastric acid secretion to generate a
stomach pH >5 (12,13). Furthermore, H. pylori may be
induced by increased gastric pH to re-enter a replicative state,
making it susceptible to antibiotics (5). Therefore, prompt, potent and
long-lasting acid suppression may be critical for H. pylori
eradication.
VPZ is a novel oral potassium-competitive acid
blocker and is being increasingly used for the treatment of ulcers
and reflux esophagitis in Japan (14,15).
Compared with LPZ, VPZ exhibits more potent and sustained
acid-inhibitory effects, and induction of greater increases in
gastric pH, consistent with the observation that VPZ accumulates to
higher concentrations and is cleared at a slower rate from gastric
glands (16,17). In addition, the intragastric pH
following VPZ treatment (pH 5.2) has been identified to be
significantly higher than that following esomeprazole treatment (pH
3.0) on day 1 of therapy (18). The
superior efficacy of VPZ compared with PPIs has been reported in
various gastrointestinal diseases (19–22).
In the current study, the eradication rate in male
patients was higher than that in female patients with VPZ-based
triple therapy. In healthy Japanese subjects, a study conducted
from the 1970s to the 1990s demonstrated that maximal gastric acid
output gradually decreased with age in male patients while
remaining constant in female patients (23). This distinction may be a reason for
the higher eradication rate observed in male patients following
VPZ-based triple therapy.
A significant difference in eradication rate was
observed in patients treated with the lower dose of CAM (400 mg)
when comparing between the two first-line drug regimens (VPZ vs.
LPZ) in the present study; this difference was not observed in
patients treated with the higher dose of CAM (800 mg). A previous
report indicated that treatment with a higher dose of CAM (800 mg)
resulted in a higher eradication rate in triple therapy using
conventional PPIs (24). The
observation of efficacy at the lower dose in the present study
presumably reflects the general efficacy of the VPZ-based triple
therapy, and not superior efficacy of the lower CAM dose. Further
study will be necessary to conclude whether the lower dose of CAM
is superior when used as part of a VPZ-based triple therapy.
Nonetheless the present results, taken together with previous
reports, indicate that the VPZ-based triple therapy is more
effective than a PPI-based therapy for resolving H. pylori
infection.
However, certain patients in the present study and
in previous work have experienced treatment failure with the
VPZ-based triple therapy. Notably, a specific H. pylori
mutation leading to CAM resistance appears to be primarily
associated with a reduced likelihood of eradication (25). For CAM-sensitive and CAM-resistant
H. pylori, the respective success rates of VPZ-based triple
therapy have been reported as 97.6–100.0% and 82.0–87.5% (25). Therefore, the current study employed a
combination of AMPC, MTZ and RPZ for second-line treatment. To
date, there have been few reports concerning the use of second-line
treatments following failures with VPZ-based first-line
therapy.
The present study demonstrated that this second-line
therapy achieved a high eradication rate, and that there was no
significant difference in eradication rate between patients who had
failed on first-line treatment with VPZ-based triple therapy or
LPZ-based triple therapy. Thus, the data indicated that failure of
first-line VPZ-based eradication therapy did not affect the outcome
of a standard second-line eradication therapy. It may be assumed
that the efficacy of second-line treatment reflects the low
proportion of H. pylori resistant to MTZ (2–5%) in Japan
(26). Thus, acid suppression may not
be a major factor of concern that influences the rate of
eradication by the second-line regimen. Although certain
small-scale studies have demonstrated high eradication rates with
VPZ-based second-line therapy (27,28), more
detailed studies comparing PPIs and VPZ will be needed to clarify
the optimum second-line treatment for PPI-failure or VPZ-failure
patients (9).
Given that VPZ exerts a potent acid inhibitory
effect, the total cost of antimicrobial drugs may be reduced by use
of VPZ (3). A sub-analysis in a
randomized controlled trial of VPZ indicated that the eradication
rate between 400 and 800 mg regimens of CAM did not differ
significantly when using VPZ (18).
In addition to the financial incentive, reductions in the treatment
time and amount of antimicrobial agent used may result in a
decrease in side effects when using CAM 400 mg (3).
The current study had a number of limitations. The
study followed an open-label, retrospective, single-center design,
and was not systematic; therefore, the effect of CYP2C19 genotype
and antibiotic resistance was not evaluated. However, regarding
this point, changes in tolerance to antibiotics would have been
presumably relatively small, since the study was performed in a
single hospital over a period of 3 years, similar to previous study
(3); and the sample size of the study
was small, meaning further larger scale study will be needed to
confirm the results.
In conclusion, the first-line H. pylori
eradication rate of the VPZ-based triple therapy was satisfactory
and significantly higher than that of the LPZ-based triple therapy
among the total patient cohort, irrespective of patient background.
Failure of the primary eradication with the VPZ-based first-line
regimen did not affect the outcome of the second-line (MTZ-based)
eradication regimen. Therefore, triple therapy with the acid
blocker VPZ should be recommended for eradication of H.
pylori.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NM and KS designed and directed the project. YN, DS,
IM, YY, YO and HI performed the clinical therapy and analyzed the
data. KT and MH interpreted the data and confirmed the findings of
this work. NM and KS wrote the article.
Ethics approval and consent to
participate
This retrospective study was approved by the Ethics
Committee of Mie Prefectural General Medical Center (Yokkaichi,
Japan). Informed consent was waived due to the retrospective nature
of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Murakami K, Sakurai Y, Shiino M, Funao N,
Nishimura A and Asaka M: Vonoprazan, a novel potassium-competitive
acid blocker, as a component of first-line and second-line triple
therapy for Helicobacter pylori eradication: A phase III,
randomised, double-blind study. Gut. 65:1439–1446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McColl KE: Clinical practice.
Helicobacter pylori infection. N Engl J Med. 362:1597–1604.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsujimae M, Yamashita H, Hashimura H, Kano
C, Shimoyama K, Kanamori A, Matsumoto K, Koizumi A, Momose K,
Eguchi T, et al: A comparative study of a new class of gastric acid
suppressant agent named vonoprazan versus esomeprazole for the
eradication of Helicobacter pylori. Digestion. 94:240–246.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okamura T, Suga T, Nagaya T, Arakura N,
Matsumoto T, Nakayama Y and Tanaka E: Antimicrobial resistance and
characteristics of eradication therapy of Helicobacter
pylori in Japan: A multi-generational comparison. Helicobacter.
19:214–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Graham DY and Shiotani A: New concepts of
resistance in the treatment of Helicobacter pylori
infections. Nat Clin Pract Gastroenterol Hepatol. 5:321–331. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hunt RH: pH and Hp-gastric acid secretion
and Helicobacter pylori: Implications for ulcer healing and
eradication of the organism. Am J Gastroenterol. 88:481–483.
1993.PubMed/NCBI
|
|
7
|
Sakurai Y, Shiino M, Okamoto H, Nishimura
A, Nakamura K and Hasegawa S: Pharmacokinetics and safety of triple
therapy with vonoprazan, amoxicillin, and clarithromycin or
metronidazole: A phase 1, open-label, randomized, crossover study.
Adv Ther. 33:1519–1535. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jung YS, Kim EH and Park CH: Systematic
review with meta-analysis: The efficacy of vonoprazan-based triple
therapy on Helicobacter pylori eradication. Aliment
Pharmacol Ther. 46:106–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanabe H, Ando K, Sato K, Ito T, Goto M,
Sato T, Fujinaga A, Kawamoto T, Utsumi T, Yanagawa N, et al:
Efficacy of vonoprazan-based triple therapy for Helicobacter
pylori eradication: A multicenter study and a review of the
literature. Dig Dis Sci. 64:3069–3076. 2017. View Article : Google Scholar
|
|
10
|
International Conference on Harmonisation
of Technical Requirements for Pharmaceuticals for Human Use: ICH
Harmonised Tripartite Guideline: Guideline for good clinical
Practice E6 (R1). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf
|
|
11
|
National Cancer Institute, National
Institutes of Health, US Department of Health and Human Services:
Common Terminology criteria for adverse events (CTCAE). Version
4.0.
|
|
12
|
Grayson ML, Eliopoulos GM, Ferraro MJ and
Moellering RC Jr: Effect of varying pH on the susceptibility of
Campylobacter pylori to antimicrobial agents. Eur J Clin
Microbiol Infect Dis. 8:888–889. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sachs G, Meyer-Rosberg K, Scott DR and
Melchers K: Acid, protons and Helicobacter pylori. Yale J
Biol Med. 69:301–316. 1996.PubMed/NCBI
|
|
14
|
Shin JM, Inatomi N, Munson K, Strugatsky
D, Tokhtaeva E, Vagin O and Sachs G: Characterization of a novel
potassium-competitive acid blocker of the gastric H,K-ATPase,
1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine
monofumarate (TAK-438). J Pharmacol Exp Ther. 339:412–420. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hori Y, Imanishi A, Matsukawa J, Tsukimi
Y, Nishida H, Arikawa Y, Hirase K, Kajino M and Inatomi N:
1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine
monofumarate (TAK-438), a novel and potent potassium-competitive
acid blocker for the treatment of acid-related diseases. J
Pharmacol Exp Ther. 335:231–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsukawa J, Hori Y, Nishida H, Kajino M
and Inatomi N: A comparative study on the modes of action of
TAK-438, a novel potassium-competitive acid blocker, and
lansoprazole in primary cultured rabbit gastric glands. Biochem
Pharmacol. 81:1145–1151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hori Y, Matsukawa J, Takeuchi T, Nishida
H, Kajino M and Inatomi N: A study comparing the antisecretory
effect of TAK-438, a novel potassium-competitive acid blocker, with
lansoprazole in animals. J Pharmacol Exp Ther. 337:797–804. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakurai Y, Mori Y, Okamoto H, Nishimura A,
Komura E, Araki T and Shiramoto M: Acid-inhibitory effects of
vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10
mg in healthy adult male subjects-a randomised open-label
cross-over study. Aliment Pharmacol Ther. 42:719–730. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashida K, Sakurai Y, Nishimura A, Kudou K,
Hiramatsu N, Umegaki E, Iwakiri K and Chiba T: Randomised clinical
trial: A dose-ranging study of vonoprazan, a novel
potassium-competitive acid blocker, vs. lansoprazole for the
treatment of erosive oesophagitis. Aliment Pharmacol Ther.
42:685–695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ashida K, Sakurai Y, Hori T, Kudou K,
Nishimura A, Hiramatsu N, Umegaki E and Iwakiri K: Randomised
clinical trial: Vonoprazan, a novel potassium-competitive acid
blocker, vs. lansoprazole for the healing of erosive oesophagitis.
Aliment Pharmacol Ther. 43:240–251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miwa H, Uedo N, Watari J, Mori Y, Sakurai
Y, Takanami Y, Nishimura A, Tatsumi T and Sakaki N: Randomised
clinical trial: Efficacy and safety of vonoprazan vs. lansoprazole
in patients with gastric or duodenal ulcers - results from two
phase 3, non-inferiority randomised controlled trials. Aliment
Pharmacol Ther. 45:240–252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kagawa T, Iwamuro M, Ishikawa S, Ishida M,
Kuraoka S, Sasaki K, Sakakihara I, Izumikawa K, Yamamoto K,
Takahashi S, et al: Vonoprazan prevents bleeding from endoscopic
submucosal dissection-induced gastric ulcers. Aliment Pharmacol
Ther. 44:583–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishimura N, Owada Y, Aimi M, Oshima T,
Kamada T, Inoue K, Mikami H, Takeuchi T, Miwa H, Higuchi K, et al:
No increase in gastric acid secretion in healthy Japanese over the
past two decades. J Gastroenterol. 50:844–852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishioka H, Mizuno M, Take S, Ishiki K,
Nagahara Y, Yoshida T, Okada H, Yokota K and Oguma K: A better cure
rate with 800 mg than with 400 mg clarithromycin regimens in
one-week triple therapy for Helicobacter pylori infection in
cigarette-smoking peptic ulcer patients. Digestion. 75:63–68. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Francesco V, Margiotta M, Zullo A,
Hassan C, Troiani L, Burattini O, Stella F, Di Leo A, Russo F,
Marangi S, et al: Clarithromycin-resistant genotypes and
eradication of Helicobacter pylori. Ann Intern Med.
144:94–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kato M, Yamaoka Y, Kim JJ, Reddy R, Asaka
M, Kashima K, Osato MS, El-Zaatari FA, Graham DY and Kwon DH:
Regional differences in metronidazole resistance and increasing
clarithromycin resistance among Helicobacter pylori isolates
from Japan. Antimicrob Agents Chemother. 44:2214–2216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katayama Y, Toyoda K, Kusano Y, Suda T,
Adachi S, Terauchi I, Oka S, Takahashi M and Tamano M: Efficacy of
vonoprazan-based second-line Helicobacter pylori eradication
therapy in patients for whom vonoprazan-based first-line treatment
failed. Gut. 66:752–753. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakurai K, Suda H, Ido Y, Takeichi T,
Okuda A, Hasuda K and Hattori M: Comparative study: Vonoprazan and
proton pump inhibitors in Helicobacter pylori eradication
therapy. World J Gastroenterol. 23:668–675. 2017. View Article : Google Scholar : PubMed/NCBI
|