Introduction
As the use of immunotherapy for treating various
types of cancer becomes more widespread, several issues require
investigation to determine their possible impact on the outcome of
cancer patients treated with anti-PD-1/PD-L1 immune checkpoint
inhibitors (CKIs) (1). Thus, the
respective effects of concomitant medications, concurrent
treatments and other possible immunomodulatory events in the
clinical history of patients prior to the initiation of
immunotherapy, or during its course, have been largely explored in
the recent years, obtaining a wide range of controversial evidence
(1-5).
For example, it seems that the use of corticosteroids or
antibiotics before or with CKIs may decrease efficacy of the
latter, whereas the use of influenza vaccine may be beneficial
irrespective of its anti-infectious efficacy (2-4).
Amongst all the topics explored in relation to immunotherapy,
radiotherapy (RT) is a considerably important issue, since the
interest in the abscopal effect has recently been rediscovered, and
described in relation to immunotherapy (6). The abscopal effect of local RT is
considered as a systemic anti-tumor immune response which reflects
the regression of non-irradiated metastatic lesions at a distance
from the primary site of irradiation (6). The relationship between the abscopal
effect and the immune system, particularly regarding lymphocytes,
has been known since 1969(7). As long
as the immune checkpoint blockade has been used to enhance the
immune response, their possible synergy with RT has been
investigated (6,7). Although the exact underlying mechanism
of the abscopal effect remains unclear, the administration of CKIs
can enhance the anti-tumor immunogenicity of RT, by preventing
PD-1/PD-L1 induced T cell anergy (6).
Nevertheless, the abscopal effect is known to be
uncommon and RT is generally unable to subvert the immune tolerance
towards the tumor (8). Combination of
RT with anticancer immunotherapy aims to shift the balance of the
immunosuppressive tumor microenvironment to achieve tumor
rejection, inducing the positive effects of RT to overcome the
possible negative effects.
Retrospective studies have been performed to
investigate the potential effect of RT when administered before or
during immunotherapy. Kiess et al (9) evaluated 46 patients with 113 brain
metastases from a melanoma, who were treated with ipilimumab and
single-fraction stereotactic radiosurgery (SRS) together, and found
that patients treated with SRS before or during administration of
ipilimumab had significantly improved overall survival (OS) and
good local disease control after 1 year compared with patients
treated with SRS following treatment with ipilimumab (9). Following retrospective studies examining
the effects of anti-CTLA-4 CKIs (9),
other retrospective reports regarding stereotactic RT/SRS during
immunotherapy confirmed its likely positive interaction with
anti-PD-1/PD-L1 CKIs (10).
Furthermore, a recent randomized prospective trial, assessed
whether stereotactic RT on a single tumor site prior to treatment
with pembrolizumab treatment enhanced tumor response in patients
with metastatic non-small cell lung cancer (NSCLC), and
demonstrated a doubling of objective response rates (ORR) with RT
immediately prior to immunotherapy compared with pembrolizumab
alone, although the results did not meet the study's prespecified
end point criteria for meaningful clinical benefit (11).
A secondary retrospective analysis of a subset of
patients treated with the anti-PD-1 pembrolizumab in the
prospective phase 1 KEYNOTE-001 study was performed by Shaverdian
et al (12). They found that
patients who had previously (at any time) received RT for the
treatment of NSCLC, before the initiation of systemic treatment
with pembrolizumab, had significantly longer survival, compared
with patients who had not received previous RT (12). This study currently represents the
largest clinical evidence about the effect of previous RT on the
outcome of patients to immunotherapy, although such data need to be
furtherly validated with prospective trials.
Together, previous studies have highlighted the
possibility of triggering an abscopal effect, particularly in cases
with high-dose low-volume RT. To investigate the effect of
different types of RT used in clinical practice, a
Palliative Radiotherapy in Advanced
Cancer patients Treated with
Immune-ChEckpoint inhibitors (PRACTICE)
retrospective analysis was performed, to compare the clinical
outcome of patients who underwent palliative RT (pRT) prior to the
initiation of anti-PD-1/PD-L1 CKIs, with patients who did not
receive RT or patients receiving pRT during the course of
immunotherapy.
Patients and methods
Patients
The present study included patients with advanced
cancer with histologically confirmed diagnosis of a tumor of any
primary origin, whom consecutively underwent treatment with single
agent anti-PD-1/PD-L1, regardless of the treatment line, at the
Medical Oncology Departments of three Italian centers, the
University Hospital of Parma (Parma, Italy), University Hospital of
L'Aquila (L,Auila, Italy) and the University Hospital of Chieti
(Chieti, Italy), between August 2015 and September 2017. A total of
192 patients with advanced cancer were recruited for the present
study and their median age was 68.6 years (range, 32-87), with 143
male and 49 female participants. Patients were stratified according
to whether they received pRT, received pRT prior to immunotherapy,
or received pRT during immunotherapy. The administration of
high-dose RT for non-palliative use was an exclusion criterion. All
patients provided written, informed consent for treatment with
immunotherapy.
Study design
A multicenter retrospective analysis of patients
with advanced cancer treated with anti-PD-1/PD-L1 CKIs, regardless
of treatment line, was performed. The primary aim of the present
analysis was to compare the clinical outcomes of patients who
received pRT prior to immunotherapy (6-months before initiation of
immunotherapy) compared with patients who did not receive any RT
within 6 months prior to initiation of immunotherapy, or during
immunotherapy. The ORR, disease control rate (DCR), progression
free survival (PFS) in months, time to treatment failure (TTF) in
months, OS in months, and rate of immune-related adverse events
(irAEs) were compared between the groups.
In order to overcome a potential positive selection
bias of patients who did not require RT in their clinical history,
the secondary aim of the study was to compare the clinical outcomes
of patients who received pRT within 6 months prior to immunotherapy
initiation and patients who received pRT during immunotherapy, in
terms of ORR, PFS, TTF, OS and rate of irAEs.
ORR was defined as the proportion of patients who
experienced an objective response (complete response or partial
response) as the best response to immunotherapy according to RECIST
criteria, version 1.1(13). DCR was
defined as the proportion of patients who experienced an objective
response or stabilization of the disease as the best response to
treatment. TTF was defined as the time from treatment's start to
discontinuation for any reason, including disease progression,
treatment toxicity, patient preference or death. PFS was defined as
the time from CKI treatment initiation to documented disease
progression or death, or to the last contact for alive patients. OS
was calculated as the time from the beginning of CKI treatment and
death, or to the last contact for alive patients.
The following covariates were analyzed: Primary
tumor (NSCLC, melanoma, renal cell carcinoma, others), age (<70
years vs ≥70 years old, based on previous studies) (14-16),
sex (male vs. female), Eastern Cooperative Oncology Group
Performance Status (ECOG PS; 0-1 vs. ≥2) (17), treatment line (first vs. further
lines), presence of bone metastases (yes vs. no, defined as
‘baseline bone metastases’) and presence of central nervous system
(CNS) metastases (yes vs. no, defined as ‘baseline CNS metastases’)
at baseline before immunotherapy.
IrAEs were graded according to the National Cancer
Institute Common Toxicity Criteria for Adverse Events (version 5.0)
(18) and cumulatively reported as
crude incidence.
Radiotherapy
The time-window for defining ‘previous pRT’ was set
at 6-months before initiation of immunotherapy, in accordance with
an arbitrary choice of the investigators, as it was determined to
be a reasonable compromise between the fact that events too far
from the beginning of therapy may not have an impact on treatment
outcome, and the well-demonstrated long-lasting effect of RT on the
immune system (19,20).
Patients who had received RT >6 months prior to
immunotherapy initiation and patients who received RT after
permanent discontinuation of immunotherapy were included in the
control group, together with patients who had never received RT.
Patients receiving a high-dose of RT for non-palliative reasons
were excluded from the present study.
Palliative RT treatments were performed (including
stereotactic RT or SRS) according to the clinical practice of the
participating centers and were defined as conventional radiation
therapy administered without curative intent, for the local control
of symptoms to metastatic sites of advanced tumors. RT was
categorized according to the treated organ/region as follows: CNS,
bone, lymph-node, visceral and other. Dose (Gy) and duration were
collected, with a median dose of 20 Gy and a mean dose of 23 Gy;
the dose range was 8-40 Gy. Patients were categorized into three
groups according to the timing of RT, as follows: patients who had
received pRT within 6 months prior to the initiation of
immunotherapy (previous pRT), patients who received pRT during
immunotherapy (concurrent pRT) and patients who did not receive RT
prior to or during immunotherapy (no RT group).
Statistical analysis
χ2 and Fisher's exact test were used to
evaluate ORR, DCR and the incidence of irAEs among the groups,
according to the sample size in contingency tables for each
comparison (21-23).
Given the well-known poor prognostic impact of CNS metastases and
bone metastases (24,25), the differences among subgroups
according to such characteristics were evaluated with a
χ2 and Fisher's exact test, respectively.
In the multivariate analysis, logistic regression
was used to evaluate the parameters which were significantly
different in the univariate analysis of DCR (26). Median PFS (PFS) and median OS (OS)
were evaluated using the Kaplan-Meier method (27). Median follow-up was calculated
according to the reverse Kaplan-Meier method (28). Cox proportional hazard models were
used to evaluate predictive variables in the univariate and
multivariate analysis for median TTF (TTF) and mOS as described
previously (29). The data cut-off
period was set as September 2018. All statistical analyses were
performed using MedCalc Statistical Software version 18.6 (MedCalc
Software bvba).
Results
Patient characteristics
A total of 192 patients with advanced cancer were
included in the present analysis. Their characteristics are
summarized in Table I. The primary
tumors reported in patients were: NSCLC, 118 patients (61.4%);
melanoma, 38 patients (19.8%), renal cell carcinoma, 23 patients
(12%); and others, 13 patients (6.8%).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
|
Characteristics | Overall
population | No RT | Previous pRT | Concurrent pRT |
|---|
| Age, years |
|
Median | 68.6 | 69 | 71 | 67 |
|
Range | 32-87 | 32-87 | 41-85 | 43-83 |
|
Elderly, ≥70
years old (%) | 88 (45.8) | 66 (48.2) | 14 (53.8) | 8 (30.8) |
| Number of patients
(%) | 192(100) | 137 (71.4) | 26 (13.5) | 29 (15.1) |
| Sex (%) |
|
Male | 143 (74.5) | 104 (75.9) | 18 (69.2) | 21 (72.4) |
|
Female | 49 (25.5) | 33 (24.1) | 8 (30.8) | 8 (27.6) |
| ECOG PS (%) |
|
0-1 | 149 (77.6) | 110 (80.3) | 17 (65.4) | 22 (75.9) |
|
≥2 | 43 (22.4) | 27 (19.7) | 9 (34.6) | 7 (24.1) |
| Primary tumor
(%) |
|
NSCLC | 118 (61.4) | 82 (59.8) | 18 (69.2) | 18 (62.1) |
|
Melanoma | 38 (19.8) | 30 (21.9) | 1 (3.9) | 7 (24.1) |
|
Renal cell
carcinoma | 23(12) | 19 (13.9) | 3 (11.5) | 1 (3.5) |
|
Others | 13 (6.8) | 6 (4.4) | 4 (15.4) | 3 (10.3) |
| Baseline bone
metastases (%) |
|
No | 124 (64.6) | 101 (73.7) | 8 (30.8) | 15 (51.7) |
|
Yes | 68 (35.4) | 36 (26.3) | 18 (69.2) | 14 (48.3) |
| Baseline CNS
metastases (%) |
|
No | 162 (84.4) | 120 (87.6) | 22 (84.6) | 20(70) |
|
Yes | 30 (15.6) | 17 (12.4) | 4 (15.4) | 9(30) |
| Anti-PD-1/PD-L1
(%) |
|
Pembrolizumab | 23(12) | 19 (13.9) | 2 (7.7) | 2 (6.9) |
|
Nivolumab | 154 (80.2) | 110 (80.3) | 20 (76.9) | 24 (82.8) |
|
Atezolizumab | 12 (6.2) | 5 (3.6) | 4 (15.4) | 3 (10.3) |
|
Avelumab | 3 (1.6) | 3 (2.2) | - | - |
| Line of
immunotherapy (%) |
|
First
line | 30 (15.6) | 26(19) | 1 (3.8) | 3 (10.3) |
|
Second or
subsequent line | 162 (84.4) | 111(81) | 25 (96.2) | 26 (89.7) |
A total of 26 patients had received pRT within 6
months prior to initiation of immunotherapy (13.5%) and were
classified as previous pRT; 29 patients (15.1%) received pRT during
the course of immunotherapy and were classified as concurrent pRT;
137 patients (71.4%) were classified as no RT (Table I).
In the previous pRT, concurrent pRT and no RT
groups, 18 (69.2%), 14 (48.3%) and 36 (26.3%) patients had baseline
bone metastases, and 4 (15.4%), 9 (30%) and 17 (12.4%) patients had
baseline CNS metastases, respectively (Table I). The incidence of bone metastases at
baseline was significantly higher in the previous pRT group
compared with the no-RT groups (P<0.0001) and the concurrent pRT
group (P=0.0193). The incidence of CNS metastases at baseline was
significantly higher in the concurrent pRT group compared with the
no RT group (P=0.0124), whereas no significant difference was
observed between the previous pRT group and the other groups in
regard to CNS metastases (Table
I).
Table II summarizes
the characteristics of patients who received RT. Among the 26
patients in the previous pRT group, a total of 27 pRT treatments
were performed, 15 (55.6%) of which were for bone metastases. The
median dose of RT treatments was 20 Gy and the mean dose was 23 Gy
(range, 8-40). Among the 29 patients in the concurrent RT group, a
total of 36 treatments were performed, 21 (58.4%) of which were for
bone metastases, with a median dose of 8 Gy and a mean dose of 14
Gy (range, 8-40).
 | Table IICharacteristics of patients who
received RT. |
Table II
Characteristics of patients who
received RT.
|
Characteristics | Previous pRT,
n=26 | Concurrent pRT,
n=29 |
|---|
| Total number of pRT
treatments | 27 | 36 |
| Body site (%) |
|
CNS | 4 (14.8) | 7 (19.4) |
|
Bone | 15 (55.6) | 21 (58.4) |
|
Lymph
nodes | 2 (7.4) | 1 (2.8) |
|
Visceral | 4 (14.8) | 7 (19.4) |
|
Others | 2 (7.4) | - |
| Dose of RT, Gy |
|
Median | 20 | 8 |
|
Mean | 23 | 14 |
|
Range | 8-40 | 8-40 |
Treatment outcome
Among the 177 evaluable patients, 50 showed partial
response and 33 had stable disease; ORR was 28.2% [95% confidence
interval (CI), 20.9-37.2] and DCR was 46.9% (95% CI, 37.3-58.1) in
the overall population (data not shown).
At the median follow-up of 20.3 months, mOS for the
overall population was 9.4 months (95% CI, 6.7-12.4; 68 patients
censored), median PFS was 4.3 months (95% CI, 3.4-5.6; 23 patients
censored) and median TTF was 5.0 months (95% CI, 3.9-6.1; 16
patients censored). In the overall population, 67 patients (34.9%)
experienced irAEs of any grade (data not shown).
Comparisons between the previous pRT
and no RT groups
Among the patients in the previous pRT group, ORR to
immunotherapy was 18.2% (95% CI, 4.9-46.5; 4 responses out of 22
evaluated patients), whereas in the no RT group it was 32.3% (95%
CI, 23.1-43.8; 41 responses out of 127 evaluated patients) and the
difference was not significant (P=0.2173). The DCR was
significantly higher in the no RT group compared with the previous
pRT group (52.8% vs. 18.2%, P=0.0026). Multivariate analysis
confirmed the significantly higher DCR among patients who did not
receive RT (P=0.0477). Table SI
summarizes the univariate and multivariate analyses for DCR
according to different patient characteristics. Baseline bone
metastases and ECOG PS were significantly associated with DCR both
in the univariate and multivariate analyses, whereas CNS metastases
were not.
The median OS of the no RT group was 12.1 months
(95% CI, 8.1-16.5), compared with 3.6 months (95% CI, 2.05-7.2) in
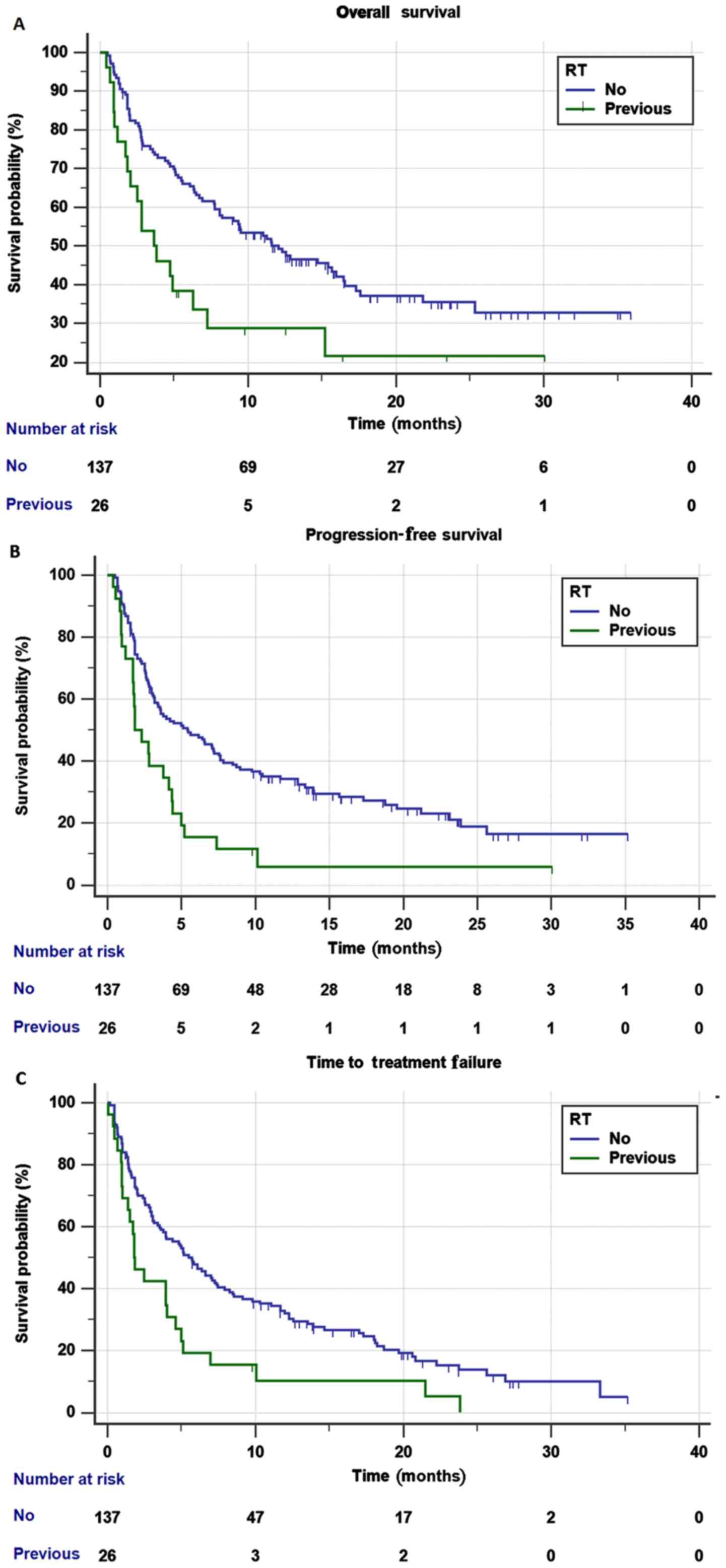
the previous pRT groups (Fig. 1A),
which was significantly shorter (HR=1.94; 95% CI, 1.17-3.22;
P=0.0095). The statistical significance of this difference was not
confirmed in the multivariate analysis (HR=1.64; 95% CI, 0.94-2.86;
P=0.0775), whereas ECOG PS and baseline bone metastases were
significantly associated with OS (Table
III).
 | Table IIIUnivariate and multivariate analysis
of overall survival. |
Table III
Univariate and multivariate analysis
of overall survival.
| Variables | Univariate
analysis, HR (95% CI); P-value | Multivariate
analysis, HR (95% CI); P-value |
|---|
| Previous pRT, Yes
vs. No | 1.94 (1.17-3.22);
P=0.0095 | 1.64 (0.94-2.86);
P=0.0775 |
| Primary tumor,
NSCLC vs. |
|
Melanoma | 0.54 (0.31-0.97);
P=0.0398 | 0.65 (0.36-1.18);
P=0.6581 |
|
Kidney | 0.89 (0.50-1.59);
P=0.7030 | 1.05 (0.58-1.92);
P=0.8586 |
|
Others | 1.12 (0.45-2.79);
P=0.7999 | 1.10 (0.43-2.79);
P=0.8399 |
| Sex, male vs.
female | 1.25 (0.78-2.01);
P=0.3377 | - |
| Age, ≥70 years old
vs. <70 years old | 1.61 (1.08-2.39);
P=0.0184 | 1.34 (0.89-2.04);
P=0.1525 |
| Treatment line,
second and subsequent lines vs. first line | 1.75 (0.96-3.21);
P=0.0673 | - |
| ECOG PS, ≥2 vs.
0-1 | 3.87 (2.53-5.93);
P<0.0001 | 3.82 (2.44-5.96);
P<0.0001 |
| Baseline CNS
metastases, yes vs. no | 1.17 (0.66-2.07);
P=0.5750 | - |
| Baseline bone
metastases, yes vs. no | 1.67 (1.12-2.49);
P=0.0117 | 1.54 (1.01-2.37);
P=0.0456 |
The median PFS of the no RT group was 5.4 months
(95% CI, 3.4-7.6), compared with 1.8 months (95% CI, 1.7-4.1) in
the previous pRT group (Fig. 1B) and
the difference was significant (HR=2.06; 95% CI, 1.31-3.24;
P=0.0016). The statistical significance of this difference was not
confirmed in the multivariate analysis (HR=1.58; 95% CI, 0.94-2.65;
P=0.0810). Baseline bone metastases and ECOG PS were significantly
associated with PFS in the univariate and multivariate analysis,
whereas CNS metastases were not (Table
SII).
The median TTF of the no RT group was 5.7 months
(95% CI, 3.7-7.4) compared with 1.8 months (95% CI, 1.3-4.0) of the
previous pRT group (Fig. 1C) and this
difference resulted in being statistically significant in both the
univariate (HR=1.92; 95% CI, 1.23-2.98; P=0.0035) and multivariate
analyses (HR=1.76; 95% CI, 1.12-2.77; P=0.0132). Baseline CNS
metastases and bone metastases were not significantly associated
with TTF in the multivariate analysis (Table SIII).
Primary tumor (NSCLC vs. others), sex, age and
treatment line, were confirmed in the multivariate analyses as
significantly associated with OS, DCR, PFS and TTF (Tables
SI-IV and III).
In the previous pRT group, 26.9% of patients
experienced irAEs of any grade, compared with 40.1% of patients in
the no RT group. The difference, which may be clinically
meaningful, was not statistically significant.
Comparisons between the previous pRT
and concurrent pRT groups
ORR and DCR in the concurrent pRT group were 17.9%
(95% CI, 5.8-41.6) and 42.9% (95% CI, 22.1-74.8), respectively.
There were no statistically significant differences with the ORR
and DCR rates of the previous pRT group (18.2 and 18.2%,
respectively; P=1.0000 and P=0.0761, respectively) (data not
shown).
The median OS of the concurrent pRT group was 8.5
months (95% CI, 5.5-12.4), whereas the median OS of the previous
pRT group was 3.6 months (HR=1.31; 95% CI, 0.71-2.41; P=0.3742) and
the difference was not significant (Fig.
S1A).
The median PFS of the concurrent pRT group was 5.0
months (95% CI, 3.3-7.2), and in the previous pRT group, the median
PFS was 1.8 months (HR=1.58; 95% CI, 0.91-2.74; P=0.1045) and the
difference was not significant (Fig.
S1B).
The median TTF of the concurrent pRT group was 5.8
months (95% CI, 4.1-10.4) compared with 1.8 months (95% CI,
1.3-4.0) of the previous pRT. The difference was significant in
both the univariate (HR=1.99; 95% CI, 1.12-3.53; P=0.0187) and
multivariate analyses (HR=2.42; 95% CI, 1.32-4.42; P=0.0040)
(Fig. S1C; Table SIV).
In the concurrent pRT group, 5 out of 29 patients
(17.2%) experienced irAEs of any grade although this was not
significantly lower compared with the 26.9% incidence rate in the
previous pRT group (P=0.3898) (data not shown).
Finally, the concurrent pRT and no RT groups were
compared (data not shown), and similar results were observed for
ORR (17.9 vs. 32%; P=0.1316), DCR (42.9 vs. 52.8%; P=0.3445), OS
(8.4 months vs. 12.1 months; P=0.0578) and TTF (5.6 vs. 5.7 months;
P=0.7501), respectively, although PFS was slightly but
significantly increased in the concurrent pRT group (5.0 vs. 5.4
months; P=0.0459).
Discussion
The results of the PRACTICE study suggest a possible
negative impact of receiving pRT within 6 months prior to
immunotherapy initiation, at least in terms of DCR, but likely also
of PFS (3x higher in the no RT group) and OS (>3x higher in the
no RT). Beyond the statistical significance, not always confirmed
at multivariate analyses (possibly due to the small sample size),
the survival differences observed among subgroups may be clinically
meaningful. Additionally, there was a trend towards less
CKI-related toxicity for patients who received pRT suggesting lower
immune-reactivity in these patients. The irAEs incidence rate was
the lowest in the concurrent pRT group, low in the previous pRT
group and the highest in the no RT group. This is consistent with
the previous studies: The higher the effectiveness, the higher the
toxicity of CKIs (30,31). Additionally, these findings also
confirm the relative safety of pRT during immunotherapy with CKIs,
possibly due to the low median dose (8 Gy in the concurrent pRT
group).
The possible negative impact of pRT on immunotherapy
effectiveness may be mitigated in the concurrent pRT group, as they
exhibited improved TTF periods compared with both the no RT and
previous pRT group, which both exhibited similar TTF periods. Thus,
it is hypothesized that a positive selection bias of
oligo-progressive patients, continuing immunotherapy with clinical
indication to loco-regional pRT on a single progressing/painful
lesion, may have resulted in the improved TTF periods observed in
the concurrent pRT group.
Several studies have demonstrated the
immunosuppressive effect of RT (particularly on T lymphocytes,
which are considered the most radiosensitive cells of the
hematopoietic system), from old preclinical models to more recent
clinical studies (32-35).
Lymphocyte count has been demonstrated to decrease after
administration of low-doses of pRT in patients with lung cancer
(35). Additionally,
radiation-induced reduction of circulating lymphocyte count and
eventual lymphocyte infiltration of tumors demonstrated a
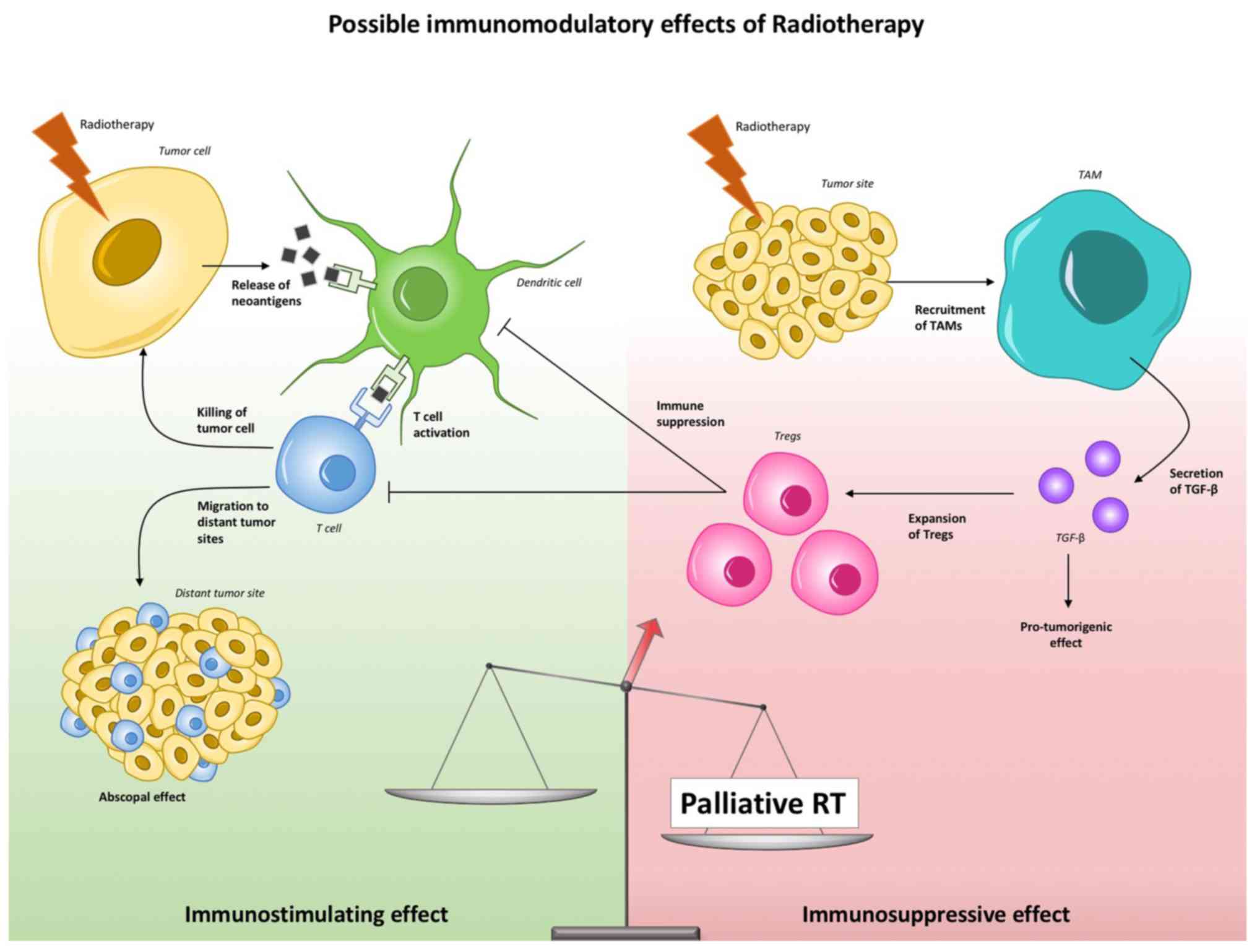
significant negative impact on OS (32). Radiation is able to activate
tumor-growth factors, such as TGF-β, and to possibly favor tumor
progression through the enhancement of M2 macrophages and the
increase of T-regulatory cells, which are the most radio-resistant
T cells (8). Thus, taking into
account the fact that the abscopal effect is not a common event and
is likely dependent on a number of factors, the negative findings
of this study agree with our current understanding of palliative RT
and immunotherapy.
A major limitation of this retrospective analysis is
that the selection of patients receiving pRT at any time in their
clinical history constitutes a selection bias, particularly with
the inclusion of subjects likely suffering from bone or CNS
metastases, both of which are well-known poor prognostic factors
for advanced cancer patients (36-39).
To verify and to reduce the impact of this
limitation, a more reliable comparison between the previous pRT and
concurrent pRT groups was performed, which were more likely to be
affected by bone or CNS metastases with a similar incidence.
Moreover, a further analysis to weight the selection bias
throughout the direct comparison of patients' characteristics,
comparing the incidence of bone and CNS metastases across subgroups
was performed.
The incidence of bone metastases at baseline was
significantly higher in the previous pRT group compared with both
the other groups. The presence of bone metastases at diagnosis is
well-known as poor prognostic factor for lung cancer, melanoma and
renal cancer (36-38),
confirming a probable selection bias in the present study. However,
the presence of CNS metastases was not a further selection bias for
the previous pRT group as the incidence was not significantly
greater.
The incidence of CNS metastases at baseline were
significantly higher in the concurrent pRT group compared with the
no RT group and this is a well-known prognostic factor predicting a
less favorable outcome (24), it did
not result in a clinically meaningful difference in outcome among
these two subgroups.
There were no statistically significant differences
observed between the previous pRT group and concurrent pRT group in
terms of PFS and OS, although this may be the result of the small
sample size, potentially clinically meaningful trends were noticed,
including a doubling of survival times in the concurrent pRT group.
Furthermore, TTF was significantly shorter for patients in the
previous pRT group, suggesting that a selection bias did not
influence the results, thus highlighting the reliability of the
comparison between irradiated and non-irradiated patients.
Taking into account the limitations, the negative
selection bias alone likely did not affect the results, as all the
other retrospective studies on the impact of RT in patients with
advanced cancer treated with CKIs were similarly affected by the
same selection bias (12), and the
results of these studies almost always show the beneficial effect
of receiving RT (9-12),
contrasting with the results of the present study.
Comparing the characteristics of patients and of
radiation treatments in previous studies, highlighted crucial
differences; the type and dose of RT. Frequently, patients reported
as receiving ‘palliative care’ in the literature often received
high-dose hypofractioned RT (40),
thus preventing confirmation of whether the immunosuppressive
effect may be the result of purely palliative RT. To the best of
our knowledge, there are no studies examining palliative RT
treatments during and before immunotherapy.
In this analysis, the median dose of RT was 20 Gy
for the previous pRT group and 8 Gy for the concurrent pRT group,
demonstrating that our study population was different from those of
previous studies. In the studies suggesting a possible synergy
between RT and immunotherapy, stereotactic RT was predominantly
used. In other studies reporting positive results, the patient
population was heterogeneous, with both palliative and curative
radiation approaches (9-12).
In the previously cited KEYNOTE-001 sub-analysis (12), comparing RT prior to immunotherapy
with no RT, 36% of patients received definitive (curative) RT
(stereotactic body RT or SRS). The weight of such a subgroup could
have shifted the balance of the final impact in favor of the
abscopal effect of RT, instead of the immunosuppressive one,
explaining the positive results of that study. This happens despite
a possible selection bias, in fact, in such a study, patients with
previous RT had a significantly greater frequency of brain
metastases (data concerning bone metastases were not reported)
(12).
Therefore, it can be hypothesized that, irrespective
of the clear limitations of the present and previous retrospective
studies, there are differences in the immunomodulatory effects of
different RT approaches, with high-dose, low-volume irradiations
providing more favorable results compared with lower-dose, purely
palliative RT treatments.
Additionally, the site of irradiation is likely to
affect the value of RT. For example a previous study included cases
of definitive thoracic RT on pulmonary or nodal lesions (12), whereas in another study, patients
receiving bone RT accounted for >50% of cases, thus it may be
the case that the abscopal effect may be more readily initiated by
irradiating soft lesions, in which the immune-infiltrating context
would be more conspicuous, offering greater probability of
containing antigen-presenting cells (11).
Interpretation of the present study suggests a
negative shift in the balance between favorable and unfavorable
immune-modulating effects of RT in the case of pRT, a hypothesis
that is presented in Fig. 2.
The limitations of the present analysis, with the
use of a retrospective cohort, the presence of a selection bias and
the small size of the groups receiving pRT, in addition to the lack
of details regarding RT volumes and techniques, prevent conclusions
from being drawn regarding the use of pRT during immunotherapy.
Nevertheless, the present study highlights the need for future
prospective analysis to determine the clinical efficacy of pRT by
stratifying the population based on RT dose, fractioning, planning
and timing in relation to immunotherapy.
Supplementary Material
Kaplan Meier survival curves of
patients in the previous RT group and patients in the concurrent RT
group. (A) Overall survival, (B) Progression free survival, (C)
Time to treatment failure. RT, radiotherapy; Previous RT, received
pRT within 6 months prior to initiation of immunotherapy;
concurrent RT, received pRT during immunotherapy.
Univariate and multivariate analyses
of DCR.
Univariate and multivariate analyses
of progression free survival.
Univariate and multivariate analyses
of time to treatment failure.
Univariate and multivariate analysis
for time to treatment failure.
Acknowledgements
We would like to thank the Consorzio
Interuniversitario Nazionale per la Bio-Oncologia for their support
in this study.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MB and AC conceived and designed the study and
drafted the manuscript. MB, EL, NDA, SB, AL, MGC, ST, GG, GP, KC,
PDM, AG, NT, MDT, EG, MM, PB, FP, LC, MT, DG, CN, CF, MT and AC
acquired the data. MB, SB, MT, AC and NDA analyzed and interpreted
the data. SB, MT, NDA, CN, DG, GG and CF critically revised the
manuscript. All authors read and approved the manuscript. All
authors have agreed both to be personally accountable for the
author's own contributions and to ensure that questions related to
the accuracy or integrity of any part of the work, even ones in
which the author was not personally involved, are appropriately
investigated, resolved, and the resolution documented in the
literature.
Ethics approval and consent to
participate
All patients provided written, informed consent for
treatment with immunotherapy. The present study was approved by the
respective Ethical Committees on Human Experimentation of each
institute, after previous approval by the coordinating center
(University of L'Aquila, Via Vetoio, Italy; approval no. 32865,
approved on July 24th, 2018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chung C: To do or not to do: A concise
update of current clinical controversies in immune checkpoint
blockade. J Oncol Pharm Pract. 25:663–673. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Derosa L, Hellmann MD, Spaziano M,
Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC,
Chaft JE, et al: Negative association of antibiotics on clinical
activity of immune checkpoint inhibitors in patients with advanced
renal cell and non-small-cell lung cancer. Ann Oncol. 29:1437–1444.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Garant A, Guilbault C, Ekmekjian T,
Greenwald Z, Murgoi P and Vuong T: Concomitant use of
corticosteroids and immune checkpoint inhibitors in patients with
hematologic or solid neoplasms: A systematic review. Crit Rev Oncol
Hematol. 120:86–92. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bersanelli M, Giannarelli D, Castrignanò
P, Fornarini G, Panni S, Mazzoni F, Tiseo M, Rossetti S, Gambale E,
Rossi E, et al: INfluenza vaccine indication during therapy with
immune checkpoint inhibitors: A transversal challenge. The INVIDIa
study. Immunotherapy. 10:1229–1239. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lesueur P, Escande A, Thariat J, Vauléon
E, Monnet I, Cortot A, Lerouge D, Danhier S, Dô P, Dubos-Arvis C,
et al: Safety of combined PD-1 pathway inhibition and radiation
therapy for non-small-cell lung cancer: A multicentric
retrospective study from the GFPC. Cancer Med. 7:5505–5513.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu Y, Dong Y, Kong L, Shi F, Zhu H and Yu
J: Abscopal effect of radiotherapy combined with immune checkpoint
inhibitors. J Hematol Oncol. 11(104)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nobler MP: The abscopal effect in
malignant lymphoma and its relationship to lymphocyte circulation.
Radiology. 93:410–412. 1969.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Formenti SC and Demaria S: Combining
radiotherapy and cancer immunotherapy: A paradigm shift. J Natl
Cancer Inst. 105:256–265. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kiess AP, Wolchok JD, Barker CA, Postow
MA, Tabar V, Huse JT, Chan TA, Yamada Y and Beal K: Stereotactic
radiosurgery for melanoma brain metastases in patients receiving
ipilimumab: Safety profile and efficacy of combined treatment. Int
J Radiat Oncol Biol Phys. 92:368–375. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lehrer EJ, Peterson J, Brown PD, Sheehan
JP, Quiñones-Hinojosa A, Zaorsky NG and Trifiletti DM: Treatment of
brain metastases with stereotactic radiosurgery and immune
checkpoint inhibitors: An international meta-analysis of individual
patient data. Radiother Oncol. 104–112. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Theelen WSME, Peulen HMU, Lalezari F, van
der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I,
Niemeijer AN, de Langen AJ, et al: Effect of pembrolizumab after
stereotactic body radiotherapy vs pembrolizumab alone on tumor
response in patients with advanced non-small cell lung cancer:
Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA
Oncol. 5:1276–1282. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shaverdian N, Lisberg AE, Bornazyan K,
Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P:
Previous radiotherapy and the clinical activity and toxicity of
pembrolizumab in the treatment of non-small-cell lung cancer: A
secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol.
18:895–903. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ciocan D, Barbe C, Aubin F, Granel-Brocard
F, Lipsker D, Velten M, Dalac S, Truchetet F, Michel C, Mitschler
A, et al: Distinctive features of melanoma and its management in
elderly patients: A population-based study in France. JAMA
Dermatol. 149:1150–1157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gridelli C, Balducci L, Ciardiello F, Di
Maio M, Felip E, Langer C, Lilenbaum RC, Perrone F, Senan S and de
Marinis F: Treatment of elderly patients with non-small cell lung
cancer: Results of an international expert panel meeting of the
Italian association of thoracic oncology. Clin Lung Cancer.
16:325–333. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Azawi NH, Joergensen SM, Jensen NV, Clark
PE and Lund L: Academy of Geriatric Cancer Research (AgeCare):
Trends in Kidney cancer among the elderly in Denmark, 1980-2012.
Acta Oncol. 55 (Suppl 1):S79–S84. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Datta SS, Ghosal N, Daruvala R,
Chakraborty S, Shrimali RK, van Zanten C, Parry J, Agrawal S,
Atreya S, Sinha S, et al: How do clinicians rate patient's
performance status using the ECOG performance scale? A
mixed-methods exploration of variability in decision-making in
oncology. Ecancermedicalscience. 13(913)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Common terminology criteria for adverse
events (ctcae) version 5.0.
https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
Accessed November 27, 2017.
|
|
19
|
Walle T, Martinez Monge R, Cerwenka A,
Ajona D, Melero I and Lecanda F: Radiation effects on antitumor
immune responses: Current perspectives and challenges. Ther Adv Med
Oncol. 10(1758834017742575)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Verastegui EL, Morales RB, Barrera-Franco
JL, Poitevin AC and Hadden J: Long-term immune dysfunction after
radiotherapy to the head and neck area. Int Immunopharmacol.
3:1093–1104. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mantel N: Chi-square tests with one degree
of freedom; extensions of the Mantel-Haenszel procedure. J Am Stat
Assoc. 58:690–700. 1963. View Article : Google Scholar
|
|
22
|
Fisher RA: On the interpretation of
χ2 from contingency tables, and the calculation of P. J
Royal Stat Soc. 85:87–94. 1992. View Article : Google Scholar
|
|
23
|
Mosteller F: Association and estimation in
contingency tables. J Am Stat Assoc. 63:1–28. 1968. View Article : Google Scholar
|
|
24
|
Cagney DN, Martin AM, Catalano PJ, Redig
AJ, Lin NU, Lee EQ, Wen PY, Dunn IF, Bi WL, Weiss SE, et al:
Incidence and prognosis of patients with brain metastases at
diagnosis of systemic malignancy: A population-based study. Neuro
Oncol. 19:1511–1521. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gdowski AS, Ranjan A and Vishwanatha JK:
Current concepts in bone metastasis, contemporary therapeutic
strategies and ongoing clinical trials. J Exp Clin Cancer Res.
36(108)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hosmer DW Jr, Lemeshow S and Sturdivant
RX: Applied Logistic Regression. 3rd edition. John Wiley
& Sons (eds). Hoboken, NJ. 2013.
https://doi.org/10.1002/9781118548387.
|
|
27
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
28
|
Schemper M and Smith TL: A note on
quantifying follow-up in studies of failure time. Control Clin
Trials. 17:343–346. 1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cox DR: Regression models and life tables.
J Royal Stat Soc B (Method). 34:187–220. 1972.
|
|
30
|
Freeman-Keller M, Kim Y, Cronin H,
Richards A, Gibney G and Weber JS: Nivolumab in resected and
unresectable metastatic melanoma: Characteristics of immune-related
adverse events and association with outcomes. Clin Cancer Res.
22:886–894. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Haratani K, Hayashi H, Chiba Y, Kudo K,
Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M and
Nakagawa K: Association of immune-related adverse events with
nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol.
4:374–378. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
McDermott CE and Gengozian N: The effect
of low exposure-rate gamma irradiation on T and B lymphocyte
function in the mouse. Int J Radiat Biol Relat Stud Phys Chem Med.
37:415–428. 1980.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gerber M, Pioch Y, Dubois JB and Serrou B:
Effects of low doses of irradiation on the T-cell-mediated
cytotoxic response. Ann Immunol (Paris). 134C:149–157.
1983.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Venkatesulu BP, Mallick S, Lin SH and
Krishnan S: A systematic review of the influence of
radiation-induced lymphopenia on survival outcomes in solid tumors.
Crit Rev Oncol Hematol. 123:42–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wolny-Rokicka E, Brzeźniakiewicz-Janus K,
Wydmański J, Tukiendorf A and Zembroń-Łacny A: Analysis of
haemostasis biomarkers in patients with advanced stage lung cancer
during hypofractionated radiotherapy treatment. J Int Med Res.
46:1876–1883. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fujimoto D, Ueda H, Shimizu R, Kato R,
Otoshi T, Kawamura T, Tamai K, Shibata Y, Matsumoto T, Nagata K, et
al: Features and prognostic impact of distant metastasis in
patients with stage IV lung adenocarcinoma harboring EGFR
mutations: Importance of bone metastasis. Clin Exp Metastasis.
31:543–551. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Barth A, Wanek LA and Morton DL:
Prognostic factors in 1,521 melanoma patients with distant
metastases. J Am Coll Surg. 181:193–201. 1995.PubMed/NCBI
|
|
38
|
Ruatta F, Derosa L, Escudier B, Colomba E,
Guida A, Baciarello G, Loriot Y, Fizazi K and Albiges L: Prognosis
of renal cell carcinoma with bone metastases: Experience from a
large cancer centre. Eur J Cancer. 107:79–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lee EQ: Nervous system metastases from
systemic cancer. Continuum (Minneap Minn). 21:415–428.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Aboudaram A, Modesto A, Chaltiel L,
Gomez-Roca C, Boulinguez S, Sibaud V, Delord JP, Chira C, Delannes
M, Moyal E and Meyer N: Concurrent radiotherapy for patients with
metastatic melanoma and receiving anti-programmed-death 1 therapy:
A safe and effective combination. Melanoma Res. 27:485–491.
2017.PubMed/NCBI View Article : Google Scholar
|