Introduction
Aerobic organisms produce reactive oxygen species
(ROS) during normal physiological processes, including cellular
respiration (1), and as a means to
combat foreign microorganisms during inflammation (2). However, if produced in mass quantities,
ROS may cause considerable tissue damage (3). Therefore, aerobic organisms have evolved
several mechanisms that antagonize the potentially damaging effects
of ROS (4), including glutathione
(5), superoxide dismutase (6) and catalase (6) systems. The above defense mechanisms can
become overwhelmed by the ROS surplus leading to a state commonly
referred to as ‘oxidative stress’ (7). This state is considered to contribute to
the development and maintenance of several chronic disease
conditions, including cancer (8),
Alzheimer's disease (9), traumatic
brain injury (10,11), and insulin resistance (12), and to ageing (7,13).
In view of the central role of ROS in the etiology
of several pathologies, the mechanism by which ROS produce cellular
damage has been and remains the subject of extensive investigation.
Although the exact mechanism remains to be elucidated, several
lines of evidence from several research groups in the field
indicate that ROS can trigger cellular damage through i) the
peroxidation of polyunsaturated fatty acids of cellular membranes
(14), ii) the generation of DNA
mutations through the nitration and deamination of DNA (15), and iii) protein nitration and
carbonylation (16), which eventually
disrupts cellular function. Noteworthy, the majority of the ROS are
involved in chain reactions that result in the formation of new ROS
species and further propagation of the initial damage (17). In addition, several of the cell
damaging effects of ROS species can be attributed to the
decomposition of the parent ROS into other markedly reactive
radicals (18). Given the above
discussion, therapeutic interventions that interfere with ROS
production and/or propagation may be of benefit for the treatment
of several chronic diseases linked to ‘oxidative stress’. In the
intravascular compartment, pharmacological agents that reduce the
cell damaging effects of peroxynitrite are a suitable target for
therapy for several reasons; peroxynitrite is the most regularly
produced reactive species in the intravascular compartment
(19), and the formation of
peroxynitrite in the intravascular compartment has been linked to
the etiology of several pathologies (19).
In our two previous reports, an in vitro
system was discussed that permitted the investigation of the
damaging effects of peroxynitrite on the proteins and lipids of the
intravascular compartment; specifically on blood plasma and
isolated platelets. In the first report, it was shown that tempol,
a drug that catalytically inactivates peroxynitrite-derived free
radicals, antagonizes the effects of peroxynitrite on blood plasma
and on platelet lipids and proteins (20). In the second report, it was shown that
phenelzine, a scavenger of reactive aldehyde species, produced
comparable effects to that of tempol (21). However, although the results in these
two previous reports were encouraging, the degree of inhibition of
the cell damaging effects via using tempol or phenelzine were
modest and we were not able to completely reverse the oxidative
damage induced by peroxynitrite on the examined endpoints; namely,
thiobarbituric acid reactive substances (TBARS) and protein
carbonylation. In the present study, it was hypothesized that, by
using a different antioxidant that targets a different step of the
proposed model by which peroxynitrite induces its cytotoxic
effects, it is possible to achieve superior protection from
peroxynitrite and completely reverse its cell damaging effects.
Specifically, the present study examined the protective effects of
U-83836E, a scavenger of lipid peroxyl radicals (22), against the peroxynitrite-mediated
oxidative damage of blood proteins and lipids. The mechanism/s by
which U-83836E protects against the cell damaging effects of
peroxynitrite is further discussed.
Materials and methods
Description of study participants and
sample collection
The present study was a prospective study performed
in the family medicine clinics of King Abdullah University Hospital
(KAUH; Ramtha, Jordan). KAUH is a tertiary teaching hospital
affiliated with Jordan University of Science and Technology (Irbid,
Jordan). Following approval of the study from the Institutional
Review Board (IRB) of KAUH (IRB no. 1/105/2017), 1 unit of blood
was collected from each of three eligible participants. The
selection of study participants was based on the criteria of being
of Jordanian descent and 22–25 years of age. Any individual who was
a smoker or an ex-smoker, suffered from chronic illnesses
associated with increased ROS, including hypertension, type 1 or
type 2 diabetes mellitus, atherosclerosis or dyslipidemia, or
received anti-histamines or nonsteroidal anti-inflammatory drugs
prior to blood collection (up to 2 weeks) were not invited to
participate in the study. A total of 2 males and 1 female consented
to participate. Blood withdrawal was performed between October and
December, 2014. Bags containing CPDA-1 anticoagulant were used for
blood collection. In order to separate blood plasma and platelets,
whole blood collected in the above bags was divided into six
individual CPDA1 tubes. This number corresponds to the number of
experimental groups.
Isolation of plasma and platelets from
whole blood
The methods used to recover plasma or platelets were
as previously described (20). In
brief, whole blood was centrifuged at 450 × g for 6.20 min at room
temperature to recover platelet-rich plasma. The platelet-rich
plasma recovered from the previous step was then subjected to
centrifugation at 2,500 × g for 5.40 min at room temperature, which
caused the sedimentation of a platelet rich pellet. This pellet was
then washed twice with Tyrode's buffer and then re-suspended in the
same buffer. The final platelet number was measured with an
automated cell counter (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and adjusted to a final platelet count of
109/ml.
Sample treatment
The experimental design and sample treatments were
as previously described (20). In
brief, the plasma or isolated platelets were distributed into
six-well plates (2 ml/well). Each well corresponded to one of the
CPDA1 tubes described above. The samples in the wells were then
treated with either vehicle (2 µl 0.3 M NaOH), 100 μM peroxynitrite
(cat. no. 81565; Cayman Chemical, Ann Arbor, MI, USA), or a
combination of peroxynitrite with different doses of U-83836E (25,
50, 75 or 100 µM; Biomol International, LP, Plymouth Meeting, PA,
USA). All treatments lasted for 20 min at 37°C.
Estimation of total plasma
proteins
The protein concentration of the plasma samples was
measured using a Bradford protein assay (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) according to the manufacturer's protocol.
The absorbance was determined at 595 nm on an ELx800 ELISA reader
(BioTek Instruments, Inc., Winooski, VT, USA).
Detection of carbonyl group and
TBARS
The methods used for the detection of carbonyl
groups and TBARS were detailed previously (20) and are thus only briefly discussed in
the present study. In terms of protein carbonylation, an
enzyme-linked immunosorbent assay (ELISA)-based assay was used to
determine the quantity of carbonyl groups in each sample (cat. no.
STA-310; Cell Biolabs, Inc., San Diego, CA, USA). In this assay,
the carbonyl groups of proteins were allowed to react with
dinitrophenyl hydrazine (DNPH), which produces a dinitrophenyl
hydrazone derivative. The excess DNPH was then washed with a
PBS/ethanol solution. The quantity of the resulting hydrazone
derivative was then quantified through its reaction with anti-DNP
antibody (rabbit immunoglobulin G; cat. no. 231002). A total of 100
µl of the primary antibody was used at a final dilution of 1:1,000.
The antibody was incubated for 1 h at room temperature on an
orbital shaker. The absorbance was measured at 450 nm on an ELx800
microplate reader (BioTek Instruments, Inc.). The TBARS assay
quantifies the levels of malondialdehyde (MDA) and other minor
aldehyde species through their reaction with thiobarbituric acid
(TBA). MDA is a reactive aldehyde species and a byproduct of lipid
peroxidation. In the present study, a colorimetric based assay was
used to measure the levels of TBA-MDA adduct of each sample (cat.
no. ab118970; Abcam, Cambridge, UK). In this assay, the samples
were first allowed to react with H2SO4 and
phosphotungstic acid. This step causes precipitation of the lipids
in the sample. Following a centrifugation step (13,000 × g for 3
min at room temperature), the lipid pellet was collected and then
resuspended with water. TBA solution (600 µl) was then added to the
dissolved pellet and boiled for 1 h. The reaction was then cooled
down in an ice bath for 10 min. The quantity of MDA-TBA adduct was
finally measured spectrophotometrically at 530 nm on an ELx800
microplate reader (BioTek Instruments, Inc.).
Statistical analysis
Statistical analysis was performed using Statistical
Package for Social Studies software version 17 (SPSS, Inc. Chicago,
IL, USA). One way analysis of variance was used to compare between
the different experimental groups, followed by Fisher's post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference between the groups.
Results
U83836E antagonizes the oxidative
damage induced by peroxynitrite on the proteins of blood plasma and
platelets
In our previous study, it was demonstrated that
peroxynitrite induced oxidative damage of blood plasma and platelet
proteins when protein oxidative damage was evaluated by measuring
carbonyl group formation following peroxynitrite treatment
(20). The present study aimed to
determine whether U83836E has the ability to antagonize the
oxidative damaging effects of peroxynitrite on blood plasma and
platelets proteins. Therefore, blood plasma and platelet samples
were treated with 100 µM of peroxynitrite alone or in combination
with the different doses of U-83836E (25, 50, 75 or 100 µM), and
protein carbonyl formation was then measured in the treated samples
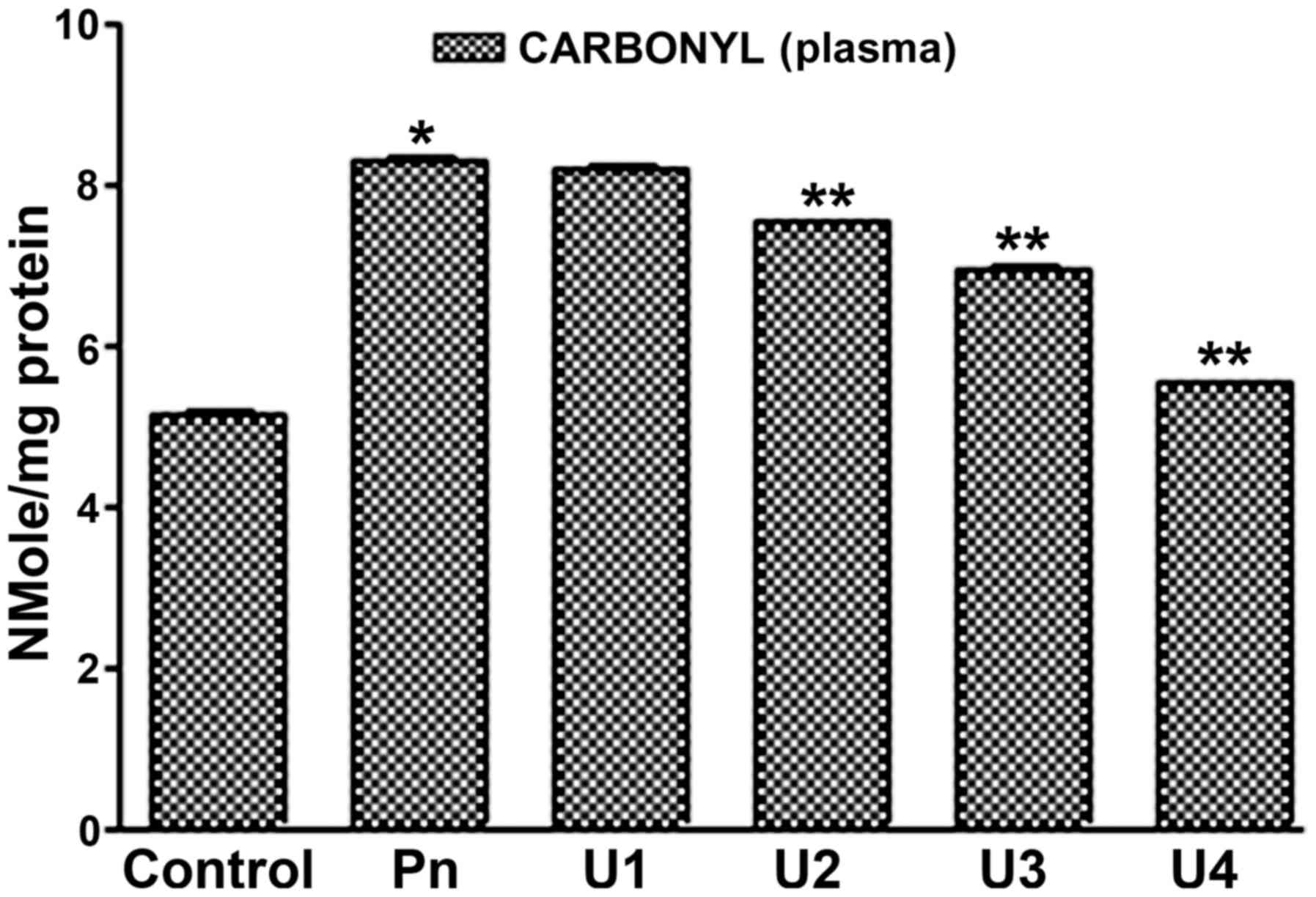
(Figs. 1 and 2). It was found that U-83836E significantly
(P<0.05) and in a dose-dependent manner reduced the rate of
carbonyl group formation of plasma proteins induced by
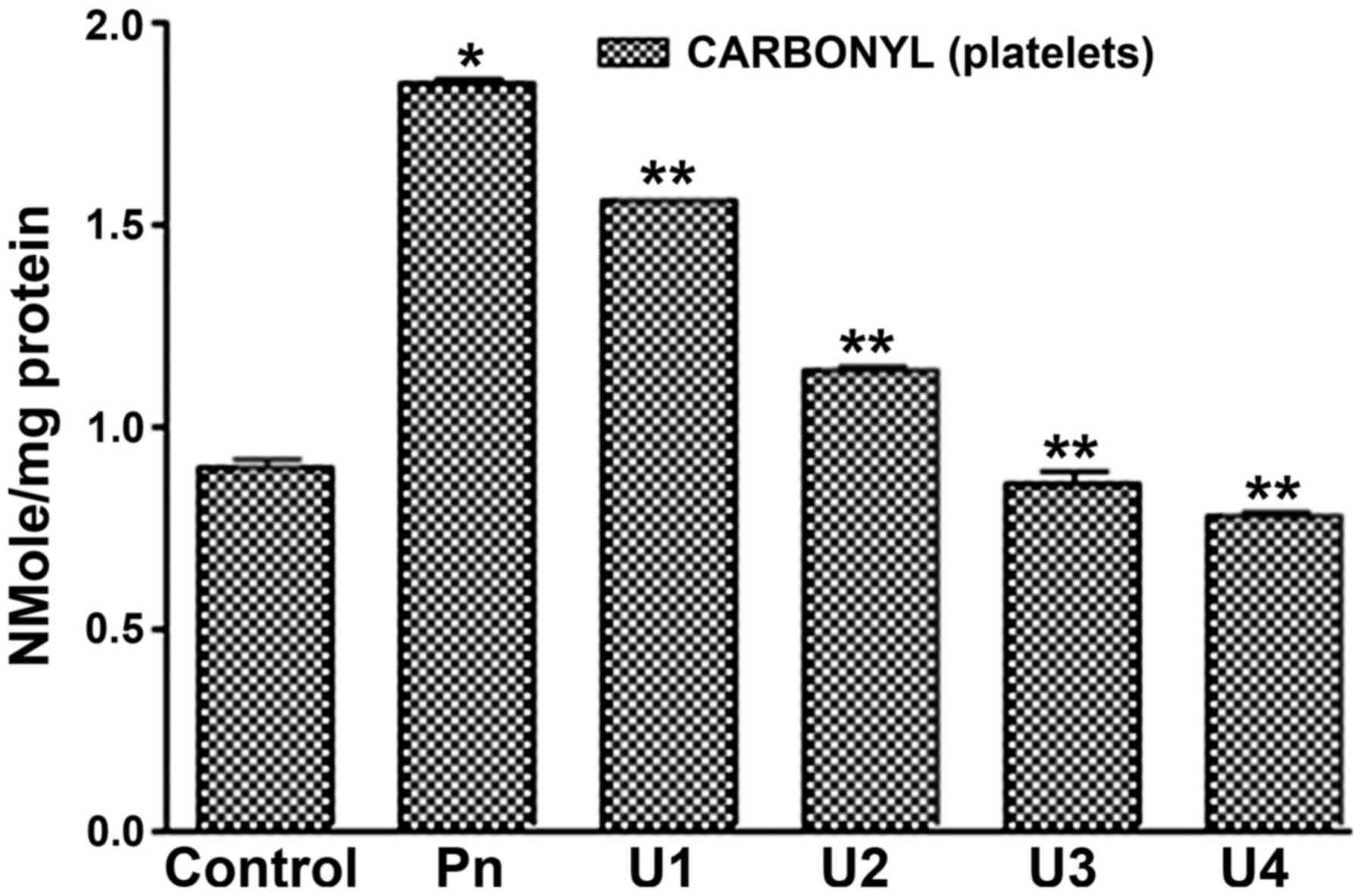
peroxynitrite treatment at doses of 50, 75 or 100 µM (Fig. 1). In addition, it was found that
U-83836E treatment significantly (P<0.05) reduced the effects of
peroxynitrite treatment on platelet proteins at all the doses
assessed (25, 50, 75 or 100 µM). Of note, in contrast to the
plasma-treated samples, U-83836E was able to completely reverse the
effect of peroxynitrite treatment at U-83836E doses of 75 or 100
µM.
U83836E antagonizes the oxidative
damage induced by peroxynitrite on the lipids of blood plasma and
platelets
An increase in TBARS concentration is a hallmark of
lipid peroxidative damage. In our previous study, it was shown that
peroxynitrite at a dose of 100 µM significantly increased the TBARS
concentration in blood plasma and platelets, indicating an increase
in lipid peroxidation (20). The
present study aimed to evaluate whether U-83836E can antagonize the
oxidative effects of peroxynitrite on blood plasma and platelet
lipids using TBARS concentration as an endpoint. Therefore, blood
plasma and platelet samples were treated with 100 µM of
peroxynitrite alone or in combination with different doses of
U-83836E (25, 50, 75 or 100 µM), and the TBARS concentrations in
the treated samples were then then measured (Figs. 3 and 4).
It was found that U-83836E significantly (P<0.05) and in a
dose-dependent manner reduced the TBARS concentration in the blood
plasma (Fig. 3) and platelet lipids
(Fig. 4) induced by peroxynitrite
treatment at U-83836E doses of 50, 75 or 100 µM. Of note, the
antagonizing effect of U-83836E, in terms of the percentage
reduction in TBARS concentration, was more marked in the blood
plasma-treated samples (Fig. 3)
compared with that in the platelet samples (Fig. 4) at U-83836E doses of 50, 75 and 100
µM.
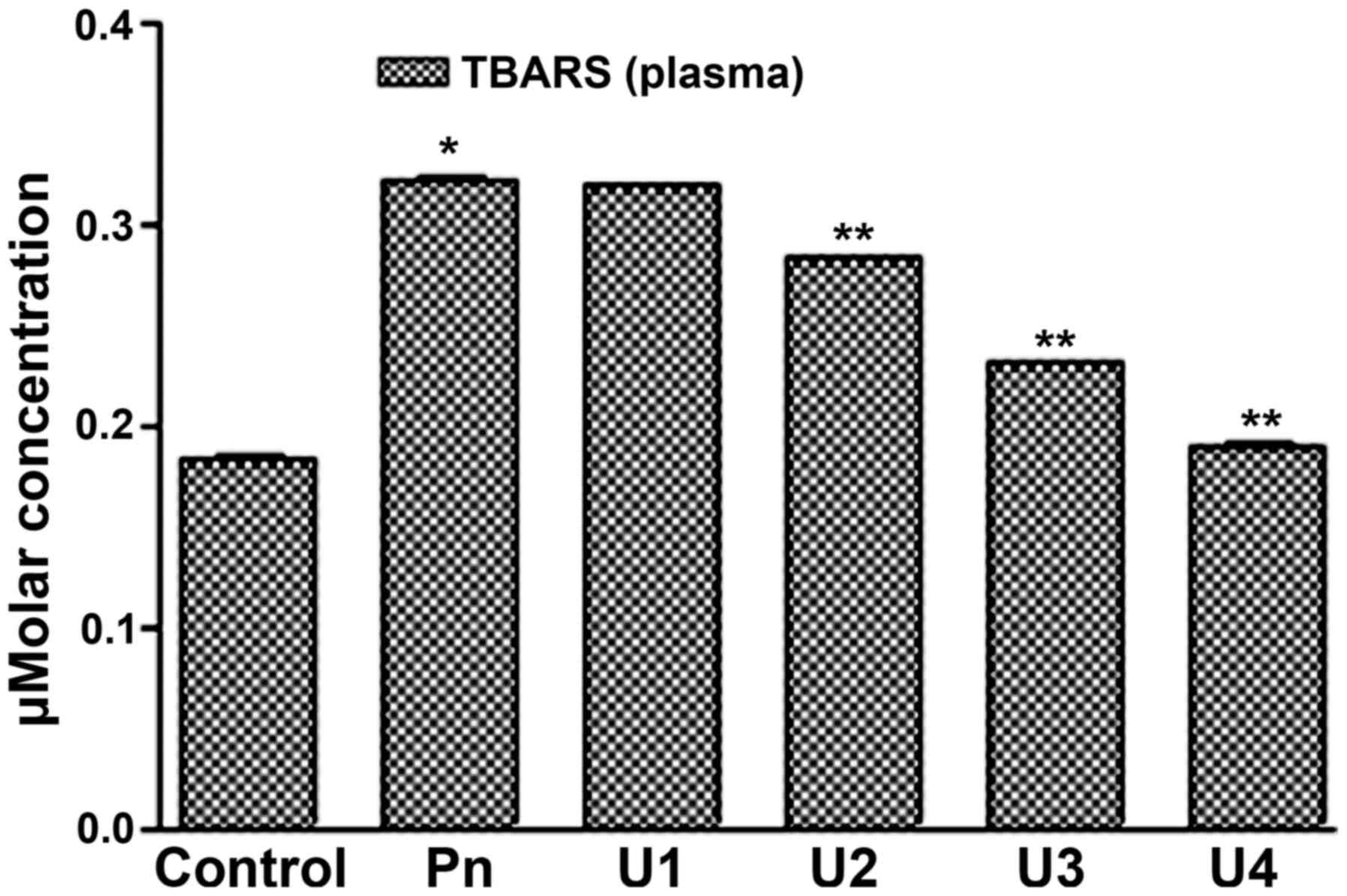 | Figure 3.U83836E reduces the ability of
peroxynitrite to induce TBARS formation in plasma lipids. Plasma
samples were treated with 100 µM of Pn alone or in combination with
U83836E at concentrations of 25, 50, 75 or 100 µM, designated as
U1, U2, U3 and U4, respectively. TBARS formation was then measured
using a colorimetric based assay. The results are expressed as μM
concentration of TBARS and are representative of three independent
experiments performed in triplicate. The concentration of TBARS is
expressed as the mean ± standard deviation. *P<0.05, compared
with the control (no U83836E) group; **P<0.05, compared with the
Pn group. TBARS, thiobarbituric acid reactive substances; Pn,
peroxynitrite. |
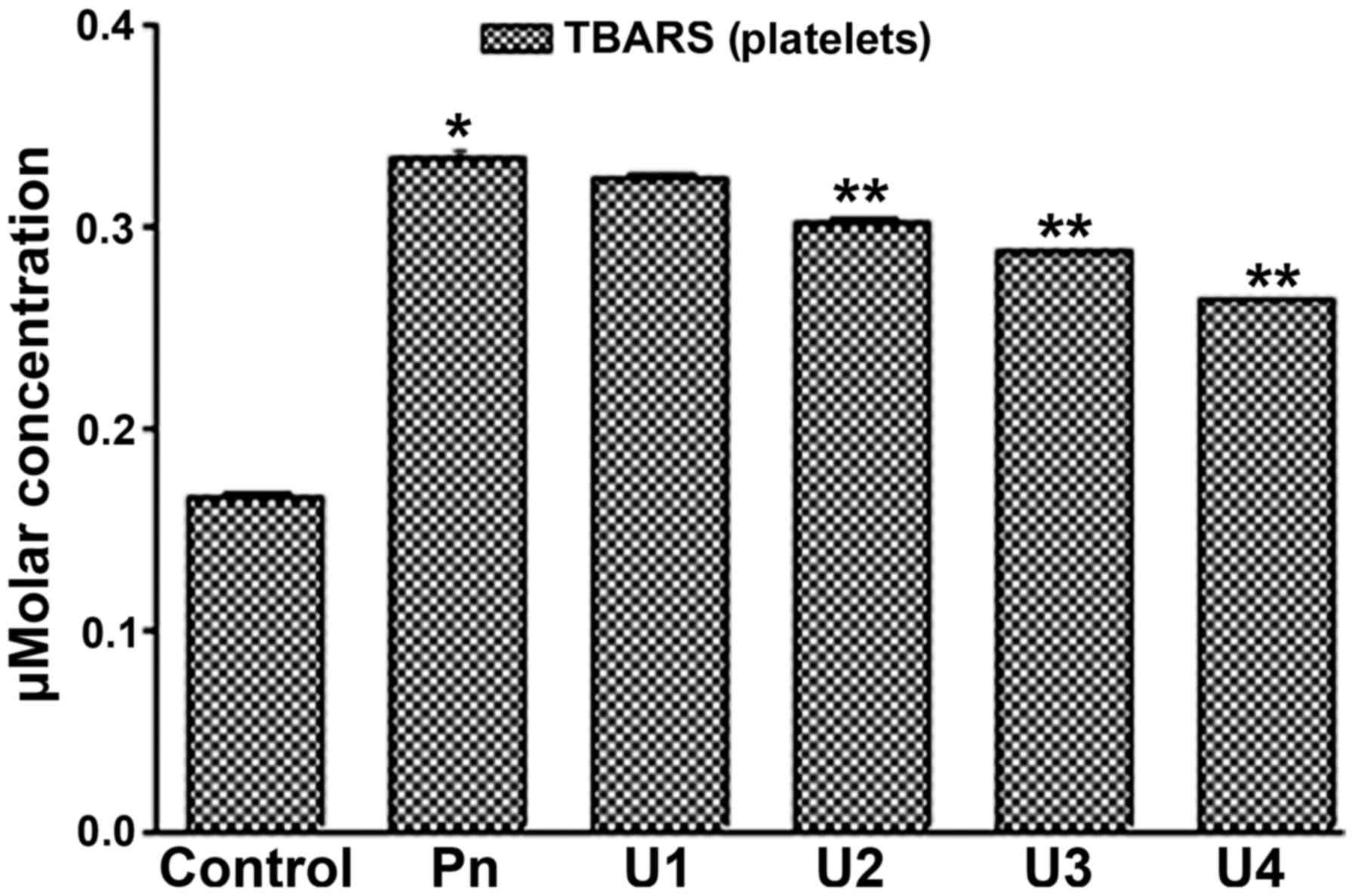 | Figure 4.U83836E reduces the ability of Pn to
induce TBARS formation in platelet lipids. Platelet samples were
treated with 100 µM of Pn alone or in combination with U83836E at
concentrations of 25, 50, 75 or 100 µM, designated as U1, U2, U3
and U4, respectively. TBARS formation was then measured using a
colorimetric based assay. The results are expressed as μM
concentration of TBARS and are representative of three independent
experiments performed in triplicate. The concentration of TBARS is
expressed as the mean ± standard deviation. *P<0.05, compared
with the control (no U83836E) group; **P<0.05, compared with the
Pn group. TBARS, thiobarbituric acid reactive substances; Pn,
peroxynitrite. |
Discussion
The production of peroxynitrite in the intravascular
compartment is a hallmark and a consistent feature of a number of
chronic diseases (19). Accordingly,
attenuation of the oxidative damage induced by peroxynitrite in the
vasculature remains one of the most important clinical challenges
(23). However, despite the clear
medical requirement for potent inhibitors of the oxidative effects
of peroxynitrite on the blood, a review of the literature in the
area of reactive oxygen and nitrogen species shows that the
majority of the studies have been performed in the context of
inhibiting the oxidative damage of peroxynitrite in the nervous
system (24). Our previous studies
reported on the potential clinical utility of two important
pharmacological agents as inhibitors of the cytotoxic effects of
peroxynitrite on blood plasma and on platelet lipids and proteins;
namely, tempol (20) and phenelzine
(21). Although, these two agents
significantly inhibited the oxidative damage induced by
peroxynitrite on blood plasma and on platelet lipids and proteins.
The inhibitory effects of the two agents were modest in certain
endpoints investigated; for example, there remained a significant
difference between the antioxidant treatment groups and the control
group. Therefore, potential remains for identifying superior
inhibitors. The present study hypothesized that, by investigating
other antioxidant agents that interfere with other steps of the
proposed model of how peroxynitrite induces its cytotoxic effect,
it is possible to identify more potent inhibitors. Additionally,
investigating other inhibitors is likely to provide further insight
on the biochemical mechanisms underlying how peroxynitrite causes
cell, body fluid and tissue damage. To this end, using an in
vitro system previously developed for investigating the
protective effects of tempol (20)
and phenelzine (21), the present
study investigated whether another antioxidant (U-83836E) was also
able to inhibit the oxidizing effects of peroxynitrite on blood
plasma and platelet lipids and proteins.
The structure of U-83836E, as reviewed previously
(25), shows that it has the
advantage of combining two different mechanisms for attenuating
oxidative damage in one single structure. U-83836E is a
2-methylaminochroman, which combines the chroman ring of
α-tocopherol (vitamin E) with a bis-pyrrolo pyrimidine. The chroman
ring component of U-83836E can effectively scavenge lipid peroxyl
radicals. However, this results in the formation of the
particularly weak chroman radical. Involvement in the subsequent
‘scavenging cycle’ requires the chroman ring to be reduced back to
the non-radical form by the action of the glutathione system. By
contrast, the bis-pyrrolo pyrimidine component of U-83836E can
repeatedly (catalytically) scavenge lipid peroxyl radicals, the
detailed mechanism of which has been reviewed previously (25). The dual mechanism by which U-83836E
scavenges lipid peroxyl radicals makes it one of the most potent
anti-oxidizing agents available.
The generation of TBARS is a well-established index
of lipid peroxidation (26). The
formation of lipid peroxyl radicals is considered one of the
‘dogmas’ of how lipid peroxidation is propagated (27). Lipid peroxidation is reported to be
induced by peroxynitrite through its decomposition into hydroxyl
radicals (28). Therefore, it is not
surprising that the scavenging of lipid peroxyl radicals via the
use of U-83836E inhibited the TBARS formation induced by
peroxynitrite treatment. The results of the present study
demonstrated that the inhibition of TBARS production by U-83836E
was more pronounced in the plasma compared with that in the
platelet samples. There are several reasons that may explain this
finding: i) Platelet samples may have a higher lipid content than
plasma samples and thus may produce more lipid peroxidation
products upon their treatment with peroxynitrite compared to plasma
samples; ii) the assay used to measure TBARS formation only
estimates the level of free MDA, therefore, protein-bound MDA does
not react with TBA using the assay described (21). It is possible that a scenario exists
in which there is a higher free MDA to protein-bound MDA ratio in
the platelet samples compared with that in the plasma samples upon
their combined treatment with the same dose of peroxynitrite and
U-83836E. As the TBARS assay is only able to detect the free MDA,
the results suggest a weaker inhibition of TBARS production by
U-83836E in the platelet samples; iii) inadvertent overheating of
the platelet samples compared with the plasma may produce secondary
MDA from the breakdown of hydroperoxides (21); this pool of MDA may evade the
antagonizing effects of U-83836E in the platelet samples causing
the apparent superior inhibition of TBARS formation by U-83836E in
the plasma samples.
By contrast, protein carbonylation is a
well-established index of protein oxidation. An increase in the
rate of protein carbonylation by peroxynitrite can result from i)
oxidation of the side chains of threonine, lysine and proline into
carbonyl groups following their reaction with the reactive oxygen
or nitrogen species that result from peroxynitrite treatment,
including lipid peroxyl radicals (a ROS) (29); or ii) the reaction of the side chains
of histidine, cysteine, and lysine with reactive aldehyde species,
for example, 4-hydroxynonenal (4-HNE) and MDA, which result from
lipid peroxidation (29). Given the
above discussion, it is not surprising that scavenging lipid
peroxyl radicals by U-83836E reversed the protein carbonylation of
blood plasma and platelets induced by peroxynitrite, as lipid
peroxyl radicals are ROS by chemical definition (17) and can increase protein carbonylation
directly, as explained above, and/or indirectly, as the formation
of lipid peroxyl radicals is an intermediate of the biochemical
pathway through which lipid peroxidation occurs, which ends in the
formation of 4-HNE and MDA.
In conclusion, the present study provided evidence
that scavenging lipid peroxyl radicals via the use U-83836E may be
a viable alternative to attenuating the cytotoxic effects of
peroxnitrite on blood plasma and on platelet lipids and
proteins.
Acknowledgements
Not applicable.
Funding
Funding for this the present study was provided
through a Sabbatical Leave Research Grant awarded by the Deanship
of Research at Jordan University of Science and Technology (grant
no. 154/2017).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
All the authors were involved in the design,
analysis of the data, and final review of the manuscript. AGM and
OA conceived the study; AGM, MAA, OA and ANA assisted in data
collection; MAA performed the statistical analysis; AGM, OA and ANA
performed all biochemical measurements; MAA and AGM drafted the
manuscript. All authors read and approved the final manuscript.
Ethical approval and consent to
participate
All procedures performed in experiments involving
human participants were in accordance with the ethical standards of
Jordan University of Science and Technology and King Abdullah
University Hospital IRB, and with the 1964 Helsinki declaration and
its later amendments or comparable ethical standards. Informed
consent was obtained from all individual participants included in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
PUFA
|
polyunsaturated fatty acids
|
|
DNA
|
deoxyribonucleic acid
|
|
TBARS
|
thiobarbituric acid reactive
substances
|
|
IRB
|
Institutional Review Board
|
|
CPDA
|
citrate phosphate dextrose adenine
|
|
SPSS
|
Statistical Package for Social
Studies
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
4-HNE
|
4-hydroxynonenal
|
|
MDA
|
malondialdehyde
|
References
|
1
|
Turrens JF: Mitochondrial formation of
reactive oxygen species. J Physiol. 552:335–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hakim J: Reactive oxygen species and
inflammation. C R Seances Soc Biol Fil. 187:286–295. 1993.(In
French). PubMed/NCBI
|
|
3
|
Bergamini CM, Gambetti S, Dondi A and
Cervellati C: Oxygen, reactive oxygen species and tissue damage.
Curr Pharm Des. 10:1611–1626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu BP: Cellular defenses against damage
from reactive oxygen species. Physiol Rev. 74:139–162. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Foyer CH and Noctor G: Ascorbate and
glutathione: The heart of the redox hub. Plant Physiol. 155:2–18.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pigeolet E, Corbisier P, Houbion A,
Lambert D, Michiels C, Raes M, Zachary MD and Remacle J:
Glutathione peroxidase, superoxide dismutase, and catalase
inactivation by peroxides and oxygen derived free radicals. Mech
Ageing Dev. 51:283–297. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Waris G and Ahsan H: Reactive oxygen
species: Role in the development of cancer and various chronic
conditions. J Carcinog. 5:14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benzi G and Moretti A: Are reactive oxygen
species involved in Alzheimer's disease? Neurobiol Aging.
16:661–674. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Werner C and Engelhard K: Pathophysiology
of traumatic brain injury. Br J Anaesth. 99:4–9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hall ED, Vaishnav RA and Mustafa AG:
Antioxidant therapies for traumatic brain injury.
Neurotherapeutics. 7:51–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Houstis N, Rosen ED and Lander ES:
Reactive oxygen species have a causal role in multiple forms of
insulin resistance. Nature. 440:944–948. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maurya PK, Noto C, Rizzo LB, Rios AC,
Nunes SO, Barbosa DS, Sethi S, Zeni M, Mansur RB, Maes M, et al:
The role of oxidative and nitrosative stress in accelerated aging
and major depressive disorder. Prog Neuropsychopharmacol Biol
Psychiatry. 65:134–144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gutteridge JM and Halliwell B: The
measurement and mechanism of lipid peroxidation in biological
systems. Trends Biochem Sci. 15:129–135. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hemnani T and Parihar MS: Reactive oxygen
species and oxidative DNA damage. Indian J Physiol Pharmacol.
42:440–452. 1998.PubMed/NCBI
|
|
16
|
Bandyopadhyay U, Das D and Banerjee RK:
Reactive oxygen species: Oxidative damage and pathogenesis. Curr
Sci. 77:658–666. 1999.
|
|
17
|
Halliwell B: Reactive oxygen species in
living systems: Source, biochemistry, and role in human disease. Am
J Med. 91(3C): 14S–22S. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beckman JS, Beckman TW, Chen J, Marshall
PA and Freeman BA: Apparent hydroxyl radical production by
peroxynitrite: Implications for endothelial injury from nitric
oxide and superoxide. Proc Natl Acad Sci USA. 87:1620–1624. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pacher P, Beckman JS and Liaudet L: Nitric
oxide and peroxynitrite in health and disease. Physiol Rev.
87:315–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mustafa AG, Bani-Ahmad MA, Jaradat AQ and
Allouh MZ: Tempol protects blood proteins and lipids against
peroxynitrite-mediated oxidative damage. Exp Biol Med (Maywood).
240:109–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mustafa AG, Al-Shboul O, Alfaqih MA,
Al-Qudah MA and Al-Dwairi AN: Phenelzine reduces the oxidative
damage induced by peroxynitrite in plasma lipids and proteins. Arch
Physiol Biochem. Dec 19–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Campo GM, Squadrito F, Campo S, Altavilla
D, Avenoso A, Ferlito M, Squadrito G and Caputi AP: Antioxidant
activity of U-83836E, a second generation lazaroid, during
myocardial ischemia/reperfusion injury. Free Radic Res. 27:577–590.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Willcox JK, Ash SL and Catignani GL:
Antioxidants and prevention of chronic disease. Crit Rev Food Sci
Nutr. 44:275–295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hall ED, Detloff MR, Johnson K and Kupina
NC: Peroxynitrite-mediated protein nitration and lipid peroxidation
in a mouse model of traumatic brain injury. J Neurotrauma. 21:9–20.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mustafa AG, Singh IN, Wang J, Carrico KM
and Hall ED: Mitochondrial protection after traumatic brain injury
by scavenging lipid peroxyl radicals. J Neurochem. 114:271–280.
2010.PubMed/NCBI
|
|
26
|
Esterbauer H and Cheeseman KH:
Determination of aldehydic lipid peroxidation products:
malonaldehyde and 4-hydroxynonenalMethods in enzymology. 186.
Elsevier; New York, NY: pp. 407–421. 1990, View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buettner GR: The pecking order of free
radicals and antioxidants: Lipid peroxidation, α-tocopherol, and
ascorbate. Arch Biochem Biophys. 300:535–543. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Radi R, Beckman JS, Bush KM and Freeman
BA: Peroxynitrite-induced membrane lipid peroxidation: The
cytotoxic potential of superoxide and nitric oxide. Arch Biochem
Biophys. 288:481–487. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suzuki YJ, Carini M and Butterfield DA:
Protein carbonylation. Antioxid Redox Signal. 12:323–325. 2010.
View Article : Google Scholar : PubMed/NCBI
|