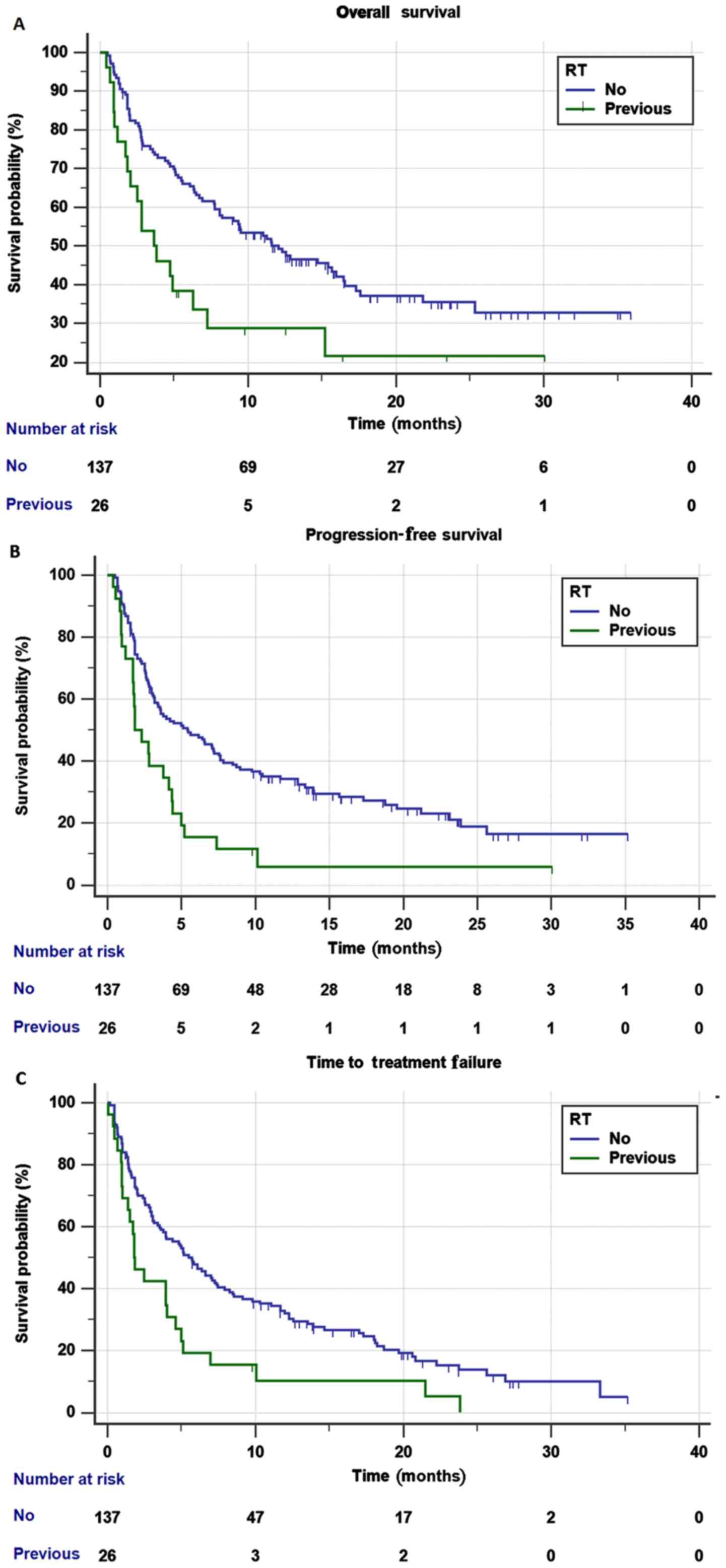
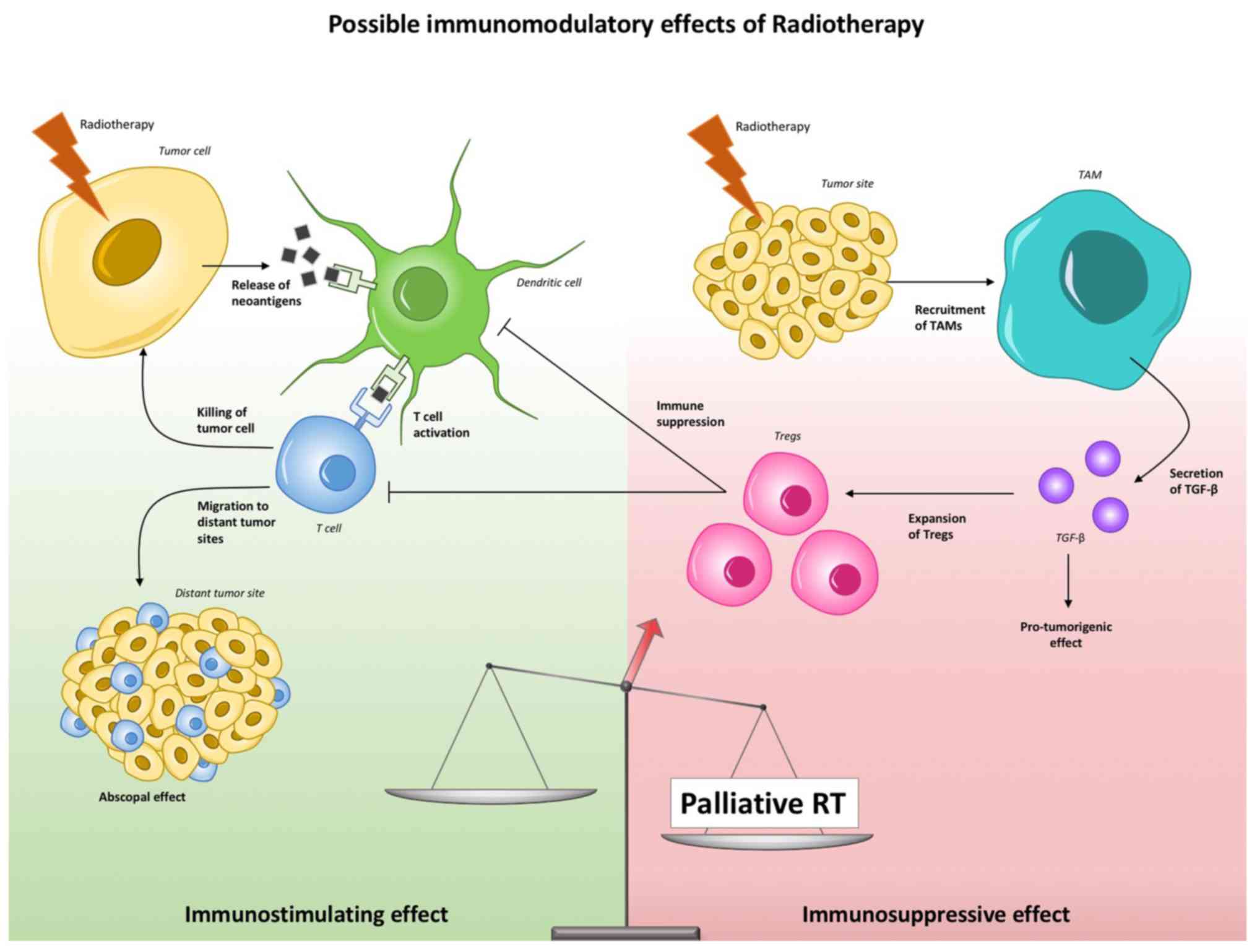
|
1
|
Chung C: To do or not to do: A concise
update of current clinical controversies in immune checkpoint
blockade. J Oncol Pharm Pract. 25:663–673. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Derosa L, Hellmann MD, Spaziano M,
Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC,
Chaft JE, et al: Negative association of antibiotics on clinical
activity of immune checkpoint inhibitors in patients with advanced
renal cell and non-small-cell lung cancer. Ann Oncol. 29:1437–1444.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Garant A, Guilbault C, Ekmekjian T,
Greenwald Z, Murgoi P and Vuong T: Concomitant use of
corticosteroids and immune checkpoint inhibitors in patients with
hematologic or solid neoplasms: A systematic review. Crit Rev Oncol
Hematol. 120:86–92. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bersanelli M, Giannarelli D, Castrignanò
P, Fornarini G, Panni S, Mazzoni F, Tiseo M, Rossetti S, Gambale E,
Rossi E, et al: INfluenza vaccine indication during therapy with
immune checkpoint inhibitors: A transversal challenge. The INVIDIa
study. Immunotherapy. 10:1229–1239. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lesueur P, Escande A, Thariat J, Vauléon
E, Monnet I, Cortot A, Lerouge D, Danhier S, Dô P, Dubos-Arvis C,
et al: Safety of combined PD-1 pathway inhibition and radiation
therapy for non-small-cell lung cancer: A multicentric
retrospective study from the GFPC. Cancer Med. 7:5505–5513.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu Y, Dong Y, Kong L, Shi F, Zhu H and Yu
J: Abscopal effect of radiotherapy combined with immune checkpoint
inhibitors. J Hematol Oncol. 11(104)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nobler MP: The abscopal effect in
malignant lymphoma and its relationship to lymphocyte circulation.
Radiology. 93:410–412. 1969.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Formenti SC and Demaria S: Combining
radiotherapy and cancer immunotherapy: A paradigm shift. J Natl
Cancer Inst. 105:256–265. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kiess AP, Wolchok JD, Barker CA, Postow
MA, Tabar V, Huse JT, Chan TA, Yamada Y and Beal K: Stereotactic
radiosurgery for melanoma brain metastases in patients receiving
ipilimumab: Safety profile and efficacy of combined treatment. Int
J Radiat Oncol Biol Phys. 92:368–375. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lehrer EJ, Peterson J, Brown PD, Sheehan
JP, Quiñones-Hinojosa A, Zaorsky NG and Trifiletti DM: Treatment of
brain metastases with stereotactic radiosurgery and immune
checkpoint inhibitors: An international meta-analysis of individual
patient data. Radiother Oncol. 104–112. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Theelen WSME, Peulen HMU, Lalezari F, van
der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I,
Niemeijer AN, de Langen AJ, et al: Effect of pembrolizumab after
stereotactic body radiotherapy vs pembrolizumab alone on tumor
response in patients with advanced non-small cell lung cancer:
Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA
Oncol. 5:1276–1282. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shaverdian N, Lisberg AE, Bornazyan K,
Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P:
Previous radiotherapy and the clinical activity and toxicity of
pembrolizumab in the treatment of non-small-cell lung cancer: A
secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol.
18:895–903. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ciocan D, Barbe C, Aubin F, Granel-Brocard
F, Lipsker D, Velten M, Dalac S, Truchetet F, Michel C, Mitschler
A, et al: Distinctive features of melanoma and its management in
elderly patients: A population-based study in France. JAMA
Dermatol. 149:1150–1157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gridelli C, Balducci L, Ciardiello F, Di
Maio M, Felip E, Langer C, Lilenbaum RC, Perrone F, Senan S and de
Marinis F: Treatment of elderly patients with non-small cell lung
cancer: Results of an international expert panel meeting of the
Italian association of thoracic oncology. Clin Lung Cancer.
16:325–333. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Azawi NH, Joergensen SM, Jensen NV, Clark
PE and Lund L: Academy of Geriatric Cancer Research (AgeCare):
Trends in Kidney cancer among the elderly in Denmark, 1980-2012.
Acta Oncol. 55 (Suppl 1):S79–S84. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Datta SS, Ghosal N, Daruvala R,
Chakraborty S, Shrimali RK, van Zanten C, Parry J, Agrawal S,
Atreya S, Sinha S, et al: How do clinicians rate patient's
performance status using the ECOG performance scale? A
mixed-methods exploration of variability in decision-making in
oncology. Ecancermedicalscience. 13(913)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Common terminology criteria for adverse
events (ctcae) version 5.0.
https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
Accessed November 27, 2017.
|
|
19
|
Walle T, Martinez Monge R, Cerwenka A,
Ajona D, Melero I and Lecanda F: Radiation effects on antitumor
immune responses: Current perspectives and challenges. Ther Adv Med
Oncol. 10(1758834017742575)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Verastegui EL, Morales RB, Barrera-Franco
JL, Poitevin AC and Hadden J: Long-term immune dysfunction after
radiotherapy to the head and neck area. Int Immunopharmacol.
3:1093–1104. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mantel N: Chi-square tests with one degree
of freedom; extensions of the Mantel-Haenszel procedure. J Am Stat
Assoc. 58:690–700. 1963. View Article : Google Scholar
|
|
22
|
Fisher RA: On the interpretation of
χ2 from contingency tables, and the calculation of P. J
Royal Stat Soc. 85:87–94. 1992. View Article : Google Scholar
|
|
23
|
Mosteller F: Association and estimation in
contingency tables. J Am Stat Assoc. 63:1–28. 1968. View Article : Google Scholar
|
|
24
|
Cagney DN, Martin AM, Catalano PJ, Redig
AJ, Lin NU, Lee EQ, Wen PY, Dunn IF, Bi WL, Weiss SE, et al:
Incidence and prognosis of patients with brain metastases at
diagnosis of systemic malignancy: A population-based study. Neuro
Oncol. 19:1511–1521. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gdowski AS, Ranjan A and Vishwanatha JK:
Current concepts in bone metastasis, contemporary therapeutic
strategies and ongoing clinical trials. J Exp Clin Cancer Res.
36(108)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hosmer DW Jr, Lemeshow S and Sturdivant
RX: Applied Logistic Regression. 3rd edition. John Wiley
& Sons (eds). Hoboken, NJ. 2013.
https://doi.org/10.1002/9781118548387.
|
|
27
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
28
|
Schemper M and Smith TL: A note on
quantifying follow-up in studies of failure time. Control Clin
Trials. 17:343–346. 1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cox DR: Regression models and life tables.
J Royal Stat Soc B (Method). 34:187–220. 1972.
|
|
30
|
Freeman-Keller M, Kim Y, Cronin H,
Richards A, Gibney G and Weber JS: Nivolumab in resected and
unresectable metastatic melanoma: Characteristics of immune-related
adverse events and association with outcomes. Clin Cancer Res.
22:886–894. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Haratani K, Hayashi H, Chiba Y, Kudo K,
Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M and
Nakagawa K: Association of immune-related adverse events with
nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol.
4:374–378. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
McDermott CE and Gengozian N: The effect
of low exposure-rate gamma irradiation on T and B lymphocyte
function in the mouse. Int J Radiat Biol Relat Stud Phys Chem Med.
37:415–428. 1980.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gerber M, Pioch Y, Dubois JB and Serrou B:
Effects of low doses of irradiation on the T-cell-mediated
cytotoxic response. Ann Immunol (Paris). 134C:149–157.
1983.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Venkatesulu BP, Mallick S, Lin SH and
Krishnan S: A systematic review of the influence of
radiation-induced lymphopenia on survival outcomes in solid tumors.
Crit Rev Oncol Hematol. 123:42–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wolny-Rokicka E, Brzeźniakiewicz-Janus K,
Wydmański J, Tukiendorf A and Zembroń-Łacny A: Analysis of
haemostasis biomarkers in patients with advanced stage lung cancer
during hypofractionated radiotherapy treatment. J Int Med Res.
46:1876–1883. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fujimoto D, Ueda H, Shimizu R, Kato R,
Otoshi T, Kawamura T, Tamai K, Shibata Y, Matsumoto T, Nagata K, et
al: Features and prognostic impact of distant metastasis in
patients with stage IV lung adenocarcinoma harboring EGFR
mutations: Importance of bone metastasis. Clin Exp Metastasis.
31:543–551. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Barth A, Wanek LA and Morton DL:
Prognostic factors in 1,521 melanoma patients with distant
metastases. J Am Coll Surg. 181:193–201. 1995.PubMed/NCBI
|
|
38
|
Ruatta F, Derosa L, Escudier B, Colomba E,
Guida A, Baciarello G, Loriot Y, Fizazi K and Albiges L: Prognosis
of renal cell carcinoma with bone metastases: Experience from a
large cancer centre. Eur J Cancer. 107:79–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lee EQ: Nervous system metastases from
systemic cancer. Continuum (Minneap Minn). 21:415–428.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Aboudaram A, Modesto A, Chaltiel L,
Gomez-Roca C, Boulinguez S, Sibaud V, Delord JP, Chira C, Delannes
M, Moyal E and Meyer N: Concurrent radiotherapy for patients with
metastatic melanoma and receiving anti-programmed-death 1 therapy:
A safe and effective combination. Melanoma Res. 27:485–491.
2017.PubMed/NCBI View Article : Google Scholar
|