Introduction
Pruritus is one of the common symptoms of chronic
liver disease (1) and it impairs
patients' quality of life (2-4).
Therefore, the assessment of pruritus is important in the clinical
management of chronic liver disease. Hepatitis B is a liver disease
caused by hepatitis B virus (HBV) infection. The World Health
Organization estimates that ~257 million people have chronic
hepatitis B (CHB) infections worldwide (5). The clinical course and outcome of HBV
infections vary according to immune balance between the viral
replication and host immune responses (6). Although interferon-α (IFN-α) or
nucleoside analogues are available against chronic infection of
HBV, a complete elimination or cure is still difficult with current
treatment strategies (7), suggesting
that CHB infection-associated comorbidities will remain issues in
patients.
Pruritus is observed in the patients with HBV
infection and previous studies reported on the prevalence of
pruritus in these patients (8,9). Bonacini
(8) reported that 8.0% of patients
with HBV infection had pruritus. Another previous study showed the
prevalence differed according to the phase of HBV infection: It is
significantly lower in the inactive CHB phase than in the hepatitis
B e antigen (HBe-Ag)-positive/negative immune-active phase (22 vs.
41%), indicating that immune response and disease phase are
associated with pruritus (9).
However, it remains unclear whether the phase of HBV infection
independently affects the prevalence of pruritus.
A study of the patients with chronic hepatitis C
showed that liver fibrosis is an independent factor associated with
pruritus (10). Furthermore, a study
of primary biliary cholangitis, serum alkaline phosphatase was
identified as an independent predictor of pruritus (11). These studies suggest that the
pathogenesis of pruritus is different among chronic liver diseases
and may be multifactorial. Therefore, an appropriate procedure is
required to test statistical independence of factors that are
potentially associated with pruritus in clinic. The current study
aimed to investigate the effects of HBV infection phase on the
prevalence of pruritus in patients with HBV infection using
propensity score-matching.
Materials and methods
Patients
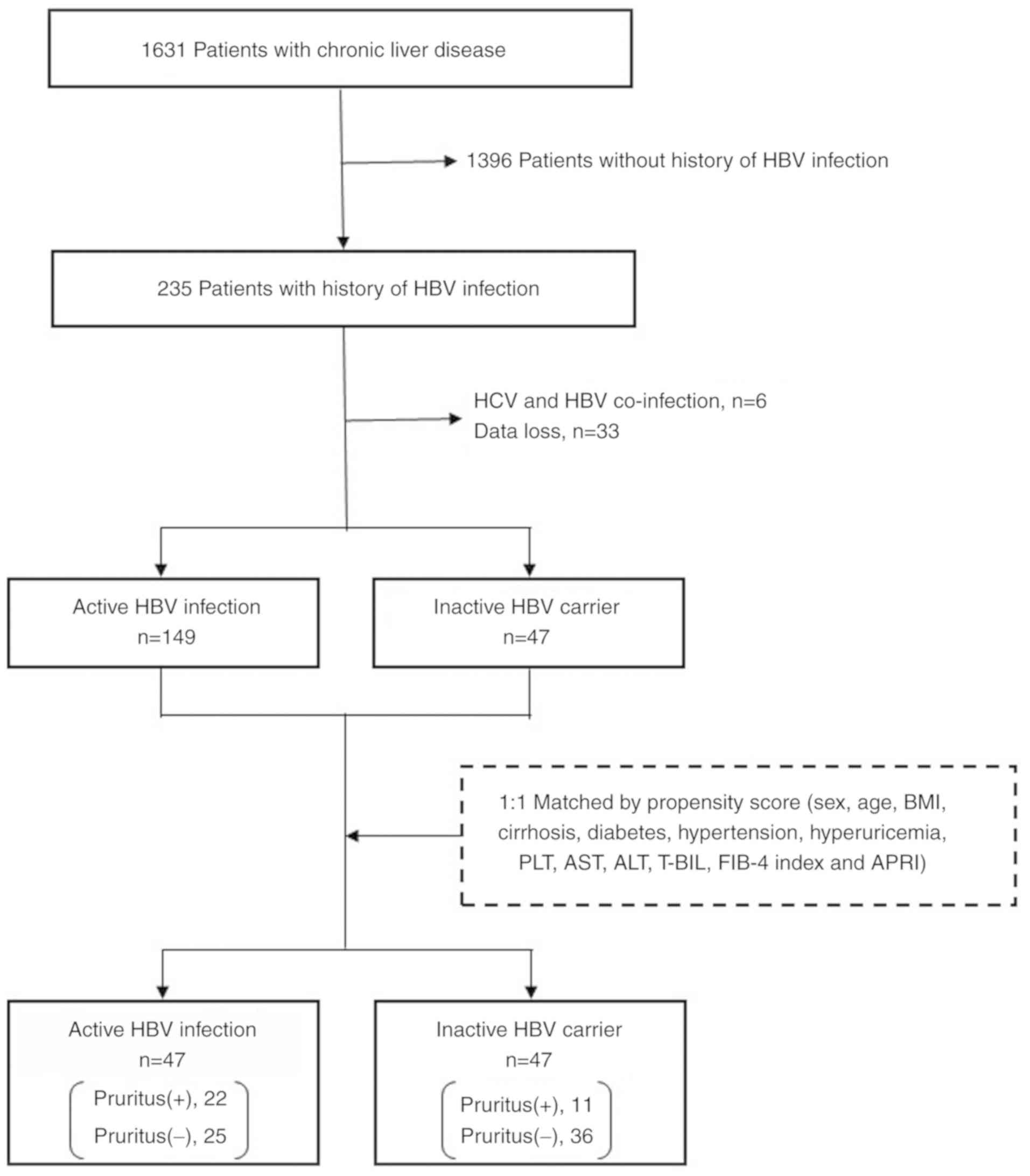
The current study performed a retrospective subgroup
analysis of the cohort included in a previous multi-center,
cross-sectional study (9). Continuous
outpatients with various chronic liver diseases who attended the
joint research facilities at Saga University Hospital, Yokohama
City University Hospital, Kochi Medical School Hospital, JA
Hiroshima General Hospital, Kurume University Hospital, Osaka City
Juso Hospital, Kawasaki Hospital or Nara City Hospital between
January and June 2016 were interviewed regarding pruritus. Patients
with overt skin disease, including eczema and atopic dermatitis,
were excluded from the survey and a total of 1,631 patients were
included. Out of these patients, 235 were infected with HBV, of
whom 39 patients were excluded due to missing data (n=33) or
co-infection with hepatitis C virus (n=6). A total of 196 patients
were enrolled in the current study and divided into two groups
according to their HBV disease phase, as defined in the American
Association for the Study of Liver Diseases guidelines (6). Patients in HBe-Ag-positive/negative
immune-active phase were classified as belonging to the active HBV
infection (n=149) group, and those in inactive CHB phase were
classified as belonging to the inactive HBV carrier (n=47) group.
No patients were in the immune tolerant phase. To minimize
differences in patient baseline characteristics between the groups,
a one-to-one nearest-neighbor matching without replacement was
performed based on propensity score techniques (12). No caliper was set, meaning that the
propensity score distance allowed for matching in order to prevent
a decrease in the number of cases. The propensity score was
calculated using 13 variables: Sex, age, body mass index,
cirrhosis, diabetes, hypertension, hyperuricemia, platelet count,
aspartate aminotransferase (AST), alanine aminotransferase (ALT),
total bilirubin, FIB-4 index (13)
and AST-to-platelet ratio index (APRI) (14). Well-balanced groups were obtained
after matching; the data from 47 inactive HBV carriers and 47
matched patients with active HBV infection were included in the
final analysis (Fig. 1).
Liver cirrhosis, diabetes, hypertension and
hyperuricemia were diagnosed by the physician in charge of the case
on the basis of the patient's clinical background and history.
Liver cirrhosis was definitively diagnosed on the basis of liver
histologic assessment, liver stiffness measured by transient
elastography (15), fibrosis-4 index,
APRI and overt findings of cirrhosis, such as ascites, jaundice,
hepatic encephalopathy and esophagogastric varices. Diabetes and
hyperuricemia were diagnosed by blood tests.
Evaluation of pruritus
Data from the interviews were recorded by physicians
using a standardized interview form, which contained questions
regarding the existence of pruritus, the location of any itch, the
duration of itching and its circadian variation and seasonality.
The severity of pruritus was evaluated using the visual analogue
scale (VAS) (16), which quantified
the degree of pruritus from 0 (no pruritus) to 10 (maximum
pruritus), according to the patients' subjective perception.
Differences in the VAS scored between day and night were
evaluated.
Statistical analysis
Two statistical experts (SK and AK) analyzed the
data. Patient characteristics were compared according to the HBV
infection phase and the presence of itching. The χ2 and
Fisher's exact test were used to perform intergroup comparisons of
categorical variables. The Mann-Whitney U or Wilcoxon signed-rank
tests were used for intergroup comparisons of continuous variables.
To identify independent predictors of pruritus, a univariate
analysis was performed using a logistic regression model in
patients after propensity score-matching using the ‘Matching’
package in R (version 3.3.2; https://www.r-project.org/index.html). All statistical
analyses were performed using R. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of the study
group
Patient characteristics are summarized in Table I. Before propensity score-matching,
the prevalence of pruritus was significantly lower in inactive HBV
carriers than in patients with active HBV infection (23 vs. 41%;
P=0.045). Prior to matching, liver cirrhosis was significantly less
prevalent in inactive HBV carriers than in patients with active HBV
infection (8.5 vs. 25%; P=0.022). The prevalence of pruritus in
inactive HBV carriers was also significantly lower than in patients
with active HBV infection, after propensity score-matching (23 vs.
47%; P=0.031).
 | Table IPatient characteristics according to
HBV infection phase before and after propensity score-matching. |
Table I
Patient characteristics according to
HBV infection phase before and after propensity score-matching.
| | Before matching | | After matching | |
|---|
| | Active HBV infection
(n=149) | Inactive HBV carrier
(n=47) | P-value | Active HBV infection
(n=47) | Inactive HBV carrier
(n=47) | P-value |
|---|
| Sexa, female | 66(44) | 16(34) | 0.283 | 16(34) | 16(34) | 1.000 |
| Age
(years)b | 83 26-97 | 61 35-85 | 0.792 | 61 39-81 | 61 35-85 | 0.971 |
| BMIb | 22.4 (15.0-34.7) | 22.5 (18.6-31.4) | 0.624 | 23.2 (18.0-29.8) | 22.5 (18.6-31.4) | 0.694 |
| Pruritusa | 61(41) | 11(23) | 0.045 | 22(47) | 11(23) | 0.031 |
|
Cirrhosisa | 37(25) | 4 (8.5) | 0.022 | 5(11) | 4 (8.5) | 1.000 |
|
Diabetesa | 27(18) | 5(11) | 0.325 | 5(11) | 5(11) | 1.000 |
|
Hypertensiona | 36(24) | 7(15) | 0.256 | 7(15) | 7(15) | 1.000 |
|
Hyperuricemiaa | 7 (4.7) | 3 (6.4) | 0.705 | 3 (6.4) | 3 (6.4) | 1.000 |
| PLT
(x104/µl)b | 18.7
(5.4-94.9) | 21.1
(12.5-34.0) | 0.063 | 20.3
(7.4-94.9) | 21.1
(12.5-34.0) | 0.480 |
| AST
(U/l)b | 24 13-182 | 24 13-58 | 0.870 | 23 14-63 | 24 13-58 | 0.534 |
| ALT
(U/l)b | 20 5-275 | 24 5-97 | 0.737 | 20 5-84 | 24 5-97 | 0.796 |
| T–BIL
(mg/dl)b | 0.8 (0.4-9.0) | 0.8 (0.5-1.5) | 0.589 | 0.8 (0.4-1.7) | 0.8 (0.5-1.5) | 0.678 |
| FIB-4
indexb | 1.800
(0.331-12.573) | 1.508
(0.400-5.057) | 0.107 | 1.490
(0.530-5.227) | 1.508
(0.400-5.057) | 0.932 |
| APRIb | 0.363
(0.096-3.233) | 0.336
(0.146-0.969) | 0.100 | 0.323
(0.105-1.154) | 0.336
(0.146-0.969) | 0.906 |
Features of pruritus
A total of 33 patients with pruritus (active HBV
infection, 22; inactive HBV carrier, 11) were identified in the
propensity-matched cohort and their itch locations are summarized
in Table II. Patients with pruritus
selected all applicable locations out of 15 listed areas. For all
patients, the most common location was the back (73%) and the least
common locations included the head (9.1%), hands (9.1%), groin
(9.1%), foot (9.1%) and fingers (3.0%); no patients reported toe
pruritus. The most common itch location in both groups was the
back, but the proportion with back pruritus was significantly
higher in patients with active HBV infection than in inactive HBV
carriers (86 vs. 46%; P=0.038). No significant differences were
observed for any other locations.
 | Table IILocation of the itch in
propensity-matched patients with pruritus, according to HBV
infection phase. |
Table II
Location of the itch in
propensity-matched patients with pruritus, according to HBV
infection phase.
| | All (n=33) | Active HBV
infection (n=22) | Inactive HBV
carrier (n=11) | P-value |
|---|
| Head | 3(9) | 2 (9.1) | 1 (9.1) | 1.000 |
| Face | 6(18) | 4(18) | 2(18) | 1.000 |
| Neck | 7(21) | 5(23) | 2(18) | 1.000 |
| Upper arm | 5(15) | 4(18) | 1 (9.1) | 0.864 |
| Lower arm | 5(15) | 1 (4.5) | 4(36) | 0.059 |
| Hands | 3 (9.1) | 1 (4.5) | 2(18) | 0.521 |
| Fingers | 1 (3.0) | 0 (0) | 1 (9.1) | 0.720 |
| Abdomen | 8(24) | 5(23) | 3(27) | 1.000 |
| Back | 24(73) | 19(86) | 5(46) | 0.038 |
| Lower back | 7(21) | 5(23) | 2(18) | 1.000 |
| Groin | 3 (9.1) | 2 (9.1) | 1 (9.1) | 1.000 |
| Thigh | 7(21) | 5(23) | 2(18) | 1.000 |
| Calf | 11(33) | 8(36) | 3(27) | 0.896 |
| Foot | 3 (9.1) | 2 (9.1) | 1 (9.1) | 1.000 |
| Toes | 0 (0) | 0 (0) | 0 (0) | - |
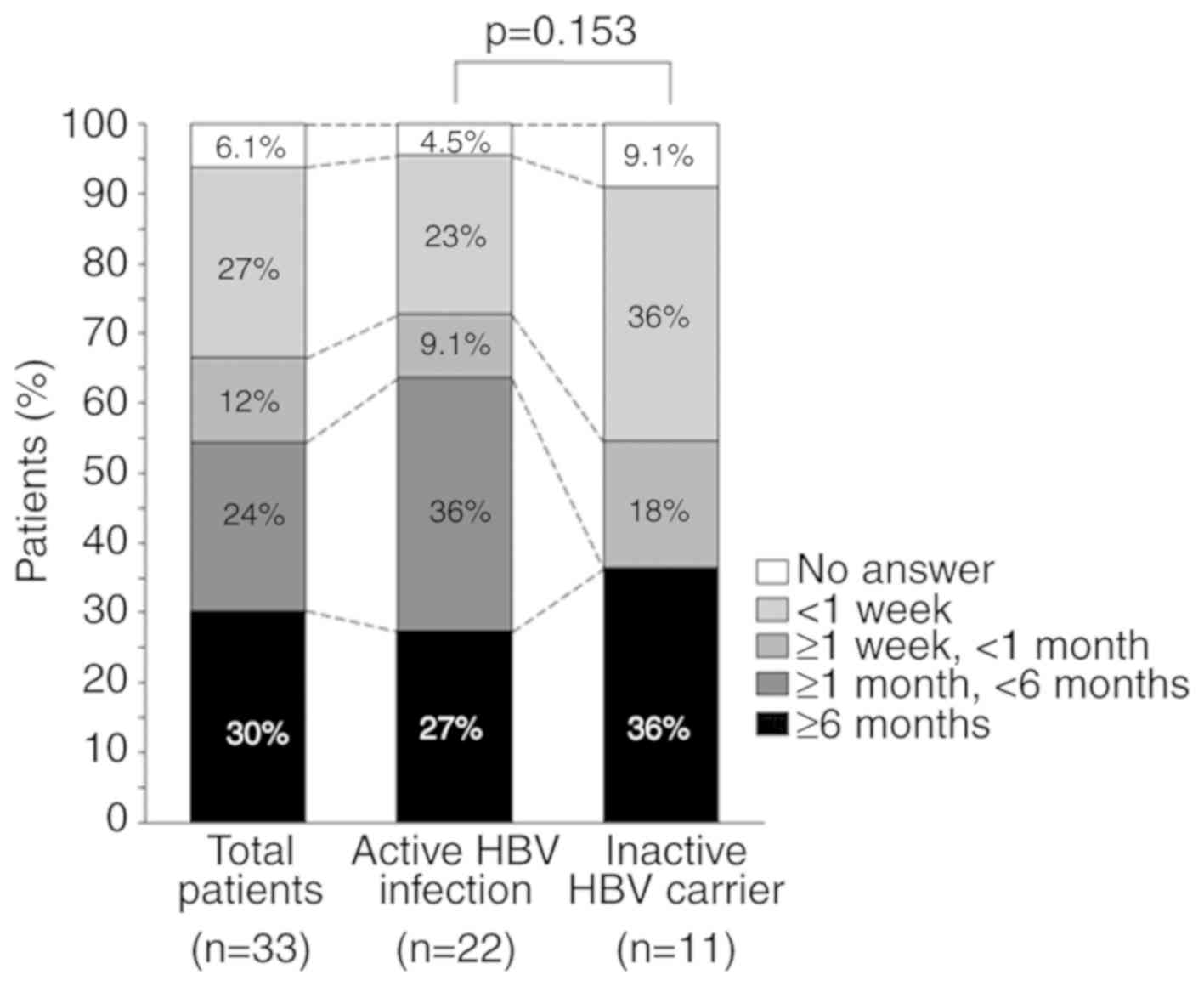
The duration for which pruritus lasted is shown in
Fig. 2. Itching lasted ≥6 months in
30, 27 and 36% of all patients, patients with active HBV infection
and in inactive HBV carriers, respectively. No patients in the
inactive HBV carrier group reported itching of a duration ≥1 and
<6 months, and there was no significant difference in the
duration of pruritus between the two groups (P=0.153).
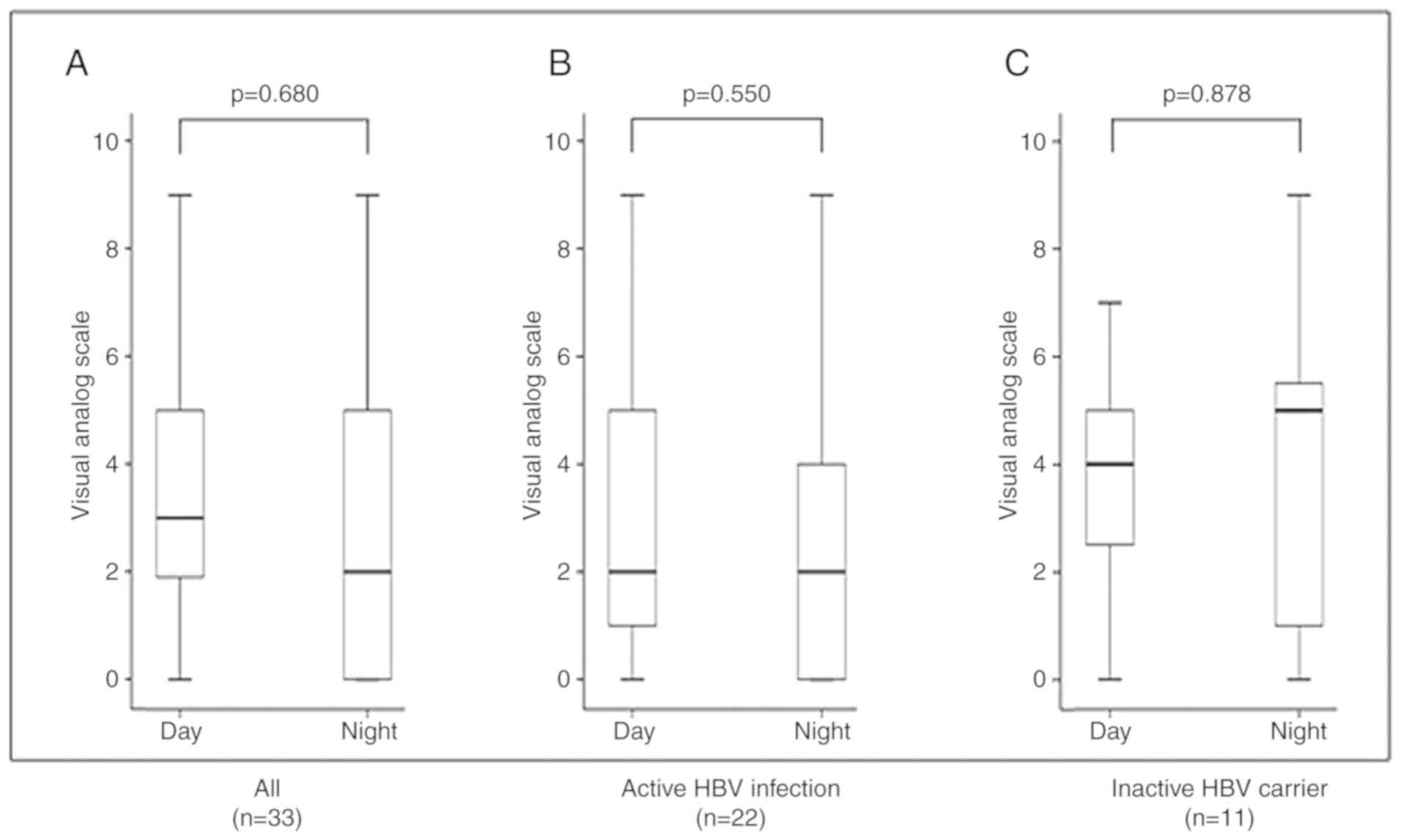
Fig. 3 shows the
severity of itching according to VAS score and the difference in
severity between day and night in the 33 patients with pruritus.
There were no differences in VAS score between day and night across
all the patients (P=0.680), in patients with active HBV infection
(P=0.550) or in inactive HBV carriers (P=0.878). Seasonal
exacerbation was identified at interview in 49% (16/33) of
patients; 88% (14/16) reported exacerbation during the winter and
13% (2/16) reported exacerbation during the summer (data not
shown).
Independent factors associated with
pruritus
In the propensity-matching cohort, patient
characteristics according to the presence of pruritus are presented
in Table III. The proportion of
inactive HBV carriers in patients without pruritus was higher than
that in patients with pruritus (59 vs. 33%; P=0.031). AST and APRI
in patients without pruritus were significantly higher than in
patients with pruritus (23 vs. 25 U/l; P=0.031; and 0.286 vs.
0.337; P=0.030). Being an inactive HBV carrier was identified as
the only independent factor associated with pruritus (odds ratio,
0.35; 95% confidence interval, 0.143-0.842; P=0.019; Table IV).
 | Table IIIPatient characteristics of
propensity-matched patients by presence or absence of pruritus. |
Table III
Patient characteristics of
propensity-matched patients by presence or absence of pruritus.
| | Pruritus
(+)(n=33) | Pruritus
(-)(n=61) | P-value |
|---|
| Sexa, female | 12(36) | 20(33) | 0.903 |
| Age
(years)b | 59 35-81 | 61 35-81 | 0.252 |
| BMIb | 21.2
(18.0-31.43) | 23.1
(19.0-30.5) | 0.106 |
| Phase of HBV
infectiona | | | 0.031 |
|
Active HBV
infection | 22(67) | 25(41) | |
|
Inactive HBV
carrier | 11(33) | 36(59) | |
|
Cirrhosisa | 2 (6.1) | 7(12) | 0.628 |
|
Diabetesa | 4(12) | 6(10) | 1.000 |
|
Hypertensiona | 5(15) | 9(15) | 1.000 |
|
Hyperuricemiaa | 2 (6.1) | 4 (6.6) | 1.000 |
| PLT
(x104/µl)b | 20.9
(9.2-94.9) | 20.5
(7.4-34.0) | 0.440 |
| AST
(U/l)b | 23 13-50 | 25 14-63 | 0.031 |
| ALT
(U/l)b | 19 5-84 | 24 8-97 | 0.173 |
| T-BIL
(mg/dl)b | 0.7 (0.4-1.4) | 0.8 (0.4-1.7) | 0.667 |
| FIB-4
indexb | 1.193
(0.516-5.227) | 1.569
(0.400-5.057) | 0.070 |
| APRIb | 0.286
(0.105-1.060) | 0.337
(0.172-1.154) | 0.030 |
 | Table IVFactors associated with pruritus in
propensity-matched patients with HBV infection. |
Table IV
Factors associated with pruritus in
propensity-matched patients with HBV infection.
| Variables | OR | 95% CI | P-value |
|---|
| Sex | | | 0.727 |
|
Male | 1 | Referent | |
|
Female | 0.85 | 0.351-2.075 | |
| Age (years) | 0.98 | 0.944-1.011 | 0.177 |
| BMI | 0.91 | 0.793-1.040 | 0.164 |
| Phase of HBV
infection | | | 0.019 |
|
Active HBV
infection | 1 | Referent | |
|
Inactive HBV
carrier | 0.35 | 0.143-0.842 | |
| Cirrhosis | 0.50 | 0.097-2.546 | 0.402 |
| Diabetes | 1.26 | 0.330-4.842 | 0.732 |
| Hypertension | 1.03 | 0.315-3.377 | 0.959 |
| Hyperuricemia | 1.09 | 0.188-6.277 | 0.925 |
| PLT (x104/µl) | 1.04 | 0.983-1.108 | 0.167 |
| AST (U/l) | 0.96 | 0.906-1.014 | 0.139 |
| ALT (U/l) | 0.99 | 0.967-1.019 | 0.572 |
| T–BIL (mg/dl) | 0.86 | 0.197-3.800 | 0.847 |
| FIB-4 index | 0.72 | 0.445-1.164 | 0.180 |
| APRI | 0.09 | 0.006-1.502 | 0.094 |
Discussion
The prevalence of pruritus was significantly lower
in inactive HBV carriers than in patients with active HBV
infection. Furthermore, it was found that the phase of HBV
infection was significantly associated with the presence of
pruritus in the propensity score-matched cohort. Previous studies
have characterized the predictors of pruritus in various liver
diseases, including HBV infection (17,18);
however, the effects of HBV infection per se and the phase
of HBV infection on pruritus have not been assessed. A previous
report suggested that active HBV infection is an independent
positive risk factor for pruritus in patients with various chronic
liver diseases (9). To the best of
our knowledge, there have been no studies that established a
predictor of pruritus in patients with HBV infections. The current
study demonstrated that in patients with HBV, matched by propensity
score, being an inactive HBV carrier was an independent negative
risk factor for pruritus.
A previous study indicated the clinical features of
pruritus in various chronic liver diseases. The following
observations have been reported: i) The most frequent itch location
is the back; ii) the duration of pruritus is ≥6 months in 38% of
patients; and iii) the severity of pruritus is significantly higher
during the day than during the night, and seasonal exacerbation
occurs in the winter (9). In the
present study, the back was identified as the most frequent itch
location in both groups, while the frequency itself was
significantly higher in patients with active HBV infection than in
inactive HBV carriers. No HBV infection phase-associated
differences in the duration of itching or the severity of pruritus
were observed.
The mechanism of pruritus in HBV infection remains
unclear. The association between HBV infection and skin lesions has
been studied in a previous systematic review, in which ~2% of
patients with HBV were found to have skin lesions and essential
mixed cryoglobulinemic vasculitis was the most frequent type
(19). It is well known that acute
HBV infection causes Gianotti Crosti syndrome (GCS), which causes
papular acrodermatitis, but skin lesions in GCS are not generally
accompanied by itching (20,21). Therefore, the mechanism and
pathogenesis of skin lesions in GCS may be different from those
causing pruritus in the cases reported here. However, the presence
of skin lesions in acute HBV infections suggested that HBV affected
the skin independent of chronic liver damage or cirrhosis.
Immune complexes targeting hepatitis B surface
antigen are considered to cause immune reactions that result in the
skin lesions in GCS (22).
Additionally, a study described cutaneous changes with itching
following IFN administration to treat HBV infection or hepatitis B
vaccination (23). Lupus, lupus-like
lesions and bullous pemphigoid are generally accompanied by itching
and have been observed during or after IFN treatment (23-25);
while lichen planus, lichen-planus-like lesions and granuloma
annulare have been described following hepatitis B immunization
(26,27). These pieces of evidence suggest that
extraordinary immune signaling is involved in the development of
the skin lesions and itching present in HBV infection, and the
immune response to HBV is likely to be key to pruritus. Liver
dysfunction in HBV infection is caused by the host immune response,
because HBV is a non-cytotoxic virus that does not cause liver
damage (28,29). Therefore, the adverse effects of HBV
infection depend on the extent of the immune response, which might
also influence the development of skin lesions and pruritus.
Indeed, the results of the current study suggested that HBV
infection phase significantly affected the prevalence of
pruritus.
The current study had few limitations. It evaluated
a limited number of patients after matching (n=94) and was not
conducted longitudinally. The collected data did not include
quantitative HBV-DNA, HBe-Ag or detailed information on
medications. Therefore, the impact of antiviral treatment, such as
with nucleic acid analogs or IFN, on HBV-DNA levels was not
evaluated. Serum AST and/or ALT levels in some patients were
normalized due to antiviral treatment may have been included in the
active HBV infection group, and some with abnormal AST and/or ALT
levels resulting from fatty liver may have been included in the
inactive HBV carrier group. Certain medications for concomitant
diseases, including bezafibrate in primary biliary cholangitis, are
known to affect pruritus in HBV infection (30). Further studies are required to
identify the link between these medications and pruritus in HBV
infection in more detail.
In conclusion, the current study identified that the
HBV infection phase was a predictor of pruritus in patients with
HBV infection. The prevalence of pruritus was significantly lower
in patients with inactive CHB phase than in those with
HBe-Ag-positive/negative immune-active phase, implying that an
inactive CHB phase suppressed pruritus. Thus, the progression to an
inactive CHB phase may contribute to the amelioration of pruritus
in patients with HBV infection.
Acknowledgements
The authors would like to thank Dr Mark Cleasby
(Edanz Group) for language editing this manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets of the present study are available from
the corresponding author on reasonable request.
Authors' contributions
YE conceived and designed the study. SO, HT, HI, KI,
MY, MO, HH, TK, HF, MK, YS, ST, HK, TT, TS, AN and YE collected and
interpreted clinical data. SO curated data. SK and AK statistically
analyzed data. SO wrote the first draft of the manuscript and HT
and YE contributed to the writing of the manuscript. HI, KI, MY,
MO, HH, TK, HF, MK, YS, ST, HK, TT, TS and AN reviewed and revised
the manuscript. All authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
The original study was conducted in accordance with
the Helsinki Declaration of 1964, as revised in 2013. The original
study protocol was approved by the Clinical Research Ethics Review
Committee of each facility. Opt-out informed consent was obtained
from all participants at time of hospitalization.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yamada T, Alpers DH, Kalloo AN, Kaplowitz
N, Owyang C and Powell DN: editor. Text of gastroenterology, 5th
ed. Oxford: Blackwell Publishing Ltd. 2009. View Article : Google Scholar
|
|
2
|
Cheung AC, Patel H, Meza-Cardona J, Cino
M, Sockalingam S and Hirschfield GM: Factors that influence
health-related quality of life in patients with primary sclerosing
cholangitis. Dig Dis Sci. 61:1692–1699. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Benito de Valle M, Rahman M, Lindkvist B,
Björnsson E, Chapman R and Kalaitzakis E: Factors that reduce
health-related quality of life in patients with primary sclerosing
cholangitis. Clin Gastroenterol Hepatol. 10:769–775.e2.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gutteling JJ, de Man RA, Busschbach JJ and
Darlington AS: Overview of research on health-related quality of
life in patients with chronic liver disease. Neth J Med.
65:227–234. 2007.PubMed/NCBI
|
|
5
|
World Health Organization. Global
hepatitis report, 2017. Geneva, Switzerland: World Health
Organization, 2017.
|
|
6
|
Chalasani N, Younossi Z, Lavine JE, Diehl
AM, Brunt EM, Cusi K, Charlton M and Sanyal AJ: The diagnosis and
management of non-alcoholic fatty liver disease: Practice Guideline
by the American Association for the Study of Liver Diseases,
American College of Gastroenterology, and the American
gastroenterological association. Hepatology. 55:2005–2023.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hoofnagle JH, Doo E, Liang TJ, Fleischer R
and Lok AS: Management of hepatitis B: Summary of a clinical
research workshop. Hepatology. 45:1056–1075. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bonacini M: Pruritus in patients with
chronic human immunodeficiency virus, hepatitis B and C virus
infections. Dig Liver Dis. 32:621–625. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Oeda S, Takahashi H, Yoshida H, Ogawa Y,
Imajo K, Yoneda M, Koshiyama Y, Ono M, Hyogo H, Kawaguchi T, et al:
Prevalence of pruritus in patients with chronic liver disease: A
multicenter study. Hepatol Res. 48:E252–E262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cacoub P, Poynard T, Ghillani P, Charlotte
F, Olivi M, Piette JC and Opolon P: Extrahepatic manifestations of
chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C.
Arthritis Rheum. 42:2204–2212. 1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Talwalkar JA, Souto E, Jorgensen RA and
Lindor KD: Natural history of pruritus in primary biliary
cirrhosis. Clin Gastroenterol Hepatol. 1:297–302. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jupiter DC: Propensity score matching:
Retrospective randomization? J Foot Ankle Surg. 56:417–420.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT,
Thomas DL, et al: Development of a simple noninvasive index to
predict significant fibrosis in patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wai CT, Greenson JK, Fontana RJ,
Kalbfleisch JD, Marrero JA, Conjeevaram HS and Lok AS: A simple
noninvasive index can predict both significant fibrosis and
cirrhosis in patients with chronic hepatitis C. Hepatology.
38:518–526. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sandrin L, Fourquet B, Hasquenoph JM, Yon
S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, et
al: Transient elastography: A new noninvasive method for assessment
of hepatic fibrosis. Ultrasound Med Biol. 29:1705–1713.
2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aitken RC: Measurement of feelings using
visual analogue scales. Proc R Soc Med. 62:989–993. 1969.PubMed/NCBI
|
|
17
|
Akuta N, Kumada H, Fujiyama S, Kawamura Y,
Sezaki H, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Suzuki Y,
et al: Predictors of pruritus in patients with chronic liver
disease and usefulness of nalfurafine hydrochloride. Hepatol Res.
48:45–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sumi R, Fukuda K, Irishio K, Hattori M,
Sawai Y, Kogita S, Igura S, Yamaguchi Y, Matsumoto Y, Nakahara M,
et al: Current status of pruritus in chronic liver diseases and
efficacy of nalfurafine hydrochloride. Kanzo. 58:486–493. 2017.(In
Japanese).
|
|
19
|
Grigorescu I and Dumitrascu DL:
Spontaneous and antiviral-induced cutaneous lesions in chronic
hepatitis B virus infection. World J Gastroenterol. 20:15860–15866.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gianotti F: Papular acrodermatitis of
childhood. An Australia antigen disease. Arch Dis Child.
48:794–799. 1973.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ishimaru Y, Ishimaru H, Toda G, Baba K and
Mayumi M: An epidemic of infantile papular acrodermatitis
(Gianotti's disease) in Japan associated with hepatitis-B surface
antigen subtype ayw. Lancet. 1:707–709. 1976. View Article : Google Scholar
|
|
22
|
Kacprzak-Bergman I and Halasa J: Frequency
of complement third component (C3) and properdin factor (BF)
phenotypes in patients with various clinical manifestations of HBV
infection. Eur J Immunogenet. 23:1–6. 1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kartal ED, Alpat SN, Ozgunes I and Usluer
G: Adverse effects of high-dose interferon-alpha-2a treatment for
chronic hepatitis B. Adv Ther. 24:963–971. 2007.PubMed/NCBI
|
|
24
|
Yilmaz S and Cimen KA: Pegylated
interferon alpha-2B induced lupus in a patient with chronic
hepatitis B virus infection: Case report. Clin Rheumatol.
28:1241–1243. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
García-Porrúa C, González-Gay MA,
Fernández-Lamelo F, Paz-Carreira JM, Lavilla E and González-López
MA: Simultaneous development of SLE-like syndrome and autoimmune
thyroiditis following alpha-interferon treatment. Clin Exp
Rheumatol. 16:107–108. 1998.PubMed/NCBI
|
|
26
|
Criado PR, de Oliveira Ramos R,
Vasconcellos C, Jardim Criado RF and Valente NY: Two case reports
of cutaneous adverse reactions following hepatitis B vaccine:
Lichen planus and granuloma annulare. J Eur Acad Dermatol Venereol.
18:603–606. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mérigou D, Léauté-Labrèze C, Louvet S,
Bioulac-Sage P and Taïeb A: Lichen planus in children: Role of the
campaign for hepatitis B vaccination. Ann Dermatol Venereol.
125:399–403. 1998.(In French). PubMed/NCBI
|
|
28
|
Guidotti LG and Chisari FV: Immunobiology
and pathogenesis of viral hepatitis. Annu Rev Pathol. 1:23–61.
2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Guidotti LG, Matzke B, Schaller H and
Chisari FV: High-level hepatitis B virus replication in transgenic
mice. J Virol. 69:6158–6169. 1995.PubMed/NCBI
|
|
30
|
Reig A, Sesé P and Parés A: Effects of
Bezafibrate on outcome and pruritus in primary biliary cholangitis
with suboptimal ursodeoxycholic acid response. Am J Gastroenterol.
113:49–55. 2018.PubMed/NCBI View Article : Google Scholar
|