Introduction
Progesterone is a steroid hormone that has been
identified to inhibit contraction of smooth muscle in various
regions in the gastrointestinal (GI) tract (1–5). It has
been indicated that changes in the levels of steroid hormones in
the plasma, including of estrogen and progesterone, leads to GI
motility disturbances in pregnant women. Specifically, pregnancy,
which is characterized by high plasma steroid hormonal levels, has
been associated with decreased gallbladder contractility (6), lowered esophageal sphincter pressure
(7), reduced gastric emptying
(8–10), and reduced small intestinal (11) and colonic transit (9). However, the exact molecular mechanisms
for such steroid hormone-associated GI motility disorders remain
poorly understood.
Progesterone can impair the actions of agonists that
are G protein receptor dependent. In the context of muscle
function, progesterone has been observed to downregulate
Gαi and Gαq proteins, which mediate
contraction, and upregulate Gαs proteins, which mediate
relaxation (1,2). Furthermore, it has been suggested that
progesterone may lead to activation of tyrosine kinases (12) and mitogen-activated protein kinases
(13), and inhibition of membrane
transport systems (14). Researchers
have previously identified that progesterone inhibited
agonist-induced contraction in dissociated colonic muscle cells,
mediated by Ca2+ release from intracellular stores
(15). The same group later reported
that progesterone decreased the basal colon motility in vivo
by altering the levels and actions of prostaglandins (16). Our group previously demonstrated that
progesterone may rapidly affect the contractile activity of
isolated gastric smooth muscle cells (GSMCs) in rats via inhibition
of the Rho kinase II pathway (17).
Physiologically, smooth muscle is an important component of the GI
tract, and maintaining its normal contractile behavior is essential
for proper GI functions. Smooth muscle relaxation is initiated by
targeting dephosphorylation of the 20-kDa regulatory myosin light
chain (MLC20). Most agents cause relaxation by
stimulating the production of cyclic adenosine monophosphate (cAMP)
or cyclic guanosine monophosphate (cGMP) (18). cAMP-activated protein kinase A and
cGMP-activated protein kinase G are the main enzymes that induce
relaxation in smooth muscle (19).
Nitric oxide (NO) induces the production of cGMP from guanosine
triphosphate via activating the soluble guanylyl cyclase (sGC)
(20). cGMP is then rapidly degraded
by cGMP-specific phosphodiesterases (PDEs) (21).
Although numerous studies (9,15,16) have examined the effect of progesterone
on GI smooth muscle, its effect on the gastric NO/cGMP pathway and
thus muscle contraction has not yet been investigated to our
knowledge. Therefore, the present study was designed to investigate
the action of progesterone on the NO/cGMP pathway in smooth muscle
cells of the stomach. Insights into the molecular basis of
progesterone effects on gastric smooth muscle function would be an
important step for improved understanding of certain GI motility
disturbances and complaints that complicate pregnancy, and of
certain female functional disorders such as female colonic inertia,
colonic slow transit and delayed gastric emptying.
Materials and methods
Materials
A DC protein assay kit for measuring protein
concentration was obtained from Bio-Rad Laboratories, Inc. (cat.
no. 500–0116; Hercules, CA, USA). A cGMP colorimetric ELISA kit
(cat. no. STA-505) was obtained from Cell BioLabs, Inc., San Diego,
CA, USA. 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; cat. no.
ab120022) and Nω-Nitro-L-arginine (L-NNA; cat. no. ab141312) were
obtained from Abcam (Cambridge, UK). A 500-µm Nitex mesh was
purchased from Sefar AG, Thal, Switzerland. All remaining chemicals
were from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Stock
solution of progesterone was prepared in 100% ethanol. Stock
solutions of ODQ and L-NNA were prepared in dimethylsulfoxide. The
final concentration of ethanol and DMSO was 1% (v/v).
Isolation of GSMCs
All experimental protocols were approved by the
Animal Care and Use Committee at Jordan University of Science and
Technology, Irbid, Jordan and all procedures were conducted in
accordance with the guidelines set by this committee. A total of 20
female Sprague-Dawley rats (12 weeks of age; 250–300 g) were
provided by the animal house of the Jordan University of Science
and Technology. They were housed under standardized conditions
(temperature 20–22°C, humidity 50–60% and a 12-h light/dark cycle)
and allowed free access to food and tap water throughout the
experiments. Animals were euthanized by inhalation of
CO2 for at least 5 min. For confirmation of euthanasia
an incision was made through the chest cavity with a scalpel blade.
Following euthanasia the stomach was immediately excised. Smooth
muscle cells were isolated from the stomachs of the rats by
sequential enzymatic digestion, filtration and centrifugation as
described previously (22,23). In brief, strips of muscle from the
stomach were dissected and incubated at 31°C for 30 min in
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer
composed of: 120 mM NaCl, 4 mM KCl, 2.0 mM CaCl2, 2.6 mM
KH2PO4, 0.6 mM MgCl2, 25 mM HEPES,
14 mM glucose, 2.1% Eagle's essential amino acid mixture, 0.1%
collagenase and 0.01% soybean trypsin inhibitor with pH adjusted to
7.4. The partly digested strips were washed twice with 50 ml
enzyme-free HEPES medium and the muscle cells were allowed to
disperse spontaneously for 30 min. The cells were harvested by
filtration through 500-µm Nitex mesh and centrifuged twice at 350 ×
g for 10 min at 4°C to eliminate broken cells and organelles. Cells
were maintained at room temperature and experiments were performed
within 2–3 h of cell collection.
Measurement of contraction in
dispersed GSMCs
Contraction of isolated muscle cells was measured by
scanning micrometry as described previously (23,24). In
brief, aliquots of cell suspension from 10 of the rats each
containing ~104 cells/ml were added to HEPES medium and
randomly distributed into either control or progesterone-treated
groups. Cells in the treatment groups were incubated at room
temperature for 10 min with progesterone (1 µM), progesterone and
ODQ (GC inhibitor; 1 µM), or progesterone and L-NNA (NO synthase
inhibitor; 1 µM). A progesterone concentration of 1 µM was
effective in our previous research (17); in addition, after reviewing the
progesterone dose response curve reported in other studies
(25,26), the concentration of 1 µM occurred in
the middle of the curve and was thus deemed suitable. Cells were
then stimulated for 10 min with acetylcholine (ACh; 0.1 µM) in the
presence or absence of treatment agents at room temperature. Cells
in the control groups were treated with or without ACh (0.1 µM).
Cells in the control group not treated with ACh (treated only with
distilled water) were considered as the negative control and used
for measuring the basal cell length. The reaction was terminated
with acrolein (0.1% final concentration). The cells were viewed
using a ×10 or ×20 objective of an inverted Nikon TMS-f microscope
(Nikon Corporation, Tokyo, Japan), and cell images were acquired
using a Canon digital camera (Canon Inc., Tokyo, Japan) and ImageJ
acquisition software (version 1.45s; National Institutes of Health,
Bethesda, MA, USA). The length of 50 muscle cells treated with the
contractile agent (ACh) was measured at random by scanning
micrometry (23,24). This was then compared with the length
of untreated cells. Contraction was expressed as the percentage
decrease of mean cell length, as compared with the control
group.
Measurement of smooth muscle NO and
cGMP
In the remaining rats (n=10), the concentration of
NO in smooth muscle samples was indirectly measured by determining
nitrite and nitrate levels utilizing an NO
(NO2−/NO3−) assay kit
(cat. no. 23479; Sigma-Aldrich; Merck KGaA) following the
manufacturer's protocol. The level of cGMP in smooth muscle samples
was also measured using the cGMP ELISA kit according to the
manufacturer's protocol. NO and cGMP levels were measured in cells
treated with progesterone, and in cells not treated with
progesterone which represented the basal levels.
Detection of progesterone receptor
(PR) expression by reverse transcription-polymerase chain reaction
(RT-PCR)
RT-PCR was performed on cDNA samples synthesized
from total RNA isolated from stomach muscle cells and PCR
conditions were optimized via preliminary runs with a BioRad T100
PCR Thermal Cycler (Bio-Rad Laboratories, Inc.). Total RNA was
isolated from freshly dispersed smooth muscle cells with a
Quick-RNA MiniPrep kit (Zymo Research Corp., Irvine, CA, USA). A
total of 2 µg RNA from each preparation was reverse transcribed
using a PrimeScript RT Master Mix (Takara Bio, Inc., Otsu, Japan)
in a 10 µl reaction volume. The following time and temperature
profile was used for the PCR reactions: 95°C for 3 min; 40 cycles
of a series consisting of 3 sec at 95°C, 20 sec at 60°C and 30 sec
at 72°C; and a final extension for 5 sec at 85°C. The optimal
annealing temperatures were determined empirically for each primer
set. The sequences of specific primers for PR isoforms A and B were
forward, 5′-TGGTTCCGCCACTCATCA-3′ and reverse,
5′-TGGTCAGCAAAGAGCTGGAAG-3′ (NM_022847.1); and for GAPDH (internal
control) were forward, 5′-TGGTGGACCTCATGGCCTAC-3′ and reverse
5′-CAGCAACTGAGGGCCTCTCT-3′. The identity and integrity of the
products were confirmed by electrophoresis on 2% agarose gel
containing 0.1 µg/ml ethidium bromide.
Statistical analysis
Results are expressed as the mean ± standard error
of the mean. Statistical analysis of all experiments was performed
using Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA). Statistical differences between two means were determined by
Student's t-test. Statistical differences between multiple groups
were determined using one-way analysis of variance followed by
Tukey's post-hoc test. Differences were considered significant at
P<0.05.
Results
Expression of PR
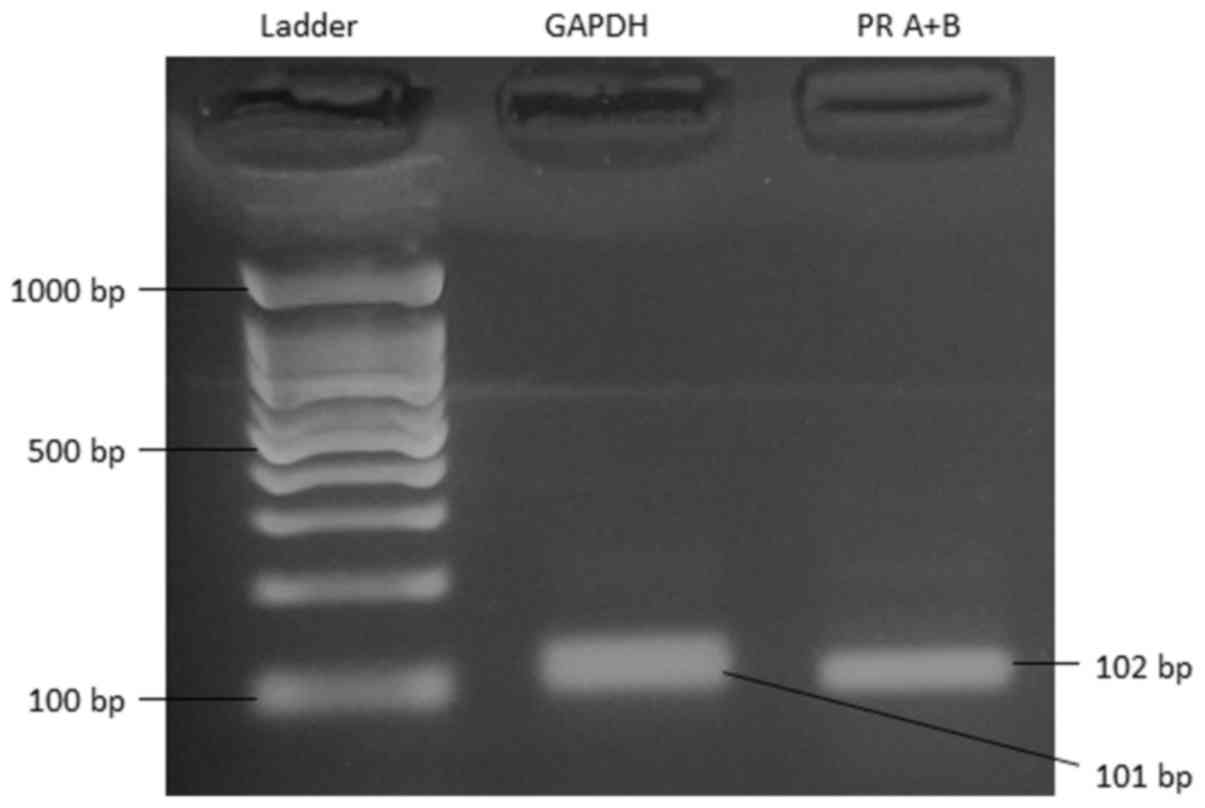
Primers aligning to a common interior sequence of PR
isoform A and B mRNA amplified a 102 bp product in RT-PCR. The
identity and integrity of the product was confirmed by
electrophoresis in agarose gel in the presence of ethidium bromide
(Fig. 1). PCR yielded the expected
product sizes (GAPDH at 101 bp and PR A+B at 102 bp) based on prior
Basic Local Alignment Search Tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi)
calculations.
Effect of progesterone on NO and cGMP
formation in single GSMCs
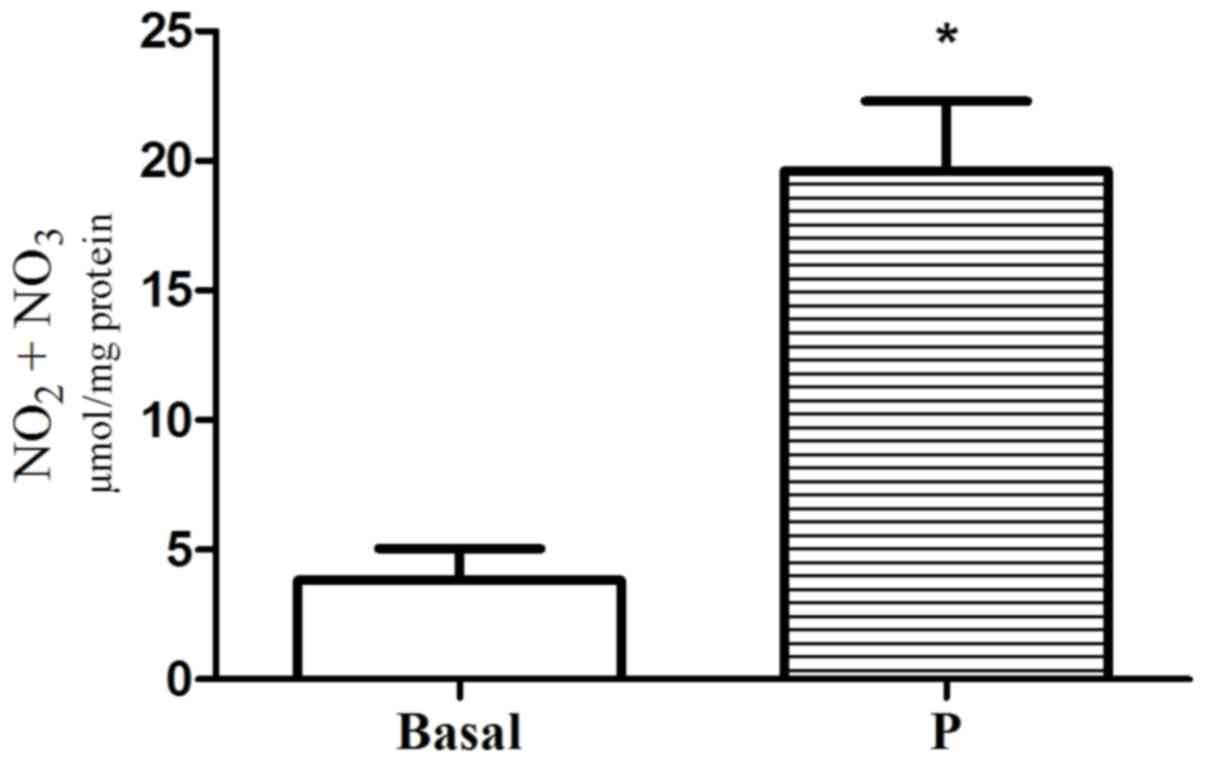
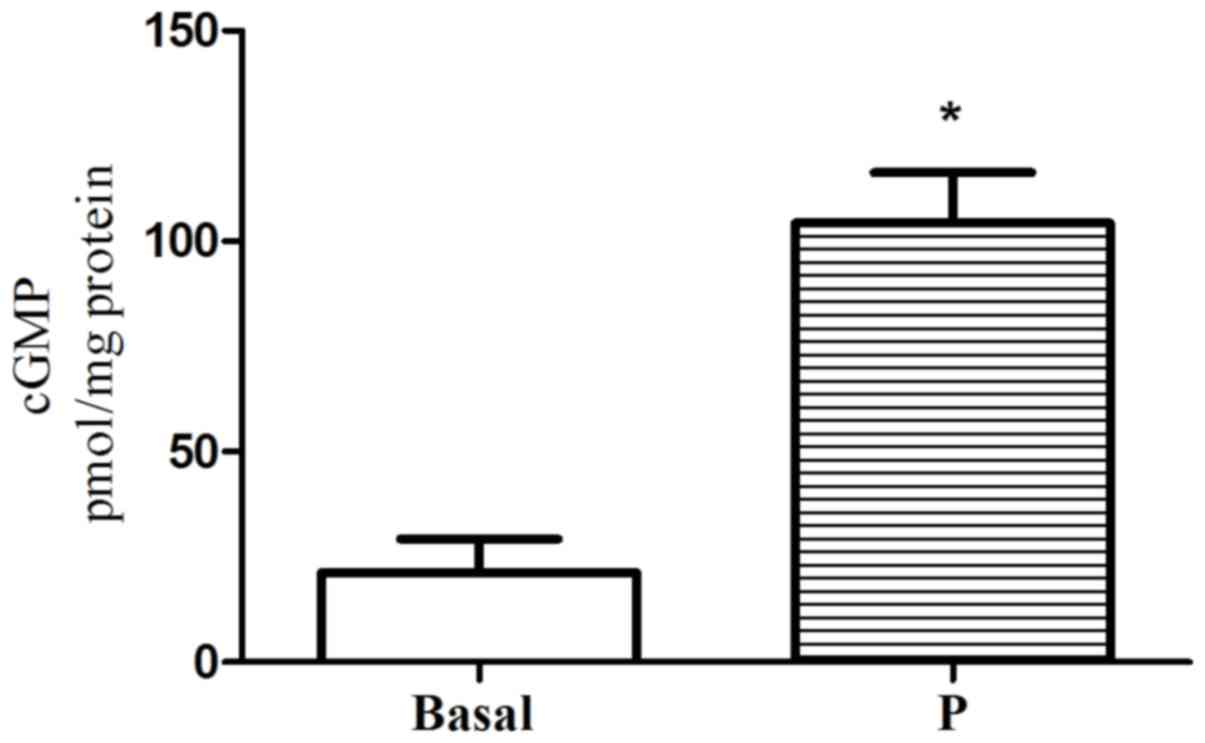
Incubation of GSMCs with progesterone significantly
increased NO and cGMP above basal levels (5.12-fold for
NO2−/NO3−and 4.88-fold
for cGMP; P<0.05; Figs. 2 and
3, respectively).
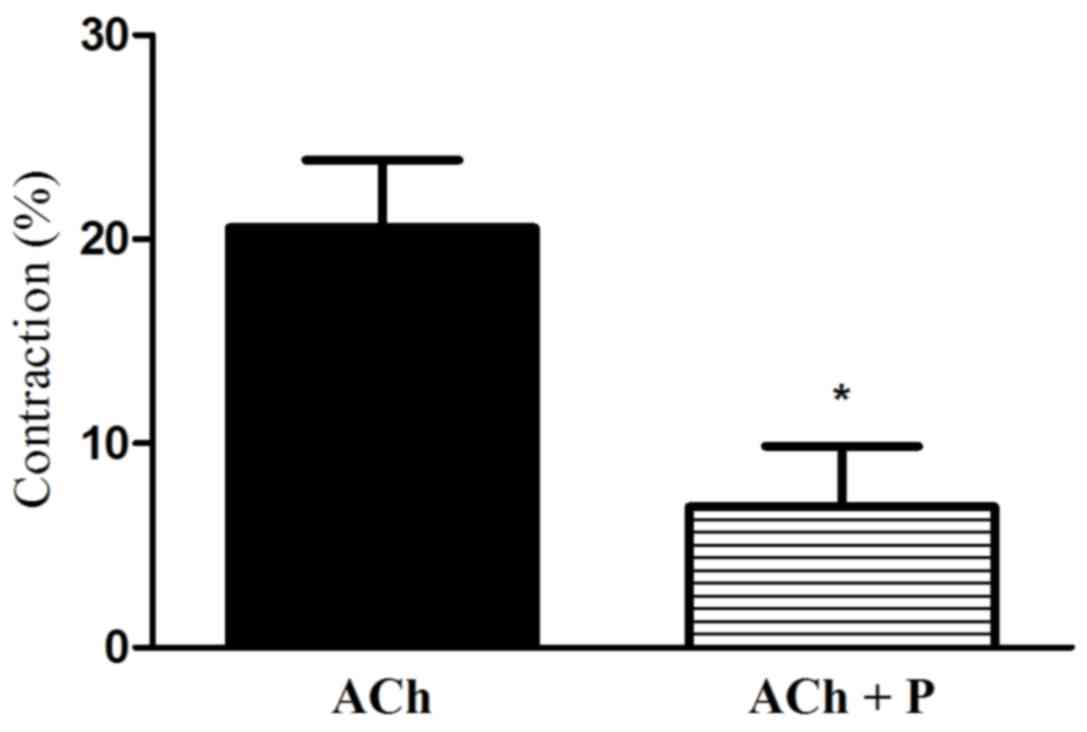
Effect of progesterone on ACh-induced
gastric muscle contraction
Treatment with ACh lead to muscle cell contraction.
More notably, treatment of GSMCs with progesterone significantly
reduced the ACh-stimulated contraction of cells (66.54% reduction;
P<0.05; Fig. 4).
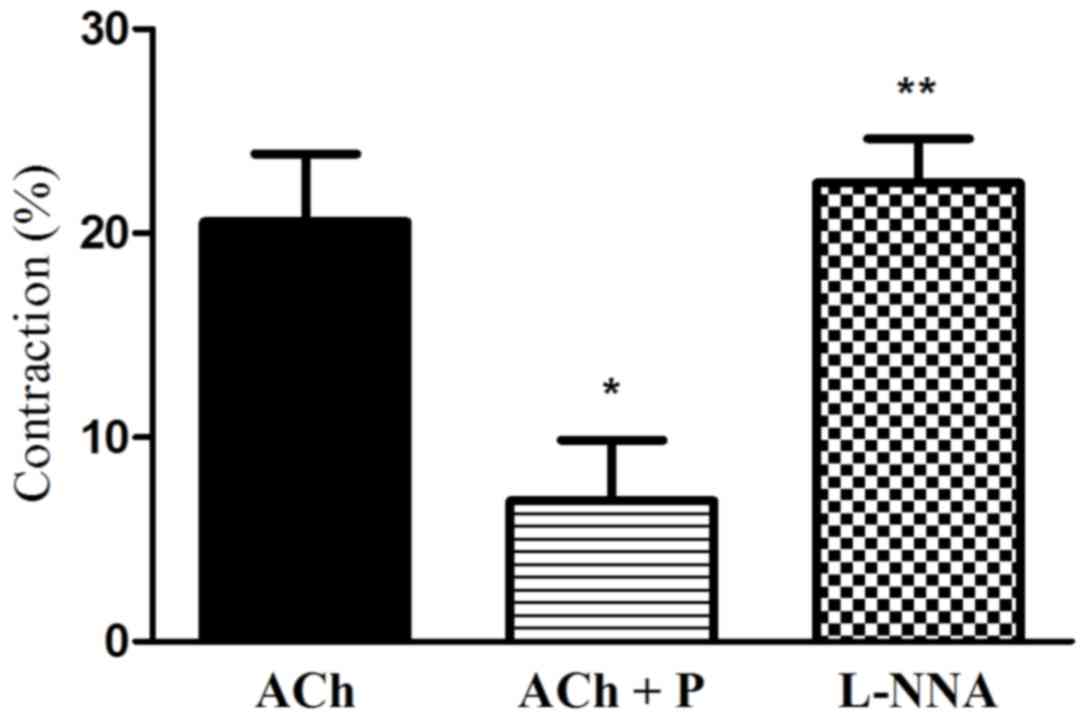
Effect of the blockade of NO synthase
on progesterone-induced relaxation
To investigate the role of NO in
progesterone-induced inhibition of muscle contraction, the effect
of NO synthase blocker (L-NNA) on progesterone-induced inhibition
of muscle contraction was examined. It was observed that L-NNA
significantly attenuated the progesterone-induced inhibition of
muscle cell contraction (2.4-fold increase in contraction vs. ACh
plus progesterone; P<0.05; Fig.
5).
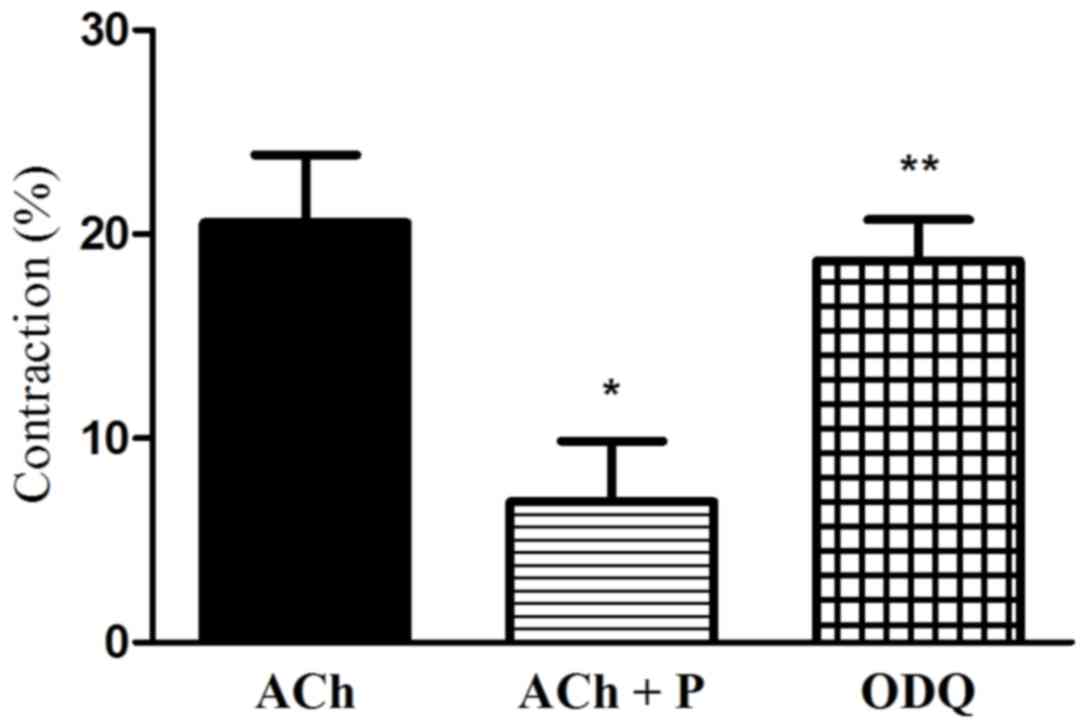
Effect of the blockade of sGC on
progesterone-induced relaxation
To investigate the role of cGMP in
progesterone-induced inhibition of muscle contraction, the effect
of sGC blocker (ODQ) on progesterone-induced inhibition of muscle
contraction was examined. ODQ alleviated the progesterone-induced
inhibition of muscle cell contraction (2.5-fold increase in
contraction vs. ACh plus progesterone; P<0.05; Fig. 6).
Discussion
The present study was designed to investigate the
mechanisms involved in the progesterone-induced effect on
agonist-stimulated contraction of smooth muscle cells in the
stomach. The results confirmed the expression of PR in GSMCs and
suggest that progesterone inhibits agonist-induced gastric muscle
contraction in rats. Such effect may be produced via stimulation of
the NO/cGMP pathway. This conclusion is supported by the following
observations: i) Progesterone inhibited ACh-induced contraction in
single GSMCs; ii) the blockade of NO synthase abolished this effect
of progesterone on gastric muscle cell contraction; and ii) the
blockade of guanylyl cyclase also attenuated this effect of
progesterone on gastric muscle cell contraction.
The present findings are in agreement with previous
studies which reported an inhibitory action of progesterone on GI
muscle contraction. For instance, Liu et al (9) identified that high doses of progesterone
could decrease gastric emptying. Similarly, Coşkun's group
(10) reported that chronic
progesterone treatment exerted inhibitory effects on gastric
emptying in conscious rats. Another study suggested that
progesterone may inhibit the contractile activity of isolated
gastric strips in rats (27).
Furthermore, a study on gallbladder muscle cells from adult guinea
pigs observed that progesterone treatment impaired the contractile
response to agonist (1). There is
data to suggest that high serum sex hormone concentration during
pregnancy is associated with alternations in the motor activity of
the GI tract, with include decreased gallbladder contractility and
lower esophageal sphincter pressure, reduced gastric emptying of
liquids, and reduced small intestine and colonic transit (5,7,8,11).
Contrary to the current findings, Xiao et al (15) reported that progesterone failed to
affect colonic muscle contraction induced by ACh in guinea pigs.
However, ACh application in their study was for 30 sec, and
considering that different receptor agonists may generate both
initial/transient (<1 min) Ca2+-dependent and
sustained (>5 min) Ca2+-independent contraction in GI
smooth muscle cells (18), it is
possible that ACh induced different signaling machinery with 30 sec
of treatment compared with the presently tested 10-min
treatment.
Treatment with progesterone for 10 min markedly
inhibited the ACh-induced contraction in gastric muscle cells. It
may be proposed that this potent hormonal effect on muscle
contraction represents mostly a nongenomic action of progesterone.
Nongenomic actions are defined as those occurring within 10 min of
hormonal exposure in a variety of tissue types (28,29). These
nongenomic actions of progesterone are mostly not blocked by
progesterone antagonists, which impede genomic actions of
progesterone and other progestins (15,25,30).
Whether progesterone affects an independent non-genomic cell
surface receptor distinct from the classical nuclear PR that is
part of the transcription-activating superfamily or affects other
membrane receptors such as G protein receptors remains unknown.
Previous studies have demonstrated the production of
NO in isolated gastric muscle cells (31) and the role of the NO/cGMP pathway in
the control of GI smooth muscle tone (18). Generally, NO induces smooth muscle
relaxation mainly through the activation of sGC and subsequent
increase in cGMP levels (18). NO can
also induce relaxation via a mechanism independent of cGMP by
acting on ion channels (32). The
NO/cGMP pathway has been reported to be involved in the relaxation
response to progesterone in various smooth muscle tissue regions
including the mesenteric arteries (33), endometrium (34), myometrium (35) and pig bladder neck smooth muscle
(36). The current results suggest
that progesterone produces relaxation in single GSMCs via the
NO/cGMP pathway, since progesterone-induced relaxations were
reduced by inhibitors of NO synthase and sGC. These findings are in
agreement with those obtained in pig bladder neck smooth muscle
(36) and rabbit pulmonary arteries
(37). The levels of cAMP and cGMP in
GI smooth muscle depend on the rates of their synthesis by cyclases
and degradation by PDEs (38,39). In addition to degradation by
phosphodiesterases, cyclic nucleotide elimination pathways include
active export into the extracellular space via members of the
multidrug resistance protein family (also known as the ATP-binding
cassette transporter family) (40). A
limitation of the current study is that focus was on the NO/cGMP
pathway and the effect of progesterone on these eliminatory
pathways was not examined. As an effect of progesterone on cyclic
nucleotide synthesis and generation pathways can be expected, this
should be investigated in future research.
Similar to the effect of progesterone on GSMCs, our
group recently reported on a reduction in the contraction of female
GSMCs following treatment with the sex steroid hormone estrogen,
and greater activation of the NO/cGMP pathway (41). These parallel findings strengthen the
hypothesis that these sex steroid hormones affect stomach muscle
cell contraction.
Previous study by our group has also indicated that
progesterone may rapidly affect the contractile activity of stomach
muscle via inhibition of the Rho kinase pathway (17). Moreover, we recently reported lower
RhoA/Rho-associated protein kinase pathway activation and lower
levels of MLC20 phosphorylation in female stomach muscle
cells compared with in male cells (22,23). These
reported differences may be related to differences in progesterone
action in each sex. Future studies on progesterone may further
uncover any other signaling pathways that are targeted by
progesterone to induce smooth muscle relaxation.
As progesterone may target various types of cells in
the stomach, studying its effect on the NO/cGMP pathway and muscle
contraction in multicellular preparations as in previous studies
(27,42) could be difficult and non-specific. For
this reason, all experiments in the present study were performed on
single gastric muscle cells to avoid the effect of other non-muscle
cell types. Indeed, the relatively high concentration of
progesterone (1 µM) required to produce relaxation of gastric
smooth muscle in the present experiments was considerably greater
than the picomolar-nanomolar levels of circulating steroids in the
plasma under normal (non-pregnant) physiological conditions
(43). However, the concentration
tested here agrees with the micromolar (0.1–10 µM) concentrations
of progesterone required to elicit significant relaxation in smooth
muscle of the GI tract in vitro (25,26). In
future studies, investigating the effect of progesterone on GSMCs
by constructing dose-response curves for a wide concentration range
would strengthen the present findings.
In conclusion, it was indicated in the present study
that progesterone reduced ACh-induced contraction in rat GSMCs and
that this progesterone-induced effect may be mediated by the
NO/cGMP pathway. Further understanding of the role of progesterone
and other sex hormones in modulating the normal physiological and
abnormal functions of the GI tract may enable more effective and
sex-dependent treatments for many of the known GI disturbances.
Acknowledgements
Not applicable.
Funding
The present work was supported by Jordan University
of Science and Technology, Irbid, Jordan (grant no. 20150178).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conception and design of the study were performed by
OAA. Acquisition of data and drafting of the manuscript were
performed by OAA, AGM and AAO. Analysis and interpretation of data
were performed by OAA, AGM, AAO, ANA, MSN and MoAA. Critical
revision of the manuscript for important intellectual content was
performed by OAA, AAO, MaAA and RAA. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Animal Care
and Use Committee of Jordan University of Science and Technology,
Irbid, Jordan.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen Q, Chitinavis V, Xiao Z, Yu P, Oh S,
Biancani P and Behar J: Impaired G protein function in gallbladder
muscle from progesterone-treated guinea pigs. Am J Physiol.
274:G283–G289. 1998.PubMed/NCBI
|
|
2
|
Chen Q, Xiao ZL, Biancani P and Behar J:
Downregulation of Galphaq-11 protein expression in guinea pig
antral and colonic circular muscle during pregnancy. Am J Physiol.
276:G895–G900. 1999.PubMed/NCBI
|
|
3
|
Datta S, Hey VM and Pleuvry BJ: Effects of
pregnancy and associated hormones in mouse intestine, in vivo and
in vitro. Pflugers Arch. 346:87–95. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ryan JP and Pellecchia D: Effect of
progesterone pretreatment on guinea pig gallbladder motility in
vitro. Gastroenterology. 83:81–83. 1982.PubMed/NCBI
|
|
5
|
Ryan JP and Bhojwani A: Colonic transit in
rats: Effect of ovariectomy, sex steroid hormones, and pregnancy.
Am J Physiol. 251:G46–G50. 1986.PubMed/NCBI
|
|
6
|
Everson GT, McKinley C, Lawson M, Johnson
M and Kern F Jr: Gallbladder function in the human female: Effect
of the ovulatory cycle, pregnancy, and contraceptive steroids.
Gastroenterology. 82:711–719. 1982.PubMed/NCBI
|
|
7
|
Brock-Utne JG, Dow TG, Dimopoulos GE,
Welman S, Downing JW and Moshal MG: Gastric and lower oesophageal
sphincter (LOS) pressures in early pregnancy. Br J Anaesth.
53:381–384. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parkman HP, Wang MB and Ryan JP: Decreased
electromechanical activity of guinea pig circular muscle during
pregnancy. Gastroenterology. 105:1306–1312. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu CY, Chen LB, Liu PY, Xie DP and Wang
PS: Effects of progesterone on gastric emptying and intestinal
transit in male rats. World J Gastroenterol. 8:338–341. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coşkun T, Sevinç A, Tevetoğlu I, Alican I,
Kurtel H and Yeğen BC: Delayed gastric emptying in conscious male
rats following chronic estrogen and progesterone treatment. Res Exp
Med. 195:49–54. 1995. View Article : Google Scholar
|
|
11
|
Ryan JP: Effect of pregnancy on intestinal
transit: Comparison of results using radioactive and
non-radioactive test meals. Life Sci. 31:2635–2640. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martinez F, Tesarik J, Martin CM, Soler A
and Mendoza C: Stimulation of tyrosine phosphorylation by
progesterone and its 11-OH derivatives: Dissection of a
Ca2+-dependent and a Ca2+-independent
mechanism. Biochem Biophys Res Commun. 255:23–27. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luconi M, Krausz C, Barni T, Vannelli GB,
Forti G and Baldi E: Progesterone stimulates p42 extracellular
signal-regulated kinase (p42erk) in human spermatozoa. Mol Hum
Reprod. 4:251–258. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Metherall JE, Waugh K and Li H:
Progesterone inhibits cholesterol biosynthesis in cultured cells.
Accumulation of cholesterol precursors. J Biol Chem. 271:2627–2633.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao ZL, Cao W, Biancani P and Behar J:
Nongenomic effects of progesterone on the contraction of muscle
cells from the guinea pig colon. Am J Physiol Gastrointest Liver
Physiol. 290:G1008–G1015. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao ZL, Biancani P and Behar J: Effects
of progesterone on motility and prostaglandin levels in the distal
guinea pig colon. Am J Physiol Gastrointest Liver Physiol.
297:G886–G893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Al-Shboul O, Mustafa A and Al-hashimi F:
Non-genomic effects of progesterone on Rho kinase II in rat gastric
smooth muscle cells. J Smooth Muscle Res. 49:55–62. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murthy KS: Signaling for contraction and
relaxation in smooth muscle of the gut. Annu Rev Physiol.
68:345–374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Francis SH and Corbin JD: Cyclic
nucleotide-dependent protein kinases: Intracellular receptors for
cAMP and cGMP action. Crit Rev Clin Lab Sci. 36:275–328. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Denninger JW and Marletta MA: Guanylate
cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta.
1411:334–350. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Somlyo AP and Somlyo AV: Ca2+
sensitivity of smooth muscle and nonmuscle myosin II: Modulated by
G proteins, kinases, and myosin phosphatase. Physiol Rev.
83:1325–1358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al-Shboul O: The role of the RhoA/ROCK
pathway in gender-dependent differences in gastric smooth muscle
contraction. J Physiol Sci. 66:85–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al-Shboul OA, Al-Dwairi AN, Alqudah MA and
Mustafa AG: Gender differences in the regulation of MLC20
phosphorylation and smooth muscle contraction in rat stomach.
Biomed Rep. 8:283–288. 2018.PubMed/NCBI
|
|
24
|
Al-Shboul O and Mustafa A: Effect of
oxidative stress on Rho kinase II and smooth muscle contraction in
rat stomach. Can J Physiol Pharmacol. 93:405–411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bielefeldt K, Waite L, Abboud FM and
Conklin JL: Nongenomic effects of progesterone on human intestinal
smooth muscle cells. Am J Physiol. 271:G370–G376. 1996.PubMed/NCBI
|
|
26
|
Gill RC, Bowes KL and Kingma YJ: Effect of
progesterone on canine colonic smooth muscle. Gastroenterology.
88:1941–1947. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang F, Zheng TZ, Li W, Qu SY and He DY:
Action of progesterone on contractile activity of isolated gastric
strips in rats. World J Gastroenterol. 9:775–778. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sager G, Ørbo A, Jaeger R and Engström C:
Non-genomic effects of progestins--inhibition of cell growth and
increased intracellular levels of cyclic nucleotides. J Steroid
Biochem Mol Biol. 84:1–8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blackmore PF, Neulen J, Lattanzio F and
Beebe SJ: Cell surface-binding sites for progesterone mediate
calcium uptake in human sperm. J Biol Chem. 266:18655–18659.
1991.PubMed/NCBI
|
|
30
|
Minshall RD, Pavcnik D, Browne DL and
Hermsmeyer K: Nongenomic vasodilator action of progesterone on
primate coronary arteries. J Appl Physiol (1985). 92:701–708. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grider JR, Murthy KS, Jin JG and Makhlouf
GM: Stimulation of nitric oxide from muscle cells by VIP:
Prejunctional enhancement of VIP release. Am J Physiol.
262:G774–G778. 1992.PubMed/NCBI
|
|
32
|
Perez-Zoghbi JF, Bai Y and Sanderson MJ:
Nitric oxide induces airway smooth muscle cell relaxation by
decreasing the frequency of agonist-induced Ca2+ oscillations. J
Gen Physiol. 135:247–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chan HY, Yao X, Tsang SY, Chan FL, Lau CW
and Huang Y: Different role of endothelium/nitric oxide in
17beta-estradiol- and progesterone-induced relaxation in rat
arteries. Life Sci. 69:1609–1617. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khorram O and Han G: Influence of
progesterone on endometrial nitric oxide synthase expression.
Fertil Steril. 91:2157–2162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bulbul A, Yağci A, Altunbaş K, Sevimli A,
Celik HA, Karadeniz A and Akdağ E: The role of nitric oxide in the
effects of ovarian steroids on spontaneous myometrial contractility
in rats. Theriogenology. 68:1156–1168. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fernandes VS, Ribeiro AS, Martínez-Sáenz
A, Blaha I, Serrano-Margüello D, Recio P, Martínez AC, Bustamante
S, Vázquez-Alba D, Carballido J, et al: Underlying mechanisms
involved in progesterone-induced relaxation to the pig bladder
neck. Eur J Pharmacol. 723:246–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li HF, Zheng TZ, Li W, Qu SY and Zhang CL:
Effect of progesterone on the contractile response of isolated
pulmonary artery in rabbits. Can J Physiol Pharmacol. 79:545–550.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rybalkin SD, Yan C, Bornfeldt KE and Beavo
JA: Cyclic GMP phosphodiesterases and regulation of smooth muscle
function. Circ Res. 93:280–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Al-Shboul O: Contraction and relaxation
signaling in gastrointestinal smooth muscle. EC Gastroenterol Dig
Syst. 5:315–321. 2018.
|
|
40
|
Sager G: Cyclic GMP transporters.
Neurochem Int. 45:865–873. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Al-Shboul OA, Nazzal MS, Mustafa AG,
Al-Dwairi AN, Alqudah MA, Abu Omar A, Alfaqih MA and Alsalem MI:
Estrogen relaxes gastric muscle cells via a nitric oxide- and
cyclic guanosine monophosphate-dependent mechanism: A
sex-associated differential effect. Exp Ther Med. 16:1685–1692.
2018.PubMed/NCBI
|
|
42
|
Ryan JP, Bhojwani A and Wang MB: Effect of
pregnancy on gastric motility in vivo and in vitro in the guinea
pig. Gastroenterology. 93:29–34. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zou S, Li X, Feng Y, Sun S, Li J,
Egecioglu E, Billig H and Shao R: Comparison of the diagnostic
values of circulating steroid hormones, VEGF-A, PIGF, and ADAM12 in
women with ectopic pregnancy. J Transl Med. 11:442013. View Article : Google Scholar : PubMed/NCBI
|