Introduction
Luteinizing hormone (LH) is essential for normal
follicular development and oocyte maturation. It is established
that an essential part of ovarian stimulation in in vitro
fertilization (IVF) is to prevent premature luteinization (1). There are two protocols for pituitary
desensitization, either lengthening daily administration of
gonadotropin-releasing hormone (GnRH) agonists, or immediately
blocking the secretion of the pituitary LH with GnRH antagonist.
Each procedure can effectively block premature LH surges (2,3).
In recent decades, GnRH antagonists have been
applied in clinical practice for ovarian stimulation protocols in
IVF. Compared with GnRH agonists, GnRH antagonists are advantageous
in that they require a shorter duration of treatment, a shorter
duration for follicle stimulating hormone (FSH) stimulation, and
are associated with a relatively lower risk of ovarian
hyperstimulation syndrome (OHSS) (4).
One particular advantage in the prevention of OHSS with the GnRH
antagonist protocol is that GnRH agonists act as trigger, inducing
a transient endogenous LH surge, rather than a human chorionic
gonadotropin (hCG) trigger that induces an extended exogenous LH
surge, particularly in patients with an expected or known high
response (5,6).
At present, there remains to be debate regarding the
safety and efficacy of GnRH antagonists and agonists for IVF embryo
transfer (IVF-ET). Individualization of ovarian stimulation for IVF
is increasingly common to tailor the stimulation protocol to the
patient's specific preconditions. To determine the advantages and
disadvantages of antagonist and agonist regimens, investigation is
required in specific classifications of IVF patients, such as in
patients with diminished ovarian reserve, in the general
population, and in patients with polycystic ovary syndrome (PCOS).
This could be relevant as these women may be particularly different
in ovarian response, particularly in relation to agonists and
antagonists. It has been reported that in general IVF patients,
ongoing pregnancy rate was significantly lower in the antagonist
group compared with in the agonist group (7). By contrast, in women with PCOS and in
women with poor ovarian response, there was no significant
difference in ongoing pregnancy between the two groups (7).
Therefore, the aim of the present study was to
evaluate the influence of GnRH antagonist compared with GnRH
agonist on IVF cycle outcome in patients in the IVF unit of Linyi
People's Hospital (Linyi, China). Furthermore, the area under the
curve (AUC) of receiver operating characteristic (ROC) curves was
used to evaluate whether the endometrial thickness, estradiol
(E2) level and progesterone (P) level on the day of hCG
administration (hCG day) had the ideal sensitivity and specificity
for predicting a clinical pregnancy in the fresh ET cycle. Overall
the results were hoped to aid reproductive specialists
indecision-making for infertile patients.
Materials and methods
Patients
The computerized files (clinical medicine
reproductive management system) of the patients who were admitted
to the IVF unit of Linyi People's Hospital from January 31, 2013 to
December 31, 2016, all of whom had reached the ET step, were
retrospectively reviewed. The study was approved by the Ethics
Committee of Linyi People's Hospital. Patients with primary or
secondary infertility were included in the study, while those with
high or low ovarian reserve (8,9) were
excluded. All of the patients underwent controlled ovarian
hyperstimulation using either the midluteal long GnRH agonist
[triptorelin; Ferring GmbH, Kiel, Germany; 0.1 mg daily,
subcutaneously (SC)] suppressive protocol (agonist group, group
GnRH-a) or the multiple-dose fixed GnRH antagonist [ganirelix
(ganirelix acetate); Merck-Serono, Ltd., Aubonne, Switzerland; 0.25
mg daily, SC] protocol (antagonist group, group GnRH-A).
The selection of the type of analog to be used was
the decision of the treating physicians. Data on the baseline
characteristics of patients in the agonist and antagonist groups
were collected from the files (Table
I). ELISA kits were used to measure serum E2 (pg/ml;
cat. no. 10491445), P (ng/ml; cat. no. 01586287), FSH (cat. no.
01360521) and LH (UI/l; cat. no. 02212941; all from Siemens AG,
Munich, Germany). Estimated ROC curves for the performance of
endometrial thickness and E2 and P levels on the hCG day
in the prediction of clinical pregnancy were also established.
 | Table I.Baseline characteristics of patients
in the GnRH-A and GnRH-a groups. |
Table I.
Baseline characteristics of patients
in the GnRH-A and GnRH-a groups.
| Variable | GnRH-A group (n=557
cycles) | GnRH-a group (n=654
cycles) | P-value |
|---|
| Age, years | 36.06±5.41 | 31.51±4.57 | <0.001 |
| BMI,
kg/m2 | 24.06±3.05 | 23.65±3.23 | 0.025 |
| FSH, UI/l | 8.23±2.63 | 6.90±1.88 | <0.001 |
| LH, UI/l | 4.25±2.35 | 4.38±2.47 | 0.646a |
| E2,
pg/ml | 47.49±19.50 | 44.19±18.43 | 0.224a |
| P, ng/ml | 0.44±0.26 | 0.42±0.22 | 0.119 |
All of the patients underwent baseline transvaginal
sonography on day 2 or 3 of the menstrual cycle to check the antral
follicle count and the thickness of the endometrium (cm). The
protocol for agonist and antagonist treatments were as reported
previously (10). Outcomes including
the mean number of total oocytes retrieved, the mean number of two
pronuclei (2PN) oocytes, the mean number of embryos available, the
mean number of embryos transferred and live birth rate (11-13)
were recorded.
The results of the influence of endometrial
thickness, P levels and E2 levels on clinical pregnancy,
based on individual ROC curves (AUCs) and the respective Youden
index [sensitivity-(1-specificity)] (14), were used to demonstrate sensitivity
and specificity in predicting clinical pregnancy.
Statistical analysis
SPSS 23.0 software for Windows (IBM Corp., Armonk,
NY, USA) was used for statistical analysis. The data were reported
as the mean ± standard deviation. Differences in the variables
between the two groups were statistically analyzed using Student's
t-test or Wilcoxon-Mann-Whitney test and Pearson's χ2
test at a two-sided significance level of 0.05. In the present
study, the AUC of ROC curves was used to evaluate whether the
endometrial thickness and E2 and P levels on the hCG day had high
sensitivity and specificity for predicting clinical pregnancy.
Results
Baseline characteristics and clinical
outcomes in the GnRH A and GnRH a groups on IVF
A total of 1,231 fresh cycles of reproductive women
with normal ovarian reserve were evaluated: 577 fresh cycles in the
GnRH-A group and 654 fresh cycles in the GnRH-a group. There were
significant differences in the baseline parameters of age, body
mass index (BMI) and FSH basal hormone profile between the GnRH-A
and GnRH-a groups (Table I). The mean
age in years was 36.08±5.40 for the GnRH-A group and 31.51±4.57 for
the GnRH-a group (P<0.001), and the mean BMI was 24.06±3.05 for
the GnRH-A group and 23.65±3.23 for the GnRH-a group (P=0.025). The
mean basal hormone profile of FSH was 8.23±2.63 UI/l for the GnRH-A
group and 6.90±1.88 UI/l for the GnRH-a group (P<0.001).
Meanwhile, the LH level did not differ significantly between the
groups: the mean LH level was 4.25±2.35 UI/l for the GnRH-A group
and 4.38±2.47 UI/l for the GnRH-a group (P=0.646). Additionally,
mean E2 level was 47.49±19.50 pg/ml for the GnRH-A group
and 44.19±18.43 pg/ml for the GnRH-a group (P=0.224), and the mean
P level was 0.44±0.26 ng/ml for the GnRH-A group and 0.42±0.22
ng/ml for the GnRH-a group (P=0.119; Table I).
When comparing the GnRH-A and GnRH-a groups on
treatment parameters, there were significant differences in the
stimulation duration (rFSH days of usage: 9.26±1.60 vs. 10.54±1.62,
respectively, P<0.001), dose of gonadotrophins on hCG day
(2,021.53±590.87 vs. 2,279.28±553.46 IU, respectively, P<0.001),
the endometrial thickness on hCG day (9.93±2.26 vs. 10.89±2.42 cm,
respectively, P<0.001), the E2 level on hCG day
(1,516.58±955.91 vs. 3,049.90±1,250.36 pg/ml, respectively,
P<0.001), the P level on hCG day (0.61±0.30 vs. 0.71±0.27 ng/ml,
respectively, P<0.001), the mean number of total oocytes
retrieved (5.31±3.26 vs. 8.63±3.73, respectively, P<0.001), the
mean number of two pronuclei (2PN) oocytes (3.71±2.66 vs.
6.21±4.21, respectively, P<0.001), the mean number of embryos
available (2.54±1.71 vs. 3.76±2.24, respectively, P<0.001) and
the mean number of embryos transferred (1.74±0.69 vs. 1.92±0.40,
respectively, P<0.001; Table
II).
 | Table II.Comparison of the groups on
stimulation characteristics and outcomes in in vitro
fertilization. |
Table II.
Comparison of the groups on
stimulation characteristics and outcomes in in vitro
fertilization.
| Characteristics | GnRH-A group (n=577
cycles) | GnRH-a group (n=654
cycles) | P-value |
|---|
| Stimulation duration,
days | 9.26±1.60 | 10.54±1.62 | <0.001 |
| Dose of
gonadotrophins, IU | 2,021.53±590.87 | 2,279.28±553.46 | <0.001 |
| E2 on hCG day,
pg/ml | 1,516.58±955.91 | 3,049.90±1250.36 | <0.001 |
| P on hCG day,
ng/ml | 0.61±0.30 | 0.71±0.27 | <0.001 |
| EM on hCG day,
cm | 9.93±2.26 | 10.89±2.42 | <0.001 |
| Mean no. of total
oocytes retrieved | 5.31±3.26 | 8.63±3.73 | <0.001 |
| Mean no. of 2PN
oocytes | 3.71±2.66 | 6.21±4.21 |
<0.001a |
| Mean no. of embryos
available | 2.54±1.71 | 3.76±2.24 |
<0.001a |
| Mean no. of embryos
transferred | 1.74±0.69 | 1.92±0.40 |
<0.001a |
| Clinical pregnancy
rate, n (%) | 185 (32.06) | 379 (57.95) | <0.001 |
| Ectopic pregnancy
rate, n (%) | 9/185 (4.86) | 17/379 (4.49) | 0.840 |
| Live birth rate, n
(%) | 144 (24.96) | 303 (46.33) | <0.001 |
| OHSS rate, n (%) | 7 (1.21) | 19 (2.91) | 0.039 |
There was significant differences in the pregnancy
outcomes between the GnRH-A and GnRH-a groups, including in
clinical pregnancy rate [185 (32.06%) vs. 379 (57.95%),
respectively, P<0.001). The group of patients who received
GnRH-A exhibited a lower rate of live births compared with that
recorded for the GnRH-a group [144 (24.96%) vs. 303 (46.33%),
P<0.001] and lower OHSS rate [7 (1.21%) vs. 19 (2.91%),
P=0.039]. However, there was no significant difference in the
outcome of ectopic pregnancy rate [9 (4.86%) vs. 17 (4.49%),
P=0.840].
Estimated ROC curves for the
performance of endometrial thickness and E2 and P levels
on the hCG day in the prediction of clinical pregnancy
The plots of the sensitivity-specificity
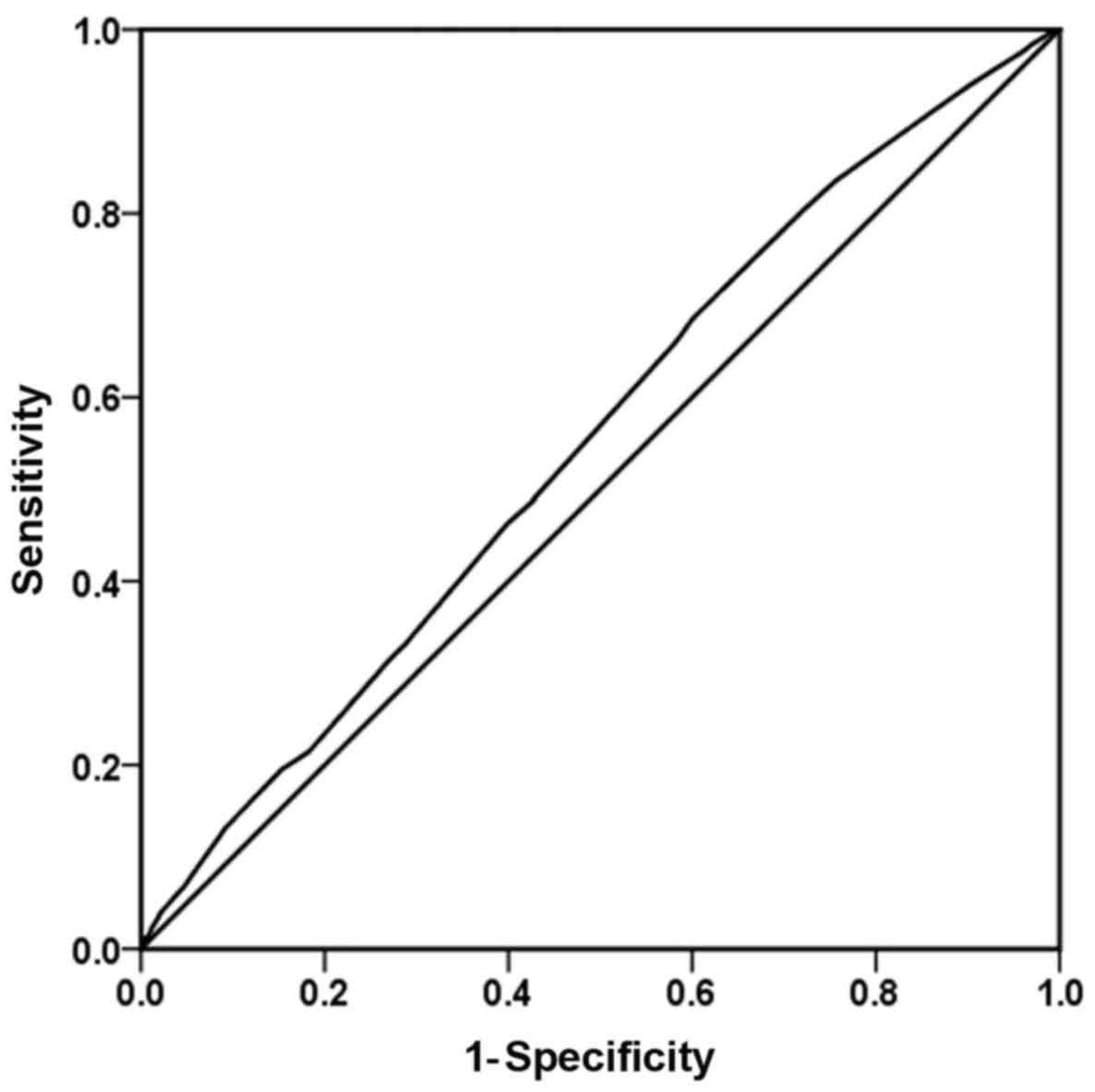
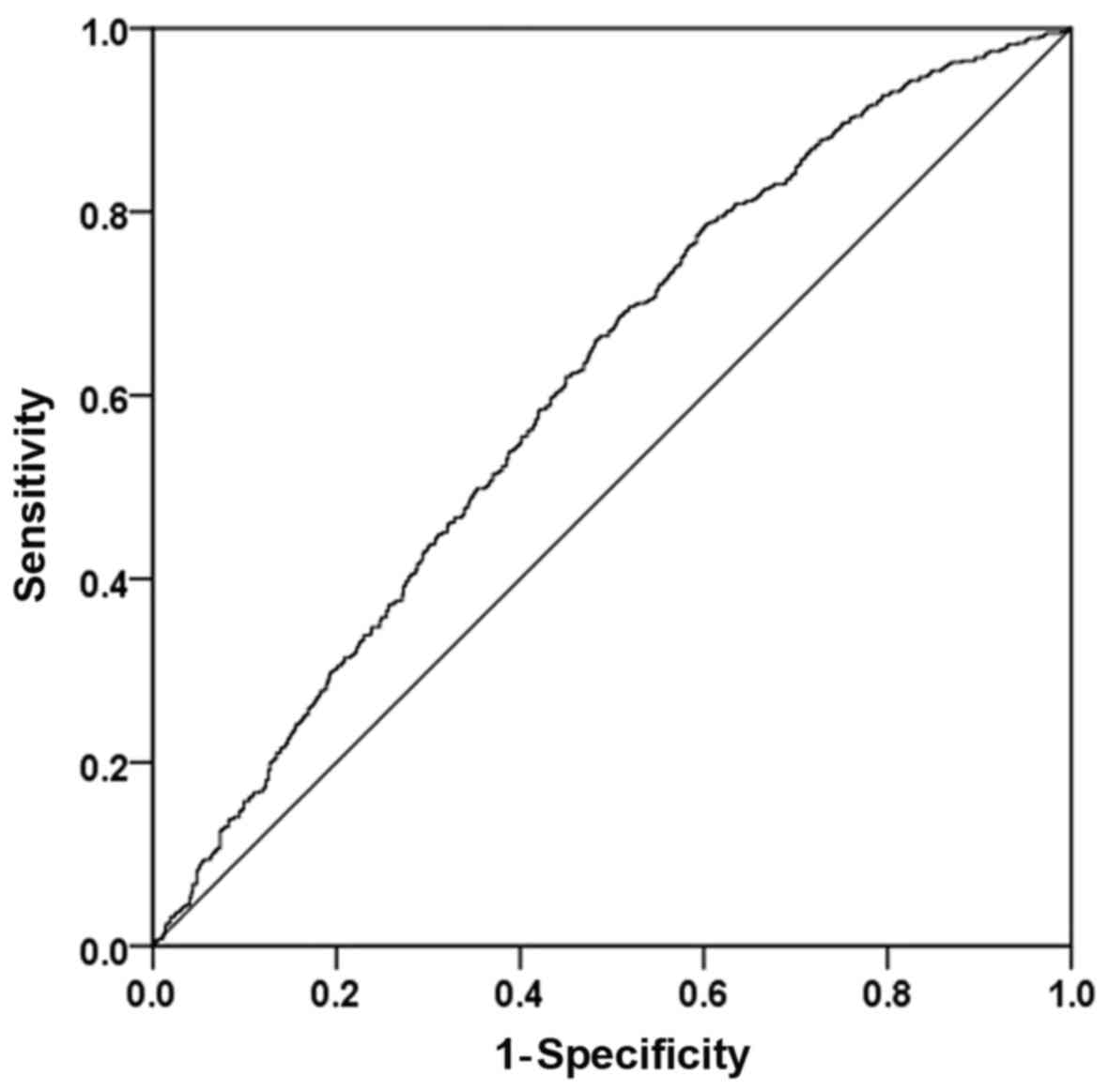
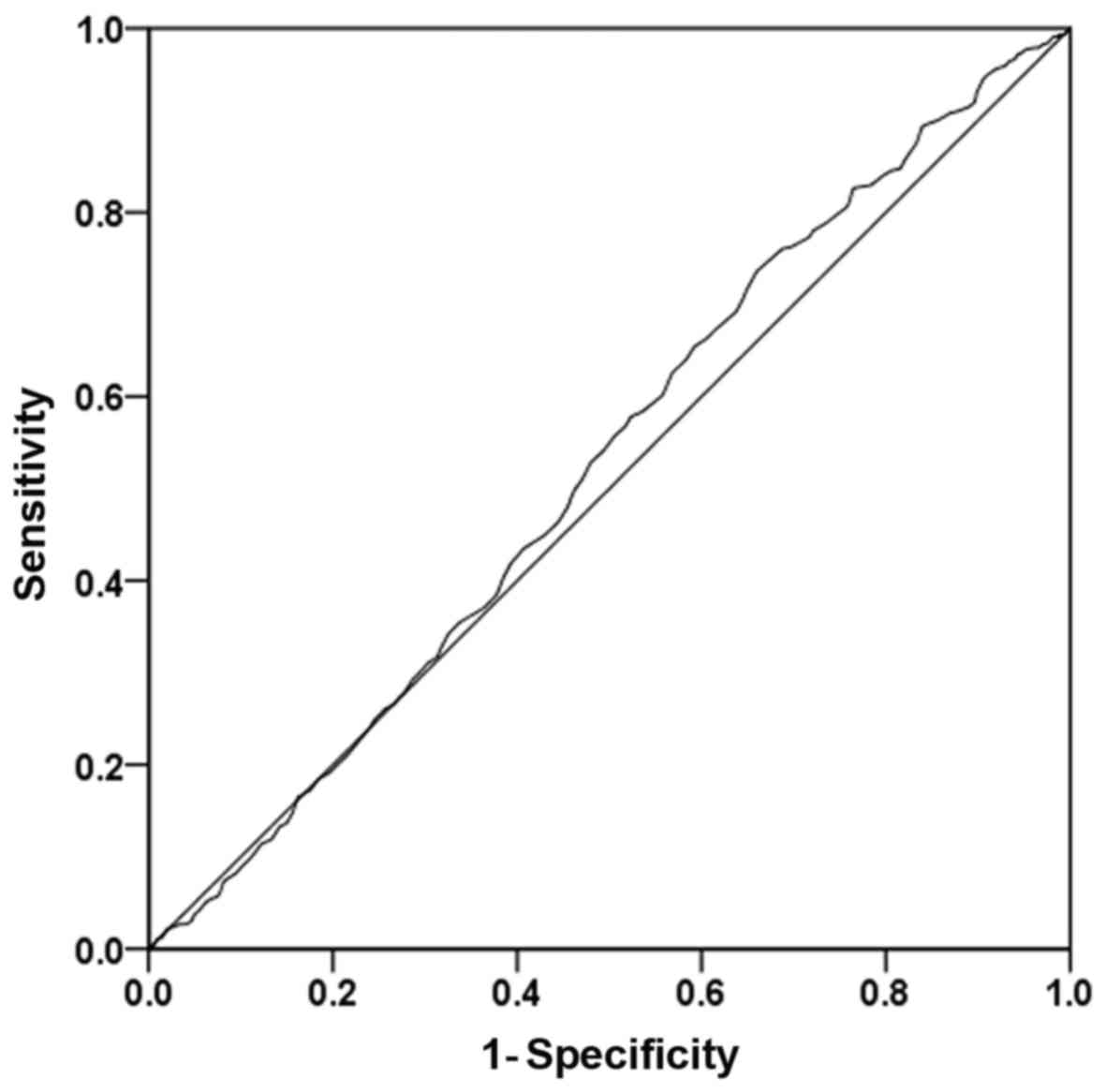
combinations in an ROC space are presented in Figs. 1-3.
The results of the influence of endometrial thickness on clinical
pregnancy, based on the ROC curve (AUC), demonstrated that the AUC
was 0.553 [95% confidence interval (CI): 0.521-0.585], and with a
cutoff of 9.25 cm, the sensitivity was 68.6% and the specificity
was 39.9%. Additionally, the Youden index was 0.085 (Fig. 1). The results of the influence of the
E2 level on hCG day on the clinical pregnancy rate, from
the ROC curve (AUC), demonstrated that the AUC was 0.613 (95% CI:
0.581-0.644), and with a cutoff of 1,520 pg/ml, the sensitivity was
78.4% and the specificity was 40.0%. Additionally, the Youden index
was 0.184 (Fig. 2). The results of
the influence of the P level on hCG day (ng/ml) on the clinical
pregnancy rate, based on the ROC (AUC), showed that the AUC was
0.526 (95% CI: 0.494-0.558), and with a cutoff of 0.415 ng/ml, the
sensitivity was 82.6% and the specificity was 23.5%. Additionally,
the Youden index was 0.061 (Fig. 3).
These results of the ROC curves demonstrated that neither the
endometrial thickness on day of ET nor the E2 and P
levels on hCG day had the best sensitivity and specificity for
predicting the clinical pregnancy.
Discussion
The present study compared the efficacy of two
different ovarian stimulation protocols (GnRH antagonist vs. GnRH
agonist) during IVF treatment in reproductive normal responder
women. It has been reported that overall GnRH antagonists do not
compromise effectiveness and significantly prevent OHSS (15). When antagonists have been used in
patients with normal ovarian function, the clinical pregnancy rate
has been reported as lower than that for the agonist protocol,
potentially due to the antagonist exerting a negative effect
directly or indirectly on endometrial receptivity, for instance by
promoting premature maturation of the endometrium (16-18).
It is undesirable that in the present study, there were significant
differences in the baseline parameters of age, BMI and FSH hormone
profile between the GnRH-A and GnRH-a groups, which differed from
similar previous study by our group on polycystic ovary syndrome
(10).
Cumulative studies have confirmed that an
association exists between age and fertility; ovarian aging and
reduced ovarian reserve are considered critical factors in
determining IVF outcomes (19-21).
Here, the mean age of the GnRH-a group was significantly lower than
that of the GnRH-A group, which may indicate that the groups were
not representative of a single general population. This would limit
the conclusions of the present study.
It is generally known that extremely high levels of
E2 are harmful to embryo condition and endometrial
receptivity, which may impact on embryo implantation. From the
above data, in the present study, there were significantly higher
E2 levels on hCG day in the GnRH-a group compared with
in the GnRH-A group. The GnRH-a protocol was also associated with
significantly higher rates of clinical pregnancy and live birth
compared with those rates in the GnRH-A group. Nevertheless, there
was no significant difference in ectopic pregnancy rate between the
groups.
Implantation is necessary for successful pregnancy
and requires adequate endometrial receptivity, which is among the
most important factors in predicting pregnancy following IVF-ET. In
particular the endometrial thickness has been documented as an
individual indicator for endometrial receptivity (22,23).
Previous study observed that the rates of clinical pregnancy and
live birth were markedly lower in patients with an endometrium
thickness of ≤7 mm, compared within those patients with an
endometrium thickness of >7 mm; however, there was no
significant difference in the rates of clinical pregnancy and
implantation with endometrial thicknesses in the 8-14 mm range
(24). In the present study, it was
identified that the endometrial thickness on the hCG day ranged
from 5.5 to 18.5 mm, and the group of patients who received the
GnRH-A regimen exhibited significantly reduced thickness compared
with those receiving the GnRH-a regimen. The influence of the
endometrial thickness on the clinical pregnancy rate was also
evaluated via ROC curve analysis. The results of the influence of
the endometrial thickness (EM) on the clinical pregnancy, using the
ROC (AUC), showed that the EM was a poor predictor of clinical
pregnancy. At present, no conclusive cut-off value for the
endometrial thickness has been established. Additionally in the
current study, the results of the ROC curves demonstrated that
neither E2 nor P level on hCG day had the best
sensitivity and specificity for predicting clinical pregnancy.
Further larger studies should be performed to elucidate the
contribution of the endometrial thickness and hormone levels on the
day of hCG administration to clinical pregnancy rate in patients
receiving GnRH antagonist or agonist.
In conclusion, in the present series of women
undergoing IVF-ET cycles, GnRH antagonist decreased oocyte
retrieval, oocyte cleavage, the number of embryos available and the
potential for embryo transfer. Furthermore, compared with the
agonist regimen, the rates of clinical pregnancy and live birth
with the antagonist regimen were significantly reduced. However,
the risk of OHSS was lower with the GnRH antagonist. In the present
study, the results of the ROC curves demonstrated that neither E2
nor P level on the day of hCG administration had ideal sensitivity
or specificity for predicting clinical pregnancy, in accordance
with our previous study (10).
Therefore, it appears that in predicting clinical pregnancy rate,
the endometrial thickness and estradiol and progesterone levels are
not the ideal predictors. It may be that embryo quality itself has
a greater impact on pregnancy outcomes. Nonetheless, more in-depth
studies are required to confirm the predictors of clinical
pregnancy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Fund of China (grant no. 81501620), the Shandong
Provincial Natural Science Foundation of China (grant nos.
ZR2014HP026 and ZR2016HP37) and the Shandong Provincial Medical and
Health Science and Technology Development Project (grant no.
2015WS0377).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ACW was principally responsible for conception and
design of the study. YX and YSZ participated in the acquisition of
the reported data. DYZ and XHZ participated in the processing and
interpretation of the reported data. FXW contributed to the writing
and revising of the manuscript. All authors read and approved the
final manuscript to be published.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants. The study was approved by the Ethics Committee of
Linyi People's Hospital [approval no. (2017) IRB NO. (0012)].
Patient consent for publication
Informed consent was obtained for publication of the
participants' data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lévy DP, Navarro JM, Schattman GL, Davis
OK and Rosenwaks Z: The role of LH in ovarian stimulation:
exogenous LH: let's design the future. Hum Reprod. 15:2258–2265.
2000.PubMed/NCBI
|
|
2
|
Huirne JA, van Loenen AC, Schats R,
McDonnell J, Hompes PG, Schoemaker J, Homburg R and Lambalk CB:
Dose-finding study of daily gonadotropin-releasing hormone (GnRH)
antagonist for the prevention of premature luteinizing hormone
surges in IVF/ICSI patients: Antide and hormone levels. Hum Reprod.
19:2206–2215. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huirne JA, van Loenen AC, Schats R,
McDonnell J, Hompes PG, Schoemaker J, Homburg R and Lambalk CB:
Dose-finding study of daily GnRH antagonist for the prevention of
premature LH surges in IVF/ICSI patients: Optimal changes in LH and
progesterone for clinical pregnancy. Hum Reprod. 20:359–367.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Weiss JM, Ludwig M, Ortmann O and Diedrich
K: GnRH antagonists in the treatment of infertility. Ann Med.
35:512–522. 2003.PubMed/NCBI
|
|
5
|
Raju GA, Chavan R, Deenadayal M,
Gunasheela D, Gutgutia R, Haripriya G, Govindarajan M, Patel NH and
Patki AS: Luteinizing hormone and follicle stimulating hormone
synergy: A review of role in controlled ovarian hyper-stimulation.
J Hum Reprod Sci. 6:227–234. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Serafini P, Yadid I, Motta EL, Alegretti
JR, Fioravanti J and Coslovsky M: Ovarian stimulation with daily
late follicular phase administration of low-dose human chorionic
gonadotropin for in vitro fertilization: A prospective, randomized
trial. Fertil Steril. 86:830–838. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lambalk CB, Banga FR, Huirne JA, Toftager
M, Pinborg A, Homburg R, van der Veen F and van Wely M: GnRH
antagonist versus long agonist protocols in IVF: A systematic
review and meta-analysis accounting for patient type. Hum Reprod
Update. 23:560–579. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
te Velde ER and Pearson PL: The
variability of female reproductive ageing. Hum Reprod Update.
8:141–154. 2002.PubMed/NCBI
|
|
9
|
Loutradis D, Drakakis P, Vomvolaki E and
Antsaklis A: Different ovarian stimulation protocols for women with
diminished ovarian reserve. J Assist Reprod Genet. 24:597–611.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhai XH, Zhang P, Wu FX, Wang AC and Liu
PS: GnRH antagonist for patients with polycystic ovary syndrome
undergoing controlled ovarian hyperstimulation for in vitro
fertilization and embryo transfer in fresh cycles. Exp Ther Med.
13:3097–3102. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Edwards RG, Bavister BD and Steptoe PC:
Early stages of fertilization in vitro of human oocytes matured in
vitro. Nature. 221:632–635. 1969.PubMed/NCBI
|
|
12
|
Lopata A, McMaster R, McBain JC and
Johnston WI: In-vitro fertilization of preovulatory human eggs. J
Reprod Fertil. 52:339–342. 1978.PubMed/NCBI
|
|
13
|
Younis JS, Radin O, Mirsky N, Izhaki I,
Majara T, Bar-ami S and Ben-ami M: First polar body and nucleolar
precursor body morphology is related to the ovarian reserve of
infertile women. Reprod Biomed Online. 16:851–858. 2008.PubMed/NCBI
|
|
14
|
Bertran EA, Berlie HD, Taylor A, Divine G
and Jaber LA: Diagnostic performance of HbA1c for diabetes in Arab
vs. European populations: A systematic review and meta-analysis.
Diabet Med. 34:156–166. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Al-Inany HG, Youssef MA, Ayeleke RO, Brown
J, Lam WS and Broekmans FJ: Gonadotrophin-releasing hormone
antagonists for assisted reproductive technology. Cochrane Database
Syst Rev. 4(CD001750)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Olivennes F, Cunha-Filho JS, Fanchin R,
Bouchard P and Frydman R: The use of GnRH antagonists in ovarian
stimulation. Hum Reprod Update. 8:279–290. 2002.PubMed/NCBI
|
|
17
|
Rackow BW, Kliman HJ and Taylor HS: GnRH
antagonists may affect endometrial receptivity. Fertil Steril.
89:1234–1239. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kolibianakis E, Bourgain C, Albano C,
Osmanagaoglu K, Smitz J, Van Steirteghem A and Devroey P: Effect of
ovarian stimulation with recombinant follicle-stimulating hormone,
gonadotropin releasing hormone antagonists, and human chorionic
gonadotropin on endometrial maturation on the day of oocyte
pick-up. Fertil Steril. 78:1025–1029. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Honnma H, Baba T, Sasaki M, Hashiba Y,
Oguri H, Fukunaga T, Endo T and Asada Y: Different ovarian response
by age in an anti-Müllerian hormone-matched group undergoing in
vitro fertilization. J Assist Reprod Genet. 29:117–125.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nelson SM, Telfer EE and Anderson RA: The
ageing ovary and uterus: New biological insights. Hum Reprod
Update. 19:67–83. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bleil ME, Adler NE, Pasch LA, Sternfeld B,
Gregorich SE, Rosen MP and Cedars MI: Psychological stress and
reproductive aging among pre-menopausal women. Hum Reprod.
27:2720–2728. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Friedler S, Schenker JG, Herman A and
Lewin A; FriedlerS: The role of ultrasonography in the evaluation
of endometrial receptivity following assisted reproductive
treatments: A critical review. Hum Reprod Update. 2:323–335.
1996.PubMed/NCBI
|
|
23
|
Dekel N, Gnainsky Y, Granot I and Mor G:
Inflammation and implantation. Am J Reprod Immunol. 63:17–21.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yoeli R, Ashkenazi J, Orvieto R, Shelef M,
Kaplan B and Bar-Hava I: Significance of increased endometrial
thickness in assisted reproduction technology treatments. J Assist
Reprod Genet. 21:285–289. 2004.PubMed/NCBI View Article : Google Scholar
|