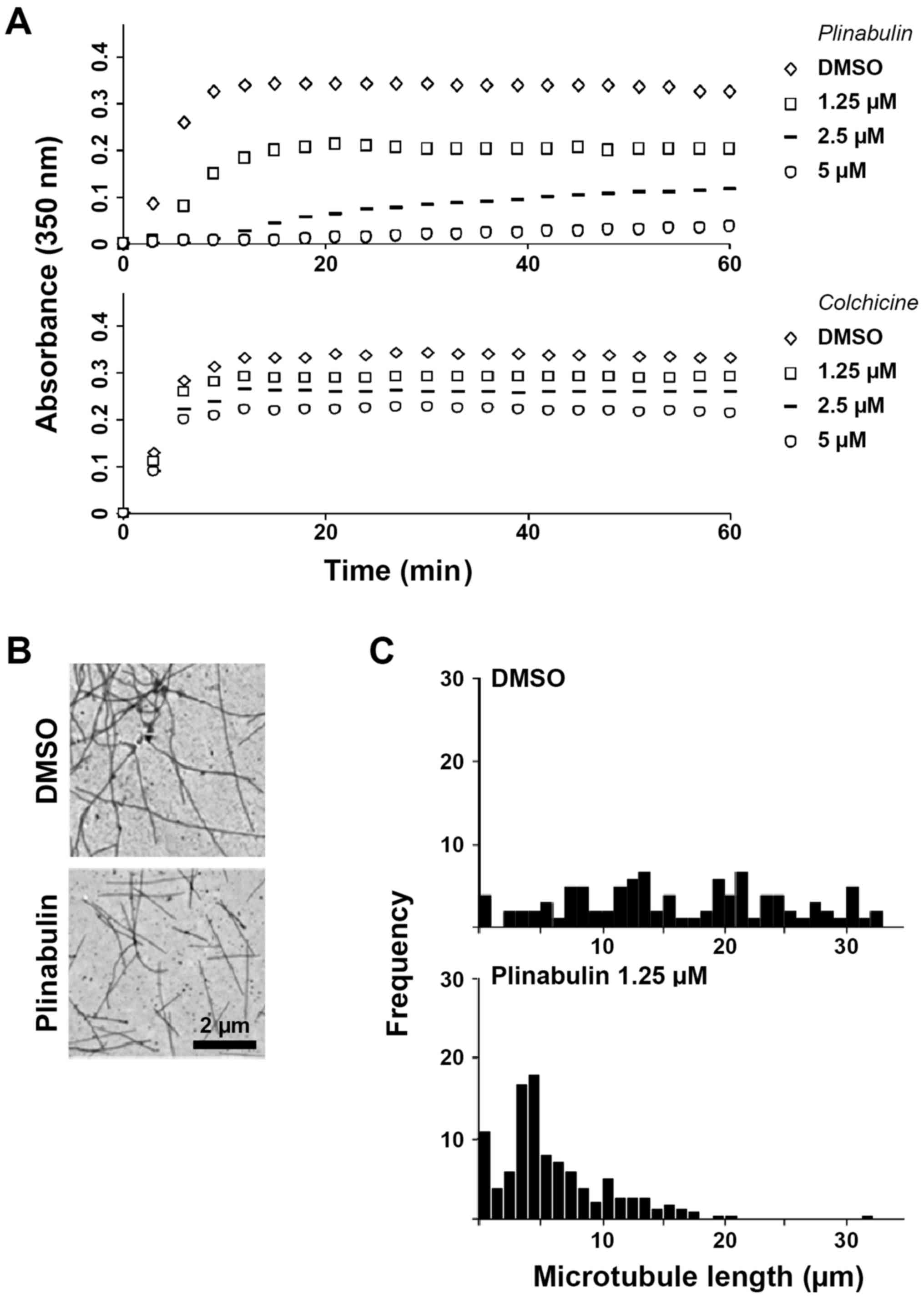
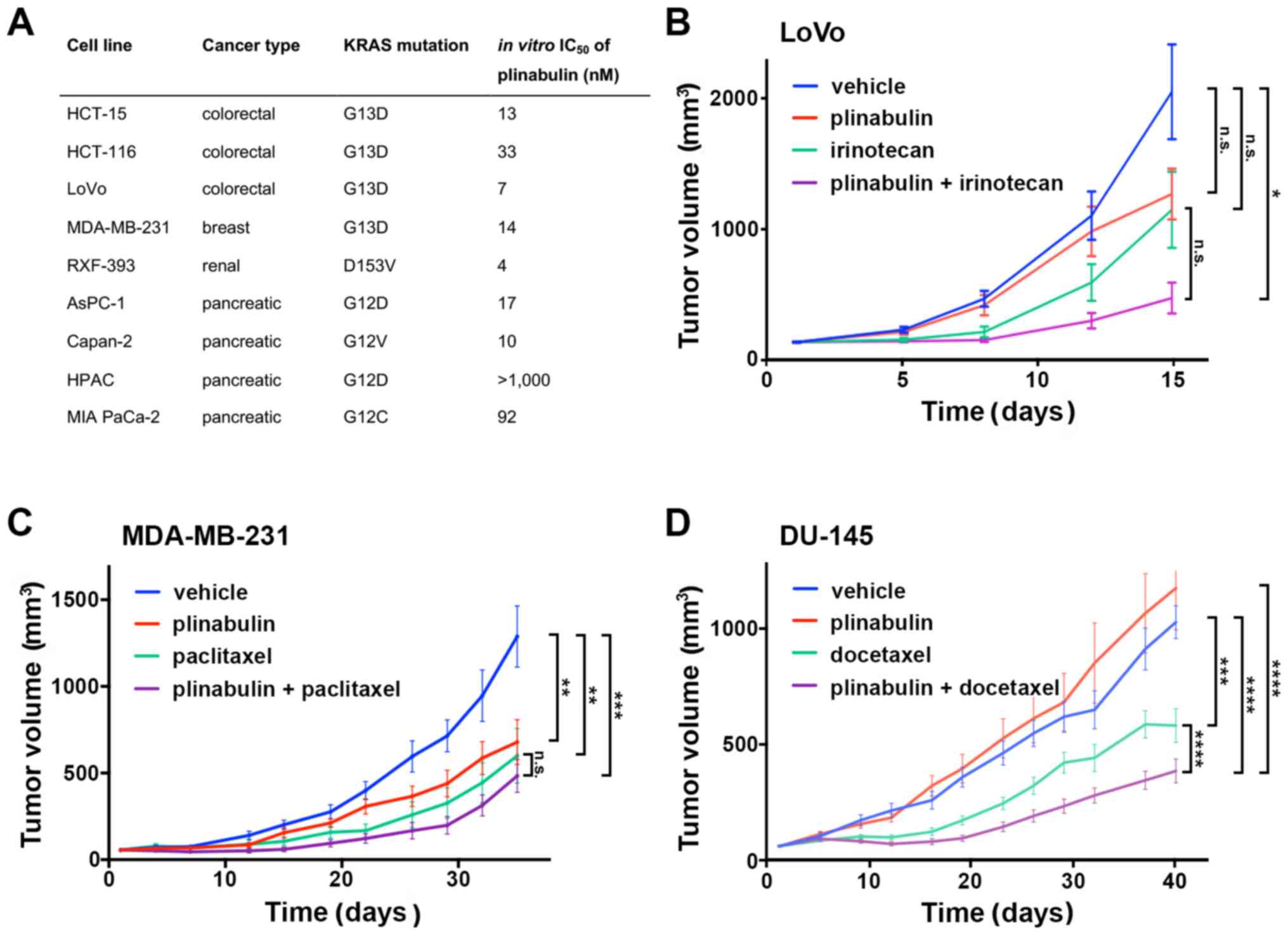
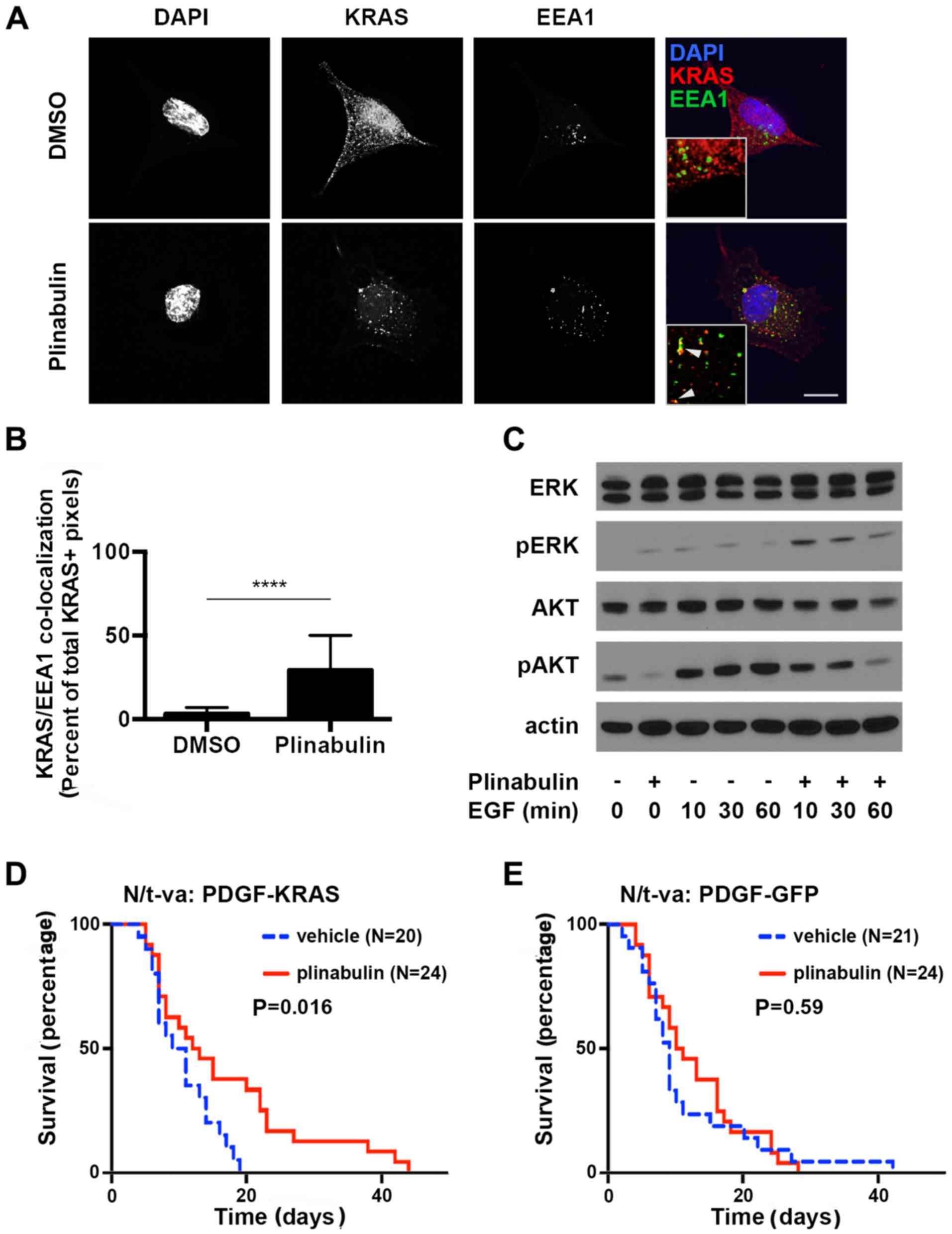
Spandidos Publications style
Cimino PJ, Huang L, Du L, Wu Y, Bishop J, Dalsing-Hernandez J, Kotlarczyk K, Gonzales P, Carew J, Nawrocki S, Nawrocki S, et al: Plinabulin, an inhibitor of tubulin polymerization, targets KRAS signaling through disruption of endosomal recycling. Biomed Rep 10: 218-224, 2019.
APA
Cimino, P.J., Huang, L., Du, L., Wu, Y., Bishop, J., Dalsing-Hernandez, J. ... Wirsching, H. (2019). Plinabulin, an inhibitor of tubulin polymerization, targets KRAS signaling through disruption of endosomal recycling. Biomedical Reports, 10, 218-224. https://doi.org/10.3892/br.2019.1196
MLA
Cimino, P. J., Huang, L., Du, L., Wu, Y., Bishop, J., Dalsing-Hernandez, J., Kotlarczyk, K., Gonzales, P., Carew, J., Nawrocki, S., Jordan, M. A., Wilson, L., Lloyd, G. K., Wirsching, H."Plinabulin, an inhibitor of tubulin polymerization, targets KRAS signaling through disruption of endosomal recycling". Biomedical Reports 10.4 (2019): 218-224.
Chicago
Cimino, P. J., Huang, L., Du, L., Wu, Y., Bishop, J., Dalsing-Hernandez, J., Kotlarczyk, K., Gonzales, P., Carew, J., Nawrocki, S., Jordan, M. A., Wilson, L., Lloyd, G. K., Wirsching, H."Plinabulin, an inhibitor of tubulin polymerization, targets KRAS signaling through disruption of endosomal recycling". Biomedical Reports 10, no. 4 (2019): 218-224. https://doi.org/10.3892/br.2019.1196