Introduction
Mesenchymal stem cells (MSCs) are adult stem cells
that have a promising role in regenerative medicine (1). MSCs are multipotent and self-renewing
cells and can differentiate into several types of cells (2). These cells are suitable for use in
clinical applications because of their various properties, such as
low immunogenicity, immunomodulatory effects (3), migration potential to sites of injury
(4) and regenerative potential
(5). MSCs can be isolated from
different sources, such as bone marrow (6), adipose tissue (7), umbilical cord (8), and dental pulp (9).
MSCs were first isolated from bone marrow (10). Then, research focused on the
identification of a less invasive source of MSCs than bone marrow.
Harvesting adipose-derived stem cells (ASCs) is less invasive than
harvesting bone marrow stem cells (BM-SCs) and can be performed
during liposuction and preferably in cases of autologous therapy
(11,12). However, ASCs face further obstacles in
the isolation steps, as they require digestion of adipose tissue
using collagenase (7), which may
affect cell viability. Therefore, identification of an alternative
noninvasive source that is suitable for autologous therapy and
requires minimal manipulation is needed.
Recently, MSCs have been successfully isolated from
the peripheral blood of rats, rabbits, canines, ovines and equines
(13-19).
The major problem with this source is the low levels of stem cells
among mononuclear cells. Researchers tried to increase the number
of stem cells in peripheral blood by injection of mobilizing
agents, such granulocyte colony-stimulating factor (20). The present study hypothesized that
MSCs are found in peripheral blood at a certain percentage in mice
and this percentage is inversely proportionate to age; its peak
should be in the first weeks after birth.
In this study peripheral blood MSCs (PB-MSCs), were
isolated from 4-week-old BALB/c white mice without using prior
mobilizing agents and compared them with bone marrow MSCs
(BM-MSCs).
Materials and methods
Ethical approval
The experimental protocol was approved by the Local
Ethical Committee of the Faculty of Medicine, Mansoura University
(Mansoura, Egypt) R/16.12.24.
Isolation of MSCs from BM and PB
MSCs were isolated from 4-week-old male BALB/c mice
(weight, 15-20 g). A total of 6 mice were provided by Medical
Experimental research Centre at the Faculty of Medicine, Mansoura
University. Animals were housed in plastic cages (3/cage) on
sawdust, with free access to food and water and were kept at a
constant temperature of 22±1˚C with 50% relative humidity and 12-h
light/dark cycles for ≥1 week before the experiment. BM was
isolated from mouse femurs and tibiae as previously described
(21). PB was isolated from the same
mouse by cardiac puncture as previously described (22). In both samples, mononuclear cells were
isolated by density gradient centrifugation (400 x g; 30 min; 20˚C)
and cultured in DMEM supplemented with 10% FBS and 1%
antibiotic-antimycotic solution (all Thermo Fisher Scientific,
Inc.) in 25 cm2 flasks. Flasks were incubated at 37˚C
with 5% CO2 and the cells were cultured until passage
4.
XTT cell proliferation assay
In the XTT assay, passage 4 MSC proliferation was
indirectly assessed by measuring metabolically active cells
(23). MSCs were seeded at 125, 250,
500 and 1,000 cells/well and allowed to grow for 7 days at
different concentrations. Optical density was measured at 450 nm
after the addition of the XTT reagent (Roche Diagnostics). The
assay was repeated three times.
Flow cytometric analysis
Passage 4 MSCs were characterized using cell surface
markers by fluorescence-activated cell sorting analyses. The cells
were stained with fluorescently labelled monoclonal antibodies
against CD29, CD44, CD105, CD90.2, CD146, Sca-1, CD45 and CD140b (1
µg/ml; Miltenyi Biotec, Inc.; Table
I) for 10 min in the dark at 2-8˚C. All data were acquired
using a flow cytometer and assessed using FACSDiva v8.0.1 (BD
Biosciences).
 | Table IFlow cytometry antibody panel used for
characterization. |
Table I
Flow cytometry antibody panel used for
characterization.
| Name | Conjugate | Clone | Cat. no. | Supplier |
|---|
| CD29 | PE | HMs1-1 | 130-102-994 | Miltenyi Biotec,
Inc. |
| CD44 | FITC | IM7.8.1 | 130-102-511 | Miltenyi Biotec,
Inc. |
| CD105 | PE | MJ7/18 | 130-102-548 | Miltenyi Biotec,
Inc. |
| CD90.2 | FITC | 30-H12 | 130-120-091 | Miltenyi Biotec,
Inc. |
| CD146 | FITC | ME-9F1 | 130-102-230 | Miltenyi Biotec,
Inc. |
| SCa-1 | PE | D7 | 130-102-832 | Miltenyi Biotec,
Inc. |
| CD45 | FITC | 30F11.1 | 130-110-658 | Miltenyi Biotec,
Inc. |
| CD140b | PE | APB5 | 130-118-457 | Miltenyi Biotec,
Inc. |
Differentiation capability
Osteogenic differentiation
Passage 4 MSCs were harvested, counted and seeded at
a density of 5x104 per well in a 6-well plate in
osteogenesis differentiation media (DMEM supplemented with 10% FBS,
0.1 µM dexamethasone, 100 µM ascorbic acid and 10 mM β-glycerol
phosphate from Sigma-Aldrich, Merck KGaA). The medium was changed
twice per week for 2-3 weeks. The differentiation potential for
osteogenesis was assessed by 40 mM Alizarin Red (pH 4.1) for 15 min
at room temperature after fixation in 10% neutral buffered formalin
for 10 min at room temperature. Quantification of calcium
deposition was performed with a commercial calcium assay kit
(Sigma-Aldrich, Merck KGaA). The assay was repeated three
times.
Adipogenic differentiation
Passage 4 MSCs were harvested, counted and seeded at
a density of 5x104 per well in a 24-well plate in
adipogenesis differentiation media (DMEM supplemented with 10% FBS,
10% HS, 0.5 mM isobutylmethylxanthine, 60 mM indomethacin and 0.5
mM hydrocortisone from Sigma-Aldrich, Merck KGaA); the medium was
changed twice per week for 2 weeks. The differentiation potential
for adipogenesis and formation of intracellular lipid droplets were
assessed by Oil red O for 15 min at room temperature after fixation
in 10% neutral buffered formalin for 10 min at room temperature
using semi quantitative scoring as described by Aldridge et
al (24). The level of
adipogenesis was evaluated by ranking 500 cells in the wells by
their fat content. Ranks were divided on the basis of the fat
proportion: Grade 1, 0-24%; grade 2, 25-49%; grade 3, 50-74%; grade
4, 75-100%. The assay was repeated three times.
Chondrogenic differentiation
Passage 4 MSCs were harvested, counted and seeded at
a density of 0.25x106 per Eppendorf tube in chondrogenic
differentiation media [high-glucose DMEM supplemented with 10 ng/ml
TGF-β3, 100 nM dexamethasone, 200 µM ascorbate-2-phosphate, 40
µg/ml proline, 1 mM pyruvate, 1 mg/ml bovine serum albumin
(Sigma-Aldrich, Merck KGaA) and 50 mg/ml ITS +3]. The medium was
replaced every 2-3 days for 21 days. Cell pellets were fixed in 10%
formalin for 1 day at room temperature and embedded in paraffin wax
at 58˚C for 15 min. Sections of the cell pellets (5 µm) were
stained with toluidine blue for 30 min at 37˚C (1% in 50%
isopropanol) to demonstrate collagen content and sulfated
proteoglycans within the extracellular matrix, indicated by blue
color. Furthermore the production of sulfated GAG was measured in
an Alcian blue binding assay (cat. no. 74240; Immunodiagnostic
Systems) following digestion in 100 µl papain solution. Absorbance
was read at 630 nm. The assay was repeated three times.
Statistical analysis
Data were presented as the mean ± standard
deviation. Statistical differences between groups were analyzed by
one-way analysis of variance followed by Tukey's post hoc test with
a Stata 7.0 software package (StataCorp LLC.). All assays were
repeated three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
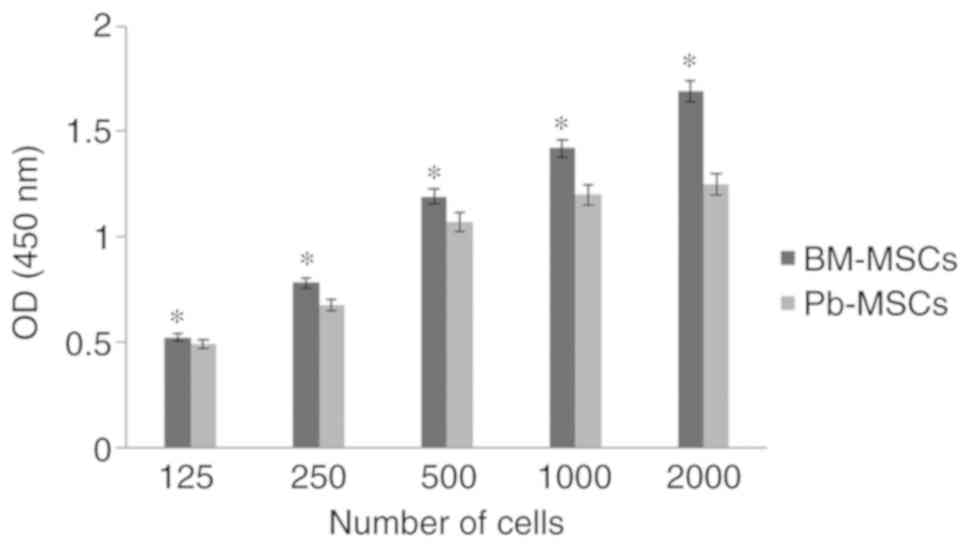
XTT assay
BM-MSCs showed a significantly greater optical
density in XTT assays compared with PB-MSCs (P<0.05) and the
difference increased as the cell number increased, indicating that
BM-MSCs have a higher proliferative rate than PB-MSCs (Fig. 1).
Flow cytometric analysis
Cultures of passage 4 BM-MSCs and PB-MSCs were
analyzed for the expression of cell-surface markers (Fig. 2). BM-MSCs were positive for CD29 and
negative for all other markers. PB-MSCs were positive for CD146,
CD29 and CD140b and negative for Sca-1, CD44, CD45, CD90 and
CD105.
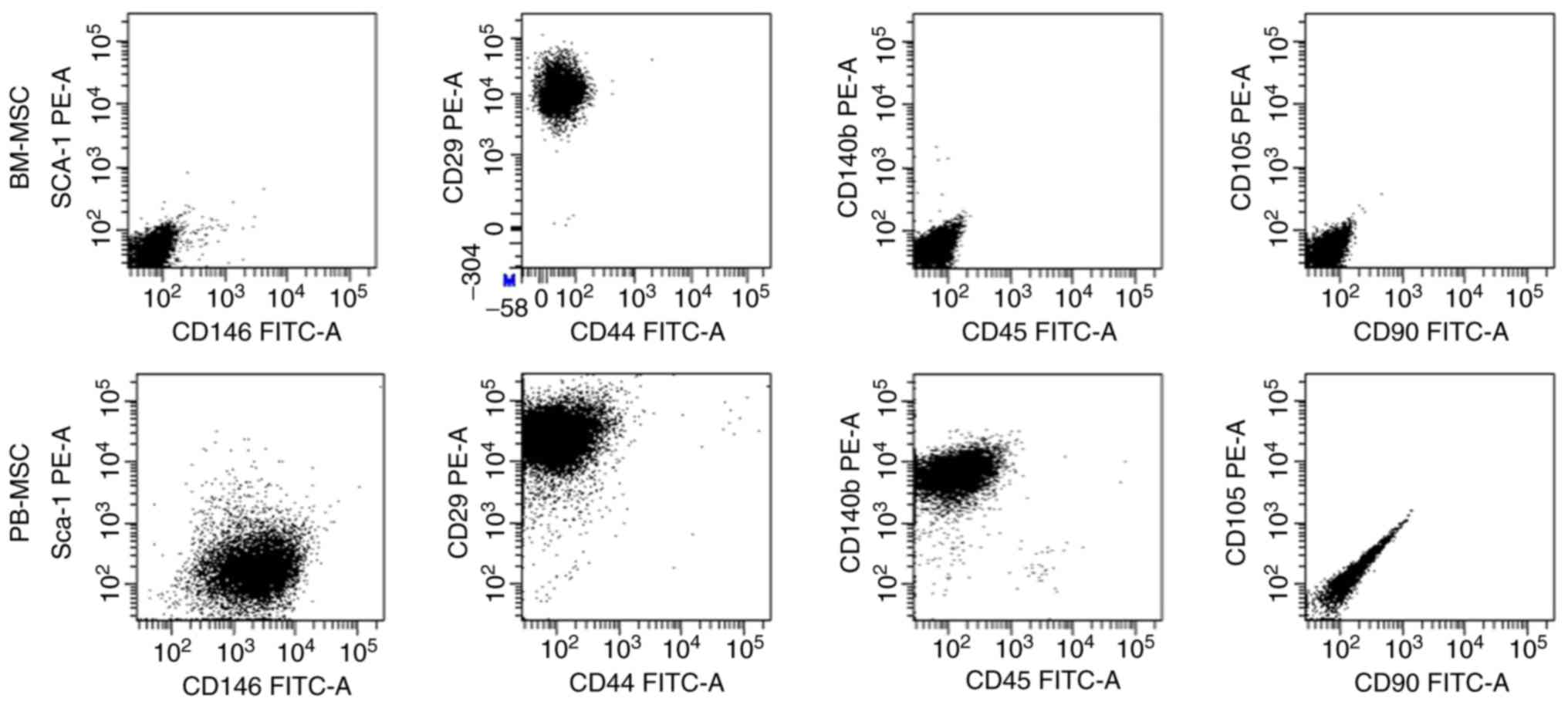 | Figure 2.Flow cytometric analysis of BM-MSCs
and PB-MSCs. BM-MSCs were positive CD29 and negative for other
markers, while PB-MSCs were positive for CD146, CD29, and CD140b
and negative for Sca-1, CD44, CD45, CD90 and CD105. CD, cluster of
differentiation; BM-MSCs, bone marrow-mesenchymal stromal cells;
PB, peripheral blood; FITC, fluorescein isothiocyanate; PE,
phycoerythrin. |
Differentiation capability
Osteogenic differentiation
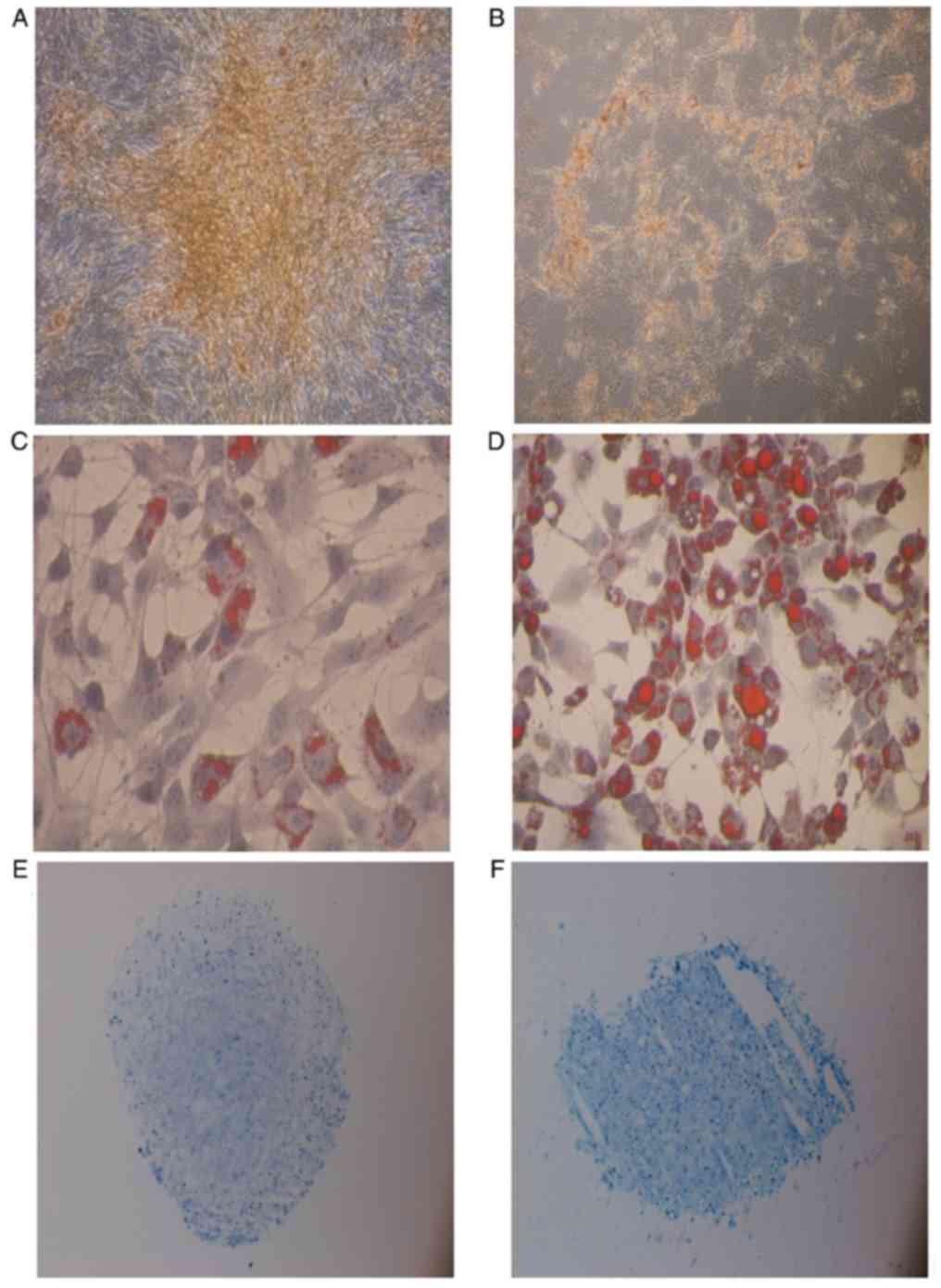
BM-MSCs and PB-MSCs differentiated into osteoblasts
(Fig. 3A and B). BM-MSCs appeared to
have a greater capability to differentiate into osteoblasts than
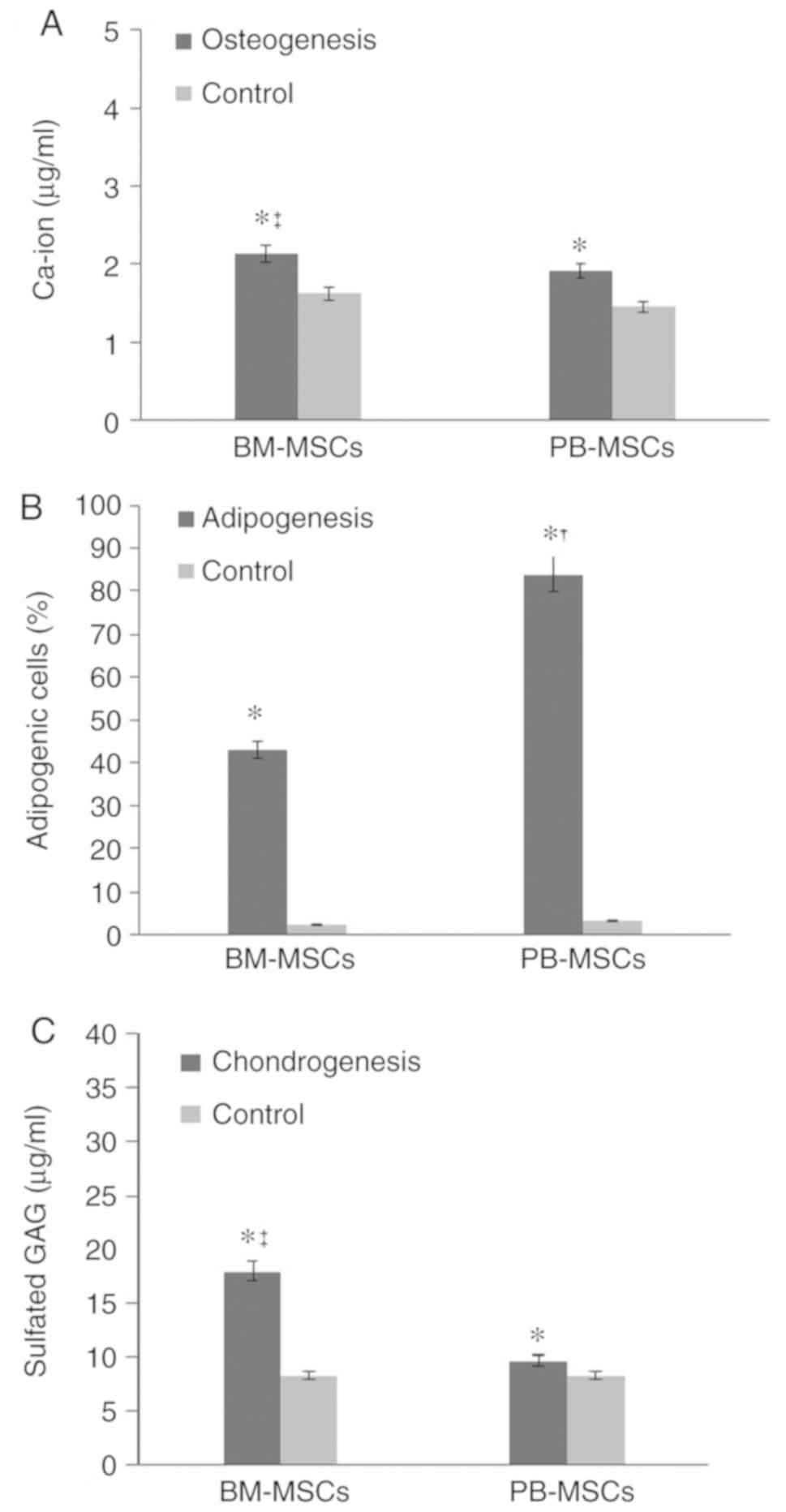
PB-MSCs. Quantification of calcium deposition showed 2.12±0.106
µg/ml differentiated BM-MSC osteoblasts and 1.91±0.6 µg/ml
differentiated PB-MSC osteoblasts (Fig.
4A).
Adipogenic differentiation
BM-MSC and PB-MSC differentiated into adipocyte
(Fig. 3C and D) and PB-MSC exhibited
a higher propensity to differentiate into adipocytes (Fig. 4B).
Chondrogenic differentiation
BM-MSCs and PB-MSCs tended to differentiate into
chondrocytes (Fig. 3E and F), but
BM-MSCs showed stronger production of sulfated GAG compared with
PB-MSCs. The concentration of sulfated GAG in differentiated
BM-MSCs was 17.93±2.44 µg/ml. The concentration of sulfated GAG in
differentiated PB-MSCs was 9.66±1.02 µg/ml (Fig. 4C).
Discussion
Bone marrow is the first and most common source of
MSCs, but because collection of bone marrow is highly invasive,
scientists have tried to find an alternative source, such as
adipose tissue. Although adipose tissue is a less invasive source
than bone marrow, there are still problems in its processing and
digestion. PB is also an easy source of MSCs. In this study, the
biological characteristics of mouse PB-MSCs and BM-MSCs were
compared with regard to the proliferation rate, surface markers and
trilineage differentiation potential (osteogenic, adipogenic and
chondrogenic).
The XTT assay showed that the proliferation rate of
BM-MSCs was compared with the PB-MSCs when cells were cultured in a
gradual concentration and were left for a week, which is consistent
with Fu et al (16), who
compared BM-MSCs and PB-MSCs in rats. Immunophenotypic
characterization also showed some differences, including CD146- and
CD140b-positive expression only in PB-MSCs. CD146 expression in the
MSC population showed heterogeneity in general and Espagnolle et
al (25) demonstrated that MSCs
with low CD146 expression had higher proliferation rates than MSCs
with high CD146, which is consistent with the present study because
PB-MSCs have high CD146 and low proliferation rates compared with
BM-MSCs.
Both PB-MSCs and BM-MSCs could differentiate into
osteoblasts, adipocytes and chondrocytes after these cells were
cultured in differentiation-induction media compared with the
control media. BM-MSCs showed higher differentiation potential to
osteoblasts and chondrocytes than PB-MSCs based on their calcium
and GAG accumulation, which is consistent with the findings of
Lyahyai et al (26) and Spaas
et al (27). In contrast to
Chong et al (28), PB-MSCs
showed higher adipogenic differentiation than BM-MSCs as assessed
by fat droplet formation. MSC circulation in the bloodstream has
been reported, but the exact tissue origin is debated. One theory
states that PB-MSCs migrate from bone marrow, but the present study
does not support this hypothesis because there are some biological
differences (13,29).
PB-MSCs are promising for autologous regenerative
therapy. Blood samples taken from healthy children could be a
source of stem cell banks for both autologous and off-shelf
allogeneic therapy.
In conclusion, PB-MSCs are easily obtained from the
PB of young mice. Although PB-MSCs and BM-MSCs have some
differences in differentiation and surface markers, they have very
similar biological characteristics. Mouse PB-MSCs are a good source
of MSCs and a parallel study of PB-MSCs and BM-MSCs in mice can
shed more light on their biology in relation to ageing.
Acknowledgements
The authors would like to thank Professor Mohamed
Sobh (Mansoura University) and Dr Jehan EL-Jawhari (University of
Leeds) for their valuable support and advise throughout the
characterization steps of the current study.
Funding
The present study was supported by the Science and
Technology Development Fund (grant no. 4223).
Availability of data and materials
The data used to support the findings of this study
are available from the corresponding author upon request.
Authors' contributions
AL, YMES, EJ and AB contributed to the study design.
AL, YMES and RC contributed data acquisition and analysis. All
authors contributed to writing and revising the manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the Local
Ethical Committee of the Faculty of Medicine, Mansoura University
(Mansoura, Egypt; no. R/16.12.24).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Si YL, Zhao YL, Hao HJ, Fu XB and Han WD:
MSCs: Biological characteristics, clinical applications and their
outstanding concerns. Ageing Res Rev. 10:93–103. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vater C, Kasten P and Stiehler M: Culture
media for the differentiation of mesenchymal stromal cells. Acta
Biomater. 7:463–477. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yagi H, Soto-Gutierrez A, Parekkadan B,
Kitagawa Y, Tompkins RG, Kobayashi N and Yarmush ML: Mesenchymal
stem cells: Mechanisms of immunomodulation and homing. Cell
Transplant. 19:667–679. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sohni A and Verfaillie CM: Mesenchymal
stem cells migration homing and tracking. Stem Cells Int.
2013(130763)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bianchi F, Sala E, Donadei C, Capelli I
and La Manna G: Potential advantages of acute kidney injury
management by mesenchymal stem cells. World J Stem Cells.
6:644–650. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Friedenstein AJ, Chailakhyan RK, Latsinik
NV, Panasyuk AF and Keiliss-Borok IV: Stromal cells responsible for
transferring the microenvironment of the hemopoietic tissues.
Cloning in vitro and retransplantation in vivo. Transplantation.
17:331–340. 1974.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang HS, Hung SC, Peng ST, Huang CC, Wei
HM, Guo YJ, Fu YS, Lai MC and Chen CC: Mesenchymal stem cells in
the Wharton's jelly of the human umbilical cord. Stem Cells.
22:1330–1337. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang
J, Xu GT, Liang A and Liu S: Concise reviews: Characteristics and
potential applications of human dental tissue-derived mesenchymal
stem cells. Stem Cells. 33:627–638. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Friedenstein AJ, Chailakhjan RK and
Lalykina KS: The development of fibroblast colonies in monolayer
cultures of guinea-pig bone marrow and spleen cells. Cell Tissue
Kinet. 3:393–403. 1970.PubMed/NCBI
|
|
11
|
Harasymiak-Krzyzanowska I, Niedojadlo A,
Karwat J, Kotula L, Gil-Kulik P, Sawiuk M and Kocki J: Adipose
tissue-derived stem cells show considerable promise for
regenerative medicine applications. Cell Mol Biol Lett. 18:479–493.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lotfy A, Salama M, Zahran F, Jones E,
Badawy A and Sobh M: Characterization of mesenchymal stem cells
derived from rat bone marrow and adipose tissue: A comparative
study. Int J Stem Cells. 7:135–142. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
He Q, Wan C and Li G: Concise review:
Multipotent mesenchymal stromal cells in blood. Stem Cells.
25:69–77. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Koerner J, Nesic D, Romero JD, Brehm W,
Mainil-Varlet P and Grogan SP: Equine peripheral blood-derived
progenitors in comparison to bone marrow-derived mesenchymal stem
cells. Stem Cells. 24:1613–1619. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zvaifler NJ, Marinova-Mutafchieva L, Adams
G, Edwards CJ, Moss J, Burger JA and Maini RN: Mesenchymal
precursor cells in the blood of normal individuals. Arthritis Res.
2:477–488. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Fu WL, Zhang JY, Fu X, Duan XN, Leung KK,
Jia ZQ, Wang WP, Zhou CY and Yu JK: Comparative study of the
biological characteristics of mesenchymal stem cells from bone
marrow and peripheral blood of rats. Tissue Eng Part A.
18:1793–1803. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Longhini ALF, Salazar TE, Vieira C, Trinh
T, Duan Y, Pay LM, Li Calzi S, Losh M, Johnston NA, Xie H, et al:
Peripheral blood-derived mesenchymal stem cells demonstrate
immunomodulatory potential for therapeutic use in horses. PLoS One.
14(e0212642)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li S, Huang KJ, Wu JC, Hu MS, Sanyal M, Hu
M, Longaker MT and Lorenz HP: Peripheral blood-derived mesenchymal
stem cells: Candidate cells responsible for healing critical-sized
calvarial bone defects. Stem Cells Transl Med. 4:359–368.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fu Q, Tang NN, Zhang Q, Liu Y, Peng JC,
Fang N, Yu LM, Liu JW and Zhang T: Preclinical study of cell
therapy for osteonecrosis of the femoral head with allogenic
peripheral blood-derived mesenchymal stem cells. Yonsei Med J.
57:1006–1015. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fruehauf S, Veldwijk MR, Seeger T,
Schubert M, Laufs S, Topaly J, Wuchter P, Dillmann F, Eckstein V,
Wenz F, et al: A combination of granulocyte-colony-stimulating
factor (G-CSF) and plerixafor mobilizes more primitive peripheral
blood progenitor cells than G-CSF alone: Results of a European
phase II study. Cytotherapy. 11:992–1001. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nadri S and Soleimani M: Isolation murine
mesenchymal stem cells by positive selection. In Vitro Cell Dev
Biol Anim. 43:276–282. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kuznetsov SA, Mankani MH, Gronthos S,
Satomura K, Bianco P and Robey PG: Circulating skeletal stem cells.
J Cell Biol. 153:1133–1140. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jones EA, English A, Henshaw K, Kinsey SE,
Markham AF, Emery P and McGonagle D: Enumeration and phenotypic
characterization of synovial fluid multipotential mesenchymal
progenitor cells in inflammatory and degenerative arthritis.
Arthritis Rheum. 50:817–827. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Aldridge A, Kouroupis D, Churchman S,
English A, Ingham E and Jones E: Assay validation for the
assessment of adipogenesis of multipotential stromal cells-a direct
comparison of four different methods. Cytotherapy. 15:89–101.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Espagnolle N, Guilloton F, Deschaseaux F,
Gadelorge M, Sensébé L and Bourin P: CD146 expression on
mesenchymal stem cells is associated with their vascular smooth
muscle commitment. J Cell Mol Med. 18:104–114. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lyahyai J, Mediano DR, Ranera B, Sanz A,
Remacha AR, Bolea R, Zaragoza P, Rodellar C and Martín-Burriel I:
Isolation and characterization of ovine mesenchymal stem cells
derived from peripheral blood. BMC Vet Res. 8(169)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Spaas JH, De Schauwer C, Cornillie P,
Meyer E, Van Soom A and Van de Walle GR: Culture and
characterisation of equine peripheral blood mesenchymal stromal
cells. Vet J. 195:107–113. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chong PP, Selvaratnam L, Abbas AA and
Kamarul T: Human peripheral blood derived mesenchymal stem cells
demonstrate similar characteristics and chondrogenic
differentiation potential to bone marrow derived mesenchymal stem
cells. J Orthop Res. 30:634–642. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hass R, Kasper C, Böhm S and Jacobs R:
Different populations and sources of human mesenchymal stem cells
(MSC): A comparison of adult and neonatal tissue-derived MSC. Cell
Commun Signal. 9(12)2011.PubMed/NCBI View Article : Google Scholar
|